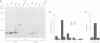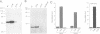Abstract
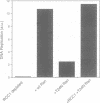
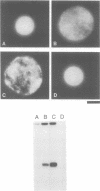
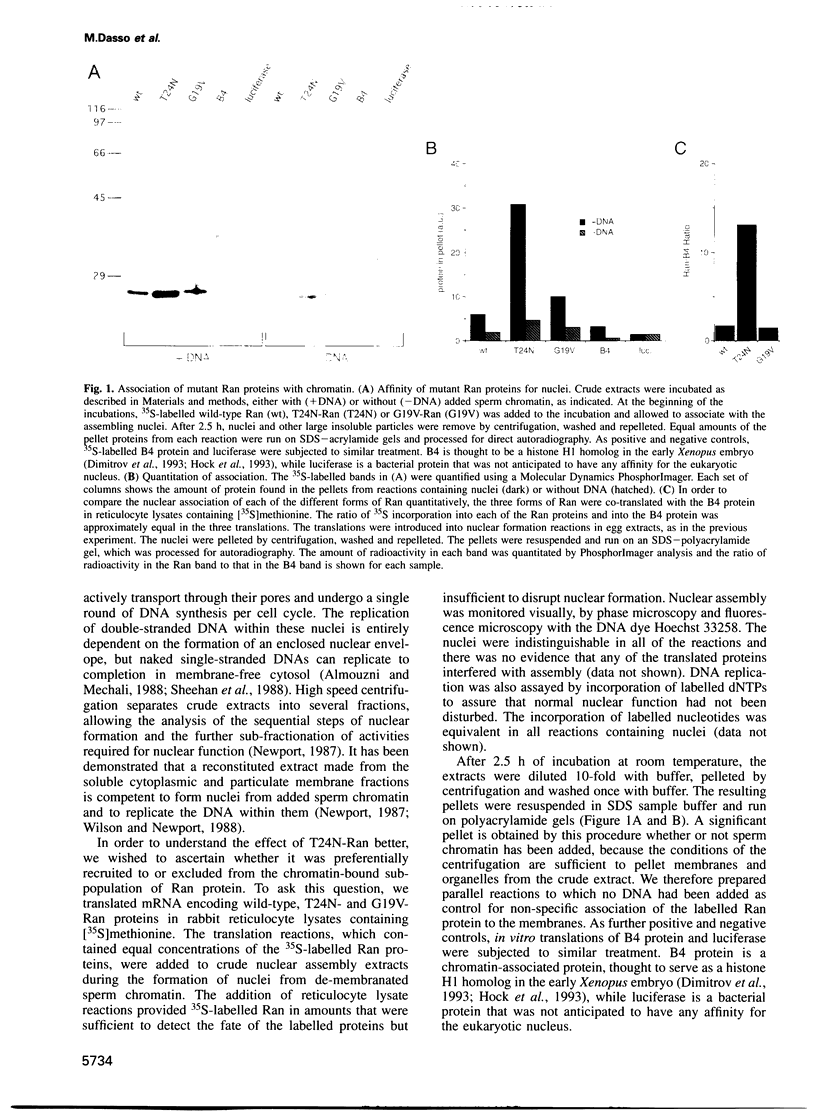
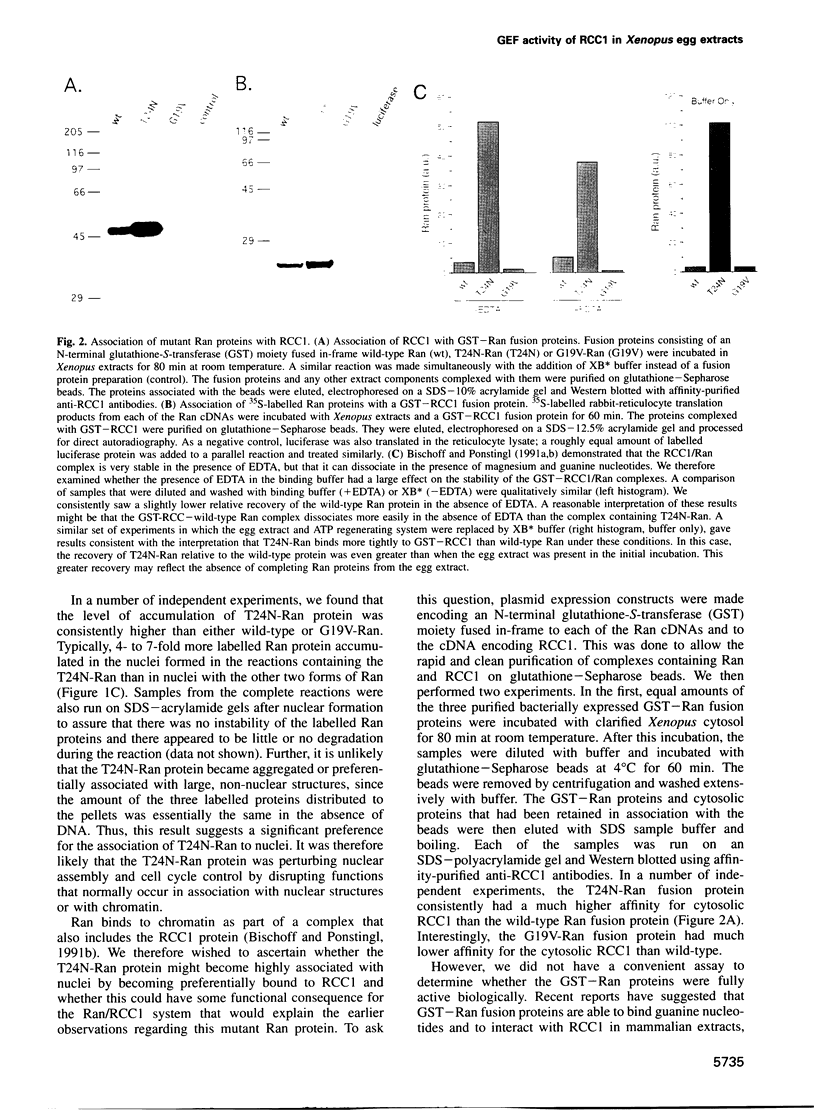
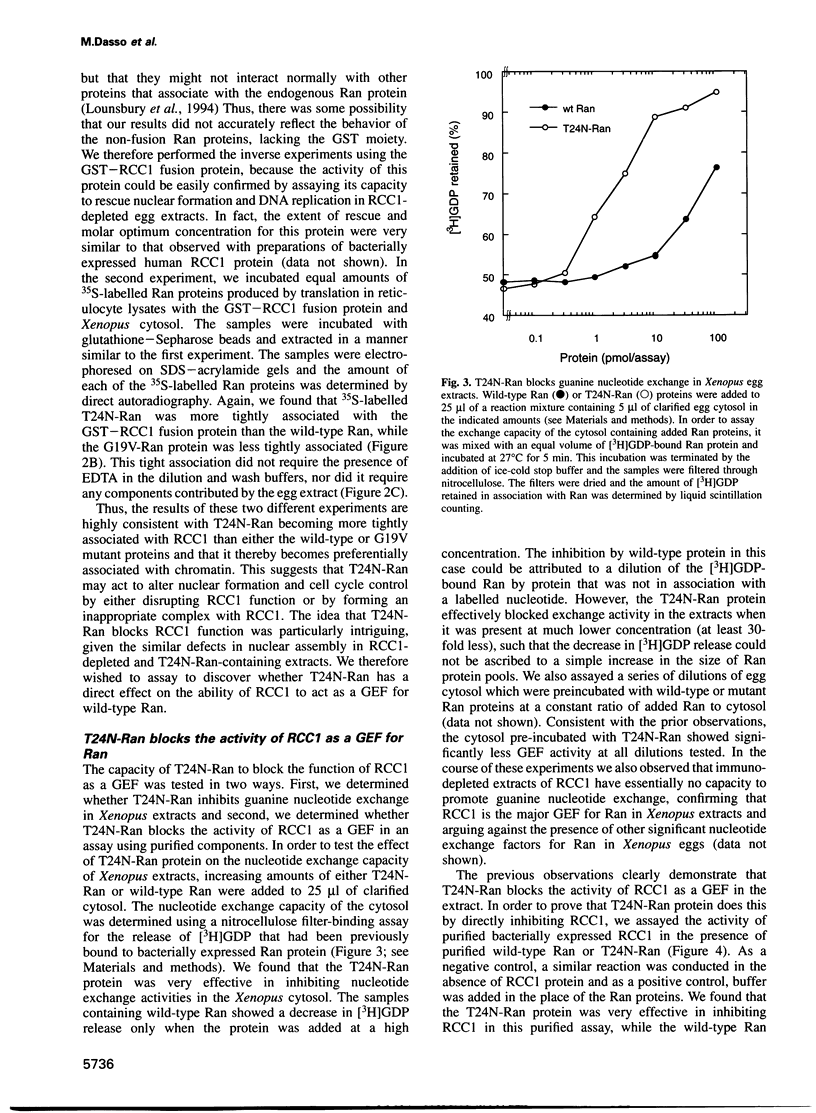
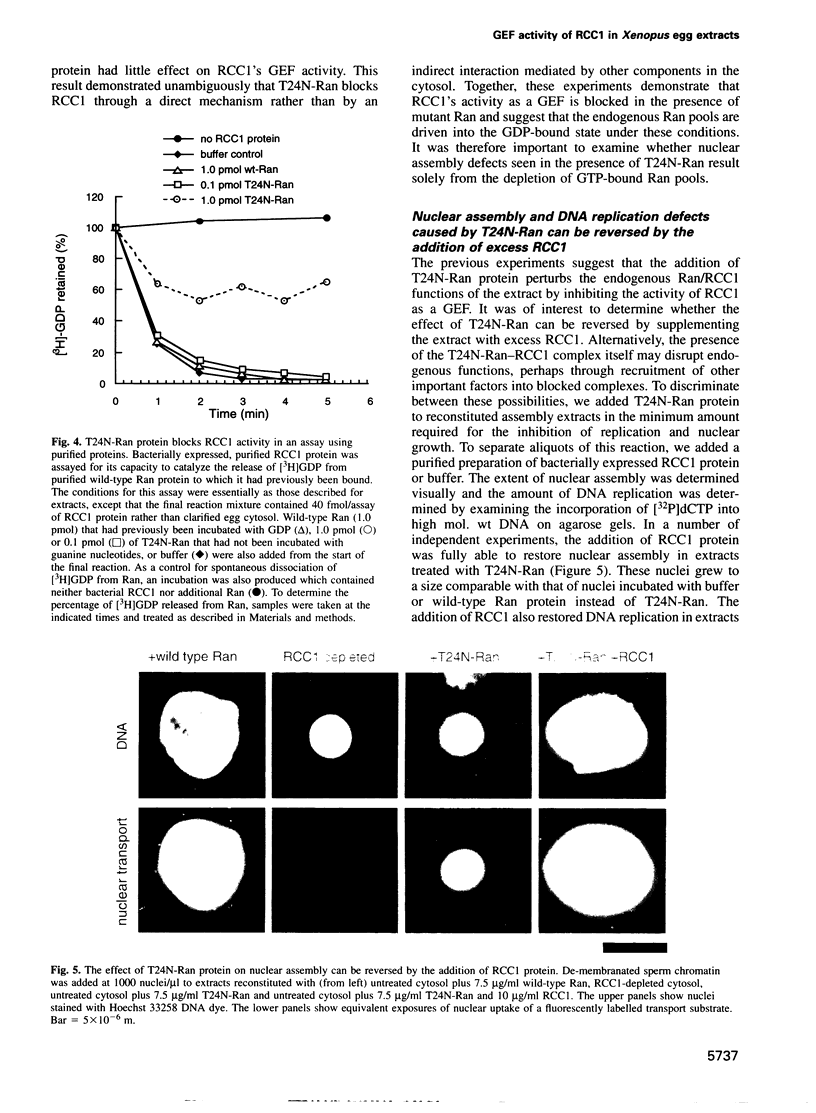
The Ran protein is a small GTPase that has been implicated in a large number of nuclear processes including transport. RNA processing and cell cycle checkpoint control. A similar spectrum of nuclear activities has been shown to require RCC1, the guanine nucleotide exchange factor (GEF) for Ran. We have used the Xenopus laevis egg extract system and in vitro assays of purified proteins to examine how Ran or RCC1 could be involved in these numerous processes. In these studies, we employed mutant Ran proteins to perturb nuclear assembly and function. The addition of a bacterially expressed mutant form of Ran (T24N-Ran), which was predicted to be primarily in the GDP-bound state, profoundly disrupted nuclear assembly and DNA replication in extracts. We further examined the molecular mechanism by which T24N-Ran disrupts normal nuclear activity and found that T24N-Ran binds tightly to the RCC1 protein within the extract, resulting in its inactivation as a GEF. The capacity of T24N-Ran-blocked interphase extracts to assemble nuclei from de-membranated sperm chromatin and to replicate their DNA could be restored by supplementing the extract with excess RCC1 and thereby providing excess GEF activity. Conversely, nuclear assembly and DNA replication were both rescued in extracts lacking RCC1 by the addition of high levels of wild-type GTP-bound Ran protein, indicating that RCC1 does not have an essential function beyond its role as a GEF in interphase Xenopus extracts.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi M., Clark M. W., Vijayraghavan U., Abelson J. A yeast mutant, PRP20, altered in mRNA metabolism and maintenance of the nuclear structure, is defective in a gene homologous to the human gene RCC1 which is involved in the control of chromosome condensation. Mol Gen Genet. 1990 Oct;224(1):72–80. doi: 10.1007/BF00259453. [DOI] [PubMed] [Google Scholar]
- Almouzni G., Méchali M. Assembly of spaced chromatin promoted by DNA synthesis in extracts from Xenopus eggs. EMBO J. 1988 Mar;7(3):665–672. doi: 10.1002/j.1460-2075.1988.tb02861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhumeur P., Lee A., Tam R., DiPaolo T., Fortin N., Clark M. W. GSP1 and GSP2, genetic suppressors of the prp20-1 mutant in Saccharomyces cerevisiae: GTP-binding proteins involved in the maintenance of nuclear organization. Mol Cell Biol. 1993 Apr;13(4):2152–2161. doi: 10.1128/mcb.13.4.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff F. R., Ponstingl H. Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature. 1991 Nov 7;354(6348):80–82. doi: 10.1038/354080a0. [DOI] [PubMed] [Google Scholar]
- Blow J. J., Laskey R. A. Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell. 1986 Nov 21;47(4):577–587. doi: 10.1016/0092-8674(86)90622-7. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Burstein E. S., Brondyk W. H., Macara I. G. Amino acid residues in the Ras-like GTPase Rab3A that specify sensitivity to factors that regulate the GTP/GDP cycling of Rab3A. J Biol Chem. 1992 Nov 15;267(32):22715–22718. [PubMed] [Google Scholar]
- Clark K. L., Sprague G. F., Jr Yeast pheromone response pathway: characterization of a suppressor that restores mating to receptorless mutants. Mol Cell Biol. 1989 Jun;9(6):2682–2694. doi: 10.1128/mcb.9.6.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasso M., Newport J. W. Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: studies in Xenopus. Cell. 1990 Jun 1;61(5):811–823. doi: 10.1016/0092-8674(90)90191-g. [DOI] [PubMed] [Google Scholar]
- Dasso M., Nishitani H., Kornbluth S., Nishimoto T., Newport J. W. RCC1, a regulator of mitosis, is essential for DNA replication. Mol Cell Biol. 1992 Aug;12(8):3337–3345. doi: 10.1128/mcb.12.8.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasso M. RCC1 in the cell cycle: the regulator of chromosome condensation takes on new roles. Trends Biochem Sci. 1993 Mar;18(3):96–101. doi: 10.1016/0968-0004(93)90161-f. [DOI] [PubMed] [Google Scholar]
- Dimitrov S., Almouzni G., Dasso M., Wolffe A. P. Chromatin transitions during early Xenopus embryogenesis: changes in histone H4 acetylation and in linker histone type. Dev Biol. 1993 Nov;160(1):214–227. doi: 10.1006/dbio.1993.1299. [DOI] [PubMed] [Google Scholar]
- Drivas G. T., Shih A., Coutavas E., Rush M. G., D'Eustachio P. Characterization of four novel ras-like genes expressed in a human teratocarcinoma cell line. Mol Cell Biol. 1990 Apr;10(4):1793–1798. doi: 10.1128/mcb.10.4.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig L. A., Cooper G. M. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol. 1988 Aug;8(8):3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W., Stutz F., Rosbash M., Wickens M. Defects in mRNA 3'-end formation, transcription initiation, and mRNA transport associated with the yeast mutation prp20: possible coupling of mRNA processing and chromatin structure. Genes Dev. 1992 Oct;6(10):1914–1926. doi: 10.1101/gad.6.10.1914. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991 Feb 1;192(2):262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Hock R., Moorman A., Fischer D., Scheer U. Absence of somatic histone H1 in oocytes and preblastula embryos of Xenopus laevis. Dev Biol. 1993 Aug;158(2):510–522. doi: 10.1006/dbio.1993.1209. [DOI] [PubMed] [Google Scholar]
- Kadowaki T., Goldfarb D., Spitz L. M., Tartakoff A. M., Ohno M. Regulation of RNA processing and transport by a nuclear guanine nucleotide release protein and members of the Ras superfamily. EMBO J. 1993 Jul;12(7):2929–2937. doi: 10.1002/j.1460-2075.1993.tb05955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth S., Dasso M., Newport J. Evidence for a dual role for TC4 protein in regulating nuclear structure and cell cycle progression. J Cell Biol. 1994 May;125(4):705–719. doi: 10.1083/jcb.125.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Tam R., Belhumeur P., DiPaolo T., Clark M. W. Prp20, the Saccharomyces cerevisiae homolog of the regulator of chromosome condensation, RCC1, interacts with double-stranded DNA through a multi-component complex containing GTP-binding proteins. J Cell Sci. 1993 Sep;106(Pt 1):287–298. doi: 10.1242/jcs.106.1.287. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Masui Y. Roles of cytosol and cytoplasmic particles in nuclear envelope assembly and sperm pronuclear formation in cell-free preparations from amphibian eggs. J Cell Biol. 1984 Apr;98(4):1222–1230. doi: 10.1083/jcb.98.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounsbury K. M., Beddow A. L., Macara I. G. A family of proteins that stabilize the Ran/TC4 GTPase in its GTP-bound conformation. J Biol Chem. 1994 Apr 15;269(15):11285–11290. [PubMed] [Google Scholar]
- Matsumoto T., Beach D. Premature initiation of mitosis in yeast lacking RCC1 or an interacting GTPase. Cell. 1991 Jul 26;66(2):347–360. doi: 10.1016/0092-8674(91)90624-8. [DOI] [PubMed] [Google Scholar]
- Melchior F., Paschal B., Evans J., Gerace L. Inhibition of nuclear protein import by nonhydrolyzable analogues of GTP and identification of the small GTPase Ran/TC4 as an essential transport factor. J Cell Biol. 1993 Dec;123(6 Pt 2):1649–1659. doi: 10.1083/jcb.123.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. S., Blobel G. A G protein involved in nucleocytoplasmic transport: the role of Ran. Trends Biochem Sci. 1994 May;19(5):211–216. doi: 10.1016/0968-0004(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Moore M. S., Blobel G. The GTP-binding protein Ran/TC4 is required for protein import into the nucleus. Nature. 1993 Oct 14;365(6447):661–663. doi: 10.1038/365661a0. [DOI] [PubMed] [Google Scholar]
- Newmeyer D. D., Forbes D. J. Nuclear import can be separated into distinct steps in vitro: nuclear pore binding and translocation. Cell. 1988 Mar 11;52(5):641–653. doi: 10.1016/0092-8674(88)90402-3. [DOI] [PubMed] [Google Scholar]
- Newport J. Nuclear reconstitution in vitro: stages of assembly around protein-free DNA. Cell. 1987 Jan 30;48(2):205–217. doi: 10.1016/0092-8674(87)90424-7. [DOI] [PubMed] [Google Scholar]
- Nishimoto T., Eilen E., Basilico C. Premature of chromosome condensation in a ts DNA- mutant of BHK cells. Cell. 1978 Oct;15(2):475–483. doi: 10.1016/0092-8674(78)90017-x. [DOI] [PubMed] [Google Scholar]
- Nishimoto T., Ishida R., Ajiro K., Yamamoto S., Takahashi T. The synthesis of protein(s) for chromosome condensation may be regulated by a post-transcriptional mechanism. J Cell Physiol. 1981 Nov;109(2):299–308. doi: 10.1002/jcp.1041090213. [DOI] [PubMed] [Google Scholar]
- Nishitani H., Kobayashi H., Ohtsubo M., Nishimoto T. Cloning of Xenopus RCC1 cDNA, a homolog of the human RCC1 gene: complementation of tsBN2 mutation and identification of the product. J Biochem. 1990 Feb;107(2):228–235. doi: 10.1093/oxfordjournals.jbchem.a123031. [DOI] [PubMed] [Google Scholar]
- Nishitani H., Ohtsubo M., Yamashita K., Iida H., Pines J., Yasudo H., Shibata Y., Hunter T., Nishimoto T. Loss of RCC1, a nuclear DNA-binding protein, uncouples the completion of DNA replication from the activation of cdc2 protein kinase and mitosis. EMBO J. 1991 Jun;10(6):1555–1564. doi: 10.1002/j.1460-2075.1991.tb07675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M., Coutavas E., D'Eustachio P., Rush M. G. Effects of mutant Ran/TC4 proteins on cell cycle progression. Mol Cell Biol. 1994 Jun;14(6):4216–4224. doi: 10.1128/mcb.14.6.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M., Drivas G., D'Eustachio P., Rush M. G. Ran/TC4: a small nuclear GTP-binding protein that regulates DNA synthesis. J Cell Biol. 1993 Jan;120(2):313–323. doi: 10.1083/jcb.120.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazer S., Nurse P. A fission yeast RCC1-related protein is required for the mitosis to interphase transition. EMBO J. 1994 Feb 1;13(3):606–615. doi: 10.1002/j.1460-2075.1994.tb06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Croy R. G., Pardee A. B. Exposure to caffeine and suppression of DNA replication combine to stabilize the proteins and RNA required for premature mitotic events. J Cell Physiol. 1987 Apr;131(1):85–91. doi: 10.1002/jcp.1041310113. [DOI] [PubMed] [Google Scholar]
- Schweighoffer F., Cai H., Chevallier-Multon M. C., Fath I., Cooper G., Tocque B. The Saccharomyces cerevisiae SDC25 C-domain gene product overcomes the dominant inhibitory activity of Ha-Ras Asn-17. Mol Cell Biol. 1993 Jan;13(1):39–43. doi: 10.1128/mcb.13.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan M. A., Mills A. D., Sleeman A. M., Laskey R. A., Blow J. J. Steps in the assembly of replication-competent nuclei in a cell-free system from Xenopus eggs. J Cell Biol. 1988 Jan;106(1):1–12. doi: 10.1083/jcb.106.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe C., Newport J. W. Systems for the study of nuclear assembly, DNA replication, and nuclear breakdown in Xenopus laevis egg extracts. Methods Cell Biol. 1991;35:449–468. doi: 10.1016/s0091-679x(08)60583-x. [DOI] [PubMed] [Google Scholar]
- Stick R., Hausen P. Changes in the nuclear lamina composition during early development of Xenopus laevis. Cell. 1985 May;41(1):191–200. doi: 10.1016/0092-8674(85)90073-x. [DOI] [PubMed] [Google Scholar]
- Tucker J., Sczakiel G., Feuerstein J., John J., Goody R. S., Wittinghofer A. Expression of p21 proteins in Escherichia coli and stereochemistry of the nucleotide-binding site. EMBO J. 1986 Jun;5(6):1351–1358. doi: 10.1002/j.1460-2075.1986.tb04366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Sekiguchi T., Nishitani H., Miyauchi K., Ohtsubo M., Nishimoto T. Premature chromosome condensation is induced by a point mutation in the hamster RCC1 gene. Mol Cell Biol. 1990 Feb;10(2):577–584. doi: 10.1128/mcb.10.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. L., Newport J. A trypsin-sensitive receptor on membrane vesicles is required for nuclear envelope formation in vitro. J Cell Biol. 1988 Jul;107(1):57–68. doi: 10.1083/jcb.107.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]