Abstract
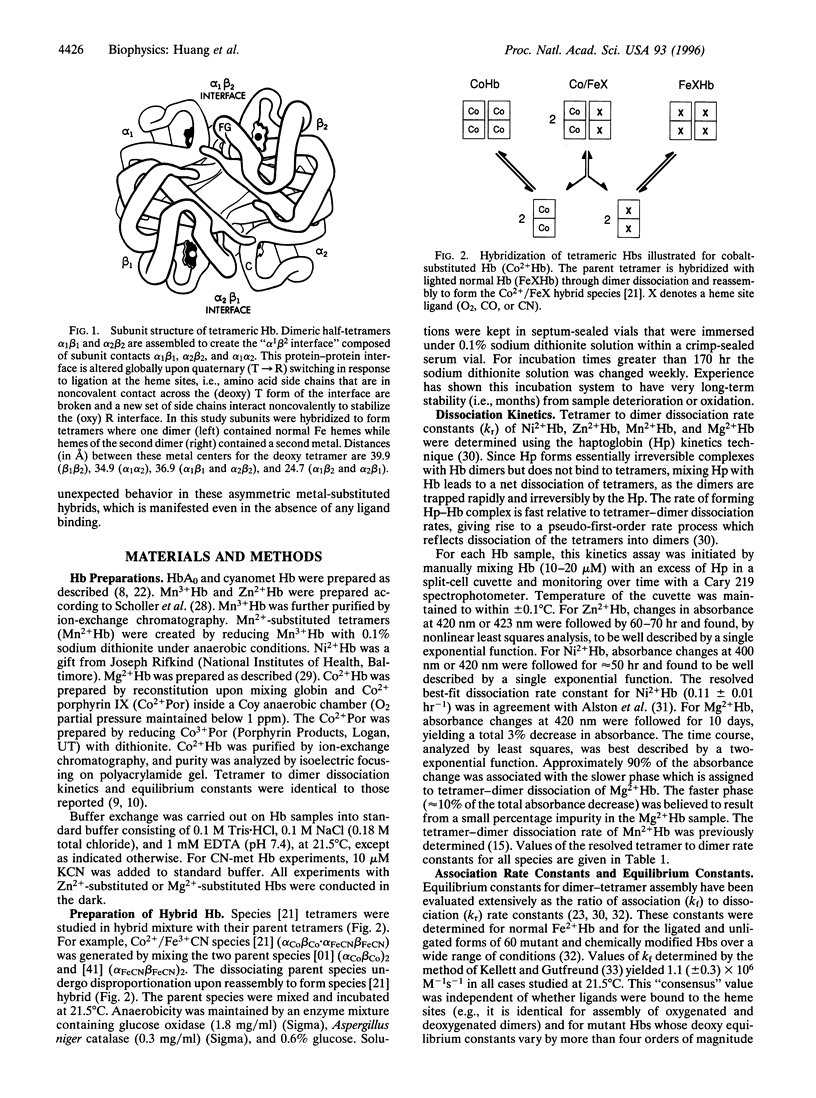
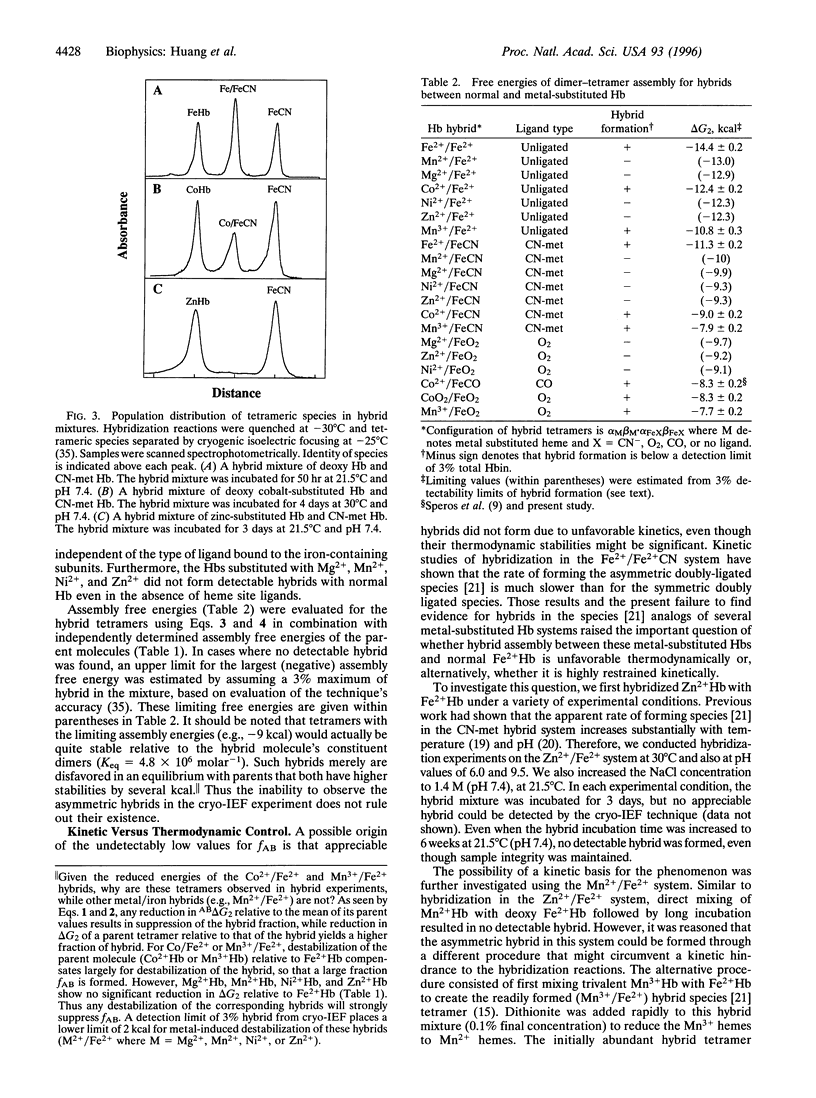
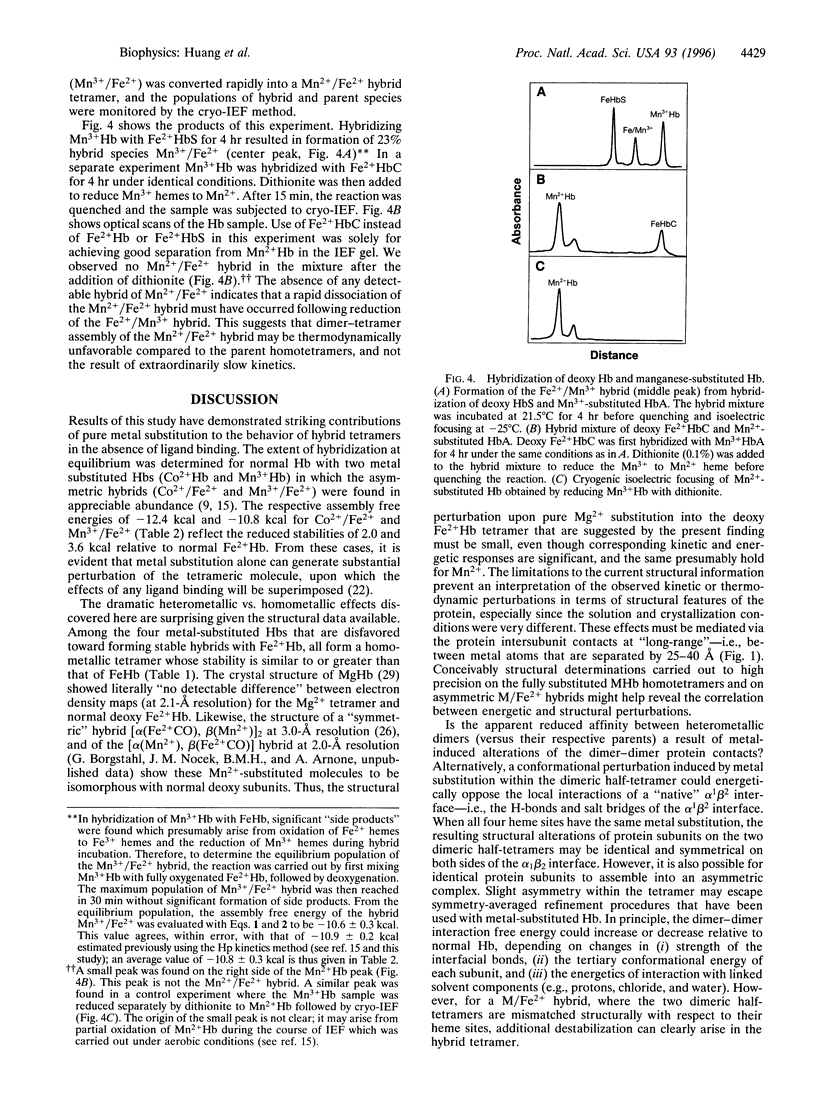
Hybridization experiments between normal Hb tetramers (Fe2+ Hb) and those with four metal-substituted hemes (i.e., replacement of Fe2+ by Co2+, Mg2+, Mn2+, Mn3+, Ni2+, or Zn2+) have revealed unexpected behavior. These homometallic Hbs have previously served as models that mimic the deoxy or oxy properties of normal Fe2+ Hb. In this study, hybrids were composed of one alpha 1 beta 1 dimer that is metal-substituted at both hemes, in association with a second dimer alpha 2 beta 2 that has normal Fe2+ hemes. Both metal-substituted subunits are unligated, whereas the two Fe2+ subunits either are both unligated or both ligated with O2, CO, or CN. It was found that four of the metal-substituted Hbs (Mg2+ Hb, Mn2+ Hb, Ni2+ Hb, and Zn2+ Hb) did not form detectable amounts of heterometallic hybrids with normal Fe2+ Hb even though (i) their homometallic parents formed tight tetrameric complexes with stabilities similar to that of Fe2+ Hb and (ii) hybrids with metal substitution at both alpha sites or both beta sites are known to form readily. This striking positional effect was independent of whether the normal Fe2+ hemes were ligated and of which ligand was used. These findings indicate that surprisingly large changes in tetramer behavior can arise from small and subtle perturbations at the heme sites. Possible origins of these effects are considered.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers G. K., Doyle M. L., Myers D., Daugherty M. A. Molecular code for cooperativity in hemoglobin. Science. 1992 Jan 3;255(5040):54–63. doi: 10.1126/science.1553532. [DOI] [PubMed] [Google Scholar]
- Alston K., Schechter A. N., Arcoleo J. P., Greer J., Parr G. R., Friedman F. K. Preparation and properties of nickel hemoglobin. Hemoglobin. 1984;8(1):47–60. doi: 10.3109/03630268408996960. [DOI] [PubMed] [Google Scholar]
- Arnone A., Rogers P., Blough N. V., McGourty J. L., Hoffman B. M. X-ray diffraction studies of a partially liganded hemoglobin, [alpha(FeII-CO)beta(MnII)]2. J Mol Biol. 1986 Apr 20;188(4):693–706. doi: 10.1016/s0022-2836(86)80015-8. [DOI] [PubMed] [Google Scholar]
- Blough N. V., Hoffman B. M. Carbon monoxide binding to the ferrous chains of [Mn,Fe(II)] hybrid hemoglobins: pH dependence of the chain affinity constants associated with specific hemoglobin ligation pathways. Biochemistry. 1984 Jun 19;23(13):2875–2882. doi: 10.1021/bi00308a005. [DOI] [PubMed] [Google Scholar]
- Daugherty M. A., Shea M. A., Ackers G. K. Bohr effects of the partially-ligated (CN-met) intermediates of hemoglobin as probed by quaternary assembly. Biochemistry. 1994 Aug 30;33(34):10345–10357. doi: 10.1021/bi00200a015. [DOI] [PubMed] [Google Scholar]
- Doyle M. L., Speros P. C., LiCata V. J., Gingrich D., Hoffman B. M., Ackers G. K. Linkage between cooperative oxygenation and subunit assembly of cobaltous human hemoglobin. Biochemistry. 1991 Jul 23;30(29):7263–7271. doi: 10.1021/bi00243a031. [DOI] [PubMed] [Google Scholar]
- Hoffman B. M., Gibson Q. H., Bull C., Crepeau R. H., Edelstein S. J., Fisher R. G., McDonald M. J. Manganese-substituted hemoglobin and myoglobin. Ann N Y Acad Sci. 1975 Apr 15;244:174–186. doi: 10.1111/j.1749-6632.1975.tb41530.x. [DOI] [PubMed] [Google Scholar]
- Hoffman B. M., Petering D. H. Coboglobins: oxygen-carrying cobalt-reconstituted hemoglobin and myoglobin. Proc Natl Acad Sci U S A. 1970 Oct;67(2):637–643. doi: 10.1073/pnas.67.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Ackers G. K. Transformation of cooperative free energies between ligation systems of hemoglobin: resolution of the carbon monoxide binding intermediates. Biochemistry. 1996 Jan 23;35(3):704–718. doi: 10.1021/bi952400i. [DOI] [PubMed] [Google Scholar]
- Imai K., Ikeda-Saito M., Yamamoto H., Yonetani T. Studies on cobalt myoglobins and hemoglobins X. Determination of microscopic oxygen-equilibrium constants of iron--cobalt hybrid hemoglobins and their parent hemoglobins. J Mol Biol. 1980 Apr 15;138(3):635–648. doi: 10.1016/s0022-2836(80)80021-0. [DOI] [PubMed] [Google Scholar]
- Johnson M. L., Halvorson H. R., Ackers G. K. Oxygenation-linked subunit interactions in human hemoglobin: analysis of linkage functions for constituent energy terms. Biochemistry. 1976 Nov 30;15(24):5363–5371. doi: 10.1021/bi00669a024. [DOI] [PubMed] [Google Scholar]
- Kellett G. L., Gutfreund H. Reactions of haemoglobin dimers after ligand dissociation. Nature. 1970 Aug 29;227(5261):921–926. doi: 10.1038/227921a0. [DOI] [PubMed] [Google Scholar]
- LiCata V. J., Dalessio P. M., Ackers G. K. Single-site modifications of half-ligated hemoglobin reveal autonomous dimer cooperativity within a quaternary T tetramer. Proteins. 1993 Nov;17(3):279–296. doi: 10.1002/prot.340170306. [DOI] [PubMed] [Google Scholar]
- LiCata V. J., Speros P. C., Rovida E., Ackers G. K. Direct and indirect pathways of functional coupling in human hemoglobin are revealed by quantitative low-temperature isoelectric focusing of mutant hybrids. Biochemistry. 1990 Oct 23;29(42):9771–9783. doi: 10.1021/bi00494a003. [DOI] [PubMed] [Google Scholar]
- Miura S., Ikeda-Saito M., Yonetani T., Ho C. Oxygen equilibrium studies of cross-linked asymmetrical cyanomet valency hybrid hemoglobins: models for partially oxygenated species. Biochemistry. 1987 Apr 21;26(8):2149–2155. doi: 10.1021/bi00382a013. [DOI] [PubMed] [Google Scholar]
- Moffat K., Loe R. S., Hoffman B. M. The structure of metmanganoglobin. J Mol Biol. 1976 Jul 5;104(3):669–685. doi: 10.1016/0022-2836(76)90128-5. [DOI] [PubMed] [Google Scholar]
- Perrella M., Benazzi L., Shea M. A., Ackers G. K. Subunit hybridization studies of partially ligated cyanomethemoglobins using a cryogenic method. Evidence for three allosteric states. Biophys Chem. 1990 Jan;35(1):97–103. doi: 10.1016/0301-4622(90)80064-e. [DOI] [PubMed] [Google Scholar]
- Perrella M., Rossi-Bernardi L. Detection of hemoglobin hybrid formation at subzero temperature. Methods Enzymol. 1981;76:133–143. doi: 10.1016/0076-6879(81)76122-6. [DOI] [PubMed] [Google Scholar]
- Scholler D. M., Wang M. Y., Hoffman B. M. Metal-substituted hemoglobin and other hemoproteins. Methods Enzymol. 1978;52:487–493. doi: 10.1016/s0076-6879(78)52053-3. [DOI] [PubMed] [Google Scholar]
- Shibayama N., Imai K., Morimoto H., Saigo S. Oxygen equilibrium properties of asymmetric nickel(II)-iron(II) hybrid hemoglobin. Biochemistry. 1993 Aug 31;32(34):8792–8798. doi: 10.1021/bi00085a009. [DOI] [PubMed] [Google Scholar]
- Smith F. R., Ackers G. K. Experimental resolution of cooperative free energies for the ten ligation states of human hemoglobin. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5347–5351. doi: 10.1073/pnas.82.16.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith F. R., Gingrich D., Hoffman B. M., Ackers G. K. Three-state combinatorial switching in hemoglobin tetramers: comparison between functional energetics and molecular structures. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7089–7093. doi: 10.1073/pnas.84.20.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speros P. C., LiCata V. J., Yonetani T., Ackers G. K. Experimental resolution of cooperative free energies for the ten ligation species of cobalt(II)/iron(II)-CO hemoglobin. Biochemistry. 1991 Jul 23;30(29):7254–7262. doi: 10.1021/bi00243a030. [DOI] [PubMed] [Google Scholar]
- Tsuneshige A., Zhou Y. X., Yonetani T. Oxygen equilibrium studies of cross-linked iron-cobalt hybrid hemoglobins. Models for partially ligated intermediates of cobalt hemoglobin. J Biol Chem. 1993 Nov 5;268(31):23031–23040. [PubMed] [Google Scholar]
- Turner G. J., Galacteros F., Doyle M. L., Hedlund B., Pettigrew D. W., Turner B. W., Smith F. R., Moo-Penn W., Rucknagel D. L., Ackers G. K. Mutagenic dissection of hemoglobin cooperativity: effects of amino acid alteration on subunit assembly of oxy and deoxy tetramers. Proteins. 1992 Nov;14(3):333–350. doi: 10.1002/prot.340140303. [DOI] [PubMed] [Google Scholar]
- Yonetani T., Yamamoto H., Woodrow G. V., 3rd Studies on cobalt myoglobins and hemoglobins. I. Preparation and optical properties of myoglobins and hemoglobins containing cobalt proto-, meso-, and deuteroporphyrins and thermodynamic characterization of their reversible oxygenation. J Biol Chem. 1974 Feb 10;249(3):682–690. [PubMed] [Google Scholar]



