Abstract
Vascularization of tissue-engineered constructs, requiring the transport of oxygen, nutrients and waste through a thick and cellular dense meshwork, continues to hamper the success of the technology in addressing the donor organ shortage crisis. Microfluidic technology has emerged as a viable alternative to traditional in vitro platforms utilized by tissue engineers, to understand how the complex cellular microenvironment directs vascular cell behavior and functionality. In this review, the essence of microfluidic technology and transport phenomenon that make them unique for vascular tissue engineering will be briefly introduced. The main scope of this review is to expose how new and innovative microfluidic fabrication techniques are being utilized for exciting applications that have allowed insight into the spatio/temporal dynamics of vascular cell behavior. Specifically, microfluidic devices which range in functionality from simultaneously controlling oxygen and shear stress levels to perfusable biopolymer networks, will be discussed in the context of how they bolster traditional in vitro platforms, by providing greater data output, accessibility, and physiological relevance.
Introduction
Richard Feynman’s There’s Plenty of Room at the Bottom is the esteemed 1959 lecture viewed by many as the inspirational seed giving rise to the field of micro and nanotechnology, whose ramifications are evident in a myriad of disciplines ranging from microelectromechanical (MEMs) systems utilized in the semiconductor industry to the advent of lab-on-a-chip technologies for tissue engineering applications. Although high-resolution features of sensors and transistors for electronic devices were being readily produced on the micron scale by chemical etching and lithography techniques, it was not until the 1979 invention of a miniaturized gas chromatographic analyzer, where the abilities to manipulate small amounts of fluids were fully realized [1]. This miniaturized functional device, recognized as the first microfluidic system, not only demonstrated portability, minimal reagent use and sensitivity to detect small volumes of analyte in a high-throughput fashion, but also showed the promise of precisely controlling fluids. Microfluidic devices with cross-sectional geometries on the order of 10–100 μm [2] are unique in that fluids exhibit laminar flow profiles described by low Reynolds numbers, the ratio of inertial to viscous forces.
Through the advance in microfabrication techniques [3], as depicted in Figure 1, microfluidic technology has emerged as a novel platform in tissue engineering, an interdisciplinary field aimed to augment or replace diseased tissues or organs, to develop in vitro models that mimic physiological conditions and to accelerate drug development [4]. Though successful clinical application of tissue engineered skin, bone, cartilage, urethra, and bladder show promise in the field [5], the challenge to engineer tissue constructs requiring extensive vasculature remains [6]. Oxygen, nutrient and waste exchange are essential to organ viability, and nearly all tissues reside within 100–200 μm from capillaries [7], blood vessels only 5–10 μm in diameter that facilitate this transfer. Engineered tissue exceeding this diffusional exchange limit fails due to ischemia [8], leading to an ever increasing need for vascular tissue engineering research for developing pre-vascularized constructs, uncovering the chemical and mechanical cues that govern the de novo (vasculogenesis), or sprouting/branching (angiogenesis) formation of blood vessels, and understanding how these cues mechanistically regulate healthy and diseased vascular physiology.
Figure 1.
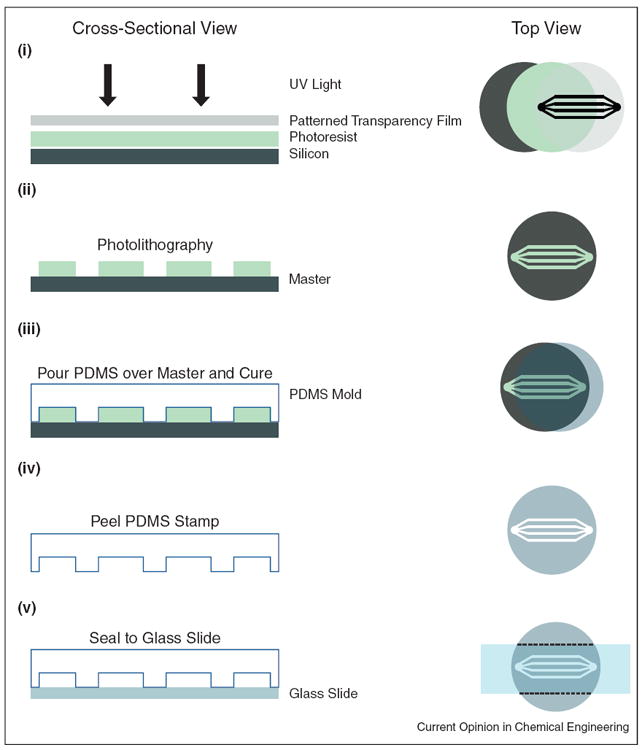
Microfluidic device fabrication by soft lithography. Left panel shows cross-sectional view while the right panel displays a top down perspective of the fabrication process. (i) Microfluidic channel geometries can be designed on a computer program and printed to a high-resolution transparency film. (ii) After applying photoresist to a silicon wafer, the drawn geometry can be transferred as a ‘master’ mold via photolithography. (iii) Polydimethylsiloxane (PDMS), a soft elastomeric material, is poured over the master mold, cured and (iv) peeled off, retaining the geometric features. (iv) Finally the new PDMS mold can be sealed to a glass slide or coverslip, leaving open channels.
Adapted from [46].
Scope of review
In this review, we discuss how vascular tissue engineering research involving microfluidic systems circumvents the downfalls of traditional in vitro platforms, providing the unprecedented capacity to address the aforementioned limitations to engineered tissue. Specifically, we demarcate studies that use exciting new fabrication techniques to model the vascular niche in two-dimensions and three-dimensions (2D; 3D), and emphasize the versatility of microfluidic tools for studying: (a) endothelial cell responses to mechanical stress and oxygen tensions, (b) angiogenic branching, sprouting and vessel network formation as well as (c) future outlooks for microfluidic investigation of vasculogenesis from embryonic and progenitor cells.
Utilizing microfluidic technology in vascular tissue engineering
Microfluidics and mechanics
The robustness of microfluidic systems over traditional in vitro platforms can be summarized through three key metrics namely physiological relevance, accessibility and data output [9]. These measures are specifically exemplified when investigating the individual or synergistic effects of mechanical cues such as confinement, surface topography, and shear stress on vascular cell behavior. Although perfusable arterial blood vessels, veins, and capillaries differ in location, role, size and extracellular composition, they all consist of a luminal monolayer of mechanosensing endothelial cells (ECs) that act as an interface between blood and underlying tissue. Physical consequences of pulsatile blood flow include a cyclic myogenic scheme, in which supporting stromal pericytes and smooth muscles cells (SMCs) contract and dilate, generating cyclic pressures and stretch which ECs experience, to maintain uniform luminal hemodynamic shear stresses (Figure 2). While traditional in vitro platforms are used to investigate mechanical forces experienced by vascular cells, they are limited in capturing the range of these stressors in a high-throughput manner. For example, depending on the location and homeostatic state, ECs can experience anywhere between 10 and 200 dyn/cm2 of shear stress [10]. Microfluidic systems are compact platforms with the capacity to capture the breadth of physiologically relevant microenvironments, requiring minimal reagents (10−6 to 10−18 l), cells and experimental time [11]. The geometry of microfluidic systems also allows for the spatial control of chemical signals via gradients, and molecular transport at interfaces through diffusion, serving as a powerful tool to create a mosaic of chemical microenvironments to study cell behavior [12].
Figure 2.
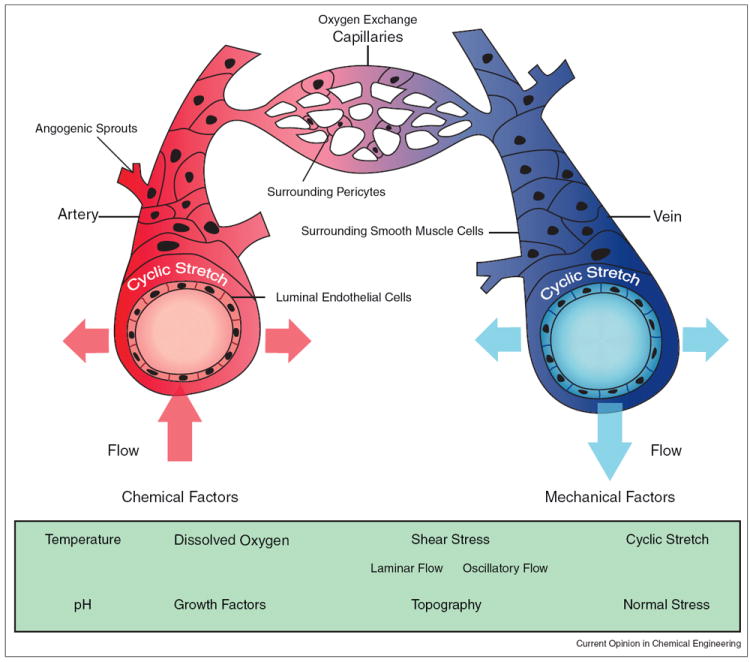
Blood vessel schematic with mechanical and chemical environmental factors. Arterial vessels (shown in red) carry oxygenated blood from the heart, narrow into capillary vessels that exchange oxygen and other nutrients to surrounding tissues, which eventually broaden into veins (shown in blue), which primarily carry deoxygenated blood toward the heart. Arteries, veins and capillaries are similar in that they contain in inner layer of endothelial cells. Capillaries contain stromal pericyte cells for vessel support while arteries and veins contain smooth muscle cells. Vascular cells not only experience parallel (shear), perpendicular (normal) and circumferential (cyclic) mechanical stressors resulting from blood flow, but also topographical forces due to the microenvironment. If blood flow is restricted within the vessels, the flow regime will shift from laminar to oscillatory or disturbed turbulent flow profiles. In addition to these mechanical factors, vascular cells also experience changes in chemical stimuli such as temperature, growth factors, oxygen levels, and pH.
There are a variety of techniques ranging from pneumatic and syringe pumps to electro-kinetics to control shear stress in microfluidic devices. A computer-controlled assembly, utilizing an array of piezoelectric, actuating Braille pins integrated into a PDMS microfluidic device, was used to the study the effects of a range of re-circulating pulsatile shear on ECs [13]. Although monumental at the time, displaying the ability of ECs to align and elongate in the presence of pulsatile flow, the system is limited to a maximum shear stress of 12 dynes/cm2. However the design was unique, introducing the utility of Braille display for fluid actuation in vascular studies.
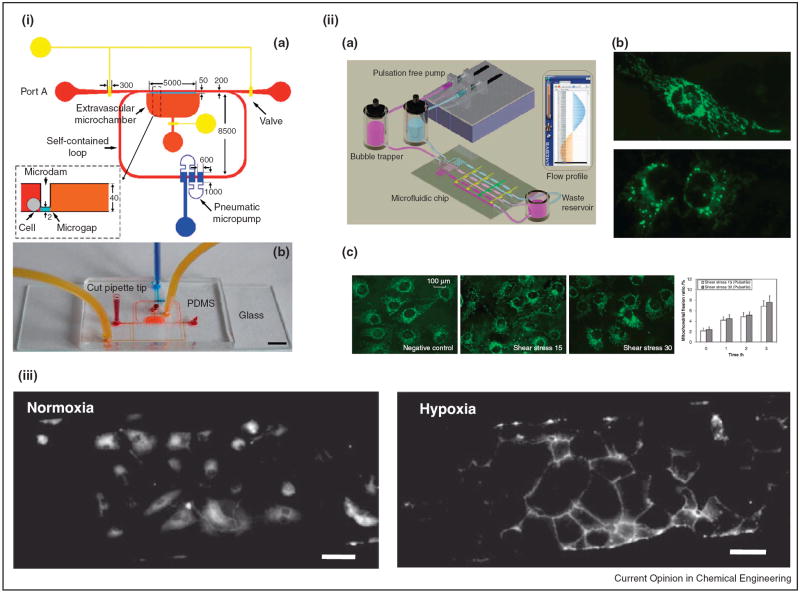
The limitation of shear stress to higher ranges was overcome by an inventive PDMS microfluidic design, consisting of a single inlet, outlet, and 10 microchannels. Unlike the previous system, only an external peristaltic pump was needed for generating shear conditions between 1 and 130 dynes/cm2, resulting in changes in EC morphology and function in non-pulsatile flow [14]. While syringe pumps have been readily used to create shear stress atop vascular cells in microfluidic devices, self-contained circulation of media through pumping platforms prevents the removal of soluble factors secreted by vascular cells that modulate cell–cell communication. To address this Shao and collogues incorporated a novel fluid actuation pneumatic micropump into a PDMS based microfluidic device, to create a self-circulating, unsteady pulsatile and oscillatory shear stress regime (Figure 3i). Although fluid flow is one directional during the pulsatile condition, the cells simultaneously experience forward (+8.15 dynes/cm2) and counter flow (−5.92 dynes/cm2) shear in the device. Interestingly ECs did not align with the direction of flow, demonstrating the sensitivity of EC behavior to steady laminar flow profiles [15]. While these aforementioned microfluidic devices recapitulate either steady or pulsatile flow, other systems are able to mimic both of these regimes in a single platform. Through a multi-tiered PDMS assembly, separate functioning layers can be built into a single microfluidic device. A slab containing pneumatic valves sandwiched in between a bottom microfluidic network for EC culture and top pneumatic control piece was fabricated to study the effects of pulsatile arterial shear levels on oxidative stress response. Mitochondrial morphology and intracellular reactive oxygen species (ROS) production, two indicators of EC response during inflammation, were evaluated between non-pulsatile shear conditions (constant 30 dynes/cm2), pulsatile resting (15 dynes/cm2 shear at 70 beats per minute (bpm)), and exercise states (30 dynes/cm2 shear at 140 bpm). An increase in a diffuse and fragmented mitochondria, compared to a filamentous reticular network morphology, as well as elevated ROS levels under pulsatile conditions were observed, elucidating the importance of introducing pulsatile flow in hemodynamic models [16] (Figure 3ii).
Figure 3.
Microfluidic systems for investigating shear and oxygen tension on endothelial cell behavior. (i) Illustration (a) and top view (b) of a PDMS based microcirculatory system containing a pneumatic micropump for delivering pulsatile and oscillatory shear stress. Scale bar is 5 mm. Reproduced from [15] with permission from the Royal Society of Chemistry. (ii) (a) Schematic of a multilayer PDMS microfluidic chip for investigating endothelial cell reactive oxygen species (ROS) production under different shear conditions. (b) Top — normal mitochondria morphology; Bottom — mitochondria morphology while undergoing apoptosis. (c). With increasing shear, endothelial cell mitochondria morphology becomes fragmented, opposed to a filamentous reticular network in static conditions. Reproduced from [16] with permission from the Royal Society of Chemistry. (iii) Immunofluorescence images of CD31 expression (shown in white) of human endothelial colony forming cells cultured in normoxic and hypoxic conditions for 24 h shows increase in membrane localization in hypoxia. Scale bars are 100 μm. Reproduced from [25••] with permission from Wiley.
Multi-tiered devices have also been used to examine the synergistic effects of cyclic stretch and fluid shear on vascular cells. Vacuum driven stretch was applied to a PDMS elastic membrane initially seeded with a bottom layer of SMCs, followed by the addition of a top layer of ECs [17]. Through application of stretch and shear to this co-culture system, differences in EC attachment to SMCs were denoted, namely ECs displaying greater spreading and adhesion to the supporting stromal cells under simultaneous shear and stretch conditions in comparison to static conditions. Furthermore, an enhanced EC alignment was observed under the two mechanical stressors, suggesting ECs can decipher between individual stretches and shear forces.
Stem cells, either from embryonic, adult, or genetically reprogrammed origin, remain an essential cell source for regenerative and vascular tissue engineering applications, in that they have the unique ability to self-renew and differentiate into mature lineages. Having the mechanosensing abilities of their mature counterparts, mesenchymal [18], endothelial progenitor [19] and embryonic stem cells [20], have been shown to functionally respond to shear stress environments through upregulation of markers related to ECs. Our lab is particularly interested in creating microfluidic platforms to uncouple the synergetic effects of shear stress and oxygen availability, a well-known contributor to angiogenesis in the developing embryo, wound, and tumor microenvironment [21,22]. With a proof of concept study, we demonstrated the initial design and fabrication of a multilayer microfluidic device constructed by micro-milling an oxygen impermeable material with the functionality of maintaining dissolved oxygen tensions in both static and dynamic shear conditions [23•]. We expanded upon this system, using soft lithography for channel fabrication, and observed morphological changes to endothelial colony forming cells (ECFCs) and stem cell derived smooth muscle like cells [24], as well as mature ECs in different shear and oxygen tension conditions [25••] (Figure 3iii).
3D microfluidics
Incorporating the ECM
In addition to cyclic stretch and shear mechanical forces, vascular cells encounter a 3D extracellular matrix (ECM), a hydrated, fibrous structural meshwork of proteins and molecules that serve to biochemically and mechanically guide vascular cell behavior. Vascular tissue engineers are interested in the reproducible development of scaffolding materials from both natural and synthetic sources that mirror physiologically relevant chemistry, bio-degradability, nutrient transport, and structural properties.
Conventional in vitro and 2D microfluidic platforms do not capture the physiologically relevant 3D vascular microenvironment consisting of multi-cellular branching lumens of circular cross-section, with integrated ECM components permissible to fluid flow and remodeling. Perfusable, synthetic poly(ethylene glycol)-diacrylate polymer (PEGDA) microgels were lithographically fabricated and manually assembled into vascular-like, tubular concentric microfluidic networks containing inner EC and outer SMC layers. This novel 3D biomimetic microfluidic device was also amenable to other geometrical configurations, demonstrating the exquisite spatial control of sequentially assembly scaffolding materials [26]. Using an adapted soft lithography technique, naturally derived type 1 collagen with encapsulated stromal cells was used as an elastomeric replicating mold, subsequently pressure sealed to matching layers to form open channels, in which after seeding with ECs resulted in 3D microvascular networks [27]. This fabricated scheme permitted the investigation of thrombosis, the unfavorable attachment and aggregation of immune blood platelets, characteristic of many disease states including heart failure and some cancers. By introducing chemical agents activating ECs from a quiescent to a stimulated state, the researchers were able to visualize the spatial and temporal dynamics of platelet adhesion, with future capabilities for testing vascular drug efficacy in fluidic models. In another study, the potential for cancer drug discovery was exemplified when the role of EC barrier function and the dynamics of tumor cell metastasis via intravasation was evaluated by coupling a 3D microfluidic system to a high-resolution imaging platform [28••].
Angiogenesis and vasculogenesis models
Developmental, regeneration and pathological states of tissues rely on angiogenesis, the extension of pre-existing vasculature in response to inadequate oxygen and nutrient supply. Although traditional in vitro platforms for studying angiogenesis, which either entailed growing ECs on a coated 2D surface with instructive adhesive proteins or coaxing monolayers of ECs to invade into 3D hydrogel scaffolds encapsulated with factors, provided substantial insight into vessel assembly [29], they lack in the ability to simultaneously introduce chemical and mechanical stimuli, with high specificity in a spatiotemporal manner.
Three channel, PDMS based microfluidic assemblies containing scaffold regions have been a powerful fabrication technique used to study angiogenesis with controlled gradients of soluble factors. Pressure differences between two microfluidic channels, sandwiching a 3D collagen gel for cell seeding, created an interstitial flow environment (27–35 μm/min), that was used to study EC angiogenesis in a co-culture system with hepatocytes, liver cells that can secrete angiogenic growth factors when clustered [30]. Under interstitial flow, hepatocytes as monolayers on one side of a collagen gel began organizing into 3D constructs, and with the addition of ECs on the opposite side, formation of capillary protrusions in which ECs extended toward the liver cells occurred, mirroring early stages of liver regeneration processes. In the absence of the hepatocytes, on the other hand, ECs formed 2D sheet like structures. Traditional cell culturing techniques are unable to capture the high surface area to volume ratios like the capability of microfluidic devices, which served advantageous in this study, allowing the direct examination of the cross-talk of soluble factors excreted by multiple cell types.
In a similar design scheme, angiogenic sprouting was observed under three different chemical gradients, namely horizontal and vertical gradients of soluble vascular endothelial growth factor (VEGF), and supplemental diffusive gradient of angiopoietin 1 (ANG-1), factors that induce EC sprouting [31] and vessel stabilization [32] respectively. With this unique platform, live-cell microscopy uncovered the distinct roles of the synergy between mechanical (type 1 collagen gel) and chemical (VEGF, ANG-1) cues on vessel morphogenetic processes in 3D [33]. In addition to collagen scaffolds, hybrid hyaluronic acid and collagen hydrogels have been used to study angiogenesis [34]. ECs that form a luminal monolayer in vivo are oriented in circular cross-sections. Using viscous finger patterning [35], and triple channel design, open lumens lined with ECs were exposed to VEGF concentration gradients to observe sprouting [36]. With the additional ability to introduce SMCs to the side channels, the migratory potential of ECs was hampered in agreement with other microfluidic studies [37].
Sacrificial molding has emerged as yet another novel fabrication methodology for developing 3D perfusible ECM microfluidic platforms. Structural lattices of sacrificial carbohydrate glass, coated with poly(d-lactide-co-glycolide) (PDLGA), were encapsulated in different biopolymers (Poly (ethylene glycol), Alginate, Agarose, Fibrin, Matrigel) containing fibroblasts, cells present in the outermost layer of arteries and veins, and degraded to form open microfluidic channels for EC seeding in a physiologically relevant 3D matrix [38••]. In this co-culture system, single and multicellular sprouts of ECs with neighboring fibroblasts formed. An additional level of complexity was added to this system, in which exponential, linear, and step gradients of cells and fluorescent beads were encapsulated into the polymeric gels to demonstrate the possibility to immobilize chemical cues such as peptides, growth factors and proteins in complex configurations. Subsequently, the same group fabricated 3D microfluidic networks composed of spatially controlled type 1 collagen diffusive gradients with angiogenic growth factors, using gelatin as a sacrificial element [39]. Through seeding a mixture of suspended human lung fibroblasts (LF) with HUVECs in a fibrinogen/collagen type 1 ECM mixture in a three channel replica molded PDMS microfluidic chip, perfusable microvascular networks spontaneously formed in a 3D microenvironment. Additionally, after four days of culture, Kim et al. were able to generate HUVEC sprouting events that lead to lumenized vessel structures, by seeding growth factor secreting LF and HUVECs in opposing channels separated by a fibrin matrix in their device [40] (Figure 4i). Using a similar EC, fibroblast co-culture system, Baker et al. were able to demonstrate the growth factor dependent sprouting of ECs in a physiologically relevant environment, with the capacity to spatially guide morphogenetic events in a high-resolution manner, posing potentials for engineering vasculature in specific locations for tissue scaffolds (Figure 4ii).
Figure 4.
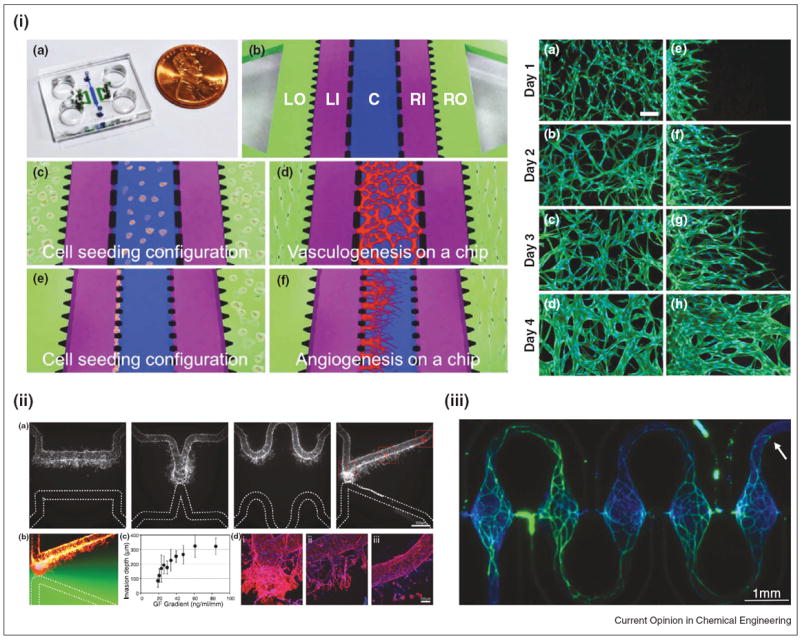
Microfluidic angiogenesis and vasculogenesis models. (i) Left — Illustration of a three-channel microfluidic system for investigating both angiogenesis and vasculogenesis. Right — Immunofluorescence images showing propagation of angiogenic sprouts and interconnected vessels. Reproduced from [40] with permission from the Royal Society of Chemistry. (ii) An array of microfluidic geometries, used to generate various growth factor gradients, displays the spatial control of endothelial cell sprouting in a 3D microfluidic platform. Reproduced from [39] with permission from the Royal Society of Chemistry. (iii) Immunofluorescence image of repeating self-assembled human microtissues within a novel microfluidic platform. Reproduced from [41••] with permission from the Royal Society of Chemistry.
Conclusion and future directions
The use of Braille display, micro-milling, multi-layer, sacrificial and replica molded microfluidics are just a few of examples of novel fabrication techniques, built upon traditional lithography, that serve as innovative platforms for vascular tissue engineering. As fabrication techniques continually evolve, concurrent control over several parameters such as chemical gradients, confinement, shear stress, oxygen, and substrate topography in vascularized microfluidic constructs with thicknesses (millimeter range) mirroring tissues found in vivo is feasible. Although much of this review focused on mature ECs and angiogenesis, new studies have leveraged microfluidics systems to study interstitial flow on vasculogenesis, the nascent formation of blood vessels through cellular maturation pathways. Multiple connected, self-assembled 3D microtissues of ECFCs, co-cultured with fibroblasts in an ECM suspension, formed when grown in physiologically relevant oxygen levels in PDMS based microfluidic channels [41••,42] (Figure 4iii). In addition, the self-organization of ECs co-cultured in suspension with stem cell derived pericytes in collagen matrices into vessels was also recently investigated using a microfluidic platform [43••]. Drug effects on this self-organization process were also explored. As methodologies to controllably guide stem cells to progenitor and mature vascular phenotypes [44,45] develop, new and exciting avenues for microfluidic platforms are emerging, greatly enhancing the field of vascular tissue engineering.
Acknowledgments
We gratefully acknowledge support for relevant studies from our laboratory presented in this review by the National Institutes of Health Grant U54CA143868 and the National Science Foundation Grant 1054415 (to S.G).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
-
•
of special interest
-
••
of outstanding interest
- 1.Terry SC, Jerman JH, Angell JB. A gas chromatographic air analyzer fabricated on a silicon wafer. IEEE Trans Electron Devices. 1979;26:1880–1886. [Google Scholar]
- 2.Stone HA, Stroock AD, Ajdari A. Engineering flows in small devices: microfluidics toward a lab-on-a-chip. Annu Rev Fluid Mech. 2004;36:381–411. [Google Scholar]
- 3.Stroock AD, Whitesides GM. Components for integrated poly (dimethylsiloxane) microfluidic systems. Electrophoresis. 2002;23:3461–3473. doi: 10.1002/1522-2683(200210)23:20<3461::AID-ELPS3461>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Griffith LG. Tissue engineering—current challenges and expanding opportunities. Science. 2002;295:1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 5.Ikada Y. Challenges in tissue engineering. J R Soc Interface. 2006;3:589–601. doi: 10.1098/rsif.2006.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khademhosseini A, Vacanti J, Langer R. Progress in tissue engineering. Sci Am Mag. 2009;300:64–71. doi: 10.1038/scientificamerican0509-64. [DOI] [PubMed] [Google Scholar]
- 7.Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev. 2009;15:353–370. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Delivery Rev. 2011;63:300–311. doi: 10.1016/j.addr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Young EWK. Advances in microfluidic cell culture systems for studying angiogenesis. J Lab Autom. 2013;18(6):417–436. doi: 10.1177/2211068213495206. [DOI] [PubMed] [Google Scholar]
- 10.Wong KHK, Chan JM, Kamm RD, Tien J. Microfluidic models of vascular functions. Annu Rev Biomed Eng. 2012;14:205–230. doi: 10.1146/annurev-bioeng-071811-150052. [DOI] [PubMed] [Google Scholar]
- 11.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 12.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A. 2006;103:2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song JW, Gu W, Futai N, Warner KA, Nor JE, Takayama S. Computer-controlled microcirculatory support system for endothelial cell culture and shearing. Anal Chem. 2005;77:3993–3999. doi: 10.1021/ac050131o. [DOI] [PubMed] [Google Scholar]
- 14.Chau L, Doran M, Cooper-White J. A novel multishear microdevice for studying cell mechanics. Lab Chip. 2009;9:1897. doi: 10.1039/b823180j. [DOI] [PubMed] [Google Scholar]
- 15.Shao J, Wu L, Wu J, Zheng Y, Zhao H, Jin Q, Zhao J. Integrated microfluidic chip for endothelial cells culture and analysis exposed to a pulsatile and oscillatory shear stress. Lab Chip. 2009;9:3118. doi: 10.1039/b909312e. [DOI] [PubMed] [Google Scholar]
- 16.Chin LK, Yu JQ, Fu Y, Yu T, Liu AQ, Luo KQ. Production of reactive oxygen species in endothelial cells under different pulsatile shear stresses and glucose concentrations. Lab Chip. 2011;11:1856. doi: 10.1039/c0lc00651c. [DOI] [PubMed] [Google Scholar]
- 17.Zheng W, Jiang B, Wang D, Zhang W, Wang Z, Jiang X. A microfluidic flow-stretch chip for investigating blood vessel biomechanics. Lab Chip. 2012;12:3441. doi: 10.1039/c2lc40173h. [DOI] [PubMed] [Google Scholar]
- 18.Dong J-d, Gu Y-q, Li C-m, Wang C-r, Feng Z-g, Qiu R-x, Chen B, Li J-x, Zhang S-w, Wang Z-g, Zhang J. Response of mesenchymal stem cells to shear stress in tissue-engineered vascular grafts. Acta Pharmacol Sin. 2009;30:530–536. doi: 10.1038/aps.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto K, Takahashi T, Asahara T, Ohura N, Sokabe T, Kamiya A, Ando J. Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress. J Appl Physiol. 2003;95:2081–2088. doi: 10.1152/japplphysiol.00232.2003. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto K. Fluid shear stress induces differentiation of flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. AJP: Heart Circ Physiol. 2004;288:H1915–H1924. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- 21.Lee YM, Jeong CH, Koo SY, Son MJ, Song HS, Bae SK, Raleigh JA, Chung HY, Yoo MA, Kim KW. Determination of hypoxic region by hypoxia marker in developing mouse embryos in vivo: a possible signal for vessel development. Dev Dyn. 2001;220:175–186. doi: 10.1002/1097-0177(20010201)220:2<175::AID-DVDY1101>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 22.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the hif system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 23•.Abaci HE, Devendra R, Smith Q, Gerecht S, Drazer G. Design and development of microbioreactors for long-term cell culture in controlled oxygen microenvironments. Biomed Microdevices. 2011;14:145–152. doi: 10.1007/s10544-011-9592-9. A microfluidic device, permissible to live cell imaging, real-time measurement of dissolved oxygen levels, while simultaneously controlling shear stress levels, was developed from a simple, low cost fabrication scheme. Results demonstrate the potential for long term cell culture studies with microenvironment monitoring capabilities. [DOI] [PubMed] [Google Scholar]
- 24.Wanjare M, Kuo F, Gerecht S. Derivation and maturation of synthetic and contractile vascular smooth muscle cells from human pluripotent stem cells. Cardiovasc Res. 2013;97:321–330. doi: 10.1093/cvr/cvs315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Abaci HE, Devendra R, Soman R, Drazer G, Gerecht S. Microbioreactors to manipulate oxygen tension and shear stress in the microenvironment of vascular stem and progenitor cells. Biotechnol Appl Biochem. 2012;59:97–105. doi: 10.1002/bab.1010. In addition to developing a unique design equation that relates microfluidic channel geometry and several cellular properties, the authors demonstrate the independent control of shear stress and oxygen tension to cultured progenitor and stem cells pertaining to vasculture. [DOI] [PubMed] [Google Scholar]
- 26.Du Y, Ghodousi M, Qi H, Haas N, Xiao W, Khademhosseini A. Sequential assembly of cell-laden hydrogel constructs to engineer vascular-like microchannels. Biotechnol Bioeng. 2011;108:1693–1703. doi: 10.1002/bit.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, Kermani P, Hempstead B, Fischbach-Teschl C, López JA, Stroock AD. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A. 2012;109:9342–9347. doi: 10.1073/pnas.1201240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc Natl Acad Sci U S A. 2012;109:13515–13520. doi: 10.1073/pnas.1210182109. In this unique study, tumor-endothelial interactions were investagited using a microfluidc assembly containing a 3D extracellular matrix hydrogel. Results demonstrate temporal dynamics of tumor cell intravasation in response to control and imparied endothelial barrier function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gagnon E, Cattaruzzi P, Griffith M, Muzakare L, LeFlao K, Faure R, Béliveau R, Hussain SN, Koutsilieris M, Doillon CJ. Human vascular endothelial cells with extended life spans: in vitro cell response, protein expression, and angiogenesis. Angiogenesis. 2002;5:21–33. doi: 10.1023/a:1021573013503. [DOI] [PubMed] [Google Scholar]
- 30.Sudo R, Chung S, Zervantonakis IK, Vickerman V, Toshimitsu Y, Griffith LG, Kamm RD. Transport-mediated angiogenesis in 3d epithelial coculture. FASEB J. 2009;23:2155–2164. doi: 10.1096/fj.08-122820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 32.Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, Shibuya M, Takakura N, Koh GY, Mochizuki N. Differential function of tie2 at cell–cell contacts and cell–substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008;10:513–526. doi: 10.1038/ncb1714. [DOI] [PubMed] [Google Scholar]
- 33.Shin Y, Jeon JS, Han S, Jung G-S, Shin S, Lee S-H, Sudo R, Kamm RD, Chung S. In vitro 3d collective sprouting angiogenesis under orchestrated ang-1 and vegf gradients. Lab Chip. 2011;11:2175–2181. doi: 10.1039/c1lc20039a. [DOI] [PubMed] [Google Scholar]
- 34.Jeong GS, Kwon GH, Kang AR, Jung BY, Park Y, Chung S, Lee S-H. Microfluidic assay of endothelial cell migration in 3d interpenetrating polymer semi-network ha-collagen hydrogel. Biomed Microdevices. 2011;13:717–723. doi: 10.1007/s10544-011-9541-7. [DOI] [PubMed] [Google Scholar]
- 35.Bischel LL, Lee S-H, Beebe DJ. A practical method for patterning lumens through ecm hydrogels via viscous finger patterning. J Lab Autom. 2012;17:96–103. doi: 10.1177/2211068211426694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bischel LL, Young EWK, Mader BR, Beebe DJ. Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials. 2013;34:1471–1477. doi: 10.1016/j.biomaterials.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung S, Sudo R, Mack PJ, Wan C-R, Vickerman V, Kamm RD. Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab Chip. 2009;9:269. doi: 10.1039/b807585a. [DOI] [PubMed] [Google Scholar]
- 38••.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen D-HT, Cohen DM, Toro E, Chen AA, Galie PA, Yu X, Chaturvedi R, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11:1–7. doi: 10.1038/nmat3357. Using a novel sacrifical molding fabrication scheme, this study demonstrated the reproducible production of perfusable biopolymer microfluidic networks, permissibile to multicellular architectures mimicking in vivo vasculature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker BM, Trappmann B, Stapleton SC, Toro E, Chen CS. Microfluidics embedded within extracellular matrix to define vascular architectures and pattern diffusive gradients. Lab Chip. 2013;13:3246. doi: 10.1039/c3lc50493j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim S, Lee H, Chung M, Jeon NL. Engineering of functional, perfusable 3d microvascular networks on a chip. Lab Chip. 2013;13:1489. doi: 10.1039/c3lc41320a. [DOI] [PubMed] [Google Scholar]
- 41••.Hsu Y-H, Moya ML, Hughes CCW, George SC, Lee AP. A microfluidic platform for generating large-scale nearly identical human microphysiological vascularized tissue arrays. Lab Chip. 2013;13(15):2990–2998. doi: 10.1039/c3lc50424g. Using circuit design analogies and soft lithography fabrication, microtissue arrays consisiting of endothelial and stromal cells seeded within a fibrin gel, were exposed to physiologically relevant interstitial flow, a key determinant in vasculogenesis. This platform has the unprecedented capability of high-throughput studies on the effects of varying physiological conditions in 3D biomimetic environment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moya ML, Hsu Y-H, Lee AP, Hughes CC, George SC. In vitro perfused human capillary networks. Tissue Eng Part C: Methods. 2013;19(9):730–737. doi: 10.1089/ten.tec.2012.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43••.van der Meer AD, Orlova VV, ten Dijke P, van den Berg A, Mummery CL. Three-dimensional co-cultures of human endothelial cells and embryonic stem cell-derived pericytes inside a microfluidic device. Lab Chip. 2013;13(18):3562–3568. doi: 10.1039/c3lc50435b. This paper investigated the role of transforming growth factor-beta (TGF-β) inhibition on the formation of three dimensional vascular structures within a PDMS microfluidic channel, generated from the mixture of precursor pericyte and human umbilical vein endothelial cells in a collagen 1 matrix. This study displays the inovation in developing in vitro assays for drug screening on organ-on-a-chip platforms. [DOI] [PubMed] [Google Scholar]
- 44.Kusuma S, Shen Y-I, Hanjaya-Putra D, Mali P, Cheng L, Gerecht S. Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proc Natl Acad Sci U S A. 2013;110(31):12601–12606. doi: 10.1073/pnas.1306562110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samuel R, Daheron L, Liao S, Vardam T, Kamoun WS, Batista A, Buecker C, Schäfer R, Han X, Au P, Scadden DT, et al. Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2013;110(31):12774–12779. doi: 10.1073/pnas.1310675110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDonald JC, Whitesides GM. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc Chem Res. 2002;35:491–499. doi: 10.1021/ar010110q. [DOI] [PubMed] [Google Scholar]