Abstract
Background
Recently, high-dose oral synthetic delta-9-tetrahydrocannabinol (THC) was shown to alleviate cannabis withdrawal symptoms. The present data describe cannabinoid pharmacokinetics in chronic daily cannabis smokers who received high-dose oral THC pharmacotherapy and later, a smoked cannabis challenge.
Methods
11 daily cannabis smokers received 0, 30, 60, or 120 mg/day THC for four 5-day medication sessions, each separated by 9-days of ad-libitum cannabis smoking. On the 5th day, participants were challenged with smoking one 5.9% THC cigarette. Plasma collected on the 1st and 5th days was quantified by GC-GC-MS for THC, 11-hydroxy-THC (11-OH-THC), and 11-nor-9-carboxy-THC (THCCOOH). Linear ranges (ng/mL) were 0.5–100 for THC, 1–50 11-OH-THC, and 0.5–200 THCCOOH.
Results
During placebo dosing, THC, 11-OH-THC and THCCOOH concentrations consistently decreased, while all cannabinoids increased dose-dependently during active dronabinol administration. THC increase over time was not significant after any dose, 11-OH-THC increased significantly during 60 and 120 mg/day doses, and THCCOOH increased significantly only during the 120 mg/day dose. THC and 11-OH-THC, and THCCOOH concentrations peaked within 0.25 h after cannabis smoking, except after 120 mg/day THC when THCCOOH peaked 0.5 h before smoking.
Conclusions
The significant withdrawal effects noted during placebo dronabinol administration were supported by significant plasma THC and 11-OH-THC concentration decreases. During active dronabinol dosing, significant dose-dependent increases in THC and 11-OH-THC concentrations support withdrawal symptom suppression. THC concentrations after cannabis smoking were only distinguishable from oral THC doses for 1 h, too short a period to feasibly identify cannabis relapse. THCCOOH/THC ratios were higher 14 h after overnight oral dronabinol abstinence, but cannot distinguish oral THC dosing from smoked cannabis intake.
Keywords: dronabinol, plasma, cannabinoids, smoking, oral administration
1. Introduction
Dronabinol (Marinol®) is a pure synthetic oral form of delta-9-tetrahydrocannabinol (THC), and since 1999, is a Schedule III drug under the Controlled Substances Act in the United States.1–2 Dronabinol is approved by the US Food and Drug Administration for the treatment of anorexia in AIDS and other wasting diseases, and as an anti-emetic agent in cancer patients undergoing chemotherapy.3 Maximum recommended doses are 20 mg/day for appetite stimulation and 15 mg/m2 4–6 times daily (175 mg/day assuming a typical 1.95 m2 body surface area) for anti-emesis.2 Dronabinol might be a useful adjunct intervention for treating cannabis dependence, as cannabis withdrawal symptoms were significantly attenuated during its administration.4–6 Oral THC administration, up to 90 mg/day, dose-dependently suppressed withdrawal in an outpatient environment.7 Levin et al. reported that 40 mg/day dronabinol promoted retention in treatment and reduced withdrawal symptoms, but failed to improve abstinence compared to placebo in patients seeking treatment for cannabis dependence.8 We also found that dronabinol dose-dependently attenuated cannabis withdrawal in our recent inpatient study.9 However, it did not alter smoked cannabis subjective effects, whereas cannabis-induced increases in heart rate were attenuated by the 60 and 120 mg doses.9
THC accumulates in the adipose tissue of chronic, frequent cannabis smokers.10–11 An individual’s smoking history including duration, frequency, and amount of cannabis determine the total body burden of stored THC. These factors contribute to variation in THC bioavailability. Thus, assessment of the pharmacokinetics of dronabinol in chronic frequent cannabis smokers is important for understanding the time course and magnitude of its subjective, physiological, and clinical effects.
The pharmacokinetic profile of dronabinol differs markedly from smoked cannabis. After smoked cannabis, THC rapidly enters the bloodstream and reaches the brain within seconds. Peak THC concentrations in plasma are achieved prior to the end of smoking, and then rapidly decline to about 10% of maximum within 1–2 h.12 Effects are perceptible within 1 min.13 After dronabinol, the onset of action is 30–60 min following ingestion, with drug effects and plasma THC concentrations concurrently peaking 2–4 h later.14–15 Dronabinol, formulated in sesame oil is almost completely absorbed (90–95%) after a single dose from the gastrointestinal tract, but owing to first-pass hepatic metabolism and rapid tissue uptake, only 6%–20% of an orally administered THC dose reaches systemic circulation.16–18
Blood cannabinoid concentrations better reflected behavioral and physiological responses during oral THC treatment than administered dose. Furthermore, as a potential pharmacotherapeutic agent for cannabis dependence, the pharmacokinetics of oral THC (dronabinol) needs to be more comprehensively elucidated. Goodwin et al.19 investigated the plasma pharmacokinetics of 7.5 mg/day dronabinol (recommended dose for appetite stimulation) for 5 consecutive days in 6 cannabis smokers. THC and 11-hydroxy-THC (11-OH-THC) were detectable for 1.5 h after the first dose, and maximum concentrations of 3.8 and 2.6 ng/mL were reached after 107 and 6.5 h, respectively. THCCOOH was detectable 1.5 h after initiating dosing and increased over the 5 days, reaching the peak concentration 1.5 h after the last 15th dose. Recently, cannabinoid pharmacokinetics were characterized following around-the-clock oral THC (dronabinol) administration (40–120 mg/day) to chronic daily cannabis smokers. Mean plasma Cmax and Tmax were 47.7 ng/mL on day 5 for THC, 23.9 ng/mL on day 7 for 11-OH-THC and 327 ng/mL on day 9 for the non-psychoactive 11-nor-9-carboxy-THC (THCCOOH) metabolite.20 However, the pharmacokinetics of multiple 10, 20, and 40 mg (t.i.d.) oral THC and placebo dosing within the same subjects for therapeutic dosing has not been studied.
The present study examined disposition of THC, 11-OH-THC and THCCOOH in chronic cannabis smokers’ plasma following continuous oral dronabinol pharmacotherapy over 5 days and an acute smoked cannabis challenge. Pharmacodynamic outcomes including cannabis withdrawal ratings during the study were previously presented.9 In this report, relationships among dose, plasma cannabinoid concentrations, and cannabis withdrawal symptoms are described. Also the possibility of detecting active cannabis smoking during oral dronabinol treatment was evaluated, and cannabinoid disposition and metabolite ratios in plasma after smoked and oral THC are characterized. These cannabinoid data suggest appropriate therapeutic doses to suppress cannabis withdrawal symptoms, and demonstrate the much higher peak concentrations achieved after smoked cannabis. These data extend current knowledge of the behavioral pharmacology and pharmacokinetics of oral dronabinol and smoked cannabis in chronic cannabis smokers.
2. Material and methods
2.1. Chemicals and reagents
THC, 11-OH-THC, and THCCOOH for calibrators and quality control samples and corresponding internal standards (d3-THC, d3-11-OH-THC, d9-THCCOOH) were purchased from Cerilliant (Round Rock, TX, USA), ZSTHC020 solid-phase extraction (SPE) columns from United Chemical Technologies (Bristol, PA, USA). Dronabinol (Marinol®) was acquired from Abbott Laboratories (Abbott Park, IL, USA). Cannabis cigarettes were obtained from the U.S. National Institute on Drug Abuse (NIDA).
2.1. Participants
Inclusion criteria were 18–55 years old, smoking cannabis at least 25 days per month during the past 12 months, and a positive urine cannabinoid sample on admission. Clinically significant medical or psychiatric disease, substance dependence other than cannabis or nicotine, pregnant or breastfeeding, history of cannabis-related psychosis or other adverse reaction, interest in substance abuse treatment, or allergy to sesame oil (ingredient of dronabinol) were exclusionary.
Participants resided 51 days on the residential research unit of the Johns Hopkins University Behavioral Pharmacology Research Unit, with 24 h medical coverage.
2.2. Study procedures
The study was approved by the John Hopkins University Institutional Review Board and all participants provided written informed consent. Ten males and 1 female participated in the study [mean (SD) age, 35.1 (8.3) years; body-mass index, 28.6 (8.2) kg/m2]. These individuals self-reported first smoking cannabis at a mean of 14.4 (2.4) years, with daily smoking of 1–9 joints. Participant demographics and self-reported use histories are detailed in Table 1. A within-subject design was utilized. All participants received double blind one of three doses of dronabinol: 30 (10 mg tid), 60 (20 mg tid), or 120 (40 mg tid) mg/day, or placebo dronabinol in a counterbalanced order during four 5-day medication sessions (Figure 1). Oral capsules were administered at 9:00, 14:00, and 19:00 each day and on the 5th (last) day (11:30) of each placebo or active dronabinol session, participants self-administered 5 controlled puffs (5 sec inhalation, 10 sec breath hold, 40 sec inter-puff interval) from one smoked cannabis cigarette containing 5.9% THC. Each medication session was separated by a 9-day washout session during which ad-libitum cannabis smoking was allowed between 12:00 and 23:00 each day.
Table 1.
Demographics and self-reported cannabis smoking characteristics for 11 chronic frequent cannabis smokers.
| Participanta | Sex | Age, yrs | BMI kg/m2 | Joints/dayb, n | Smoked in past 30 d | Age 1st smoked, yrs |
|---|---|---|---|---|---|---|
| A | M | 47 | 19.4 | 2.1 | 30 | 13 |
| B | M | 36 | 28.5 | 5.6 | 30 | 17 |
| C | M | 29 | 30.2 | 4.7 | 30 | 9 |
| E | F | 31 | 36.0 | 5.0 | 30 | 18 |
| H | M | 30 | 21.0 | 5.9 | 28 | 14 |
| I | M | 52 | 26.1 | 2.3 | 29 | 14 |
| J | M | 39 | 48.2 | 3.4 | 28 | 13 |
| K | M | 37 | 27.7 | 2.0 | 29 | 16 |
| M | M | 30 | 32.4 | 2.9 | 29 | 14 |
| N | M | 25 | 22.0 | 3.0 | 30 | 14 |
| P | M | 30 | 23.1 | 3.5 | 29 | 16 |
|
| ||||||
| Mean | 35.1 | 28.6 | 3.7 | 29.3 | 14.4 | |
| SD | 8.3 | 8.2 | 1.4 | 0.8 | 2.4 | |
All participants received four doses of dronabinol: 0 (placebo), 30 (10 mg tid), 60 mg/day (20 mg tid), or 120 mg/day (40 mg tid) in counterbalanced order during four 5-day medication sessions
Mean is calculated based on a self-reported daily use over the last 30 days
Figure 1.
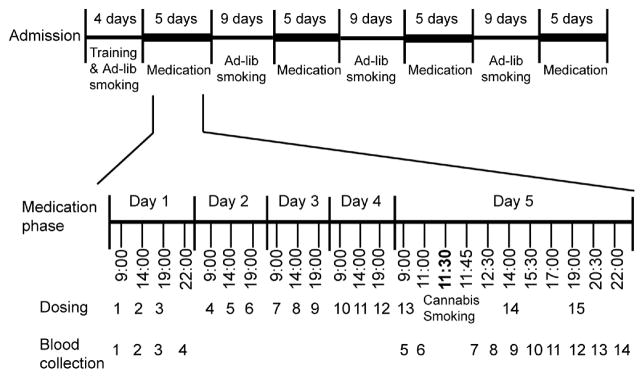
Study design timeline.
2.3. Specimen collection and analysis
In each 5-day dronabinol (placebo, 30, 60, or 120 mg/day) session, plasma specimens were collected 4 times (9:00, 14:00, 19:00, 22:00) on the first day. An additional 10 plasma specimens were obtained on the last day at 9:00, 11:00, 11:45, 12:30, 14:00, 15:30, 17:00, 19:00, 20:30, and 22:00, prior to and after the smoked cannabis challenge at 11:30. Blood specimens were collected on ice in 6 mL sodium heparin Vacutainer® tubes, centrifuged within 2 h, and plasma separated and stored frozen at −20°C until analysis.
Plasma THC, 11-OH-THC, and THCCOOH concentrations were analyzed by a previously published method with several modifications.21 Briefly, 2 mL ice-cold acetonitrile was added drop wise to 1 mL plasma to precipitate proteins. Four mL sodium acetate buffer (2N, pH=4.0) were added. The supernatant was subjected to SPE and cannabinoids eluted from the column with 5 mL hexane/ethyl acetate (80:20, v/v). Elutes were dried and derivatized with 25 μL BSTFA at 70°C for 30 min. A 3 μL splitless injection of derivatized extracts was introduced to a 2-dimensional GCMS with a Dean’s switch and cryotrap. Linear ranges were 0.5–100 ng/mL for THC, 1.0–50 ng/mL for 11-OH-THC, and 0.5–200 ng/mL for THCCOOH. Intra- and inter-assay imprecision were ≤5.2% and ≤3.8%, respectively, and analytical recoveries were 39.8–85.3%.
2.4. Data analysis
Statistical calculations utilized SPSS 14.0 for Windows (SPSS, Chicago, IL). Medians and ranges were utilized for data analysis after determining non-normal distribution by the Kolmogorov-Smirnov normality test, and homogeneity by the Levene’s test of variances, and concentration variations between collections were examined by a nonparametric related samples Wilcoxon Signed Rank test. A two-tailed p< 0.05 defined significance for all comparisons.
3. Results
Fifty-six plasma specimens were collected from each participant over four 5-day cannabis dronabinol administrations and a single cannabis cigarette challenge on the last day of each session.
3.1. Cannabinoid disposition after oral dronabinol administration
Participants received dronabinol 3 times a day, with the first dose at 9:00; participants were abstinent from smoked cannabis for at least 10 h from the preceding ad-libitum smoking day. There were no significant differences in median cannabinoid concentrations at baseline (9:00), but with high inter-individual variability for all analytes in the placebo, 30, 60, and 120 mg/day dronabinol sessions (Figure 2). During 9:00–22:00 on the first day of 30, 60, and 120 mg/day dronabinol administrations, THC (p>0.05), 11-OH-THC (p<0.001), and THCCOOH (p<0.001) concentrations increased dose-dependently; however, in the placebo session, concentrations of all cannabinoid analytes decreased (Figure 2).
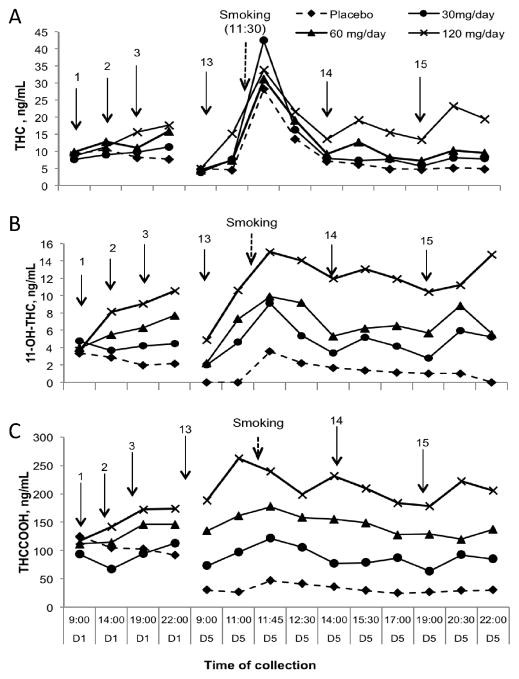
Figure 2.
Median (n=11) delta-9-tetrahydrocannabinol (THC) (A), 11-hydroxy-THC (11-OH-THC) (B) and 11-nor-9-carboxy-THC (THCCOOH) (C) plasma concentrations during 1st (D1) and 5th (D5) days of 0, 30, 60 and 120 mg/day dronabinol and a single smoked cannabis challenge. Arrows indicate dronabinol administration.
At 9:00 on the 5th day of each session, participants were abstinent overnight (14 h) from the preceding oral administration. We evaluated concentration changes between the last sample at 22:00 on Day 1 and the next sample at 9:00 on Day 5. Median THC concentrations were significantly lower at 9:00 on the 5th day than at 22:00 on the first day of all 4 dronabinol sessions (Figure 2A). 11-OH-THC concentrations also were significantly lower for all but the 120 mg/day dosing session (Figure 2B). THCCOOH concentrations decreased significantly only over the placebo session (Figure 2C).
Two hours after the 9:00 (Day 5) oral dose, all cannabinoid concentrations increased in all dosing sessions, except during the placebo session (Figure 2). By 11:00 (Day 5) median THC concentrations significantly (p<0.01) increased 2-fold after the 30 and 60 mg/day doses and 3-fold after the 120 mg/day dose (Figure 2A). Results were less consistent for 11-OH-THC and THCCOOH concentrations, with significant 2-fold increases in 11-OH-THC after the 30 and 120 mg/day doses, and 3-fold increases after the 60 mg/day dose (Figure 2B). THCCOOH concentrations significantly (p=0.021) increased only after the 60 mg/day dose (Figure 2C) over the same time period.
3.2. Cannabinoid disposition after acute smoked cannabis
All plasma THC, 11-OH-THC, and THCCOOH concentrations peaked by 0.25 h after the smoked cannabis cigarette challenge, except for THCCOOH concentrations in the 120 mg/day session, which showed higher concentrations 0.5 h before smoked cannabis. Despite different dosing regimens, median maximum THC concentrations were similar at 0.25 h after smoking (Figure 2A). Median maximum 11-OH-THC concentrations were similar in 30 and 60 mg/day sessions, but significantly higher in 120 mg/day (Figure 2B). Maximum THCCOOH concentrations dose-dependently, but non-significantly changed after the smoking challenge in the 0, 30, 60, and 120 mg/day sessions (Figure 2C).
As expected, THC concentrations significantly decreased within 1 h after smoking in all sessions, and remained low concordantly with concentrations found during oral dronabinol administration on Day 1 (Figure 2A). Median 11-OH-THC and THCCOOH concentrations decreased non-significantly after smoking, and remained relatively stable with non-significant change throughout all dosing sessions.
Over the 5-day oral dronabinol administration, THC concentrations in the last (22:00) sample on Day 1 were not significantly different from those in the last (22:00) sample on Day 5 in all active dronabinol sessions, despite the smoked cannabis challenge (11:30). 11-OH-THC concentrations were higher in 120 mg/day session, and THCCOOH concentrations did not differ significantly during any of the active dronabinol sessions in the same comparison. During the 5-day placebo administration, all cannabinoid concentrations were significantly (p<0.01) lower in the last (22:00) sample on Day 5 than in the last (22:00) sample on Day 1 (Figure 2).
3.3. 11-OH-THC/THC ratios in plasma
On Day 1 after ad-libitum cannabis smoking, median baseline 11-OH-THC/THC ratios (0.38; 0.46; 0.38; 0.47) were similar for placebo, 30, 60 and 120 mg/day dronabinol doses, respectively. Median (range) 11-OH-THC/THC ratios remained unchanged (0.44–0.57) throughout Day 1 during 30 and (0.48–0.50) in 60 mg/day dronabinol. In the 120 mg/day session, ratios significantly increased after the first oral dose, due to a rapid 11-OH-THC increase, and remained higher (0.74–0.79) thereafter, whereas in placebo, ratios gradually decreased (0.23–0.32) during the same time interval (Figure 3).
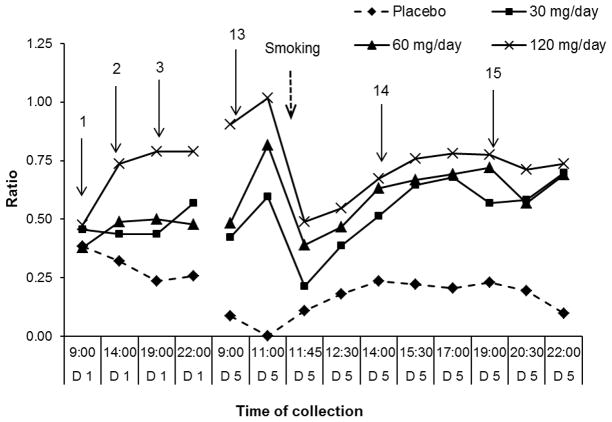
Figure 3.
Median (n=11) 11-hydroxy-THC (11-OH-THC)/delta-9-tetrahydrocannabinol (THC) ratios during 1st (D1) and 5th (D5) days of 0, 30, 60 and 120 mg/day dronabinol and single smoked cannabis challenge at 11:30 on Day 5. Arrows indicate time of dronabinol administration.
After an overnight (14 h) dronabinol abstinence, on the 5th day at 9:00, median 11-OH-THC/THC ratios (0.42; 0.48) were non-significantly different in the 30 and 60 mg/day sessions, but significantly (p<0.01) higher (0.90) for 120 mg/day than those on Day 1 at 22:00. Two h after the 9:00 oral dose (0.5 h prior to cannabis smoking), median ratios (0.59; 0.82; 1.02) peaked in all dronabinol sessions. Ratios could not be calculated in the placebo session because no 11-OH-THC was detected.
11-OH-THC/THC decreased significantly after cannabis smoking (0.25 h) (0.21; 0.39; 0.49), due to an immediate and high THC increase. However, the ratios observed up to 2.5 h post-smoking exceeded those on Day 1 (during oral administration) for 30 and 60 mg/day doses and were similar for the 120 mg/day session. In placebo, the median ratio was 0.11, as 11-OH-THC became measurable 0.25 h after smoking. The ratios during placebo reached a maximum (0.23) within 2.5 h and remained unchanged for 9 h after smoking.
3.4. THCCOOH/THC ratios in plasma
Median baseline THCCOOH/THC ratios (16.6; 14.8; 16.3; 13.7) were similar for all active and placebo dronabinol doses. After the 1st oral THC dose, all THCCOOH/THC ratios decreased in a dose-independent manner as THC increased (Figure 4).
Figure 4.
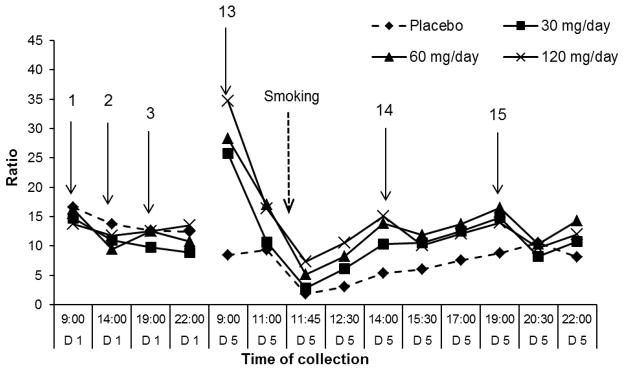
Median (n=11) 11-nor-9-carboxy-THC (THCCOOH)/delta-9-tetrahydrocannabinol (THC) ratios during 1st (D1) and 5th (D5) days of 0, 30, 60 and 120 mg/day dronabinol and single smoked cannabis challenge at 11:30 on Day 5. Arrows indicate time of dronabinol administration.
THCCOOH/THC ratios (25.7; 28.3; 34.7) were significantly higher at 9:00 on Day 5, following 14 h overnight abstinence, than at the last collection (22:00) on Day 1 in 30, 60 and 120 mg/day sessions, respectively. However, during placebo, THC and THCCOOH concentrations continued to decrease, yielding a non-significant median ratio decrease of 8.4. Two h after the 9:00 oral dose, median ratios (10.6; 17.1; 16.4) significantly (p<0.01) decreased in the active dronabinol sessions. After smoking (0.25 h), THCCOOH/THC ratios (1.9; 2.8; 5.1; 7.4) significantly decreased, as expected.
One h after the smoked cannabis challenge session, THCCOOH/THC ratios were back to levels observed during active dronabinol administrations on Day 1. During placebo, ratios continually increased for 9 h after smoking (Figure 4).
4. Discussion
Vandrey et al.9 demonstrated dronabinol’s ability to dose-dependently suppress cannabis withdrawal in chronic frequent cannabis smokers. However, demonstration of pharmacological specificity is the most important criterion for validating a true drug withdrawal syndrome. The present data extend Vandrey’s observations by concurrently demonstrating the disposition and time course of THC and metabolites in plasma following dronabinol maintenance and smoked cannabis challenge. Participants in this study were chronic frequent cannabis smokers, generally smoking cannabis multiple times a day; the safety profile and tolerability of high doses of dronabinol observed in this study may not apply to individuals with less frequent patterns of cannabis use.
The occurrence of psychological and physical withdrawal symptoms in chronic frequent cannabis smokers upon their cessation of use is supported by inpatient and outpatient studies and human experimental laboratory studies. Symptoms decrease with resumption of cannabis use. In our study, plasma THC and 11-OH-THC concentrations markedly decreased during the 5 days of placebo dronabinol administration prior to the smoking challenge session. Significant withdrawal effects during this cannabis abstinence period were reported by Vandrey,9 including self-reported ratings of decreased appetite, diarrhea, nausea, irritability, sleep disturbance, mood and alertness at morning awakening, anxiety, increased aggression and anger, headaches, and difficulty concentrating. During the active dronabinol sessions, THC and 11-OH-THC concentrations increased dose-dependently, with the most noticeable increases during 120 mg/day dosing. This level of dronabinol dosing significantly suppressed most withdrawal symptoms, consistent with CB1 receptor agonist suppression of cannabis withdrawal. This response is also consistent with outpatient clinical trials and human experimental laboratory studies showing dronabinol suppression of cannabis withdrawal.7, 22–23
As expected, smoking a single cannabis cigarette significantly increased THC plasma concentrations. Maximum concentrations are known to be reached after the first several puffs; by 15 min post smoking mean THC concentrations are approximately 60% of peak concentrations.24 In our study, 0.25 h after smoking peak THC concentrations were still significantly higher than concentrations after oral THC doses. Within 1 h, plasma THC decreased to concentrations typically observed during oral dronabinol administration, demonstrating the short window for distinguishing between oral and smoked cannabis for clinical or forensic purposes. 11-OH-THC and THCCOOH concentrations were not noticeably different following the cannabis smoking challenge in comparison to dronabinol maintenance sessions. 11-OH-THC/THC ratios were significantly different before and immediately after smoked cannabis, especially during 120 mg/day dronabinol dosing. Ratios were much higher during 120 mg/day dosing compared to those after smoking because of extensive first-pass metabolism. Contribution of smoked THC to 11-OH-THC concentrations, suggested to be 5–10% of smoked THC concentrations,12, 16 was relatively minor compared to the contribution from continuous oral THC administration. In contrast, THC was highly elevated in the bloodstream after cannabis smoking, and therefore, lower 11-OH-THC/THC ratios were observed. THCCOOH/THC ratios were comparable during active oral THC dosing and smoked cannabis, because of high THC and THCCOOH concentrations constantly present during continuous dronabinol dosing and after smoked cannabis challenge. THCCOOH/THC ratios cannot distinguish before and after smoking, so relapse to smoked cannabis cannot be identified with these ratios. However, THCCOOH/THC ratios were considerably higher following the 14 h overnight cannabis abstinence for all active dronabinol doses. Plasma THC concentrations rapidly declined after discontinuing cannabis use, due to THC’s faster elimination rate compared to THCCOOH. Chronic cannabis smokers may have residual plasma THC concentrations of ≤2 ng/mL 12 h after smoking a cannabis cigarette containing 19 mg THC,25 and ≤5.2 ng/mL 22.5 h after 37 (20 mg) dronabinol doses administered over 8 days.20 In our study, after 14 h of oral THC abstinence, THC concentrations during active dronabinol dosing matched the placebo session concentrations and were ≤5 ng/mL. Conversely, THCCOOH concentrations remained unchanged following overnight abstinence and were significantly higher than during placebo, leading to high THCCOOH/THC ratios. These THCCOOH/THC ratio changes may help monitoring medication compliance when oral THC is prescribed to non-cannabis smokers for appetite stimulation or as an anti-emetic.
In conclusion, we show THC and metabolites disposition in plasma of chronic cannabis smokers following continuous dronabinol administration over 5 days and an acute smoked cannabis challenge. Chronic frequent cannabis smokers participating in this study safely tolerated high doses of dronabinol, which may not apply to individuals with less frequent patterns of cannabis intake. The significant withdrawal effects noted during placebo dronabinol administration were supported by significant plasma THC and 11-OH-THC concentration decreases. During active dronabinol dosing, significant dose-dependent increases in THC and 11-OH-THC concentrations reflect suppression of withdrawal symptoms. THC, 11-OH-THC, and THCCOOH concentrations peaked within 0.25 h after cannabis smoking, significantly exceeding concentrations following oral THC administration across oral doses. However, the ability to identify active cannabis smoking during dronabinol treatment is only possible for about 1 h after smoking, and therefore, it is not feasible to identify cannabis relapse in clinical or forensic cases. THC and THCCOOH elimination rate differences led to median THCCOOH/THC ratios ≥26 after 14 h dronabinol abstinence; the ratios were lower both during oral THC dosing and after smoked cannabis, preventing identification of smoked cannabis relapse.
Acknowledgments
This research was supported by grant R01-DA025044 from the U.S. National Institute on Drug Abuse (NIDA) and the National Institutes of Health, Intramural Research Program of the National Institute on Drug Abuse.
We thank the nursing, recruiting, and medical staff of the Behavioral Pharmacology Research Unit (BPRU), and the Chemistry and Drug Metabolism laboratory at the NIDA Intramural Research Program for their effort and dedication in helping complete this project.
References
- 1.Drug Enforcement Administration. [Accessed February, 2013.];Federal Register 64 FR 35928. 1999 Available at: http://www.gpo.gov/fdsys/pkg/FR-1999-07-02/pdf/99-16833.pdf. [PubMed]
- 2.U.S. Food and Drug Administration. [Accessed February, 2013.];Drugs@FDA: FDA Approved Drug Products. Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails.
- 3.Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential. J Ethnopharmacol. 2006;105:1–25. doi: 10.1016/j.jep.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Vandrey RG, Budney AJ, Moore BA, et al. A cross-study comparison of cannabis and tobacco withdrawal. Am J Addict. 2005 Jan-Feb;14(1):54–63. doi: 10.1080/10550490590899853. [DOI] [PubMed] [Google Scholar]
- 5.Budney AJ, Vandrey RG, Hughes JR, et al. Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J Subst Abuse Treat Dec. 2008;35(4):362–368. doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin KH, Copersino ML, Heishman SJ, et al. Cannabis withdrawal symptoms in non-treatment-seeking adult cannabis smokers. Drug Alcohol Depend. 2010;111:120–127. doi: 10.1016/j.drugalcdep.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budney AJ, Vandrey RG, Hughes JR, et al. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 2007;86:22–29. doi: 10.1016/j.drugalcdep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Levin FR, Mariani JJ, Brooks DJ, et al. Dronabinol for the treatment of cannabis dependence: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116:142–150. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vandrey R, Stitzer ML, Mintzer MZ, et al. The dose effects of short-term dronabinol (oral THC) maintenance in daily cannabis users. Drug Alcohol Depend. 2012;128:64–70. doi: 10.1016/j.drugalcdep.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karschner EL, Schwilke EW, Lowe RH, et al. Do delta9-tetrahydrocannabinol concentrations indicate recent use in chronic cannabis users? Addiction. 2009;104:2041–2048. doi: 10.1111/j.1360-0443.2009.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergamaschi MM, Karschner EL, Goodwin RS, et al. Impact of prolonged cannabinoid excretion in chronic daily cannabis smokers’ blood on per se drugged driving laws. Clin Chem. 2013;59:519–526. doi: 10.1373/clinchem.2012.195503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol. 1992;16:276–282. doi: 10.1093/jat/16.5.276. [DOI] [PubMed] [Google Scholar]
- 13.Huestis MA, Sampson AH, Holicky BJ, et al. Characterization of the absorption phase of marijuana smoking. Clin Pharmacol Ther. 1992;52:31–41. doi: 10.1038/clpt.1992.100. [DOI] [PubMed] [Google Scholar]
- 14.Lemberger L, Weiss JL, Watanabe AM, et al. Delta-9-tetrahydrocannabinol. Temporal correlation of the psychologic effects and blood levels after various routes of administration. N Engl J Med. 1972;286:685–688. doi: 10.1056/NEJM197203302861303. [DOI] [PubMed] [Google Scholar]
- 15.Huestis MA, Elsohly M, Nebro W, et al. Estimating time of last oral ingestion of cannabis from plasma THC and THCCOOH concentrations. Ther Drug Monit. 2006;28:540–544. doi: 10.1097/00007691-200608000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Wall ME, Sadler BM, Brine D, et al. Metabolism, disposition, and kinetics of delta-9-tetrahydrocannabinol in men and women. Clin Pharmacol Ther. 1983;34:352–363. doi: 10.1038/clpt.1983.179. [DOI] [PubMed] [Google Scholar]
- 17.Sporkert F, Pragst F, Ploner CJ, et al. Pharmacokinetic investigations and delta-9-tetrahydrocannabinol and its metabolites after single administration of 10 mg marinol in attendance of a psychiatric study. The Annual Meeting of The International Association of Forensic Toxicologists; Prague, Czech Republic. 2001. p. 62. [Google Scholar]
- 18.Ohlsson A, Lindgren JE, Wahlen A, et al. Plasma delta-9 tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin Pharmacol Ther. 1980;28:409–416. doi: 10.1038/clpt.1980.181. [DOI] [PubMed] [Google Scholar]
- 19.Goodwin RS, Gustafson RA, Barnes A, et al. Delta(9)-tetrahydrocannabinol, 11-hydroxy-delta(9)-tetrahydrocannabinol and 11-nor-9-carboxy-delta(9)-tetrahydrocannabinol in human plasma after controlled oral administration of cannabinoids. Ther Drug Monit. 2006;28:545–551. doi: 10.1097/00007691-200608000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Schwilke EW, Schwope DM, Karschner EL, et al. Delta9-tetrahydrocannabinol (THC), 11-hydroxy-THC, and 11-nor-9-carboxy-THC plasma pharmacokinetics during and after continuous high-dose oral THC. Clin Chem. 2009;55:2180–2189. doi: 10.1373/clinchem.2008.122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowe RH, Karschner EL, Schwilke EW, et al. Simultaneous quantification of delta9-tetrahydrocannabinol, 11-hydroxy-delta9-tetrahydrocannabinol, and 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid in human plasma using two-dimensional gas chromatography, cryofocusing, and electron impact-mass spectrometry. J Chromatogr A. 2007;1163:318–327. doi: 10.1016/j.chroma.2007.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart CL, Haney M, Ward AS, et al. Effects of oral THC maintenance on smoked marijuana self-administration. Drug Alcohol Depend. 2002;67:301–309. doi: 10.1016/s0376-8716(02)00084-4. [DOI] [PubMed] [Google Scholar]
- 23.Haney M, Hart CL, Vosburg SK, et al. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29:158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- 24.Huestis MA. Marijuana. In: Levine B, editor. Principles of Forensic Toxicology. Washington: AACC Press; 2003. pp. 246–264. [Google Scholar]
- 25.Peat MA. Distribution of delta-9-tetrahydrocannabinol and its metabolites. In: Baselt RC, editor. Advances in Analytical Toxicology II. Chicago: Year Book Medical Publishers; 1989. pp. 186–217. [Google Scholar]