Abstract
Apathy and hypersomnia occur after stroke and, by definition, reduce participation in rehabilitation, but their effect on outcome from acute rehabilitation is not known. We performed a retrospective review of 213 patients admitted to a stroke-specialized acute rehabilitation unit in the United States. All patients had ischemic or hemorrhagic stroke, and no dementia or dependence on others pre-stroke. We diagnosed apathy and hypersomnia using standardized documentation by treating therapists. We used multiple regression analysis to control for overall impairment (combination of strength, cognitive and sensory measures), age, time since stroke, and stroke type (ischemic or hemorrhagic).
44 (21%) of patients had persistent apathy, and 12 (5.6%) had persistent hypersomnia. Both groups were more impaired in cognition, sustained attention, and more likely to be treated for depression. Patients with apathy were 2.4 times more likely to go to a nursing home, and had discharge FIM scores 12 points below the mean. Patients with hypersomnia were 10 times more likely to go to a nursing home, and had discharge FIM scores 16 points below the mean. These findings indicate that studies to prospectively define these clinical factors and potential confounds using standardized tools are indicated, and if confirmed, justify studies to identify these patients early and develop targeted interventions.
Keywords: apathy, hypersomnia, stroke, rehabilitation
INTRODUCTION
In addition to the well-recognized motor and sensory deficits in patients with stroke, apathy and hypersomnia can reduce goal-directed behavior and therefore participation in rehabilitation. If reduction in participation due to apathy or hypersomnia affects the rehabilitation process, it could explain some of the variability in recovery [1, 2], and treatment of these conditions could improve patients’ response to rehabilitation interventions, reducing disability and improving outcomes. But, as a prerequisite to initiating clinical trials of such treatments, we first need to know the prevalence of these conditions in the acute rehabilitation period, and whether they have an independent effect on outcome.
Apathy is a reduction of goal-directed behavior in the setting of intact consciousness, and can be due to impaired emotional reactivity, motor planning deficits, or inability to self-initiate behaviors [3]. In stroke, it is associated with damage or reduced blood flow to prefrontal cortex and basal ganglia [4–6]. Additionally, it may occur as a consequence of coexisting illnesses such as depression and neurodegenerative diseases, with potentially overlapping mechanisms [7–9].
Considering all etiologies, a recent meta-analysis of 24 studies found that apathy occurs in 29.5 – 40.2% of patients after stroke, and is typically associated with worse disability and enduring cognitive deficits [10]. None of the studies in the meta-analysis were from the American acute rehabilitation population; two were performed in Japan where patients enter rehabilitation > one month after stroke: Hama and colleagues found 40% of patients had apathy by patient-report and 19% by structured interview of the caregiver; Santa and colleagues found 20% with apathy by patient report [11, 12]. Studies that followed patients over time found that apathy generally remains present even up to one year [13, 14].
Hypersomnia is excessive total sleep and can be due to dysfunction of the brain’s arousal network or night-time sleep disruption [15]. It can occur after stroke from focal injury to basal forebrain or diencephalic structures [16], or be a feature of delirium in the setting of metabolic disturbances [17]. Three studies found that hypersomnia occurred between 4 and 18% of patients in the first days after stroke [18–20], and one study found it in 14% of 44 patients in acute rehabilitation [21]. A more recent study, only reported in a review article, found that in the chronic phase (21 +/− 18 months) after stroke 27% of patients had hypersomnia [15].
The published literature is difficult to apply to the American acute rehabilitation because none specifically looked at effect on outcome in this population. Furthermore, studies of apathy in similar time periods after stroke typically excluded patients with aphasia or severe cognitive deficits to allow for use of a patient-report measure such as the Apathy Scale [22]. To address these limitations, we conducted a preliminary study, using existing medical records, to study effects of apathy and hypersomnia on outcomes from acute rehabilitation. Instead of using a patient-report scale, we operationally defined the presence of these conditions using treating therapists’ observations, ensuring that all patients could be included. We hypothesized that, independent of stroke severity and other factors, apathy and hypersomnia would have a negative impact on outcome, as judged by disposition location (nursing home versus home) and disability.
METHODS
Patient Subjects
We conducted a retrospective electronic chart review of patients treated in the Stroke Program at Burke Rehabilitation Hospital in 2011. We included 362 patients admitted from an acute care hospital with a diagnosis of ischemic or hemorrhagic stroke. We then excluded 149 patients who: completed less than seven days of rehabilitation; had a diagnosis of a neurodegenerative disease; required assistance of another person for daily activities prior to the stroke; were transferred out of rehabilitation to a hospital for greater than three days; were admitted more than once within the year (we excluded the later admission); or had inadequate documentation required for analysis. This resulted in a total of 213 patients for analysis.
The study was approved by the Institutional Review Board of Burke Rehabilitation Center.
Available documentation
Data for patient behaviors and other clinical characteristics were obtained from physician and therapist electronic clinical documentation, as well as billing, laboratory and other electronic clinical databases.
Definition of apathy and hypersomnia
Patients were classified as having apathy or hypersomnia based on behaviors documented by physical and speech therapists approximately three to five times per week. This observational approach allowed us to include patients with aphasia and cognitive deficits that would be unable to complete self-report scales, and ensured that the behaviors were present during therapy sessions. To limit the effect of transient disturbances of arousal (e.g., a night of inadequate sleep or infection), we required that the behaviors were documented in at least half of the daily progress notes, with a minimum of three total notes.
To define apathy and hypersomnia, we chose from a list of terms that therapists used to document patient behavior. This list included: “hypoarousal”, “internally distracted”, “impulsivity”, “decreased initiation”, “externally distracted”, “flat affect”, “perseveration”, “restlessness”, “lability”, “confabulation.” Our operational definition of apathy was that the therapists selected the term “decreased initiation,” implying that patients did not participate in therapy unless encouraged. This was the closest term to the core of the definition of apathy - “a lack of motivation” – used by most authors [23, 24]. “Flat affect” is also a feature of apathy [23], though we only report on it here as a clinical descriptor. Our operational definition of hypersomnia was that the therapist selected the term “hypoarousal,” used to describe patients who had their eyes closed and appeared sleepy during the therapy session. Note that while this definition of hypersomnia includes patients who were sleepy due to disturbed nighttime sleep, our clinical experience is that this is an uncommon cause of persistent hypersomnia and most patients identified by the operational definition had excessive total sleep. Because this study is based on a retrospective review of routine clinical records in which most patients had a single therapist for the course of their treatment, we were unable to assess inter-rater reliability.
We also recorded speech therapist documentation of findings in the first 48 hours after admission that have been reported to co-occur with apathy, including impaired sustained attention, and impaired executive dysfunction [5, 25]. Therapists used a variety of tasks to test for these, and then documented their presence or absence based on an overall impression.
Outcome measures
Our first outcome was discharge disposition (home versus nursing home), as home discharge is a fundamental goal of an acute rehabilitation stay. Our second outcome was disability, the foremost factor in determining home discharge [26]. We defined disability as the mean discharge (final 48 hours of stay) FIM™ score (UB Foundation Activities, Inc.). The 18 items of the FIM are scored on an ordinal scale from 1 (dependent) to 7 (no assistance needed), so a total score ranges from 18 to 126. We calculated total FIM with the standard procedures, except that mobility was determined only by walking independence and not wheelchair independence.
We also performed two exploratory outcome measures: change in total FIM from admission (mean over first 48 hours) to discharge (mean over final 48 hours); and FIM at three months post-discharge. Change in FIM was not used as a primary outcome measure because patients had varied lengths of stay, and a recent study using Rasch analysis found that changes in the FIM are not comparable across different levels of the scale due to non-linearities in the tool [27]. Three month post-discharge FIM was also not used as a primary outcome measure as it was obtained by phone and was not available on approximately one-half of the patients.
We did not use change in impairment as an outcome measure, as impairment measures (e.g., Fugl-Meyer, Motricity Index) were not available at multiple time points from a sufficient number of patients.
Additional predictors
In addition to hypersomnia and apathy, we tested the outcome measures against other patient characteristics, first with univariate statistics, and then with multiple regression analyses, including those factors with p<0.1 on the univariate analyses. We decided a priori to not include length of stay in the multiple regression analyses as it was typically determined within the first week of admission based on diagnosis and level of disability, and therefore primarily reflected stroke severity.
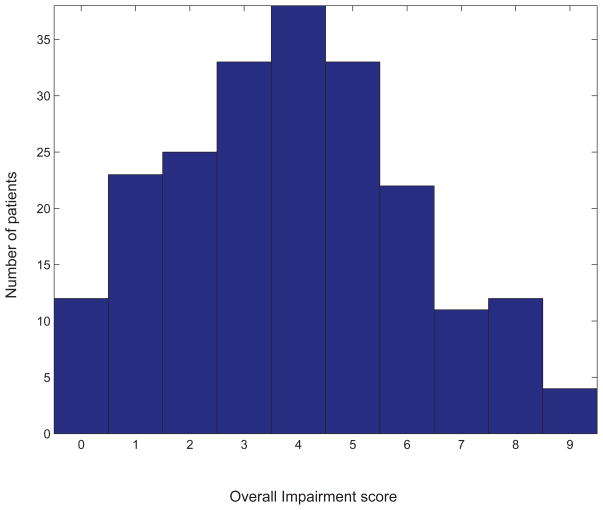
To characterize patients’ impairment we created an overall measure similar to the NIH Stroke Scale (NIHSS), using available clinical data from the first 24 hours of admission (Table 1). The NIHSS was not available in the medical records. Our measure, like the NIHSS, includes tests of motor function, language, sensation, vision, neglect, and overall cognition. We chose to use an overall impairment measure, rather than testing each component individually, as deficits typically co-occurred in moderate to severe strokes. The overall impairment measure also formed a unimodal distribution, which facilitated statistical testing (Figure 1).
Table 1.
Components of the overall impairment measure.
| Component | Examiner | Points towards total |
|---|---|---|
| Weakness (by Motricity Index*) | Physician | 0 = 4 points; 1–34 = 3 points; 35–64 = 2 points; 65–99 = 1 point; 100 = 0 points |
| Sensory loss | Physician | 1 point if present |
| Visual field cut or visual neglect | Physician | 1 point if present |
| Aphasia | Speech therapist | 1 point if present |
| Spatial neglect | Occupational therapist | 1 point if present |
| Cognitive Deficit** | Physician (MMSE) and therapists (FIM) | 1 point if MMSE<24 or admission FIM Problem Solving <4 |
Motricity Index [36, 37] is a scale that converts a subset of the Medical Research Council (1 to 5 point) scale [38] into 0 to 100 points (0 no movement, 100 normal). It has been shown to correlate with the Rivermead Motor Assessment [39], Barthel ADLs [37], and with the Fugl-Meyer (r=0.87) in the 78% of our patients who had admission Fugl-Meyer performed.
Cognitive deficit was scored as present if Mini-Mental Status Exam (MMSE) <24 or FIM Problem Solving <4, as the MMSE is not sensitive to executive function deficits [40].
Figure 1.
Histogram of our overall impairment measure (Table 1) in all 213 subjects. Note that combining the six components into one measure produces a unimodal distribution.
We did not study imaging findings of stroke location, as we did not have access to original imaging studies for most patients.
Statistical Analyses
Pearson’s chi-squared test was used to test association between binary variables (e.g., discharge location and presence of apathy). Student’s t-test was used to compare means of continuous variables between groups (e.g., age and presence vs. absence of apathy). Simple linear regression was used to compare continuous variables with each other (e.g., age and discharge FIM score). All tests were two-tailed.
To treat multiple predictors together, we used multiple logistic regression for the outcome of discharge disposition (a binary variable), and multiple linear regression for discharge total FIM (an ordinal scale that is typically treated as continuous).
Statistical tests were run with built-in and in-house Matlab (Mathworks, MA, USA) code.
RESULTS
Description of patients
Of the 213 patients who met inclusion and exclusion criteria, 44 (21%) had persistent apathy, 12 (5.6%) had persistent hypersomnia, and 9 (4.2%) had both. The remaining 148 patients (those without persistent apathy or hypersomnia) were treated as controls. We compared these groups on a range of demographics and exam findings reported by therapists and physicians from admission exams (Table 2). There were no statistically significant associations of apathy or hypersomnia with age, hemorrhagic stroke, gender, or history of previous stroke. Compared to control patients, those with apathy or hypersomnia had worse overall cognition (MMSE < 24 or FIM problem solving <4), impaired sustained attention, and flat affect. The hypersomnia patients were significantly weaker (by Motricity Index). Both groups also had more disability at admission (by FIM), longer lengths of stay, and higher rates of nursing home discharge. Patients with apathy or hypersomnia were more likely to be placed on alerting medications (modafinil or amantadine), though only a total of 6 patients were on these medications.
Table 2. Univariate comparison between patients with and without apathy/hypersomnia.
Values represent mean (25th–75th percentile) or percent (95% confidence interval by binomial). Values for patients with hypersomnia or apathy were compared to controls using t-tests or chi-squared as appropriate. ∘ are predictors used to create the overall impairment measure (Table 1). To account for multiple comparisons in comparing clinical descriptors between patient groups we used the False Discovery Rate (FDR) method [41, 42]. This changed the p≤0.05 threshold for statistical significance to p≤0.017 (represented by a * here and in Table 5). See manuscript for descriptions of individual characteristics.
| Control Patients (n=166) | Hypersomnia (n=12) | Apathy (n=44) | |
|---|---|---|---|
| Demographics | |||
| Age | 74.9 (68.0 – 82.0) | 78.2 (74.0 – 82.5) | 78.1 (73.5 – 84.0) |
| Length Of Stay | 20.2 (16.0 – 24.0) | *26.6 (23.0 – 30.5) | *23.6 (20.5 – 27.0) |
| Days Since Stroke | 8.2 (5.0 – 9.0) | 11.8 (6.0 – 15.0) | 8.8 (6.0 – 11.5) |
| Female | 52% (44 – 60%) | 33% (10 – 65%) | 59% (43 – 74%) |
| Hemorrhagic stroke | 13% (8 – 19%) | 17% (2 – 48%) | 16% (7 – 30%) |
| Previous Stroke | 23% (17 – 31%) | 33% (10 – 65%) | 30% (17 – 45%) |
| Discharged to nursing home | 26% (19 – 33%) | *83% (52 – 98%) | *61% (45 – 76%) |
| Exam findings from first 48 hours of admission | |||
| FIM | 65.4 (57.2 – 76.6) | *38.0 (26.9 – 51.0) | *47.5 (33.1 – 58.9) |
| Impaired Attention | 47% (37 – 52%) | *100% (74 – 100%) | *83% (65 – 90%) |
| Flat Affect | 8% (5 – 14%) | *67% (35 – 90%) | *66% (50 – 80%) |
| Executive Dysfunction | 58% (51 – 66%) | 92% (62 – 100%) | 70% (55 – 83%) |
| Motricity Index∘ | 64.8 (45.0 – 88.0) | *42.6 (0.0 – 100.0) | 53.9 (21.0 – 86.2) |
| Sensory Abnormality∘ | 47% (39 – 55%) | 58% (28 – 85%) | 61% (45 – 76%) |
| Visual Field Abnormality∘ | 55% (48 – 63%) | 58% (28 – 85%) | 68% (52 – 81%) |
| Aphasia∘ | 32% (25 – 40%) | 25% (5 – 57%) | 36% (22 – 52%) |
| Neglect∘ | 27% (21 – 35%) | 50% (21 – 79%) | 39% (24 – 55%) |
| Cognitive Deficit∘ | 53% (45 – 61%) | *92% (62 – 100%) | *84% (70 – 93%) |
| Treatment | |||
| Alerting Medication | 1% (0 – 4%) | *33% (10 – 65%) | *9% (3 – 22%) |
Effect of apathy and hypersomnia on outcome
On univariate analysis, the strongest correlates of nursing home disposition and discharge FIM were overall impairment (defined in Table 1), apathy and hypersomnia (all p<0.001; Table 3). Days between stroke and admission, age, and hemorrhagic stroke also correlated but less strongly.
Table 3. Univariate testing of patient descriptors against the two outcome measures.
For discharge disposition: we report odds ratio only for binary predictors; p-values are by chi-squared for binary predictors and t-test for continuous predictors. For discharge total FIM: estimated effect size is column 2 minus column 1 for those with p<0.1; p-values are by t-test for binary predictors and by simple linear regression for continuous predictors.
| Discharge Disposition
| ||||
|---|---|---|---|---|
| Nursing home discharge Odds ratio (95% CI) | Nursing home discharge % (95% CI) or value (25th to 75th percentiles) | Home discharge % (95% CI) or value (25th to 75th percentiles) | p-value | |
| Days Since Stroke | 9.8 (5.0 to 12.5) | 7.8 (5.0 to 9.0) | 0.019 | |
| Age | 78.4 (74.0 to 84.5) | 74.2 (67.0 to 81.0) | 0.002 | |
| Female | 0.81 (0.46 to 1.4) | 50% (38 to 62%) | 55% (47 to 64%) | 0.462 |
| Hemorrhagic Stroke | 2.6 (1.2 to 5.8) | 21% (12 to 32%) | 9% (5 to 15%) | 0.018 |
| Previous Stroke | 0.63 (0.32 to 1.3) | 19% (11 to 30%) | 28% (20 to 36%) | 0.19 |
| Overall Impairment | 5.4 (4.0 to 7.0) | 3.2 (2.0 to 4.0) | <0.001 | |
| Apathy | 4.4 (2.2 to 8.8) | 38% (26 to 50%) | 12% (7 to 19%) | <0.001 |
| Hypersomnia | 11 (2.4 to 53) | 14% (7 to 24%) | 1% (0 to 5%) | <0.001 |
| Discharge Total FIM
| ||||
|---|---|---|---|---|
| FIM with predictor present or at its 75th percentile (95% CI) | FIM with predictor absent or at its 25th percentile (95% CI) | Estimated Effect Size | p-value | |
| Days Since Stroke | 83.4 (78.8 – 88.0) | 87.0 (84.7 – 89.3) | 3.6 | 0.002 |
| Age | 81.4 (58.6 – 104.3) | 87.6 (68.4 – 106.7) | 6.2 | 0.001 |
| Female | 83.7 (75.0 – 99.3) | 85.4 (71.9 – 102.1) | N/A | 0.532 |
| Hemorrhagic Stroke | 77.9 (61.0 – 93.6) | 85.5 (74.9 – 101.4) | 7.5 | 0.064 |
| Previous Stroke | 85.7 (74.4 – 100.8) | 84.1 (72.0 – 101.0) | N/A | 0.607 |
| Overall Impairment | 78.2 (73.4 – 83.0) | 95.3 (93.4 – 97.2) | 17.1 | <0.001 |
| Apathy | 67.1 (51.0 – 89.6) | 89.0 (79.8 – 101.9) | 21.9 | <0.001 |
| Hypersomnia | 54.6 (37.2 – 67.0) | 86.3 (75.9 – 101.2) | 31.7 | <0.001 |
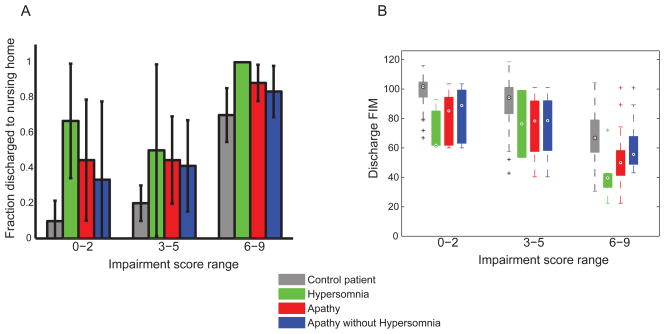
On multiple regression analysis, apathy and hypersomnia remained associated with both primary outcome measures after adjusting for all other factors (Table 4). Patients with apathy were 2.4 times more likely to go to a nursing home and had discharge FIM scores 12 points lower than the mean. Patients with hypersomnia were 10 times more likely to go to a nursing home and had discharge FIM scores 16 points lower than the mean. To highlight the independence from stroke severity, Figure 2 shows that at all ranges of our overall impairment measure, patients with apathy or hypersomnia were more likely to go to a nursing home and had lower discharge FIM.
Table 4.
Multiple regression analyses.In the first data column we report the odds ratio and beta, both in units of change in outcome per unit change of the predictor. In the second data column we report the continuous predictors (e.g., age) as difference in outcome at its 75th percentile versus its 25th percentile for easier comparison with the binary predictors (e.g., presence of apathy). Values for binary predictors represent effect on outcome when present vs. absent.
| Logistic Regression for Nursing Home Disposition
| |||
|---|---|---|---|
| Odds ratio (95% CI) | Odds ratio of 75th vs. 25th percentile or present vs. absent (95% CI) | p-value | |
| Age | 1.06 (1.02 to 1.10) | 2.17 (1.26 to 3.72) | 0.005 |
| Overall Impairment | 1.76 (1.44 to 2.14) | 5.41 (2.97 to 9.86) | <0.001 |
| Days Since Stroke | 1.04 (0.98 to 1.10) | 1.21 (0.90 to 1.63) | 0.203 |
| Hemorrhagic stroke | 1.86 (0.69 to 5.01) | 1.86 (0.69 to 5.01) | 0.218 |
| Apathy | 2.41 (1.01 to 5.74) | 2.41 (1.01 to 5.74) | 0.046 |
| Hypersomnia | 10.06 (1.33 to 75.83) | 10.06 (1.33 to 75.83) | 0.025 |
| Linear regression for Total FIM at Discharge
| |||
|---|---|---|---|
| Beta (95% CI) | Difference in discharge FIM, 75th vs. 25th percentile or present vs. absent (95% CI) | p-value | |
| Age | −0.33 (−0.5 to −0.1) | −4.4 (−6.9 to −1.8) | <0.001 |
| Overall Impairment | −4.82 (−5.7 to −4.0) | −14.0 (−17.0 to −12.0) | <0.001 |
| Days Since Stroke | −0.36 (−0.7 to −0.04) | −1.8 (−3.4 to −0.18) | 0.031 |
| Hemorrhagic Stroke | −1.42 (−6.8 to 4.0) | −1.4 (−6.8 to 4.0) | 0.609 |
| Apathy | −12.4 (−17.2 to −7.6) | −12.4 (−17.2 to −7.6) | <0.001 |
| Hypersomnia | −16.2 (−24.6 to −7.9) | −16.2 (−24.6 to −7.9) | <0.001 |
Figure 2.
Presence of apathy and hypersomnia are associated with higher nursing home discharge rates (2a) and lower discharge FIM scores (2b), after controlling for our overall impairment measure (Table 1). Error bars in 2a are 95% confidence limits by binomial statistics. 2b: Encircled dots are medians; bars represent 25th to 75th percentiles; whiskers extend to extreme data points not considered outliers.
As seen in Table 4, hypersomnia was more strongly associated with the outcome measures than apathy. Since 20% of the patients with apathy also had hypersomnia, we wondered whether the effect of apathy was merely due to the subset with hypersomnia. To address this, we calculated the same multiple regression analyses with hypersomnia patients removed. We found that apathy remained correlated with fraction discharged to nursing home, but was no longer statistically significant (odds ratio 2.19, 95% CI 0.91 to 5.29, p=0.082). Apathy remained significantly correlated with lower discharge FIM (10.73 points lower, 95% CI −15.71 to −5.75, p<0.001). We also illustrate this with separate bars in Figures 2a and 2b. Thus apathy by itself had a significant association with outcome, though not nearly as large as hypersomnia. We did not do the converse and test the effect of hypersomnia with apathy patients removed, as only 3 of 12 hypersomnia patients did not have apathy.
Exploratory outcome measures
Regarding change in total FIM from admission to discharge, in the whole sample, the mean change was 23.2 points (25th percentile 15.7; 75th percentile 30.3). On univariate analysis, only apathy (4.4 fewer points, p=0.013) and hypersomnia (7.0 fewer points, p=0.024), correlated significantly with change in FIM. Admission FIM, overall impairment, previous stroke, length of stay, age, and days between stroke and admission did not correlate with change in FIM (all p-values >0.2). On multiple regression analysis using apathy and hypersomnia only, neither remained significant predictors of change in FIM (p=0.062 for apathy and p=0.13 for hypersomnia). Interpretation of the change in FIM is problematic because, as mentioned above, the scale is not a uniform reflection of function: the same change in score at the lower end of the scale may represent a different functional improvement than at the upper end of the scale.
Regarding FIM at three months post discharge, we had data on 52% of patients (110 out of the 213 patients). 17 (15%) of this subgroup of patients had apathy, and 2 (2%) had hypersomnia during their admission. Patients were followed up at a mean of 120 days after discharge (no statistically significant difference in number of days for patients with versus without apathy, t-test p=0.3). In multiple regression analysis, using the same predictors as discharge FIM above, we found that age, overall impairment and apathy remained significant predictors of three-month FIM scores, with apathy predicting 13 points lower FIM (p<0.01). While consistent with discharge FIM scores (Table 4), these results are less reliable due to the low number of participants and because the data were obtained by telephone.
Other potential causes of apathy and hypersomnia
Apathy and hypersomnia can occur in patients with stroke due to causes other than the stroke. As this was a retrospective study, we were unable to determine the specific cause in a given patient, but we were able to test for the prevalence of three potential causes: infection, depression and sedating medications (Table 5).
Table 5. Univariate testing of other factors that can cause apathy or hypersomnia.
See Table 2 for methodology and interpretation of symbols
| Control Patients (n=166) | Hypersomnia (n=12) | Apathy (n=44) | |
|---|---|---|---|
| Infection during stay: | |||
| By Billing Code | 22% (16 – 29%) | 42% (15 – 72%) | 32% (19 – 48%) |
| By Antibiotic Initiation | 34% (27 – 42%) | 50% (21 – 79%) | *59% (43 – 74%) |
| By One Elevated WBC | 20% (15 – 27%) | 33% (10 – 65%) | *39% (24 – 55%) |
| New diagnosis of depression: | |||
| By Billing Code | 39% (31 – 46%) | *92% (62 – 100%) | 57% (41 – 72%) |
| By Antidepressant Initiation | 25% (18 – 32%) | *75% (43 – 95%) | *50% (35 – 65%) |
| Sedating Medication | 8% (4 – 13%) | 8% (0 – 38%) | 14% (5 – 27%) |
We tested for infection using billing codes, initiation of antibiotics, and presence of a single elevated white blood cell count (WBC). Apathy was associated with infection as defined by initiation of antibiotic and an elevated WBC, though there was no association between hypersomnia and infection. We tested for depression by billing code and initiation of treatment with an antidepressant. Both apathy and hypersomnia were associated with both criteria for depression (the association of apathy and depression by billing code was close to statistical significance). We found no association of either condition with use of sedating medications (antipsychotics, benzodiazepines, meclizine, tizanidine and cyclobenzaprine).
DISCUSSION
We found that 21 percent of the patients admitted to an acute rehabilitation hospital for ischemic or hemorrhagic stroke had apathy, and six percent had hypersomnia. Both apathy and hypersomnia were associated with higher rates of nursing home discharges and higher disability (by FIM score) at discharge, even after controlling for stroke severity at admission by a multi-system impairment measure, age, stroke type (hemorrhagic vs. ischemic), and time since stroke. To our knowledge, this is the first study to assess the effect of apathy and hypersomnia on outcome from the American acute rehabilitation population, and confirms the relevance of these disorders in this population.
Our findings on the prevalence of apathy and hypersomnia are generally in accord with previous studies, and reported differences are likely due to differences in patient population and methodology [10, 21]. Other studies of patients in similar time periods after stroke also found more disability in patients with apathy [13, 28, 25, 12] and hypersomnia [21]. We were unable to find other studies correlating apathy or hypersomnia with disposition location from acute rehabilitation.
We found that patients with both apathy and hypersomnia had more impaired overall cognition (abnormal MMSE or FIM problem solving) and were more likely to have deficits of sustained attention as reported by treating therapists (Table 2). Multiple other studies also found correlation of apathy with low MMSE [13, 28, 25], though [12] did not. Hypersomnia also correlated with motor impairment. These associations could reflect proximity of lesions underlying these conditions to those causing other impairments, or that the patients did not perform at their peak ability on impairment measures (i.e., inadequate effort devoted to task performance).
Limitations
The primary limitation of this study is that the data were collected retrospectively using a clinical database, rather than prospectively using formal research scales, and multiple raters to check reliability. Nevertheless, clinical records of individual therapists can be a meaningful and valid measure, as previous work on depression has shown that clinician impression can be as good as formal scales [29]. Clinician impression also offers the ability to assess patients with aphasia or cognitive deficits who cannot respond to the typical questionnaires used for apathy.
Other limitations include the role of possibly confounding factors such as infection and depression. Infections can modify the linkage between stroke and hypersomnia or apathy, as more severe strokes lead to infections (e.g., by dysphagia or urinary retention), which can lead to delirium, a disorder of arousal and attention [17]. We found no association of infections and hypersomnia. We found an unclear association of infection with apathy, as these patients were more likely to have an elevated WBC and be started on an antibiotic, but were no more likely to have a billing code reflecting an infection (Table 5). One interpretation that could be tested in a prospective study is that apathetic patients were more likely to be tested and treated for presumed infections that turned out to not be actual infections.
Depression is a potential confounder, as it is associated with worse stroke outcomes [30, 31], and can present with apathy [7]. Our database did not include formal testing of depression, but both groups were more likely to have billing codes consistent with depression (trend for apathy, significant for hypersomnia), and more than twice as likely to be treated with antidepressants (Table 5). Multiple previous studies found no association between depression and apathy after stroke [28, 32, 11, 12], suggesting that many of these patients were not actually depressed, but were treated as if they were. More studies are needed to disambiguate depression from apathy and hypersomnia in stroke patients, especially as there are reports of some antidepressants worsening apathy [33, 34].
IMPLICATIONS
While we have shown that apathy and hypersomnia are strongly associated with outcome from acute rehabilitation, we do not address the mechanism of this effect. There are several possibilities, not mutually exclusive. One possibility is that the behavioral abnormalities of these conditions result in more dependence on others, and therefore need for institutional care. This is supported by our finding of lower FIM score after controlling for overall impairment (Figure 2b and Table 4). A second possibility is that patients with these conditions have slower rates of recovery due to decreased participation in therapy. This could be formally evaluated in a prospective study with serial measurements of impairment as well as participation. A third possibility is that both apathy and hypersomnia are signs of under-aroused brains, which are not performing as well as they could (as proposed in [35]).
Our findings demonstrate that apathy and hypersomnia are common in patients undergoing acute rehabilitation after stroke. Both conditions contributed to explaining the range of outcomes of patients in acute rehabilitation, and should be added as covariates in prospective observational and interventional studies. Both can be measured by purely observational means, allowing inclusion of patients with language and cognitive disorders [21, 32], and should use validated and blinded measures to the extent possible. If prospective trials confirm their relevance, targeted treatments should be developed based on studies of underlying mechanism. Adequate treatment at an early stage could potentially improve patient response to acute rehabilitation, thereby lowering costs of care by increasing the fraction of patients discharged home.
Acknowledgments
We thank Cathy Dwyer, Janet Herbold and Michael Reding from the Burke Rehabilitation Hospital for assistance with acquiring and interpreting the original data. We gratefully acknowledge funding support from the Burke Medical Research Institute.
Footnotes
Compliance with Ethics Requirements
Ari Harris declares that he has no conflict of interest.
Jessica Elder declares that she has no conflict of interest.
Nicholas Schiff declares that he has no conflict of interest.
Jonathan Victor declares that he has no conflict of interest.
Andrew Goldfine declares that he has no conflict of interest.
Contributor Information
Ari L. Harris, Burke Medical Research Institute, Weill Cornell Medical College, 785 Mamaroneck Ave, White Plains, NY, USA, 106051.
Jessica Elder, Department of Biostatistics and Epidemiology, Weill Cornell Medical College, Burke Medical Research Institute, 785 Mamaroneck Ave, White Plains, NY, USA, 10605.
Nicholas D. Schiff, Brain and Mind Research Institute, Weill Cornell Medical College, 1300 York Ave, New York, NY, USA, 10065.
Jonathan D. Victor, Brain and Mind Research Institute, Weill Cornell Medical College, 1300 York Ave, New York, NY, USA 10065.
Andrew M. Goldfine, Burke Medical Research Institute, Weill Cornell Medical College, 785 Mamaroneck Ave, White Plains, NY, USA, 10605.
REFERENCE LIST
- 1.Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke. 1992;23(8):1084–9. doi: 10.1161/01.str.23.8.1084. [DOI] [PubMed] [Google Scholar]
- 2.Prabhakaran S, Zarahn E, Riley C, Speizer A, Chong JY, Lazar RM, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabilitation and Neural Repair. 2008;22(1):64–71. doi: 10.1177/1545968307305302. [DOI] [PubMed] [Google Scholar]
- 3.Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex. 2006;16(7):916–28. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- 4.Murakami T, Hama S, Yamashita H, Onoda K, Kobayashi M, Kanazawa J, et al. Neuroanatomic Pathways Associated With Poststroke Affective and Apathetic Depression. Am J Geriatr Psychiatry. 2013 doi: 10.1016/j.jagp.2013.01.057. [DOI] [PubMed] [Google Scholar]
- 5.Okada K, Kobayashi S, Yamagata S, Takahashi K, Yamaguchi S. Poststroke apathy and regional cerebral blood flow. Stroke. 1997;28(12):2437–41. doi: 10.1161/01.str.28.12.2437. [DOI] [PubMed] [Google Scholar]
- 6.Onoda K, Kuroda Y, Yamamoto Y, Abe S, Oguro H, Nagai A, et al. Post-stroke apathy and hypoperfusion in basal ganglia: SPECT study. Cerebrovasc Dis. 2011;31(1):6–11. doi: 10.1159/000319771. [DOI] [PubMed] [Google Scholar]
- 7.American Psychiatric A. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR Fourth Edition. 4. Amer Psychiatric Pub; 2000. [Google Scholar]
- 8.Ligthart SA, Richard E, Fransen NL, Eurelings LSM, Beem L, Eikelenboom P, et al. Association of vascular factors with apathy in community-dwelling elderly individuals. Arch Gen Psychiatry. 2012;69(6):636–42. doi: 10.1001/archgenpsychiatry.2011.1858. [DOI] [PubMed] [Google Scholar]
- 9.Robert P, Onyike CU, Leentjens AF, Dujardin K, Aalten P, Starkstein S, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. European psychiatry: the journal of the Association of European Psychiatrists. 2009;24(2):98–104. doi: 10.1016/j.eurpsy.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 10.van Dalen JW, van Charante EPM, Nederkoorn PJ, van Gool WA, Richard E. Poststroke Apathy. Stroke. 2013 doi: 10.1161/STROKEAHA.112.674614. [DOI] [PubMed] [Google Scholar]
- 11.Hama S, Yamashita H, Shigenobu M, Watanabe A, Hiramoto K, Kurisu K, et al. Depression or apathy and functional recovery after stroke. Int J Geriat Psychiatry. 2007;22(10):1046–51. doi: 10.1002/gps.1866. [DOI] [PubMed] [Google Scholar]
- 12.Santa N, Sugimori H, Kusuda K, Yamashita Y, Ibayashi S, Iida M. Apathy and functional recovery following first-ever stroke. Int J Rehabil Res. 2008;31(4):321–6. doi: 10.1097/MRR.0b013e3282fc0f0e. [DOI] [PubMed] [Google Scholar]
- 13.Mayo NE, Fellows LK, Scott SC, Cameron J, Wood-Dauphinee S. A longitudinal view of apathy and its impact after stroke. Stroke. 2009;40(10):3299–307. doi: 10.1161/STROKEAHA.109.554410. [DOI] [PubMed] [Google Scholar]
- 14.Withall A, Brodaty H, Altendorf A, Sachdev PS. A longitudinal study examining the independence of apathy and depression after stroke: the Sydney Stroke Study. Int Psychogeriatr. 2011;23(2):264–73. doi: 10.1017/S1041610209991116. [DOI] [PubMed] [Google Scholar]
- 15.Bassetti CL, Hermann DM. Sleep and stroke. Handb Clin Neurol. 2011;99:1051–72. doi: 10.1016/B978-0-444-52007-4.00021-7. [DOI] [PubMed] [Google Scholar]
- 16.Bassetti C, Mathis J, Gugger M, Lovblad KO, Hess CW. Hypersomnia following paramedian thalamic stroke: a report of 12 patients. Annals of Neurology. 1996;39(4):471–80. doi: 10.1002/ana.410390409. [DOI] [PubMed] [Google Scholar]
- 17.Oldenbeuving AW, de Kort PLM, Jansen BPW, Algra A, Kappelle LJ, Roks G. Delirium in the acute phase after stroke: incidence, risk factors, and outcome. Neurology. 2011;76(11):993–9. doi: 10.1212/WNL.0b013e318210411f. [DOI] [PubMed] [Google Scholar]
- 18.Marquardsen J. The natural history of acute cerebrovascular disease: a retrospective study of 769 patients. Acta Neurol Scand. 1969;45(Suppl-38):11+. [PubMed] [Google Scholar]
- 19.Terént A, Andersson B. The prognosis for patients with cerebrovascular stroke and transient ischemic attacks. Ups J Med Sci. 1981;86(1):63–74. doi: 10.3109/03009738109179211. [DOI] [PubMed] [Google Scholar]
- 20.Kotila M. Declining incidence and mortality of stroke? Stroke. 1984;15(2):255–9. doi: 10.1161/01.str.15.2.255. [DOI] [PubMed] [Google Scholar]
- 21.Reding MJ, Gardner C, Hainline B, Devinsky O. Neuropsychiatric Problems Interfering with Inpatient Stroke Rehabilitation. Neurorehabilitation and Neural Repair. 1993;7(1):1–7. doi: 10.1177/136140969300700102. [DOI] [Google Scholar]
- 22.Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 1992;4(2):134–9. doi: 10.1176/jnp.4.2.134. [DOI] [PubMed] [Google Scholar]
- 23.Starkstein SE, Leentjens AFG. The nosological position of apathy in clinical practice. J Neurol Neurosurg Psychiatr. 2008;79(10):1088–92. doi: 10.1136/jnnp.2007.136895. [DOI] [PubMed] [Google Scholar]
- 24.Marin RS, Wilkosz PA. Disorders of diminished motivation. J Head Trauma Rehabil. 2005;20(4):377–88. doi: 10.1097/00001199-200507000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Brodaty H, Sachdev PS, Withall A, Altendorf A, Valenzuela MJ, Lorentz L. Frequency and clinical, neuropsychological and neuroimaging correlates of apathy following stroke - the Sydney Stroke Study. Psychol Med. 2005;35(12):1707–16. doi: 10.1017/S0033291705006173. [DOI] [PubMed] [Google Scholar]
- 26.Ween JE, Alexander MP, D’Esposito M, Roberts M. Factors predictive of stroke outcome in a rehabilitation setting. Neurology. 1996;47(2):388–92. doi: 10.1212/wnl.47.2.388. [DOI] [PubMed] [Google Scholar]
- 27.Glenny C, Stolee P, Thompson M, Husted J, Berg K. Underestimating Physical Function Gains: Comparing FIM Motor Subscale and interRAI Post Acute Care Activities of Daily Living Scale. Archives of Physical Medicine and Rehabilitation. 2012;93(6):1000–8. doi: 10.1016/j.apmr.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 28.Starkstein SE, Fedoroff JP, Price TR, Leiguarda R, Robinson RG. Apathy following cerebrovascular lesions. Stroke. 1993;24(11):1625–30. doi: 10.1161/01.str.24.11.1625. [DOI] [PubMed] [Google Scholar]
- 29.Berg A, Lönnqvist J, Palomäki H, Kaste M. Assessment of Depression After Stroke A Comparison of Different Screening Instruments. Stroke. 2009;40(2):523–9. doi: 10.1161/STROKEAHA.108.527705. [DOI] [PubMed] [Google Scholar]
- 30.Kauhanen ML, Korpelainen JT, Hiltunen P, Brusin E, Mononen H, Määttä R, et al. Poststroke Depression Correlates With Cognitive Impairment and Neurological Deficits. Stroke. 1999;30(9):1875–80. doi: 10.1161/01.STR.30.9.1875. [DOI] [PubMed] [Google Scholar]
- 31.Pohjasvaara T, Vataja R, Leppävuori A, Kaste M, Erkinjuntti T. Depression is an independent predictor of poor long-term functional outcome post-stroke. European Journal of Neurology. 2001;8(4):315–9. doi: 10.1046/j.1468-1331.2001.00182.x. [DOI] [PubMed] [Google Scholar]
- 32.Carota A, Berney A, Aybek S, Iaria G, Staub F, Ghika-Schmid F, et al. A prospective study of predictors of poststroke depression. Neurology. 2005;64(3):428–33. doi: 10.1212/01.WNL.0000150935.05940.2D. [DOI] [PubMed] [Google Scholar]
- 33.Kodela S, Venkata PD. Antidepressant induced apathy responsive to dose reduction. Psychopharmacol Bull. 2010;43(4):76–9. [PubMed] [Google Scholar]
- 34.Padala PR, Padala KP, Monga V, Ramirez DA, Sullivan DH. Reversal of SSRI-associated apathy syndrome by discontinuation of therapy. Ann Pharmacother. 2012;46(3) doi: 10.1345/aph.1Q656. [DOI] [PubMed] [Google Scholar]
- 35.Goldfine AM, Schiff ND. What is the role of brain mechanisms underlying arousal in recovery of motor function after structural brain injuries? Curr Opin Neurol. 2011;24(6):564–9. doi: 10.1097/WCO.0b013e32834cd4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demeurisse G, Demol O, Robaye E. Motor Evaluation in Vascular Hemiplegia. European Neurology. 1980;19(6):382–9. doi: 10.1159/000115178. [DOI] [PubMed] [Google Scholar]
- 37.Wade DT, Hewer RL. Motor loss and swallowing difficulty after stroke: frequency, recovery, and prognosis. Acta Neurol Scand. 1987;76(1):50–4. doi: 10.1111/j.1600-0404.1987.tb03543.x. [DOI] [PubMed] [Google Scholar]
- 38.Medical Research C. Aids to the Investigation of Peripheral Nerve Injuries. J Neurol Psychiatry. 1943;6(1–2) [Google Scholar]
- 39.Collin C, Wade D. Assessing motor impairment after stroke: a pilot reliability study. Journal of Neurology, Neurosurgery & Psychiatry. 1990;53(7):576–9. doi: 10.1136/jnnp.53.7.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB A frontal assessment battery at bedside. Neurology. 2000;55(11):1621–6. doi: 10.1212/WNL.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 41.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Statist Soc B. 1995;57(1):289–300. [Google Scholar]
- 42.Benjamini Y, Yekutieli D. The Control of the False Discovery Rate in Multiple Testing under Dependency. The Annals of Statistics. 2001;29(4):1165–88. [Google Scholar]