Abstract
We report the production, isolation and structure elucidation of the sesquiterpene isopterocarpolone from an Appalachian isolate Streptomyces species RM-14-6. While isopterocarpolone was previously put forth as a putative plant metabolite, the current study highlights the first native bacterial production of isopterocarpolone and the first full characterization of isopterocarpolone using 1D and 2D NMR spectroscopy and HR-ESI mass spectrometry. Considering the biosynthesis of closely related metabolites (geosmin or 5-epiaristolochene), the structure of isopterocarpolone also suggests the potential participation of one or more unique enzymatic transformations. In this context, this work also sets the stage for the elucidation of potentially novel bacterial biosynthetic machinery.
Keywords: terpene, natural product, biosynthesis, cyclase, isoprenoid, bacteria
1. Introduction
Bacterial terpenoids are attractive targets given the relative simplicity of bacterial genomics, genetic manipulation and corresponding enzymology studies. Consequently, there is heightened interest in the identification and isolation of bacterial terpenes (Meguro et al., 2013; Nakano et al., 2011). Examples of bacterial sesquiterpenes include geosmin (Gerber & Lechevalier, 1965), (-)-germacradien-4-ol, (-)-epi-abisabolol (Nakano et al., 2011), and kandenols (Ding et al., 2012). As part of our ongoing natural product discovery initiative focused upon unique environments within the Commonwealth of Kentucky (Shaaban et al., 2013; Wang et al., 2013), herein we report the production, isolation and structure elucidation of the sesquiterpene isopterocarpolone (Scheme 1, 3) from an Appalachian isolate designated Streptomyces species RM-14-6. While 3 was previously put forth as a putative plant metabolite (Pterocarpus santalinus) (Kumar et al., 1974), the current study highlights the first native bacterial production of this metabolite and provides the first structural characterization/validation of 1 via 1D and 2D NMR spectroscopy and HR-ESI mass spectrometry.
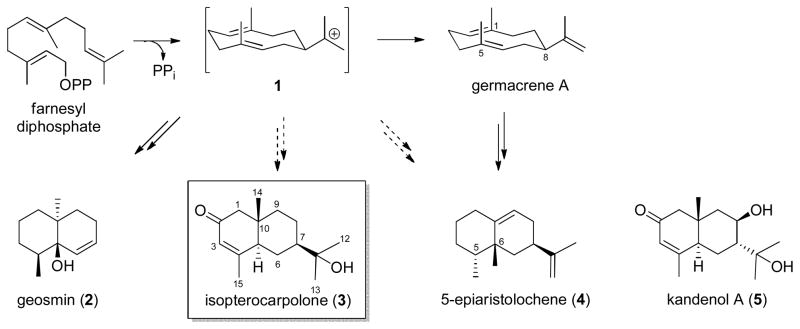
Scheme 1.
Proposed biosynthetic divergence leading to isopterocarpolone (3), 5-epiaristolochene (4) (Whitehead et al., 1989) and geosmin (2) (Jiang et al., 2006) where solid arrows represent known processes and dashed arrows represent putative routes. Kandenol A (5) (Ding et al., 2012) represents a closely related analogue of isopterocarpolone (3).
2. Results and Discussion
HPLC-HRMS analysis of a crude extract from a 50 mL fermentation revealed a peak with the molecular weight of 236 Da (237.1849 [M+H]+). The deduced molecular formula C15H24O2 from HRESIMS implied a member of the sesquiterpene class lacking a suitable match in the AntiBase (Laatsch, 2012). Scale up fermentation (8 L) of the strain followed by isolation and purification afforded 7.1 mg of 3 as a colorless oil (see supporting information). The 1H NMR spectrum of 3 in CDCl3 displayed a singlet peak of an olefinic proton at δ 5.83 along with four methyl group signals at δ 1.91, 1.28, 1.28 and 0.91. The methyl signal at δ 1.91 suggested the attachment to an sp2-carbon. Additional signals for nine aliphatic protons were observed in the range δ 2.81~ 1.34 ppm. The 13C NMR/HSQC spectra identified 3 as a sesquiterpene comprised of 15 carbon atoms including a carbonyl (δC 199.7), one oxygenated quaternary carbon signal at δ 74.7, and two olefinic carbons (δC 126.8, CH and 164.1, Cq) within an α,β-unsaturated ketone. In addition, signals for four methylenes, one quaternary carbon and four methyl groups were observed between δC 55.1-18.1 ppm. Thorough analyses of 1H, 13C, HSQC, COSY, TOCSY and HMBC spectra cumulatively established the structure of 3 as depicted in Scheme 1 and Figure S1 (Supporting Information). The NOE interactions between H-5/H-7ax and H-9ax, and between H3-14/H-6ax and H-8ax in the NOESY spectrum implicated a trans A/B ring juncture with H-5 and the C-10 methyl as axial and an equatorial C-7 hydroxyisopropyl (Supporting Information, Figure S1). Thus, 3 was deduced to be isopterocarpolone, a compound first reportedly isolated from Pterocarpus santalinus (Kumar et al., 1974) but one for which no spectroscopic or spectrometric data has been reported. The absolute structure of the isopterocarpolone was concluded to be 5R,7R,10S, and the determined optical rotation of 3 of [α]D25 = +42° (CHCl3) is consistent with the previously reported value of [α]D32 = +47° (CHCl3) (Kumar et al., 1974; Kitajima et al. 2003). Isopterocarpolone (3) was inactive in antibacterial, antifungal, and cytotoxicity assays (See supporting information).
To the best of our knowledge, this is the first reported isolation of isopterocarpolone from bacteria. While geosmin (2) and kandenol A (5) represent bacterial metabolites structurally related to isopterocarpolone (3), the plant terpenoid 5-epiaristolochene (4) shares an identical skeletal framework with divergence deriving through the intermediate 1 via C-1 to C-6 methyl migration and inversion of C-5 stereochemistry (Scheme 1) (Whitehead et al., 1989). Biosynthetically, the key cyclization event en route to 2 that results in the formation of decalene backbone proceeds with the elimination of an acetone molecule (Scheme 1; Jiang et al., 2006) while the genesis of 4 proceeds through germacrene A (Rising et al., 2000). For the latter, the addition of a proton at the C-5 of germacrene followed by C1-C6 cyclization and C-1→C-6 methyl and C-6→C-5 migrations has been proposed for the biosynthesis of 4. Interestingly, neither biosynthetic strategy provides a plausible explanation for the biosynthesis of 3. Thus, the potential participation of one or more unique enzymatic transformations in the biosynthesis of 3 is expected and, in this context, the isolation and characterization of 3 reported herein may provide an unparalleled resource for the elucidation of the underlying catalytic process.
3. Conclusions
In this study, we report the new bacterial sesquiterpene 3 from an Appalachian isolate Streptomyces species RM-14-6. While 3 was previously implicated as a plant metabolite, the structure of 1 is fully assigned here for the first time using 1D and 2D NMR spectroscopy and HR-ESI mass spectrometry. Considering the biosynthesis of closely related sesquiterpenes (geosmin or 5-epiaristolochene), the structure of isopterocarpolone (3) also suggests the potential participation of one or more unique enzymatic transformations. This work also sets the stage for the elucidation of potentially novel bacterial biosynthetic machinery.
Supplementary Material
Acknowledgments
This work was supported in part by the University of Kentucky College of Pharmacy, the University of Kentucky Markey Cancer Center and the National Center for Advancing Translational Sciences (UL1TR000117).
Footnotes
Conflict of Interest
The authors report competing interests. JST is a co-founder of Centrose (Madison, WI).
Supplementary material, including the full experimental details, bacterial strain characterization, physico-chemical properties, and full spectroscopic data of 1, are available online
References
- Ding L, Amier A, Fiebig HH, Lin WH, Peschel G, Hertweck C. Kandenols A-E, eudesmenes from an endophytic Streptomyces sp of the mangrove tree Kandelia candel. J Nat Prod. 2012;75:2223–2227. doi: 10.1021/np300387n. [DOI] [PubMed] [Google Scholar]
- Gerber NN, Lechevalier HA. Geosmin, an earthly-smelling substance isolated from actinomycetes. Appl Microbiol. 1965;13:935–938. doi: 10.1128/am.13.6.935-938.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, He X, Cane DE. Geosmin biosynthesisStreptomyces coelicolor germacradienol/germacrene D synthase converts farnesyl diphosphate to geosmin. Am Chem Soc. 2006;128:8128–8129. doi: 10.1021/ja062669x. [DOI] [PubMed] [Google Scholar]
- Kitajima J, Kamoshita A, Ishikawa T, Takano A, Fukuda T, Isoda S, Ida Y. Glycosides of Atractylodes lancea. Chem Pharm Bull. 2003;51:673–678. doi: 10.1248/cpb.51.673. [DOI] [PubMed] [Google Scholar]
- Kumar N, Ravindra B, Seshadri T. Terpenoids of Pterocarpus santalinus heartwood. Phytochemistry. 1974;13:633–636. [Google Scholar]
- Laatsch H. AntiBase 2012 The natural compound identifier. Wiley-Vch; [Google Scholar]
- Meguro A, Tomita T, Nishiyama M, Kuzuyama T. Identification and characterization of bacterial diterpene cyclases that synthesize the Cembrane Skeleton. Chembiochem. 2013;14:316–321. doi: 10.1002/cbic.201200651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano C, Kudo F, Eguchi T, Ohnishi Y. Genome mining reveals two novel bacterial sesquiterpene cyclases: (-)-germacradien-4-ol and (-)-epi-alpha-bisabolol synthases from Streptomyces citricolor. Chembiochem. 2011;12:2271–2275. doi: 10.1002/cbic.201100418. [DOI] [PubMed] [Google Scholar]
- Rising KA, Starks CM, Noel JP, Chappell J. Demonstration of germacrene A as an intermediate in 5-epi-aristolochene synthase catalysis. J Am Chem Soc. 2000;122:1861–1866. [Google Scholar]
- Shaaban KA, Wang X, Elshahawi SI, Ponomareva LV, Sunkara M, Copley GC, Hower JC, Morris AJ, Kharel MK, Thorson JS. Herbimycins D-F, Ansamycin Analogues from Streptomyces sp RM-7-15. J Nat Prod. 2013;76:1619–1626. doi: 10.1021/np400308w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Shaaban KA, Elshahawi SI, Ponomareva LV, Sunkara M, Zhang Y, Copley GC, Hower JC, Morris AJ, Kharel MK, Thorson JS. Frenolicins C–G, Pyranonaphthoquinones from Streptomyces sp RM-4-15. J Nat Prod. 2013;76:1441–1447. doi: 10.1021/np400231r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead IM, Threlfall DR, Ewing DF. 5-Epi-aristolochene is a common precursor of the sesquiterpenoid phytoalexins capsidiol and debneyol. Phytochemistry. 1989;28:775–779. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.