Abstract
Background
The production of competent oocytes depends on a bi-directional communication between the oocyte and cumulus cells. The goal of this study was to determine whether simple parameters monitored in cumulus cells from individual human oocytes have any predictive value, and thus correlate with clinically relevant parameters.
Methods
97 cumulus-oocyte complexes were recovered from 31 patients undergoing ICSI treatment. After the oocytes were denuded, cumulus cell density from individual oocytes was determined. Cells were probed for viability using propidium iodide and for apoptosis by Annexin V staining or by monitoring caspase activity. These parameters were correlated with oocyte status, fertilization ability and patient age (≤29 years old and ≥30 years old). All variables were checked for normal distribution and then compared by Kruskal-Wallis, Mann-Whitney or one-way ANOVA tests.
Results
Mature oocytes were surrounded by more cumulus cells (16073±2595, p = 0.026), which were also more viable and less apoptotic than atretic or degenerated oocytes. Mature oocytes that fertilized had higher caspase activity in the surrounding cumulus cells than those that did not fertilize. Younger patients presented lower cumulus cells density (8882±2380 vs. 15036±2143 cells; p = 0.034); and cumulus cells had higher apoptosis levels in younger patients than older ones (6775.5±1831.6 RLU vs. 2591±46.5 RLU, p = 0.002 for caspase activity).
Conclusion
The data suggests that high density and apoptosis of cumulus cells are promising parameters to indirectly predict individual oocyte status. Although more studies and a larger data set are needed, cumulus cells presented the potential to be used as simple predictors of female fertility and/or ovarian ageing.
Keywords: Annexin V, Apoptosis, Caspase activity, Cumulus cells, Cumulus-oocyte complex, Female fertility, Oocyte biomarkers, Woman age
Introduction
The cumulus-oocyte complex is a structure characteristic of higher mammals, resulting in an interesting symbiosis between an oocyte (germline) and surrounding cumulus cells (somatic) (1). Cumulus cells share an intimate communication with the oocyte through gap junctions and paracrine factors (2), allowing a bidirectional supply of nutrients and signaling molecules that regulate the simultaneous development and maturation of both cell types (3–6). It is well-described that the cumulus cells not only support oocyte maturation but also help its conduction in the oviduct and these cells participate in the mechanisms controlling sperm access (7).
The ovulated oocyte is surrounded by an expanded matrix of cumulus cells that works as a viscous matrix (8) and although these cells are progressively lost, they can accompany the oocyte for over 72 hr (9). There are many relevant studies focusing on cumulus cells, namely on oocyte communication (for review see (10), cumulus cell apoptosis (2, 4, 6, 11–17) or their transcriptomic profile (18). However, there is little mention of human cumulus cell density in single complexes, and its relationship with important oocyte and embryo aspects. The only such data indicates that, shortly after ovulation, an average of 20.000 cumulus cells surround a mature oocyte resulting in a cumulus mass of several millimeters (9). Similarly, Hashimoto et al. concluded that a bovine cumulus-oocyte complex contains 21.000 cumulus cells and this is the only report stating that a proper cumulus cells density during oocyte maturation is essential for continual development of corona-enclosed oocytes (19).
Apoptosis is a process of cellular self-destruction that occurs under physiological control and maintains system homeostasis (11, 20). Unlike cell density, apoptosis in cumulus cells is well documented. Since cumulus cells release signals important for oocyte maturation and developmental competence, it is expectable that the occurrence of apoptosis in these cells has an impact on the female gamete (15). However, contradictory reports using different approaches to monitor apoptosis, such as DNA fragmentation levels (2, 4, 6, 11–16), expression of apoptosis-associated molecules (4) or caspase activity (15) have failed to clarify the effects of cumulus cells apoptosis on oocyte function.
The apoptotic process is a sequence of events (20) and one of the earliest is the translocation of the phospholipid phosphatidylserine (PS) from the inner to the outer leaflet of the plasma membrane (21). Moreover, apoptosis is based on the activation of three principal components: Bcl-2 family proteins (22); caspases, an essential group of cystein-aspartic acid proteases required for self-destruction (23); and the Apaf-1/CED-4 protein that relays signals integrated by Bcl-2 family proteins (22).
The aim of the present study was to investigate whether simple parameters monitored in cumulus cells from individual oocytes correlated with important clinical parameters, such as oocyte maturity and quality, oocyte fertilization status, resultant embryo quality and patient age.
Methods
Chemicals
All chemicals were supplied by Sigma-Aldrich (St. Louis, MO, USA), unless stated otherwise.
Biological material
Cumulus cells were obtained from patients undergoing ICSI treatment at the Human Reproduction Service, University Hospitals of Coimbra. Informed consent was obtained from all patients, who signed forms for this purpose, approved by the Institutional Review Board (IRB) of the University Hospitals of Coimbra. All human material was used in accordance with the appropriate ethical guidelines provided by the IRB of the University Hospitals of Coimbra that also approved the study.
Cumulus cells achievement and preparation
From January 2012 to January 2013, a total of 97 cumulus-oocyte complexes from 31 patients were recovered by follicular puncture. The oocytes were denuded (separating cumulus cells from the oocyte) through micropipette manipulation and enzymatic treatment with the SynVitro Hyadase hyaluronidase (Origio, Malov, Denmark). The samples were kept in Flushing medium (Origio) until further study.
Quantification of cumulus cells number per oocyte
Cumulus cells samples were centrifuged (300 g, 5 min), the supernatant discarded and resuspended in PBS. In order to achieve concentration parameter, 10 µl of the sample was placed in a Makler chamber and the cell counting was performed. Finally, the number of cumulus cells per oocyte was estimated.
Detection assay of Caspase-3/7 activity
This Caspase-Glo 3/7 luminiscence assay was performed according to the manufacturer's instructions (24). After preparation of the Caspase-Glo 3/7 reagent, 5000 cumulus cells (in 100 µl of medium) were incubated with 100 µl of the reagent, for one hour at room temperature in the dark. After the incubation period, light emission was read using a Synergy HT Multi-Mode Microplate Reader (BioTek, Winooski, VT, USA).
Annexin V, propidium iodide and Hoechst staining
For Annexin V, staining suspensions of cumulus cells from individual oocytes were incubated with 1 µl of Annexin V (2.5 µg/ml) for 15 min at 37°C in the dark. For viability, staining cells were stained using both 50 µg/ml propidium iodide (Immunostep, Salamanca, Spain) and 10 µg/ml Hoechst 33342 (Molecular Probes, Eugene, OR, USA). No fixation or blocking was performed, as cells were alive. Samples were examined using a Zeiss Axiophot II Imaging Fluorescence microscope (Carl Zeiss, Gottingen, Germany) equipped with a triple bandpass filter, and the percentage of stained cumulus cells was determined by counting 100 cells per coverslip, in at least four different fields. A total of ±10.000 cells were placed on the slide. All experimental counts were performed blinded by at least two observers. Both positive and negative controls for Annexin V were performed as described, using apoptosis inducers such as hydrogen peroxide, and buffer with no calcium, respectively (21). Nuclear staining with Hoescht (alive) or PI (dead) was considered for viability, Annexin V staining was considered as a sign of early apoptosis.
Figure 1.
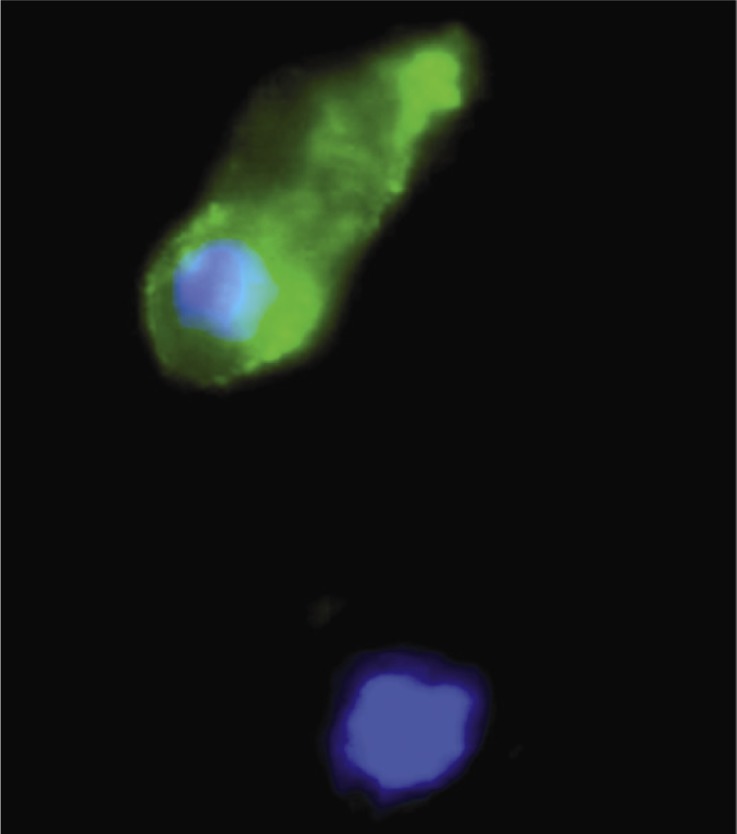
Cumulus cells positive (top) or negative (bottom) for Annexin V staining. DNA is stained with Hoescht 33342
Statistical analysis
Statistical analysis was carried out using SPSS for Windows (version 20, Chicago, IL, USA). All variables were checked for normal distribution using the one sample Kolmogorov-Smirnov test. The Kruskal-Wallis and Mann-Whitney tests were used to compare number of cumulus cells and caspase activity with oocyte quality and maturity. The Mann-Whitney test was also used to compare the number of cumulus cells with fertilization status, embryo quality score and patient age (≤29 years old and ≥30 years old); and to compare caspase activity with different patient age. The independent samples t-test was used to compare caspase activity with oocyte fertilization state and embryo quality score; to compare cumulus cells viability (live/dead cells) and apoptosis (Annexin V positive cells) relatively to different oocyte fertilization status, embryo quality score and patient age. One-way ANOVA and Tukey test were used to compare cumulus cells viability and apoptosis with oocyte quality and maturity groups. Results are presented as means ± standard error. Statistical significance was considered when p < 0.05.
Results
Cumulus cell density per oocyte
Our results indicated that a human oocyte is surrounded on average by 13583 cumulus cells (13583±1751 cells, n = 72). Cell density was dependent on oocyte quality, with mature oocytes (metaphase II oocytes-MII) presenting more surrounding cumulus cells (16073±2595 cells) than atretic (AT; the oocyte suffers atresia) and degenerated oocytes (DEG: the oocyte degenerated and only cumulus cells and sometimes the zona pellucida are present; 7737±1547 cells; p = 0.026; Table 1). Neither oocyte fertilization status nor the quality of the resultant embryo had any relationship with the density of cumulus cells per oocyte (Table 1). However, thirty year old patients or the older ones had a higher cumulus cell density per oocyte (15036±2143 cells) than patients who were twenty nine years old or younger (8882±2380 cells; p = 0.034; Table 1).
Table 1.
Number of cumulus cells per oocyte versus oocyte maturity and quality, oocyte fertilization status and patient age
| Clinical parameter | Number of cumulus cells per oocyte (M±SEM) | n |
|---|---|---|
| Oocyte maturity and quality | ||
| MII oocytes | 16073±2595 * | 41 |
| MI + GV oocytes | 15454±4992 | 11 |
| AT + DEG oocytes | 7737±1547 * | 19 |
| Oocyte fertilization status | ||
| Fertilized oocytes | 15480±3241 | 25 |
| Non fertilized oocytes | 17000±4433 | 16 |
| Patient age (year) | ||
| ≤ 29 | 8882±2380 * | 17 |
| ≥ 30 | 15036±5478 * | 55 |
p < 0.05
MII: Metaphase II; MI: Metaphase I; GV: Germinal Vesicle; AT: Atretic; DEG: Degenerated
Viability and apoptosis of cumulus cells in individual oocytes
As monitored by propidium iodide per-meability, cumulus cell viability did not seem to be related with any of the parameters monitored (data not shown), and we thus decided to focus on apoptotic markers. We were able to observe that cumulus cells surrounding mature MII oocytes not only are more viable/non-apoptotic (Annexin V−; 48.2±4.9%; 17.3±6%; p = 0.008; Table 2) but also tend to be less positive for Annexin V (11.6± 2.3%; 25.8±6.5%; p = 0.051; Table 2) than cumulus cells surrounding AT and DEG oocytes. No differences were observed between the cumulus cells surrounding MI and GV oocytes and the remaining groups (Table 2).
Table 2.
Viability and apoptosis of cumulus cells per individual oocyte relative to several clinical parameters
| Clinical parameter | Live cells (%): Annexin V−/Propidium iodide-(M±SEM) | Annexin V+cells (%) (M±SEM) | n | Caspase-3/7 activity (Mean RLU±SEM) | n |
|---|---|---|---|---|---|
| Oocyte maturity and quality | |||||
| MII oocytes | 48.2±4.9 ** | 11.6±2.6 # | 16 | 2865±614 | 37 |
| MI + GV oocytes | 27.2±12.5 | 11.2±5.8 | 4 | 5090±1808 | 12 |
| AT + DEG oocytes | 17.3±6 ** | 25.8±6.5 # | 6 | 4807±2028 | 14 |
| Oocyte fertilization status | |||||
| Fertilized oocytes | 47.6±5.3 | 13.5±3.2 | 12 | 2885±437 * | 20 |
| Not fertilized oocytes | 50.2±13.1 | 6±2.2 | 4 | 1633±367 * | 16 |
| Patient age (year) | |||||
| ≤29 | 39.3±5.4 | 9.6±2.1*** | 19 | 6775±1831 ** | 17 |
| ≥30 | 33.7±9 | 29.1±4.3*** | 7 | 2591±547 ** | 46 |
p = 0.05
p < 0.05
p ≤ 0.01
p < 0.001
MII: Metaphase II; MI: Metaphase I; GV: Germinal Vesicle; AT: Atretic; DEG: Degenerated
Cumulus cells surrounding oocytes that fertilized after ICSI had a higher caspase activity compared with cumulus cells that were around oocytes that do not fertilize (2884.9±437 RLU; 1632.7±367.1 RLU; p = 0.035; Table 2). However, there were no differences regarding viability and the percentage of Annexin V positive cells (Table 2).
In terms of patient age, we found that patients that were thirty years old or older had a lower percentage of Annexin V positive cumulus cells compared with patients who were twenty nine years old or younger (9.6±2.1; 29.1±4.3; p < 0.001; Table 2). Cumulus cell caspase activity was also lower in older patients compared with younger ones (2591±546.5 RLU; 6775.5±1831.6 RLU; p = 0.002; Table 2). However, cumulus cell viability was the same in both groups (Table 2). Furthermore, cumulus cell density, viability or apoptosis in individual oocytes had no relationship with the quality of the resulting embryo (data not shown).
Discussion
One of the main issues in assisted reproduction is the inefficiency in accurate prediction of the quality of a specific oocyte, and thus its potential to contribute to a successful pregnancy. Given that directly monitoring the oocyte might impair its status, the study of cumulus cells seems a reasonable alternative. However, the caveat is that whatever techniques proposed to evaluate cumulus cells must be quick and reliable in terms of accurately reflecting oocyte quality and must be widely applicable in the clinic. In this study, we showed that very simple and straightforward parameters may be useful in determining oocyte quality using cumulus cells in a clinical setting.
Besides a single report stating that development of bovine corona-enclosed oocytes is dependent on cumulus cells density (19), the role of human cumulus cell density on oocyte maturity and quality, fertilization status, resultant embryo quality and patient age is unknown. In fact, this is the first study which defines that the mean number of human cumulus cells that surround an oocyte collected by follicular puncture is 13583±1751 cells. Taking into account oocyte maturity status, an MII oocyte is surrounded by 16073±2595 cumulus cells, a number close to the only previously described one (20000 cumulus cells) (9), and that is clearly higher than what is found in bad quality/unviable oocytes (AT and DEG oocytes). This suggests that non-viable oocytes must be incapable of supplying cumulus cells with appropriate substances and signaling molecules, and thus that cells may not have the perfect microenvironment to survive or divide. On the other hand, cumulus cells density could be somehow influencing oocyte quality. Curiously, immature oocytes do not differ from bad quality/unviable oocytes in terms of cumulus cell density. In bovine, the developmental competence of oocytes surrounded by cumulus cells was demonstrated to be supported in a cell density-dependent manner, and the addition of proper cumulus cells density (1.6×106 to 3.2× 106 cells/ml) to the maturation medium led to an improvement in development of bovine corona-enclosed oocytes (19). Cumulus cells from MII oocytes that fertilize after ICSI appear to have the same density in comparison with cumulus cells from MII oocytes that do not fertilize. Additionally, no difference in cumulus cells density was found in oocytes that give rise to embryos with different quality. Interestingly, however, our data suggests that younger patients have fewer cumulus cells per oocyte than older ones.
Cumulus cell viability was not related to any of the parameters monitored, suggesting that the possibility of using this simple parameter in order to predict oocyte development after ICSI will be limited. On the other hand, it is known that apoptosis can occur in at least four different follicular compartments, theca cells, mural cells, cumulus cells and in the oocyte (12) and that ovarian follicles with identical morphology have different apoptosis levels in mural granulosa and cumulus cells (25). Therefore, it seems reasonable to correlate this phenomenon with oocyte fate, or quality of the resulting embryo. In fact, it was already proven that the removal of the oocyte from the cumulus-oocyte complex leads to a substantial increase in cumulus cells apoptosis due to the absence of soluble paracrine signals produced and secreted by the oocyte (26).
We observed that MII oocytes appear to be surrounded by more non-apoptotic (Annexin V negative) cumulus cells than AT and DEG oocytes, although no differences were observed regarding immature oocytes. This is in disagreement with two other studies (using DNA fragmentation levels) which stated that higher levels of apoptosis on cumulus cells surround immature oocytes rather than mature ones (13, 14).
Interestingly, cumulus cells from oocytes that fertilize after ICSI had a higher caspase activity than cumulus cells from oocytes that do not fertilize, although they have the same viability and similar levels of markers for early apoptosis. This is consistent with the results of Raman et al. who also observed a positive correlation between cumulus cells apoptosis and fertilization rate after ICSI, in this case using the Comet assay (2). The authors stated that the apoptotic cumulus cells that fall away from the oocyte seem to function as a signal that the oocyte is ready for fertilization (2).
As different MII oocytes present different levels of caspase activity, a basal level of caspase activity in surrounding cumulus cells might be needed for the oocyte to gain fertilizing ability. On the other hand, one research suggested that cumulus cell apoptosis compromises the oocyte fertilization (13) while another paper showed no relationship between these two parameters (16).
It is well known that fertility decreases with female patient age (27–29), not only because of follicle loss, but also due to a reduction in oocyte quality (28). In our data set, when patient age was considered the cumulus cells per oocyte had the same viability, but different apoptosis levels. Younger patients had cumulus cells with a higher percentage of Annexin V positive cells, as well as cells with higher caspase activity, suggesting a higher incidence of apoptosis in cumulus cells from oocytes obtained in youngest patients. Interestingly, more apoptotic cells surrounded oocytes that both fertilize and were obtained from younger patients. However, using DNA fragmentation analysis, other studies have demonstrated the opposite, with cumulus cell apoptosis increasing after 35 years (14), 38 years (4) or 40 years (11).
Conclusion
Although more studies are needed with larger number of oocytes, it seems that high cumulus cell number and an increase in apoptotic makers seem to have a relationship with oocyte quality and maturation, oocyte fertilization ability and patient age and may therefore, potentially be used as simple biomarkers for female fertility and/or ovarian aging.
Acknowledgement
We thank Professor Ana Bela Sarmento Ribeiro and Dr. Ana Cristina Gonçalves for Annexin V and all members of the Biology of Reproduction and Stem Cell Group for support and discussions. This work was done as part of the Masters Program in Biochemistry at the Department of Life Sciences, University of Coimbra (BL).
To cite this article: Lourenço B, Sousa AP, Almeida-Santos T, Ramalho-Santos J. Relation of Cumulus Cell Status with Single Oocyte Maturity, Fertilization Capability and Patient Age. J Reprod Infertil. 2014;15(1):15-21.
Conflict of Interest
JRS, APS and BL defined the project. APS and TAS processed cumulus-oocyte complexes for analysis. BL performed all experiments on cumulus cells and initial analysis. All authors analyzed the data. All authors wrote and approved the paper.
References
- 1.Zhuo L, Kimata K. Cumulus oophorus extracellular matrix: its construction and regulation. Cell Struct Funct. 2001;26(4):189–96. doi: 10.1247/csf.26.189. [DOI] [PubMed] [Google Scholar]
- 2.Raman RS, Chan PJ, Corselli JU, Patton WC, Jacobson JD, Chan SR, et al. Comet assay of cumulus cell DNA status and the relationship to oocyte fertilization via intracytoplasmic sperm injection. Hum Reprod. 2001;16(5):831–5. doi: 10.1093/humrep/16.5.831. [DOI] [PubMed] [Google Scholar]
- 3.Eppig JJ, Wigglesworth K, Pendola F, Hirao Y. Mu-rine oocytes suppress expression of luteinizing hor-mone receptor messenger ribonucleic acid by granu-losa cells. Biol Reprod. 1997;56(4):976–84. doi: 10.1095/biolreprod56.4.976. [DOI] [PubMed] [Google Scholar]
- 4.Moffatt O, Drury S, Tomlinson M, Afnan M, Sakkas D. The apoptotic profile of human cumulus cells changes with patient age and after exposure to sperm but not inrelation to oocyte maturity. Fertil Steril. 2002;77(5):1006–11. doi: 10.1016/s0015-0282(02)02951-5. [DOI] [PubMed] [Google Scholar]
- 5.Van Soom A, Tanghe S, De Pauw I, Maes D, de Kruif A. Function of the cumulus oophorus before and during mammalian fertilization. Reprod Domest Anim. 2002;37(3):144–51. doi: 10.1046/j.1439-0531.2002.00345.x. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda S, Imai H, Yamada M. Apoptosis in cumulus cells during in vitro maturation of bovine cumulus-enclosed oocytes. Reproduction. 2003;125(3):369–76. [PubMed] [Google Scholar]
- 7.Tanghe S, Van Soom A, Nauwynck H, Coryn M, de Kruif A. Minireview: Functions of the cumulus oophorus during oocyte maturation, ovulation, and fertilization. Mol Reprod Dev. 2002;61(3):414–24. doi: 10.1002/mrd.10102. [DOI] [PubMed] [Google Scholar]
- 8.Motta PM, Nottola SA, Pereda J, Croxatto HB, Familiari G. Ultrastructure of human cumulus oopho-rus: a transmission electron microscopic study on oviductal oocytes andfertilized eggs. Hum Reprod. 1995;10(9):2361–7. doi: 10.1093/oxfordjournals.humrep.a136299. [DOI] [PubMed] [Google Scholar]
- 9.Ortiz ME, Salvatierra AM, Lopez J, Fernandez E, Croxatto HB. Postovulatory aging of human ova: I. Light microscopic observations. Gamete Res. 1982;6:11–7. [Google Scholar]
- 10.Gershon E, Plaks V, Dekel N. Gap junctions in the ovary: expression, localization and function. Mol Cell Endocrinol. 2008;282(1-2):18–25. doi: 10.1016/j.mce.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Lee KS, Joo BS, Na YJ, Yoon MS, Choi OH, Kim WW. Cumulus cells apoptosis as an indicator to predict the quality of oocytes and the outcome of IVF-ET. J Assist Reprod Genet. 2001;18(9):490–8. doi: 10.1023/A:1016649026353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikkelsen AL, Host E, Lindenberg S. Incidence of apoptosis in granulosa cells from immature human follicles. Reproduction. 2001;122(3):481–6. doi: 10.1530/rep.0.1220481. [DOI] [PubMed] [Google Scholar]
- 13.Host E, Gabrielsen A, Lindenberg S, Smidt-Jensen S. Apoptosis in human cumulus cells in relation to zona pellucida thickness variation, maturation stage, and cleavage of the corresponding oocyte after intracytoplasmic sperm injecti on. Fertil Steril. 2002;77(3):511–5. doi: 10.1016/s0015-0282(01)03006-0. [DOI] [PubMed] [Google Scholar]
- 14.Corn CM, Hauser-Kronberger C, Moser M, Tews G, Ebner T. Predictive value of cumulus cell apo-ptosis with regard to blastocyst development of cor-responding gametes. Fertil Steril. 2005;84(3):627–33. doi: 10.1016/j.fertnstert.2005.03.061. [DOI] [PubMed] [Google Scholar]
- 15.Yuan YQ, Van Soom A, Leroy JL, Dewulf J, Van Zeveren A, de Kruif A, et al. Apoptosis in cumulus cells, but not in oocytes, may influence bovine embryonic developmental competence. Theriogenology. 2005;63(8):2147–63. doi: 10.1016/j.theriogenology.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 16.Abu-Hassan D, Koester F, Shoepper B, Schultze-Mosgau A, Asimakopoulos B, Diedrich K, et al. Comet assay of cumulus cells and spermatozoa DNA status, and the relationship to oocyte fertilization andembryo quality following ICSI. Reprod Biomed Online. 2006;12(4):447–52. doi: 10.1016/s1472-6483(10)61997-9. [DOI] [PubMed] [Google Scholar]
- 17.Han ZB, Lan GC, Wu YG, Han D, Feng WG, Wang JZ, et al. Interactive effects of granulosa cell apo-ptosis, follicle size, cumulus-oocyte complex mor-phology, and cumulusexpansion on the develop-mental competence of goat oocytes: a study using the well-in-drop culture system. Reproduction. 2006;132(5):749–58. doi: 10.1530/REP-06-0055. [DOI] [PubMed] [Google Scholar]
- 18.Huang Z, Wells D. The human oocyte and cumulus cells relationship: new insights from the cumulus cell transcriptome. Mol Hum Reprod. 2010;16(10):715–25. doi: 10.1093/molehr/gaq031. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto S, Saeki K, Nagao Y, Minami N, Ya-mada M, Utsumi K. Effects of cumulus cell density during in vitro maturation of the developmental competence of bovine oocytes. Theriogenology. 1998;49(8):1451–63. doi: 10.1016/s0093-691x(98)00091-0. [DOI] [PubMed] [Google Scholar]
- 20.Ameisen JC. On the origin, evolution, and nature of programmed cell death: a timeline of four billion years. Cell Death Differ. 2002;9(4):367–93. doi: 10.1038/sj.cdd.4400950. [DOI] [PubMed] [Google Scholar]
- 21.Varum S, Bento C, Sousa AP, Gomes-Santos CS, Henriques P, Almeida-Santos T, et al. Character-ization of human sperm populations using conven-tional parameters, surface ubiquitination, and apo-ptotic markers. Fertil Steril. 2007;87(3):572–83. doi: 10.1016/j.fertnstert.2006.07.1528. [DOI] [PubMed] [Google Scholar]
- 22.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–90. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 23.Yuan YQ, Peelman LJ, Williams JL, Van Zeveren A, de Kruif A, Law A, et al. Mapping and tran-scription profiling of CASP1, 3, 6, 7 nd 8 in rela-tion to caspase activity in the bovine cumulus-oocyte complex. Anim Genet. 2004;35(3):234–7. doi: 10.1111/j.1365-2052.2004.01120.x. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien M, Moravec R, Riss T. Caspase-GloTM 3/7 assay: use fewer cells and spend less time with this homogeneous assay. Cell Notes. 2003;6:13–5. [Google Scholar]
- 25.Zeuner A, Muller K, Reguszynski K, Jewgenow K. Apoptosis within bovine follicular cells and its ef-fect on oocyte development during in vitro mat-uration. Theriogenology. 2003;59(5-6):1421–33. doi: 10.1016/s0093-691x(02)01190-1. [DOI] [PubMed] [Google Scholar]
- 26.Hussein TS, Froiland DA, Amato F, Thompson JG, Gilchrist RB. Oocytes prevent cumulus cell apo-ptosis by maintaining a morphogenic paracrine gradient of bonemorphogenetic proteins. J Cell Sci. 2005;118(Pt 22):5257–68. doi: 10.1242/jcs.02644. [DOI] [PubMed] [Google Scholar]
- 27.Baird DT, Collins J, Egozcue J, Evers LH, Gianaroli L, Leridon H, et al. Fertility and ageing. Hum Reprod Update. 2005;11(3):261–76. doi: 10.1093/humupd/dmi006. [DOI] [PubMed] [Google Scholar]
- 28.Alviggi C, Humaidan P, Howles CM, Tredway D, Hillier SG. Biological versus chronological ovarian age: implications for assisted reproductive tech-nology. Reprod Biol Endocrinol. 2009;7:101. doi: 10.1186/1477-7827-7-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balasch J. Ageing and infertility: an overview. Gynecol Endocrinol. 2010;26(12):855–60. doi: 10.3109/09513590.2010.501889. [DOI] [PubMed] [Google Scholar]


