Abstract
Background
Intrauterine insemination (IUI) is one of the therapeutic approaches for infertility. The objective of this study was to evaluate DNA integrity and apoptosis role in success of IUI in both mild male and female factor infertility.
Methods
Patients were divided into two groups: M (mild male factor; n = 29) and F (female factor; n = 31) undergoing single IUI. Ejaculates were analyzed and chromatin quality was assessed using chromomycin A3 (CMA3) staining. In addition, spermatozoal apoptosis was recognized using TUNEL assay. Statistical analyses were done using t-test and Mann Whitney test for sperm apoptosis and sperm chromatin by SPSS. Data were expressed in mean±SD for variables. P < 0.05 was considered statistically significant.
Results
Sperm concentration and progressive motility were higher in F than M group. Sperm with normal morphology were statistically similar in M and F infertile patients (32.7±15.6% vs. 35.5±9.07%, p = 0.39). Sperm chromatin immaturity was higher in patients with mild male infertility, when compared with the other group (p < 0.01). Also, 32.0±5.6% and 30.8±6.1% of the spermatozoa showed signs of apoptosis in groups M and F, respectively (p = 0.49). Very low (3.4%) clinical pregnancy rates were noticed in patients with mild male factor infertility
Conclusion
Defect in sperm motility as well as high rates of DNA damage and apoptosis may be involved with very low rate of pregnancy outcomes in patients with mild male factor infertility. Therefore, it seems the application of IUI may have better outcomes in patients with female infertility compared to mild male factor infertility.
Keywords: Apoptosis, Infertility, Intrauterine transfusion, Male, Morphology, Sperm motility, Spermatozoa
Introduction
Intra-uterine insemination (IUI) was first introduced as a technique for subfertility around 200 years ago. This is a simple, inexpensive and non-invasive infertility treatment which is the most frequently used assisted repro-ductive technology (ART) worldwide (1). Proper patient selection and sperm preparation became the first step for success in IUI program (2). Several factors affect the IUI outcome, such as age, etiology and duration of infertility, endometrial thickness, time of ovulation, follicular number, time and number of insemination (3). IUI has been accepted for the treatment of infertile couples with a variety of indications including mild male factor infertility; unexplained infertility and cervical mucus hostility (4). The correlation between sperm quality and clinical outcomes has been distinguished in IUI setting. In routine sperm preparation using swim-up or density gradients techniques, sperm are selected on the basis of progressive motility, morphological characteristics, and concentration for IUI. These parameters, however, may not guarantee the selection of spermatozoa with normal DNA/chromatin integrity (5).
Sperm DNA damage and apoptosis are useful indicators for male factor fertility and have a significant relation with infertility of men (6, 7). In testicular biopsies, increased rates of apoptosis have been reported with different degrees of incompetence (8). It is not clear whether the apoptotic markers recognized in spermatozoa are the remainders of an inconclusive apoptotic process initiated before ejaculation or whether they result from apoptosis started in the post-ejaculation period (1). Apoptosis is a programmed cell death that takes place physiologically without any inflammation (9). Recent studies have indicated that protamine deficiency and sperm DNA damage are associated with poor ART outcomes (10, 11). CMA3 reversibly binds to G-C base pairs in the minor groove of DNA. CMA3 identifies sperm with defective packaging and indirectly evaluates protamine deficiency. It has been reported that sperm protamine deficiency is associated with fertilization failure (12). Considering the advantage of CMA3 in assessment of protamine, and its possible use in andrology units, this quick evaluation of sperm with CMA3 received much attention during recent years. Evenson and his associates showed that abnormal chromatin packaging appears to be linked with nuclear DNA damage (13).
The objective of this cross-sectional study was to assess the relationship of sperm parameters as well as chromatin integrity and apoptosis with IUI outcomes in two groups of patients with female or mild male factor infertility.
Methods
Patients and ovulation induction
According to infertility etiology, patients were divided into two groups with mild male factor (group M; n = 29) and female factor infertility (group F; n = 31). Women aged between 20 − 35 years were included in this study. This investigation lasted from 2010 to 2011 at Research and Clinical Center for Infertility in Yazd. This study was approved by ethics committee of our institution.
All women underwent ovarian stimulation with daily use of 100 mg clomiphene citrate (Clomifen, Leiras, Finland) given between days 3 and 7 of cycle, followed by 150 IU of gonadotrophins (IBSA Co, Switezerland) added on day 9. Follicle growth and maturation was monitored by serial transvaginal ultrasonography. Diameter of growing follicles was recorded on days 10 to 13, and 10,000 IU of hCG (IBSA Co, Switzerland) was administered when at least one or two follicles were over 18 mm in diameter. A single IUI was performed 36 hr after hCG injection.
Semen analysis
The ejaculates were collected after 2–3 days of sexual abstinence and delivered to the andrology laboratory. After semen liquefaction, sperm were analyzed for rates of progressive and non-progressive motility according to WHO criteria (14). Also sperm morphology and sperm concentration were evaluated accordingly. Subsequently each sample was processed to separate motile sperm with normal morphology for IUI.
Evaluation of sperm nuclear chromatin
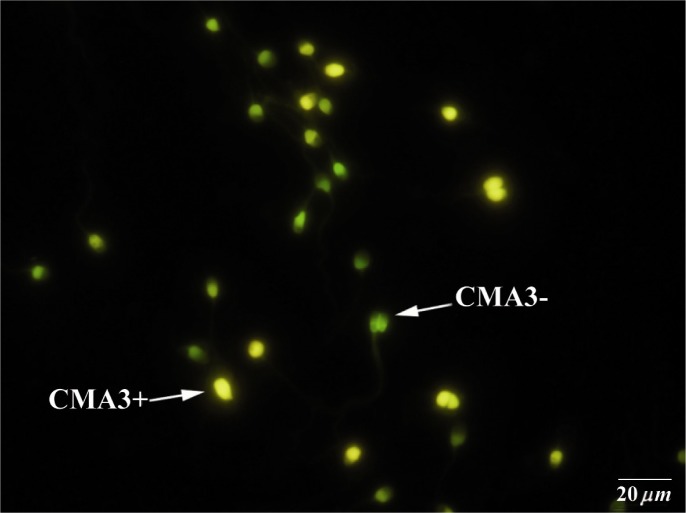
Smears were prepared from each sperm sample, dried, then fixed in Carnoys solution (methanol/glycial acetic acid, 3:1) at 4°C for 10 min. Each slide was treated with 150 µl of CMA3 (Sigma, St Louis, USA) (0.25 mg/ml in Mcvalin buffer; 7 ml citric acid, 0.l M +32.9 ml (Na2HPO4)7H2O, 0.2 M, pH = 7.0 containing 10 mM MgCl2) for 20 min. After staining, the slides were washed in buffer and mounted with buffered glycerol (Glycerol: Mcvalin, 1:1). Chromycine A3-reacted (CMA3+) sperm with protamine deficiency were bright yellow stained, and yellowish green stained ones were related to mature sperm with complete protamination (CMA3-) recognized under fluorescent microscope with a 460 nm filter (Zeiss Co, Jena, Germany) (15).
Assessment of apoptosis
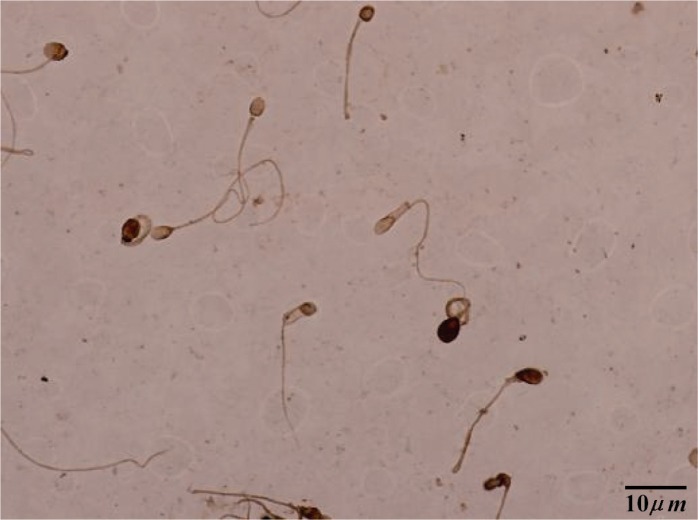
Sperm apoptosis was recognized using apoptosis detection kit (Roche Applied Sci, Germany). Smears were dried and fixed in 4% paraformaldehyde in PBS (Gibco Co, Scotland, UK) at room temperature (RT) for 1 hr. The slides were then rinsed three times with PBS and incubated with 0.3% H2O2 in methanol for 1 hr to quench endogenous peroxidase activity. The samples were treated with 0.1% Triton X-100 (Sigma Co, USA) for 5 min at 4°C and incubated with 50 µl TUNEL reaction mixture in a humidified chamber at 37°C for 1 hr. The samples were washed in PBS and exposed to DAB (3,3-diaminobezidine tetrahydrochloride) (Roche Co, Germany) as the substrate solution for color development in a dark chamber at RT for 10 min. At last, samples were dehydrated in ethanol, cleared in xylene (Sigma Co, USA), and mounted. For each sample, at least 200 nuclei were counted. For negative control, instead of TUNEL reaction mixture, slides were incubated with 50 µl of labeled solution with terminal transferase.
Statistical analysis
Statistical analyses were done using t-test and Mann Whitney test for sperm apoptosis and sperm chromatin by SPSS (version 16). Data were expressed in mean±SD for variables. P < 0.05 was considered statistically significant.
Results
The data showed that non-progressive motility as well as normal morphology of spermatozoa was similar between the M and F groups. However, significant differences in other sperm parameters were observed between the groups (Table 1). The clinical pregnancy rate was noticeably higher in F (6/31; 19.3%) than M group (1/29; 3.4%; p = 0.06). The data also showed that 28.6% (2/7) of the cases achieved multiple pregnancies. Table 2 demonstrates the variables in pregnant and non-pregnant patients. Non-pregnant group showed a significant number of immotile sperm, when compared with pregnant patients (p < 0.01). With regard to infertility duration, no relation was noticed between duration of infertility and pregnancy rates (Table 2). In addition, the results did not show any significant differences in age between pregnant and non-pregnant cases.
Table 1.
Comparisons of sperm parameters in two groups with female or male factor infertility undergoing IUI
| Sperm variable | Female factor (n = 31) | Male factor (n = 29) | p-value |
|---|---|---|---|
| Count (10 6 ) | 94.3±41.1 | 28.9±28.0 | <0.0001 |
| Progressive motility (%) | 61.8±9.7 | 37.3±10.9 | <0.01 |
| Non-progressive motility (%) | 12.3±3.4 | 12.6±10.06 | NS |
| Immotility (%) | 26.6±6.3 | 41.2±14.8 | <0.01 |
| Normal morphology (%) | 35.5±9.07 | 32.7±15.6 | NS |
| Protamine deficiency (%) | 34.2±12.9 | 49.4±16.9 | <0.01 |
| Apoptosis (%) | 30.8±6.1 | 32.0±5.6 | NS |
Data are presented as Mean±SD, NS: not significant, values inside parentheses represent (%)
Table 2.
Comparisons of sperm characteristics in pregnant and non-pregnant patients
| Variables | Pregnants (n = 7) | Non-pregnants (n = 53) | p-value |
|---|---|---|---|
| Count (106) | 60±36.05 | 63±49.8 | NS |
| Rapid progression ( % ) | 20.6±4.8 | 20.2±12.4 | NS |
| Slow progression ( % ) | 39.2±5.8 | 34.1±10.2 | NS |
| Non-progressive motility (%) | 10.4±3.9 | 12.8±7.6 | NS |
| Immotility ( % ) | 29.2±2.2 | 34.2±14.1 | <0.01 |
| Morphology ( % ) | 37.4±7.8 | 33.7±13.1 | NS |
| Infertility duration (years) | 5±2.2 | 5.7±2.7 | NS |
| Age (years) | 26.57±4.03 | 27.77±4.63 | NS |
| Chromatin immaturity ( % ) | 28.8±10.1 | 43.3±16.7 | <0.05 |
| Apoptosis ( % ) | 32.6±5.1 | 31.2±5.9 | NS |
Data are presented as Mean±SD, NS: not significant, values inside parentheses represent (%)
The findings also showed that the rate of sperm with protamine deficiency increased in sperm samples from patients with mild male infertility, when compared with the other group (p < 0.01; Figure 1). Also, the rate of protamine deficiency significantly increased in non-pregnant when compared with pregnant patients (p < 0.05; Table 2). Other findings confirmed that there was no correlation between abnormal sperm morphology and increased rate of DNA damage. However, our result showed that there were no significant differences in the rate of apoptosis in both groups of patients when compared between pregnant and not pregnant patients (Table 2; Figure 2). In addition, the result of comparison between male and female cases in correlation with sperm apoptosis was statistically similar.
Figure 1.
Chromomycin A3 (CMA3) staining: CMA3+ or protamine deficient spermatozoa appear as bright yellow, CMA3- or spermatozoa with normal protamine appear yellowish green
Figure 2.
TUNEL staining: brown stained sperm shows apoptosis+, light colored sperm indicates apoptosis−
Discussion
Some authors suggested that outcomes of semen with poor sperm morphology were comparable with normal sperm morphology in IUI setting (16). Recently, it was demonstrated that both sperm morphology and progressive motility had positive effects on IUI outcomes (17). Others found that normal sperm morphology can be considered as a predictor of IUI success (18). Also, Van Voortis et al. noticed that semen with less than 10 million motile sperm was associated with lower pregnancy rates in IUI cycles. It was shown that when the count of motile sperm was above 10 million, no significant increase would be achieved in IUI pregnancy rates (19). In this study, both sperm count and progressive motility were demonstrated to be lower in cases with mild male factor infertility when compared with female infertility. Conversely, concentration of immotile spermatozoa was observed to be higher in male factor and non-pregnant patients when compared with the other group. Prior to sperm preparation, total motile sperm of 30−50% were found to be associated with positive IUI outcomes (20). Our findings showed that the rates of normal sperm morphology were similar in both infertile groups. Therefore, sperm morphology criterion could not be a reliable predictive indication for IUI outcome. However, assessment of sperm DNA integrity can be used as a good practicability factor, particularly in patients with male infertility. The findings of this study indicates that in order to select the right candidates for clinical IUI, it is necessary to perform cytochemcial assays to assess the sperm DNA integrity of patients. These assays should be done in combination with semen analysis, especially for patients with male factor infertility. This study also showed that patients with mild male factor infertility are not suitable candidates for IUI, since the rate of chromatin immaturity in their sperm was very high. Therefore, they may benefit from other ART techniques, such as ICSI.
In one study, sperm chromatin structure and DNA integrity were known to have a critical effect on the rate of fertilization (21). The sperm chromatin condensation was shown with CMA3 assay; indicating protamine defects during histone-protamine replacement of sperm chromatin condensation in the testicular phase (21). In this regard, Saleh et al. (2003) stated that infertile men had higher rate of sperm DNA fragmentation and chromatin defects. They showed that DNA fragmentation index (DFI) has a direct relation with the overall pregnancy rate (22). Similarly, Bungum et al. also found that couples who failed pregnancy after IUI had an increased rate of sperm DNA damage (23). The aforesaid group also showed that in IUI cases in which sperm DNA damage exceeds 30%, the pregnancy success rates is close to zero (24). In agreement with Saleh et al. regarding chromatin defects, our work confirmed that the rate of sperm chromatin immaturity significantly increased in infertile men, while the rate of pregnancy decreased in cases with male infertility. CMA3 assay seems to be a more efficient assay than apoptosis which can be applied as a reliable marker for prognosis of pregnancy success in IUI program. Givercman et al. (2003) also believed that sperm chromatin structure assay (SCSA) parameter is correlated with the level of immotile spermatozoa and the percentage of chromatin packaging in ART. However, they showed that DFI parameter is independent of sperm motility (25).
In 2007, one study demonstrated that 10–20% of patients became pregnant following IUI program, which is similar to our cases (26). Recently, Lucchini and her colleagues reported 11% rate of pregnancy outcomes from their superovulated IUI cases (4). Dorjpurev et al. evaluated the effect of semen characteristics on the success of IUI. Also, they stated that some relevant characteristics of pregnancy were younger age, minimal duration of infertility and male infertility factors (17). This finding is also in agreement with Zadehmodarres et al.'s report (27). Moreover, Dorjpurev showed that sperm washing/processing did not affect the pregnancy outcomes. They achieved similar results between washed and unwashed seminal samples (17). However, it is better to wash the ejaculates in order to separate a good fraction of spermatozoa from seminal plasma, leukocyte and non-motile spermatozoa (17, 18). Also, sperm processing is suitable for preventing the transmission of infectious agents and prostaglandin to the uterus. Another finding was related to the correlation between rates of immotile sperm with lower pregnancy after IUI.
In ART, multiple pregnancies are evaluated with incidence reports of 6.5–25% (28). In our study, the rates of multiple pregnancies were higher than the aforementioned study. Our findings also showed that the rates of sperm apoptosis assay were approximately similar in two groups of mild male and female patients. This may indicate that apoptosis does not play a major role in prediction of IUI outcomes.
Conclusion
This study confirmed that there was no correlation between abnormal sperm morphology and high rate of DNA damage in IUI program. Therefore, this technology may not be recommended for patients with mild male infertility, since high rate of DNA damage was observed in their spermatozoa. Single IUI can be considered for patients with female factor infertility. Also, it might be better to plan other ART programs for male factor cases.
Acknowledgement
We would like to acknowledge the contributions of Ms. Leila Motamedzadeh. The statistical assistance of Ms. Farimah Shamsi is also well appreciated. This study was supported by a grant from Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
To cite this article: Khalili MA, Nazari S, Dehghani-Firouzabadi R, Talebi AR, Baghazadeh-Naeini Sh, Sadeghian-Nodoshan F, et al. Comparing the Roles of Sperm Chromatin Integrity and Apoptosis in Intrauterine Insemination Outcomes of Couples with Mild Male and Female Factor Infertility. J Reprod Infertil. 2014;15(1):35-40.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Lachaud C, Tesarik J, Canadas ML, Mendoza C. Apoptosis and necrosis in human ejaculated spermatozoa. Hum Reprod. 2004;19(3):607–10. doi: 10.1093/humrep/deh130. [DOI] [PubMed] [Google Scholar]
- 2.Custers IM, Steures P, Hompes P, Flierman P, van Kasteren Y, van Dop PA, et al. Intrauterine insemination: how many cycles should we perform? Hum Reprod. 2008;23(4):885–8. doi: 10.1093/humrep/den008. [DOI] [PubMed] [Google Scholar]
- 3.Kamath MS, Bhave P, Aleyamma T, Nair R, Chandy A, Mangalaraj AM, et al. Predictive factors for pregnancy after intrauterine insemination: A prospective study of factors affecting outcome. J Hum Reprod Sci. 2010;3(3):129–34. doi: 10.4103/0974-1208.74154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucchini C, Volpe E, Tocci A. Comparison of intra-follicular sperm injection and intrauterine insemination in the treatment of subfertility. J Assist Reprod Genet. 2012;29(10):1103–9. doi: 10.1007/s10815-012-9836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jayaraman V, Upadhya D, Narayan PK, Adiga SK. Sperm processing by swim-up and density gradient is effective in elimination of sperm with DNA damage. J Assist Reprod Genet. 2012;29(6):557–63. doi: 10.1007/s10815-012-9742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Melegy NT, Ali ME. Apoptotic markers in semen of infertile men: Association with cigarette smoking. Int Braz J Urol. 2011;37(4):495–506. doi: 10.1590/s1677-55382011000400009. [DOI] [PubMed] [Google Scholar]
- 7.Taylor SL, Weng SL, Fox P, Duran EH, Morshedi MS, Oehninger S, et al. Somatic cell apoptosis markers and pathways in human ejaculated sperm: potential utility as indicators of sperm quality. Mol Hum Reprod. 2004;10(11):825–34. doi: 10.1093/molehr/gah099. [DOI] [PubMed] [Google Scholar]
- 8.Jurisicova A, Lopes S, Meriano J, Oppedisano L, Casper RF, Varmuza S. DNA damage in round spermatids of mice with a targeted disruption of the Pp1cgamma gene and in testicular biopsies of patients with non-obstructive azoospermia. Mol Hum Reprod. 1999;5(4):323–30. doi: 10.1093/molehr/5.4.323. [DOI] [PubMed] [Google Scholar]
- 9.Aziz N, Said T, Paasch U, Agarwal A. The relationship between human sperm apoptosis, morphology and the sperm deformity index. Hum Reprod. 2007;22(5):1413–9. doi: 10.1093/humrep/dem016. [DOI] [PubMed] [Google Scholar]
- 10.Angelopoulou R, Plastira K, Msaouel P. Spermato-zoal sensitive biomarkers to defective protaminosis and fragmented DNA. Reprod Biol Endocrinol. 2007;5:36. doi: 10.1186/1477-7827-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasr-Esfahani MH, Aboutorabi R, Razavi S. Cred-ibility of chromomycin A3 staining in prediction of fertility. Int J Fertil Steril. 2009;9:5–10. [Google Scholar]
- 12.Tarozzi N, Nadalini M, Stronati A, Bizzaro D, Dal Prato L, Coticchio G, et al. Anomalies in sperm chromatin packaging: implications for assisted reproduction techniques. Reprod Biomed Online. 2009;18(4):486–95. doi: 10.1016/s1472-6483(10)60124-1. [DOI] [PubMed] [Google Scholar]
- 13.Evenson DP, Darzynkiewicz Z, Melamed MR. Relation of mammalian sperm chromatin heterogeneity to fertility. Science. 1980;210(4474):1131–3. doi: 10.1126/science.7444440. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. WHO laboratory manual for the examination and processing of human semen; 2010. [Google Scholar]
- 15.Talebi AR, Moein MR, Tabibnejad N, Ghasemzadeh J. Effect of varicocele on chromatin condensation and DNA integrity of ejaculated spermatozoa using cytochemical tests. Andrologia. 2008;40(4):245–51. doi: 10.1111/j.1439-0272.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- 16.Kupker W, Schulze W, Diedrich K. Ultrastructure of gametes and intracytoplasmic sperm injection: the significance of sperm morphology. Hum Reprod. 1998;13(Suppl 1):99–106. doi: 10.1093/humrep/13.suppl_1.99. [DOI] [PubMed] [Google Scholar]
- 17.Dorjpurev U, Kuwahara A, Yano Y, Taniguchi T, Yamamoto Y, Suto A, et al. Effect of semen characteristics on pregnancy rate following intrauterine insemination. J Med Invest. 2011;58(1-2):127–33. doi: 10.2152/jmi.58.127. [DOI] [PubMed] [Google Scholar]
- 18.Abdelkader AM, Yeh J. The potential use of intrauterine insemination as a basic option for infertility: a review for technology-limited medical settings. Obstet Gynecol Int. 2009;2009:584837. doi: 10.1155/2009/584837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Voorhis BJ, Barnett M, Sparks AE, Syrop CH, Rosenthal G, Dawson J. Effect of the total motile sperm count on the efficacy and cost-effectiveness of intrauterine insemination and in vitro fertilization. Fertil Steril. 2001;75(4):661–8. doi: 10.1016/s0015-0282(00)01783-0. [DOI] [PubMed] [Google Scholar]
- 20.Lee RK, Hou JW, Ho HY, Hwu YM, Lin MH, Tsai YC, et al. Sperm morphology analysis using strict criteria as a prognostic factor in intrauterine insemination. Int J Androl. 2002;25(5):277–80. doi: 10.1046/j.1365-2605.2002.00355.x. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update. 2003;9(4):331–45. doi: 10.1093/humupd/dmg027. [DOI] [PubMed] [Google Scholar]
- 22.Saleh RA, Agarwal A, Sharma RK, Said TM, Sik-ka SC, Thomas AJ., Jr Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil Steril. 2003;80(6):1431–6. doi: 10.1016/s0015-0282(03)02211-8. [DOI] [PubMed] [Google Scholar]
- 23.Bungum M, Bungum L, Humaidan P, Yding Andersen C. Day 3 versus day 5 embryo transfer: a prospective randomized study. Reprod Biomed Online. 2003;7(1):98–104. doi: 10.1016/s1472-6483(10)61736-1. [DOI] [PubMed] [Google Scholar]
- 24.Bungum M, Humaidan P, Spano M, Jepson K, Bungum L, Giwercman A. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod. 2004;19(6):1401–8. doi: 10.1093/humrep/deh280. [DOI] [PubMed] [Google Scholar]
- 25.Giwercman A, Richthoff J, Hjollund H, Bonde JP, Jepson K, Frohm B, et al. Correlation between sperm motility and sperm chromatin structure assay parameters. Fertil Steril. 2003;80(6):1404–12. doi: 10.1016/s0015-0282(03)02212-x. [DOI] [PubMed] [Google Scholar]
- 26.Ahinko-Hakamaa K, Huhtala H, Tinkanen H. Success in intrauterine insemination: the role of etiology. Acta Obstet Gynecol Scand. 2007;86(7):855–60. doi: 10.1080/00016340701416895. [DOI] [PubMed] [Google Scholar]
- 27.Zadehmodarres S, Oladi B, Saeedi S, Jahed F, Ashraf H. Intrauterine insemination with husband semen: an evaluation of pregnancy rate and factors affecting outcome. J Assist Reprod Genet. 2009;26(1):7–11. doi: 10.1007/s10815-008-9273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nuojua-Huttunen S, Tomas C, Bloigu R, Tuomivaara L, Martikainen H. Intrauterine insemination treatment in subfertility: an analysis of factors affecting outcome. Hum Reprod. 1999;14(3):698–703. doi: 10.1093/humrep/14.3.698. [DOI] [PubMed] [Google Scholar]