Abstract
Increasing interests in detecting metal ions in many chemical and biomedical fields have created demands for developing sensors and imaging agents for metal ions with high sensitivity and selectivity. This review covers recent progress in DNA-based sensors and imaging agents for metal ions. Through both combinatorial selection and rational design, a number of metal ion-dependent DNAzymes and metal ion-binding DNA structures that can selectively recognize specific metal ions have been obtained. By attaching these DNA molecules with signal reporters such as fluorophores, chromophores, electrochemical tags, and Raman tags, a number of DNA-based sensors for both diamagnetic and paramagnetic metal ions have been developed for fluorescent, colorimetric, electrochemical, and surface Raman detections. These sensors are highly sensitive (with detection limit down to 11 ppt) and selective (with selectivity up to millions-fold) toward specific metal ions. In addition, through further development to simplify the operation, such as the use of “dipstick tests”, portable fluorometers, computer-readable discs, and widely available glucose meters, these sensors have been applied for on-site and real-time environmental monitoring and point-of-care medical diagnostics. The use of these sensors for in situ cellular imaging has also been reported. The generality of the combinatorial selection to obtain DNAzymes for almost any metal ion in any oxidation state, and the ease of modification of the DNA with different signal reporters make DNA an emerging and promising class of molecules for metal ion sensing and imaging in many fields of applications.
1. Introduction
Sensing and imaging of metal ions have attracted much attention by scientists and engineers, due to the important roles of metals in many fields such as environmental, biological, and medical sciences. Traditional analytical techniques for metal ion detection, such as inductively coupled plasma mass spectrometry (ICP-MS) and atomic absorption spectroscopy (AAS), require expensive and bulky instrumentation and significant training to use properly, making it difficult for on-site and real-time detection. To overcome these limitations, significant progress has been made in developing sensors and imaging agents for the detection of metal ions, mostly based on organic molecules, peptides, proteins or cells.1–14
DNA is a biopolymer that encodes the inheritable information of many organisms. At the first glance, DNA does not appear to be a good candidate for sensing metal ions with high selectivity, as the negatively charged phosphodiester backbones of DNA are known to be capable of binding cationic metal ions with poor selectivity for any particular metal ion. While the four DNA bases, adenine (A), thymine (T), guanine (G), and cytosine (C) can also serve as ligands for metal ions,15–19 many of these DNA-metal ion interactions are non-specific and weak, making the use of DNA as sensors for metal ions very challenging because selectivity and sensitivity are required for successful detection of a specific metal ion in the presence of other potentially interfering metals in complex samples.
To meet the challenge, two approaches have been established to identify metal ion-selective DNA sequences. The first is through a combinatorial technique called in vitro selection, where random DNA libraries containing diverse DNA sequences are used to obtain desired sequences that can bind specific metal ions or use them as cofactors for catalysis.20–25 The second approach utilizes DNA sequences discovered to be able to bind specific metal ions based on the study of the DNA structures or rational design.26–33 By incorporating signal reporters such as chromophores, fluorophores, electrochemical tags and Raman tags, these metal ion-specific DNA sequences found by these two approaches have been transformed into colorimetric, fluorescent, electrochemical, and Raman sensors and imaging agents for a broad range of metal ions with high selectivity and sensitivity.23,25,34–63 This review covers the recent advances in this area (Table 1), with more focus on DNAzymes as sensors for metal ions.
Table 1.
The DNA structures discussed in this review as DNA sensors for metal ions.
| Entry | DNA structures | Mechanism of metal ion sensing | Target metal ions | Key references |
|---|---|---|---|---|
| 1 | DNAzymes | Catalytic cleavage of DNA substrates | Mg2+, Zn2+, Pb2+, Cu2+, UO22+, Hg2+, Co2+, Mn2+ | 24, 25, 91, 93, 94, 95, 96 |
| 2 | DNA mismatches | Forming stable base pairs | Hg2+, Ag+, Cu2+ | 31, 32, 218, 220 |
| 3 | DNA G-quadruplex | Stabilizing or destabilizing G-quadruplex | K+, Pb2+, Cu2+, Ag+ | 34, 35, 36, 38 |
2. Metal Ion-dependent DNAzymes for Metal Ion Recognition
In the 1990s, DNA sequences with ligand binding (called DNA aptamers) or catalytic activities (called DNAzymes, deoxyribozymes, catalytic DNA, or DNA enzymes) were discovered through combinatorial techniques including in vitro selection and systematic evolution of ligands by exponential amplification (SELEX).20,64–66 In these techniques, shown in Figure 1 as an example of in vitro selection process for UO22+-dependent DNAzymes, random DNA libraries containing 1014 or more different DNA sequences are applied under pre-defined selection pressures to isolate DNA sequences with desired properties out of the libraries; sequences thus selected are then amplified by polymerase chain reaction (PCR) to generate a new library for successive rounds of selection. After a few to a few dozen of such selection rounds, using negative selection to enhance metal ion selectivity when necessary,67,68 DNAzymes that are highly selective to use specific metal ions as cofactors to catalyze reactions can be obtained. In this way, DNAzymes that are dependent on Mg2+,69,70 Zn2+,71,72 Pb2+,20,71 Cu2+,21,73 UO22+,74 Hg2+,75 Co2+,76 and Mn2+77 for various chemical and biological reactions have been successfully discovered. Among them, DNAzymes that can cleave or ligate nucleic acids have been widely applied in the development of selective and sensitive sensors for different metal ions, after conjugating them with suitable signal reporters (Figure 2).23,25,34–63
Figure 1.
Scheme of the process for in vitro selection of UO22+-dependent DNAzymes. The random DNA library (random regions shown in green) is amplified by PCR in the presence of primers P1 to P4 and purified by polyacrylamide gel electrophoresis (PAGE) to generate an enriched pool. Then, those DNA sequences from the enriched pool that undergo UO22+-induced cleavage are isolated by PAGE and used as the starting library for the next round of selection. After a few such rounds of selection and negative selections, the DNA sequences in the final pool are cloned and sequenced. Adapted from Ref 93.
Figure 2.

A general sensor design based on nucleic acid cleaving DNAzymes for metal ion detection. The figure shows a typical fluorescent sensor. The fluorophore and quenchers may be replaced by other signal reporters, such as nanomaterials and electrochemical/Raman tags, to construct colorimetric, electrochemical, and Raman sensors. The DNAzyme may also be immobilized on a surface.
2.1 Fluorescent sensors based on metal ion-dependent DNAzymes
2.1.1 Fluorescent sensors labeled with fluorophores and quenchers
Because of the ease in labeling DNAs with fluorophores and quenchers during or after the well-established automated solid-phase synthesis of DNA, the first report of DNAzyme sensor was a fluorescent sensor for Pb2+ based on a 8–17 DNAzyme,78 that showed much higher specificity to Pb2+ over other metal ions in catalyzing the cleavage of DNA substrates with a single RNA linkage (rA) at the cleavage site (Figure 3A). Such a high selectivity, was attributed to the “lock-and-key” mode of catalysis for Pb2+-dependent activity in comparison with other metal ions such as Zn2+ and Mg2+.79–84 The key to the sensing mechanism is to take advantage of the difference of DNA melting temperatures before and after metal-dependent cleavage. In the absence of target metal ion, the enzyme strand (17E) can hybridize to its substrate strand (17DS), because the melting temperature can be designed to be above ambient temperature. Because the 17DS and 17E are labeled with a fluorophore (FAM) and a quencher (Dabcyl), respectively, the DNA hybridization resulted placing the quencher close to the fluorophore, resulting in low fluorescent signal. Upon metal-catalyzed substrate cleavage, the melting temperatures of the two product strands becomes much less than that before cleavage, which can be designed to be lower than ambient temperature. As a result, the DNA duplex dehybridizes and the fluorophore-containing fragment is released, resulting in fluorescence enhancement due to the separation of the fluorophore and quencher. Since the DNAzyme activity was dependent on the concentration of Pb2+ as the cofactor, the fluorescence enhancement rate was successfully used to determine Pb2+ concentration in water.78
Figure 3.
(A) A fluorescent sensor for Pb2+ based on a Pb2+-dependent DNAzyme using a dual-labeled approach. (B) Attachment of the fluorophore and quencher pair close to the cleavage site of DNAzymes for DNAzyme selection and sensor design. (C) Bacterial detection using metal ion-dependent DNAzymes with nearby fluorophore and quencher. (D) A Hg2+-dependent DNAzyme containing artificial nucleotides (bold U and A in the sequence, with corresponding chemical structures of modified dUaa and dUim shown on right) for the development of Hg2+ sensors. (E) Single Pb2+ ion detection using a unimolecular DNA-catalytic probe. Adapted from Refs 112, 127, 130, 94 and 136.
The above approach, named catalytic beacon, because the increase of fluorescent signal due to catalytic activity, is similar to molecular beacon,85 but possesses several advantages. First, instead of detecting DNA/RNA only in the case of molecular beacon, the catalytic beacon can detect a variety of targets such as metal ions and other molecules such as adenosine through combination of DNAzyme and aptamer.86 Second, the catalytic turnovers allow a single target to generate numerous products containing fluorophores or other labels. Allowing amplification of the signals. Finally, instead of using absolute intensity as the measure in molecular beacon, which is vulnerable to background signal fluctuations due to auto-fluorescence by many species in cells or other matrices, the catalytic beacon can rely on the rate of fluorescent increase, which is characteristic of the target-induced cleavage.
While the above catalytic beacon approach allow effective detection of metal ions, the background fluorescent signal is still relatively high, due to potential dehybridization of the substrate strand from the enzyme strand in the absence of the target at ambient conditions. While increasing the number of base pairs or GC contents can make the hybridization stronger, a compromise has to be made to allow the cleaved product DNA strands to dehybridize in the presence of the target. To overcome this limitation, a dual labeling approach was demonstrated by attaching fluorophore/quencher pairs to the two ends of the substrate DNA, and the background fluorescence of the sensor system was dramatically decreased for improved sensitivity in Pb2+ detection (Figure 3A).87
Since melting temperature is the key to the successful sensing, temperature can play a role in the detection. To eliminate temperature as a confounding effect on the performance of the sensor, a temperature-resistant sensor for Pb2+ was developed by introducing site mutations to the binding arm of the DNAzyme.88 When a classic Pb2+-dependent DNAzyme was used in the sensor design, the sensitivity and selectivity of the detection was improved due to the nature of the DNAzyme.89 Later, taking advantage of the multiple turnover characteristics of Catalytic and Molecular Beacons (CAMB), the sensitivity of DNAzyme-based sensors for Pb2+ detection was further improved, and the system became easily compatible with aptazymes (a combination of an aptamer and DNAzyme or ribozyme, whose activity can be regulated by aptamer binding to its target) for the detection of other analytes.90 In addition to the above Pb2+ sensors, using similar design concepts, UO22+-74,91,92 and Cu2+-dependent DNAzymes21,73 were also successfully transformed into fluorescent sensors for the selective and sensitive detection of UO22+ 74 and Cu2+,93 respectively. Notably, the detection limit of the UO22+ sensor was as low as 45 pM or 11 ppt,74 lower than even the corresponding detection limit of ICP-MS. This work demonstrates the promise of DNAzyme-based sensors for high-performance metal ion detection.
Instead of attaching fluorophores and quenchers to the ends of DNA strands, Li and coworkers inserted a fluorophore and a quencher close together at two nucleotides adjacent to a DNAzyme substrate’s cleavage site, obtaining metal ion-dependent DNAzymes through in vitro selection to cleave such substrate DNA for fluorogenic sensing (Figure 3B).94–98 This approach enabled the synchronization of DNAzyme catalysis and fluorescence signaling94 for many applications, including the discovery of fluorescent DNAzymes as sensors for wide pH ranges,95 specific metal ions,95 and bacteria (Figure 3C).98 One of the most prominent advantages of such sensors is the low background fluorescence due to the closely localized pairs of fluorophores and quenchers, significantly improving the signal-to-noise ratio for more sensitivity detections.94,97
Perrin and coworkers introduced unnatural DNA bases into the random DNA library during in vitro selection, and successfully selected a Hg2+-dependent DNAzyme containing 8-histaminyl-dA and 5-aminoallyl-dU that hydrolyzes nucleic acid substrates (Figure 3D).75 Based on a similarly modified DNAzyme, the Perrin group developed a sensor for Hg2+ with excellent selectivity and sensitivity via metal ion-induced inhibition, though the signal readout is not through fluorescence.99 Brennan and coworkers studied the quenching effect of heavy metal ions on fluorophore-labeled DNAs in sensor designs based on DNAzymes, and provided general guidelines for the development of more efficient fluorescence-signaling DNAzymes.100 Li and coworkers utilized a Mg2+-dependent DNAzyme with a non-classical allosteric design for the detection of metal ions and other molecules.101 To enhance the performance of DNAzyme-based sensors, Willner and coworkers introduced a ligation DNAzyme machinery coupled with peroxidase-mimic DNAzymes for sensitive chemiluminescent detection of Cu2+.102
In a typical sensor design, a nucleic acid cleaving DNAzyme and its substrate form a DNA duplex that brings close a fluorophore and quencher pair. Upon the metal ion-activated catalytic reaction, the cleavage of the substrate causes fluorescence enhancement due to the release of the fluorophore from the duplex.74,75,78,87,93 In another design, Tan and coworkers connected the DNAzyme and the substrate by a short oligonucleotide linker to construct a unimolecular form (Figure 3E).103 In this case, the ratio between the DNAzyme and the substrate was constant as 1:1, and the background signal originating from the unbound substrate was minimized. As a result, the sensitive monitoring of a single Pb2+ ion was demonstrated, using a unimolecular DNA-catalytic probe containing both a Pb2+-dependent DNAzyme fragment and a substrate motif (Figure 3E).103
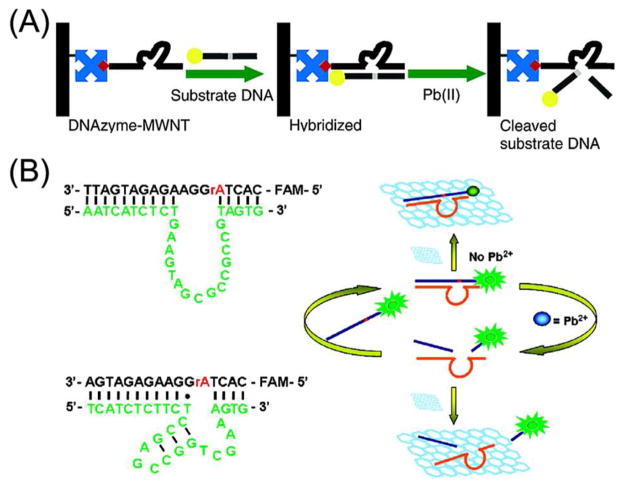
In addition to using small organic molecules as fluorophores and quenchers, other nanomaterials can also be coupled with DNAzymes to develop metal ion sensors. The Pb2+-dependent DNAzyme was modified with biotin and then conjugated to multi-walled carbon nanotubes (MWNTs) coated with streptavidin (Figure 4A).104 The catalytic cleavage of DNA substrates with multiple turnovers was maintained for the DNAzyme on the MWNTs, as compared to its un-conjugated form in solution. The MWNTs quenched the fluorescence of nearby fluorophores so that a Pb2+ sensor could be designed based on the cleavage of the fluorophore-labeled substrate by the Pb2+-dependent DNAzyme. In addition to MWNTs, many other materials such as gold nanoparticles, graphene, and single-walled carbon nanotubes also exhibit strong quenching effects on fluorophores and can take the place of quenchers in the sensor design.105–110 Pb2+ 105,107–110 and Cu2+ 106 sensors with improved performance were developed through covalent attachment or non-covalent adsorption of DNA on these nanomaterials. For example, graphene was used as efficient quenchers for the development of a Pb2+ sensor based on Pb2+-dependent DNAzymes (Figure 4B),110 where the cleavage of fluorophore-labeled substrates significantly reduced the affinity of the fluorophore-labeled DNA fragment to graphene surfaces. In addition to methods based on fluorescence intensity, gold nanoparticles111 and graphene112 were also conjugated to Cu2+-dependent DNAzymes and substrates in order to induce changes in the fluorescence anisotropy upon the Cu2+-mediated cleavage of the DNA substrates. In another work, quantum dots were conjugated with DNA to serve as fluorophores for the multiplexed detection of Pb2+ and Cu2+ in one solution.113
Figure 4.
(A) MWNTs as quenchers for fluorophores for the development of Pb2+ sensors based on Pb2+-dependent DNAzymes. (B) Graphene as an efficient quencher to bind fluorophore-labeled DNAzyme-substrate duplex for the detection of Pb2+. Adapted from Refs 138 and 144.
2.1.2 Surface immobilized fluorescent sensors
To enable regeneration and long-term storage of the sensors for more practical applications, Pb2+-dependent DNAzymes were immobilized on surfaces to develop solid sensor chips.114–120 Gold surfaces were functionalized with thiol-modified and quencher-labeled DNAzymes, and then fluorophore-labeled substrates were hybridized with the immobilized DNAzymes. Upon coming in contact with samples containing Pb2+, the substrates were cleaved by the DNAzymes and the fluorophores were released, resulting in Pb2+-dependent fluorescence enhancement for Pb2+ detection (Figure 5A).114 The immobilized sensors showed improved sensitivity over the original solution sensor while preserving the selectivity, and they could be regenerated after tests by the addition of fresh fluorophore-labeled substrate, as well as stored in the solid state.114 An internal standard was also introduced into the same sensors immobilized on nanocapillary array membranes to realize ratiometric fluorescence detection, which is more resistant to background fluctuation.115 Later, microfluidic sensor devices for Pb2+ detection were developed by conjugating the same DNAzymes on polymethylmethacrylate (PMMA) microchannel walls.116 By immobilizing DNAzymes and substrates on microarrays, Ye’s 117 and Zhao’s 118 groups constructed sensor arrays for high throughput detection of Pb2+ and Cu2+, which combined the high selectivity and sensitivity of DNAzymes and the high throughput analysis of microarrays. Sensitive flow cytometric detection of Pb2+ was successfully achieved by Guo and coworkers using magnetic beads coated with labeled DNAzymes and substrates, where the ultrahigh performance was ascribed to the use of magnetic beads and flow cytometry to abstract fluorescence signal from complicated sample matrix and reduce light scattering effects, respectively.119 Brennan and coworkers trapped different DNAzymes and substrates into sol-gel-derived matrixes as sensors for a series of metal ions. This sol-gel sensor technology reduced the interference of metal ion-induced fluorophore quenching and enabled multiplexed detection of four metal ion species using different DNAzymes in an array (Figure 5B).120
Figure 5.

(A) Immobilizing Pb2+-dependent DNAzymes and substrates on gold surfaces for fluorescent Pb2+ detection. (B) A sol-gel sensor array using different DNAzymes for simultaneous detection of four metal ion species. Adapted from Refs 148 and 154.
2.1.3 Label-free fluorescent sensors
Although covalently labeling DNAzymes and substrates with fluorophores and quenchers have been widely applied as a general strategy for the design of various metal ion sensors, such labeled DNAs are usually more complicated to synthesize and more expensive compared to DNAs without labels, and in some cases the labels may also interfere with the binding between DNAzymes and substrates or metal ions, reducing their activities. To overcome this challenge, label-free fluorescent sensors that do not require covalent labeling of DNAzymes and substrates have been developed.121–128 A number of such studies have utilized DNA intercalating dyes that exhibit distinct fluorescence characteristics when bound with double-stranded DNA (dsDNA) or single-stranded DNA (ssDNA) regions.122,124–126,128 For example, Jiang and coworkers coupled the cleavage of a DNA substrate by a Pb2+-dependent DNAzyme in the presence of Pb2+ with quantitative polymerase chain reaction, and subsequently measured the fluorescence of SYBR Green I upon its binding with the PCR products to detect the concentration of Pb2+ at a high sensitivity.122 Using graphene as the quencher for the DNA-binding GelRed dye, a label-free Cu2+ sensor was developed based on a Cu2+-dependent DNAzyme.124 Picogreen (Figure 6A)125 and SYBR Green I (Figure 6B)128 were also applied as fluorescent double-stranded DNA intercalators for the construction of label-free Pb2+ and Cu2+ sensors by Wang’s and Yao’s groups, respectively. In addition to small molecular intercalating dyes, conjugated polymers that can distinguish dsDNA and ssDNA by changes in fluorescence intensity were used to recognize the cleaved substrate by a Cu2+-dependent DNAzyme for sensitive Cu2+ detection.126
Figure 6.
(A) A label-free fluorescent sensor for Pb2+ using Picogreen. (B) A label-free fluorescent sensor for Pb2+ using SYBR Green I. (C) Binding of ATMND (receptor) to a dSpacer (AP site) opposite to a cytosine (Target base) in DNA duplex. (D) Label-free detection of small molecules using aptamers containing an AP site. (E) Label-free fluorescent sensors for Pb2+ using ATMND and DNAzyme-substrate duplex containing a vacant site. Adapted from Refs 159, 162, 163, 169 and 157.
Teramae and coworkers found that fluorescent compounds, such as derivatives of 2-amino-5,6,7-trimethyl-1,8-naphthyridine (ATMND) and riboflavin, could selectively bind to AP sites (e.g. dSpacers and C3 spacers) in a DNA duplex and result in its fluorescence quenching via complementary hydrogen bonding with the opposite bases and pi-pi stacking with the flanking bases (Figure 6C).129–133 By designing a target-induced switch of DNA structures that caused ATMND binding or release, they also developed fluorescent sensors for DNA strands129,131,133 and organic molecules (Figure 6D).130,132,134,135 We took advantage of the specific binding between ATMND and AP sites (dSpacer or vacant sites) in a DNAzyme-substrate duplex to control the binding sites of fluorophores in the label-free metal ion sensor design (Figure 6E).121,123,127 The more defined binding sites (AP sites) of ATMND in a DNA duplex compared to non-specific binding of intercalating dyes to DNA can help in the rational design of the sensors and minimize the risk of activity reduction due to the binding of dyes to the active cores of DNAzymes. In the presence of target metal ions such as Pb2+ and UO22+, the cleavage of substrates by the DNAzymes caused the deformation of duplex regions and released ATMND from the binding site, because ATMND cannot bind to ssDNA. The metal ions were quantified by measuring the fluorescence enhancement of released ATMND.121,123 The sensitivity and selectivity of this label-free method was found to be comparable with the previously reported labeled version, and it was further combined with the catalytic molecular beacon approach to develop more efficient label-free fluorescent sensors for a broader range of analytes.127
2.2 Colorimetric sensors based on metal ion-dependent DNAzymes
2.2.1 Colorimetric sensors based on gold nanoparticles
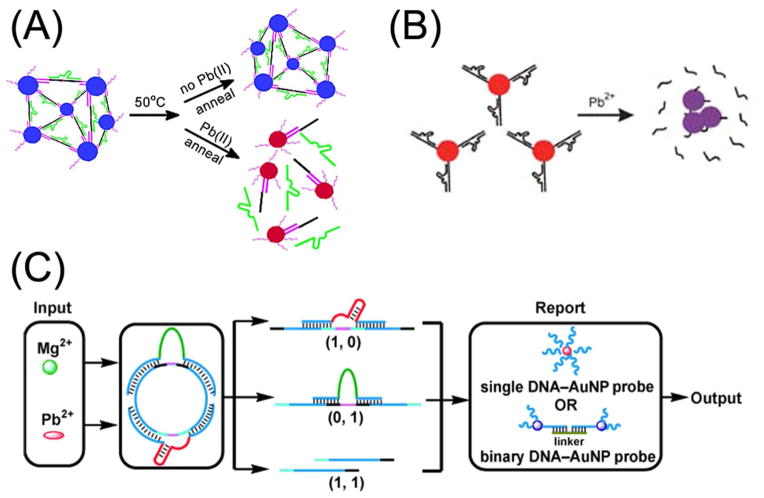
Besides fluorescence, colorimetry has also been used as the signal output for DNAzyme-based sensors, enabling the detection of metal ions by direct eye observation without any instrumentation or excitation light.136–153 Taking advantage of the color changes of DNA-functionalized gold nanoparticles when transforming between discrete and aggregated states as demonstrated by Mirkin et. al.154 and Alivisatos et. al.155, our group has developed a series of colorimetric sensors based on different DNAzymes for the detection of a series of metal ions.136–144 In 2003, the first colorimetric Pb2+ sensor based on gold nanoparticles and DNAzymes was developed (Figure 7A).136 In this approach, the gold nanoparticles were cross-linked as aggregates by the DNAzyme substrates through DNA hybridization, displaying a blue color with a broad absorption band around 700 nm. In the presence of Pb2+, the cross-linker substrates were readily cleaved, so that no aggregates could be formed and the dispersed gold nanoparticles showed a red color with an absorption band at 522 nm. The light extinction ratio E522/E700 could then be used as a measure for the quantification of Pb2+ concentrations in water. Interestingly, the dynamic range of Pb2+ detection was successfully tuned from 0.1~4 to 10~200 μM by changing the ratio of active and inactive DNAzymes.136 A follow-up study further optimized the sensor design by testing different arm lengths of DNAzymes, gold nanoparticle alignments, ratios of DNAzymes and substrates, pH, and temperatures.138 The detection of Pb2+ and formation of nanoparticle aggregates was accelerated at room temperature by the “tail-to-tail” alignment of DNA to 42 nm gold nanoparticles.137 To make the sensor system less vulnerable to environmental fluctuations, a new approach of “light-up” (assembly) detection compared to the previous “light-down” (disassembly) response was developed with the assistance of invasive DNA.139 When an improved design was applied utilizing asymmetric DNAzymes and substrates to form the gold nanoparticle aggregates, the usage of invasive DNA was avoided to further simplify the detection.140 In addition to the Pb2+-dependent DNAzyme, Cu2+- and UO22+-dependent DNAzymes were also functionalized with gold nanoparticles for the development of colorimetric sensors for Cu2+ 141 and UO22+ 143 using a similar approach, respectively. Instead of cross-linking nanoparticles by DNA, Li and coworkers demonstrated that metal ion-induced cleavage of substrates by DNAzymes on dispersed gold nanoparticles caused the aggregation of the nanoparticles, and metal ions such as Pb2+ could be detected by the color change from red to purple (Figure 7B).146 A colorimetric sensor with logic response to Pb2+ and Mg2+ was described by Zhang and coworkers based on two DNAzymes and cross-linked gold nanoparticles (Figure 7C).147 When gold nanoparticles functionalized with DNAzymes were entrapped in hydrogels via DNA cross-linking, they could also serve as a colorimetric sensor for the detection of metal ions such as Cu2+, as reported by Yang and coworkers.149 Besides color change, DNAzyme-cross-linked gold nanoparticles were also used for ultrasensitive metal ion detection via the light scattering signal change upon formation or dissolution of aggregates.150
Figure 7.
(A) A colorimetric Pb2+ sensor based on DNAzymes and functionalized gold nanoparticle assemblies that undergo disassembly in the presence of Pb2+. (B) A colorimetric Pb2+ sensor based on Pb2+-induced assembly of DNAzyme-functionalized gold nanoparticles. (C) Logic response to Pb2+ and Mg2+ using gold nanoparticle as signal output by a DNA duplex containing two DNAzymes as sensors. Adapted from Refs 170, 180 and 181.
In addition to thiol-gold interactions, DNA can bind to gold nanoparticles non-covalently via its nucleotide bases, with much higher binding affinities to gold for ssDNA than fully complementary dsDNA. Following this principle, label-free colorimetric sensors for metal ions have been developed using unmodified gold nanoparticles and DNAzymes.143–145,148,151–153 Both Wang group145 and our group144 reported the use of a label-free Pb2+-dependent DNAzyme and unmodified 13 nm gold nanoparticles as colorimetric sensors for Pb2+ detection in water. In the presence of Pb2+, the DNAzyme-substrate duplex underwent cleavage and formed ssDNA fragments, which stabilized gold nanoparticles upon salt addition. Therefore, the concentration of Pb2+ in the samples was quantified by measuring the color change from blue to red. A similar approach was also applied in our group to a UO22+-dependent DNAzyme, and the resulting label-free sensor successfully detected UO22+ in water without any modifications to the gold nanoparticles or DNAzymes.143 For a label-free colorimetric Cu2+ sensor, Yang and coworkers utilized a unimolecular self-cleaving Cu2+-dependent DNAzyme and unmodified gold nanoparticles.148 Through nanogold-seeded nucleation amplification, a sensitive label-free UO22+ sensor was developed using a UO22+-dependent DNAzyme and unmodified gold nanoparticles.151 Similar to their labeled analogues, the label-free sensors based on DNAzymes and gold nanoparticles were also capable of serving as light scattering sensors for sensitive detection of Pb2+ and Cu2+, with comparable performance as the labeled ones.152,153
2.2.2 Colorimetric “dipstick” tests using lateral flow devices
Because the molar extinction coefficients of gold nanoparticles are much larger than most organic dyes, they are ideal materials for developing colorimetric test strips for metal ion detection at low concentrations. Using lateral flow devices similar to a previous approach for aptamers,156 a Pb2+-dependent DNAzyme was coupled with gold nanoparticles to construct an easy-to-use dipstick for Pb2+ in paints (Figure 8A).157 In the presence of Pb2+, the cleavage of substrates by the DNAzyme removed biotin from the surface of gold nanoparticles, enabling the capture of these red-colored nanoparticles on the test zone for visible detection of Pb2+ concentrations. Zeng and coworkers utilized Cu2+- and Pb2+-dependent DNAzymes to fabricate a similar lateral flow device for the detection of Cu2+ (Figure 8B) and Pb2+, respectively.158,159 The introduction of a catalytic DNA circuit in the Pb2+-sensitive device dramatically enhanced the sensitivity of the sensor when compared with previous solution-based approaches.159
Figure 8.
(A) Lateral flow dipstick for visible detection of Pb2+ in paint. (B) Lateral flow dipstick for visible detection of Cu2+. Adapted from Refs 191 and 192.
2.2.3 Colorimetric sensors based on hydrogen peroxidase-mimic DNAzymes
Another strategy to develop colorimetric DNAzyme-based sensors for metal ion detection is the use of metal ion-dependent DNAzymes to recognize target metal ions and hydrogen peroxidase-mimic DNAzymes to catalyze color-generating chemical reactions for signal output.43,160–168 Willner and coworkers developed a DNAzyme cascade to transform the Pb2+-induced cleavage of substrate by the Pb2+-dependent DNAzyme into the production of colored oxidized 2,2′-azino-bis(3-ethylbenzothiazoline)-6-sulfonate (ABTS), therefore achieving colorimetric detection of Pb2+ by monitoring the increase of absorbance at 414 nm or direct observation of green color generation (Figure 9A).164 The group also demonstrated a similar design for colorimetric detection of UO22+ using a UO22+-dependent DNAzyme, and introduced one additional Mg2+-dependent DNAzyme for the construction of a logic gate.165 Tan and coworkers combined the Cu2+-dependent DNAzyme, substrate, and a hydrogen peroxidase-mimic DNAzyme into a unimolecular sensor, and achieved colorimetric detection of Cu2+ with high sensitivity (Figure 9B).166 Similar dual-DNAzyme approaches were also applied by other groups for the detection of Pb2+ and Cu2+ using Pb2+- and Cu2+-dependent DNAzymes, respectively.167,168
Figure 9.

(A) Pb2+-induced activation of a hydrogen peroxidase-mimic DNAzyme (red) by a Pb2+-dependent DNAzyme (blue) for colorimetric Pb2+ detection. (B) Colorimetric detection of Cu2+ by a unimolecular sensor containing a hydrogen peroxidase-mimic DNAzyme (blue) by a Cu2+-dependent DNAzyme (yellow). Adapted from refs 195 and 196.
2.3 Electrochemical and Raman sensors based on metal ion-dependent DNAzymes
Electrochemical and Raman signals have also been reported for developing metal ion sensors based on metal ion-dependent DNAzymes.169–184 For example, Plaxco and coworkers achieved ppb level electrochemical Pb2+ detection using an electrode-bound DNAzyme assembly, where the cleavage of substrates by Pb2+-dependent DNAzymes brought the attached electrochemical tags much closer to the electrode to enhance the electrochemical signals (Figure 10A).169 Shao and coworkers also developed an electrochemical Pb2+ sensor by immobilization of DNAzymes and DNA-gold bio bar codes on electrodes for amplified detection (Figure 10B).170 Similarly, other excellent works utilized different DNAzymes and nanomaterials to construct a series of sensors on electrodes, and have successfully detected Pb2+,173,177,179,182,184 Cu2+,172,174,181 Mg2+,178 and UO22+ 180 in various samples. In additional to the above sensors based on electrical signals, electrochemiluminescent sensors were also demonstrated for the sensitive detection of Pb2+.171,173,175,183 A study modifying DNAzyme-based sensors with Raman tags on gold nanoparticles instead of electrochemical tags enabled the detection of Pb2+ by surface enhanced Raman spectra (SERS).176
Figure 10.

(A) Electrical detection of Pb2+ by Pb2+-induced cleavage of DNAzyme substrates that decreases the distance between electrochemical tags and gold electrodes. (B) An electrochemical Pb2+ sensor based on Pb2+-induced cleavage of DNAzyme substrates that releases DNA-gold bio-bar codes containing ruthenium complexes. Adapted from Refs 200 and 201.
3. Mismatched DNA and G-quadruplex DNA for Metal Ion Binding
In addition to metal ion-dependent DNAzymes that were selected from random DNA libraries through combinatorial techniques, at least two types of DNA structures were also discovered to be efficient binding motifs for a series of metal ions. One of them is DNA mismatches that can bind specific metal ions to form stable “base pairs”.31–33 Examples include natural nucleobases such as T-T and C-C mismatches that can form stable T-Hg2+-T 185,186 and C-Ag+-C 187 structures in DNA duplexes with high specificity to Hg2+ and Ag+, respectively (Figure 11), as well as artificial bases that form stabilized pairs with Ag+ and Cu2+,15,31,33,188–191 though the latter has not been widely applied in sensors due to the lack of commercial availability of the artificial bases required.33 The other is the DNA G-quadruplex that is stabilized or destabilized by specific metal ions,43,53,55,192,193 such as K+,26,194,195 Pb2+,27,196,197 Ag+,30 and Cu2+ 28,29 (Figure 12). Taking advantage of the specific metal ion-DNA interactions, many metal ion sensors based on such DNA structures have been developed in recent years.32,43,53,55,192,193
Figure 11.

(A) Binding of Hg2+ and Ag+ by T-T and C-C mismatches in DNA. (B) A fluorescent Hg2+ sensor based on T-Hg2+-T. (C) A fluorescent Ag+ sensor based on C-Ag+-C. Adapted from Refs 31–33.
Figure 12.
Structure of G-quadruplex DNA stabilized by K+ (A), Pb2+ (B) and Cu2+ (C), while destabilized by Ag+ (D). Adapted from Refs 265, 224, 36 and 38.
3.1 Hg2+ sensors based on T-Hg2+-T-containing DNA
Upon the first discovery of stable complex formation between T-T mismatches and Hg2+ in a DNA duplex with high specificity to Hg2+ by Ono and coworkers (Figure 11A),185–187 the Hg2+-induced DNA duplex formation has been widely applied as a switch for Hg2+ sensor development (Figure 11B).58,102,142,198–230 Examples include but not limit to DNA-based sensors for Hg2+ with colorimetry,198,199,201,203,204,218 fluorescence,142,200,208,209,211,219,220,224,225 electrochemistry,210,215 SERS,213 surface plasmon resonance,217,227 and evanescent wave generation216 as signal output.
3.2 Ag+ sensors based on C-Ag+-C-containing DNA
Interestingly, in addition to the specific interaction between Hg2+ and T-T mismatches, Ono and coworkers also found that C-C mismatches in DNA could selectively bind Ag+ (Figure 11).187 Following this principle, a number of Ag+ sensors have been developed (Figure 11C),187,208,215,225,231–237 using colorimetry,231,232,235,237 fluorescence,208,225,233,236 light scattering,234 electrochemistry,215 and atomic force microscopy.238
3.3 Sensors for K+, Pb2+, Cu2+ and Ag+ based on G-quadruplex DNA
K+ has been known to stabilize G-quadruplex motifs in DNA.26 Fluorescent or FRET-based K+ sensors were developed by labeling G-quadruplex DNA sequences with fluorophores (Figure 12A)195,239,240 or using label-free intercalating dyes.241–243 Conjugated polymers that could distinguish K+-bound and non-bound G-quadruplex DNA were also used for amplified fluorescence detection of K+ in homogeneous solution.244,245 Colorimetric246–249 and electrochemical250,251 methods were also demonstrated based on G-quadruplex DNA for K+ detection.
In addition to K+, studies have also shown that Pb2+ is also capable of stabilizing the DNA G-quadruplex.27,196 A colorimetric sensor (Figure 12B),197 followed by a fluorescent version,252 was developed by Wang and coworkers for selective Pb2+ detection, as an alternative of sensors based on Pb2+-dependent DNAzymes. Since then, many other Pb2+ sensors have been reported using a similar DNA G-quadruplex, spanning between colorimetric,253–255 fluorescent,214,226,256–263 electrochemical,215,264,265 and resonance scattering sensors.266,267
Compared to the roles of K+ and Pb2+ in stabilizing DNA G-quadruplexes, the role of Cu2+ is less well understood, but most likely provides stabilization in the form of a metal-ligand complex (Figure 12C).28,29 Two studies by Wang and coworkers demonstrated success in developing fluorescent Cu2+ sensors based on this property.29,268
Unlike the above 3 metal ions, instead of stabilization, Ag+ was found by Kong and coworkers to destabilize DNA G-quadruplexes (Figure 12D).30 Through this approach, colorimetric30,269 and fluorescent270–272 sensors were also developed for selective Ag+ detection.
4. Portable Sensors Using Widely Available Devices
In point-of-interest applications, such as in-field or at-home detections of metal ions, laboratory-based analytical instruments such as spectrometers and electrochemical workstation are not available, thus demanding new metal ion sensors compatible with portable devices for quantitative detection. Although the lateral flow devices mentioned above (section 2.2.2) can realize fast detection without the aid of instruments, this detection is only semi-quantitative and based on observation by eye, which may suffer from human error. Quantitative detection, therefore, is difficult. To address this challenge, interesting technologies have been developed to design sensors based on publically commercialized devices, which would enable not only trained personnel but also the general public to monitor metal ions at almost any point of interest.
Yu and coworkers immobilized Pb2+-dependent DNAzymes and substrates with nanomaterials on the surface of computer-readable discs (Figure 13A).273 The nanomaterials cause error signals during laser reading of the disc. When samples containing Pb2+ were applied to such discs, the Pb2+-induced cleavage of the substrates caused the loss of nanomaterials, thus removing error signals. Using software that counted error numbers, Pb2+ concentration could be successfully quantified. Any portable computer equipped with a CD drive should be able to use this method for Pb2+ detection.
Figure 13.
(A) Detection of Pb2+ using computer-readable discs based on a Pb2+-dependent DNAzyme (17E). (B) UO22+ sensor based on UO22+-dependent DNAzyme (39E) conjugated to invertase and magnetic beads for PGM detection of UO22+. Adapted from Refs 299 and 300.
In our group, we developed a new technology to take advantage of the most successfully commercialized public diagnosis device, personal glucose meters (PGMs), for metal ion detection (Figure 13B).274,275 In this approach, invertase, an enzyme that converts PGM-inert sucrose into PGM-detectable glucose was conjugated with a UO22+-dependent DNAzyme-substrate duplex and immobilized on the surface of magnetic beads. When samples containing UO22+ were applied to the sensor, the cleavage of the substrates disrupted the duplex structure and released invertase from the surface into solution. After removal of magnetic beads by a magnet, the released invertase was allowed to catalyze the production of glucose, whose concentration was proportional to that of UO22+ in the sample. Finally, the concentration of UO22+ was successfully quantified via PGM measurement.274 To minimize the interaction between metal ions and functional materials on the surface of magnetic beads, we further demonstrated an invasive DNA approach that separated the DNAzyme reaction and the invertase release/catalysis, and achieved sensitive detection of both Pb2+ and UO22+ at concentrations well below the regulated levels by the EPA.275 Xiang and coworkers also developed another approach using the PGM to detect Cu2+ in water via the ability of Cu2+ to catalyze azide-alkyne reactions,276 which was previously applied by Mirkin and coworkers for colorimetric detection of Cu2+ using gold nanoparticles.277
5. Sensing and Imaging of Metal Ions in Living Cells
In contrast to the large amount of work about metal ion detection in vitro using DNA-based sensors, the detection and imaging of metal ions in biological systems are of great significance for medical and biological studies. However, there are only very limited examples of DNA-based sensors for metal ion detection in living cells. The difficulties include the delivery of sensor DNA into desired locations in cells, maintaining the stability of DNA strands against enzymatic degradation in living cells, and the controlled “activation” of sensors at specific locations inside cells. Our group has recently developed a fluorescent sensor for UO22+ utilizing DNAzymes for UO22+ recognition and gold nanoparticles for efficient cellular delivery (Figure 14).278 The sensor was cell-compatible and efficiently delivered into living cells for UO22+ imaging, demonstrating the promise of DNAzyme-based sensors for cellular applications for the first time. By fluorescence microscopy analysis, the sensor was localized in lysosomes and indicated the accumulation of UO22+ in lysosomes when the living cells were in a UO22+-polluted media. Further work is still needed to further investigate the mechanism of uptake and properties of the sensor in more detail and develop other sensors for cellular imaging of other metal ions. Although this study just scratches the surface of metal ions detection in vivo, it is apparent that DNA-based sensors will play larger and more important roles in in vivo applications in the future.
Figure 14.
Live cell imaging of UO22+ by fluorophore-labeled uranyl-specific DNAzymes and substrates immobilized on gold nanoparticles. Adapted from Ref 304.
6. Summary and Perspective
Through both combinatorial selection and rational design, a number of DNAzymes and DNA structures have been found to display highly selective responses to specific metal ions. Using these DNA sequences as the basis, many metal ion sensors have been developed utilizing various analytical techniques, including colorimetry, fluorescence, electrochemistry, SERS, and light scattering. For on-site and point-of-care applications, metal ion sensors that can be used with commercially available portable devices, such as computer CD drives and personal glucose meters, are already available for quantitative detection, whereas lateral flow devices are ideal for instrument-free semi-quantitative analysis of metal ions by direct color observation.
6.1 Advantages of DNA molecules as sensors and imaging agents for metal ions
Although DNA was not the first choice as sensors for metal ions due to perceived non-specific electrostatic interactions between the phosphodiester backbone of the DNA and metal ions, reports from many labs around the world in the past decade have now firmly established that some DNA molecules can be effective sensors and imaging agents for metal ions. In the process, these studies have also demonstrated distinct advantages of DNA-based methods, particularly DNAzyme-based methods, over other methods.
The first advantage is that DNAzymes that are specific for almost any metal ion in specific oxidation states may be obtained using the same in vitro selection protocol, most notably even without prior knowledge of how the sensing molecules can bind selectively to that certain metal ion.74,75,87,93,200 In contrast, it is usually difficult for most other techniques to apply successful strategies in designing sensors for one metal ion towards sensing other metal ions, and thus extensive trial and error is required for sensor development for each additional metal ion. Until recently, antibodies were considered a general method to obtain sensing molecules for a broad range of targets. However, due to the need to elicit immune responses, it is often very difficult to generate antibodies selective for targets as small as metal ions. DNAzymes, since they are obtained in vitro, do not have the same issue as antibodies.
Another major challenge in designing sensors for metal ions is lack of selectivity; the initially designed small molecule designed to bind one metal ion can often end up binding to other metal ions even more strongly. When this occurs, more work is required to redesign the molecules to better bind the intended target, which again is largely a process of trial and error. The in vitro selection method mentioned above is not immune to this problem when DNAzymes selected to be specific for one metal ion are more active in the presence of another metal ion. To meet this challenge, a “negative selection” strategy has been developed to remove the population of DNA sequences that bind competing metal ions, resulting in DNAzymes that are more selective for the target metal ion.67,68 The reason why this strategy works for in vitro selection is that, instead of starting with one design and having to repeat the process of redesign, it starts with a large DNA sequence library (up to 1015 variations). Even though the “negative selection” removes a large percentage of sequences, there are enough sequences left to perform the function in the presence of target metal ions.
Even though many molecules, especially biomolecules (e.g., proteins), are known to bind metal ions strongly and selectively, they are not metal sensors yet, as another major component is signal transduction. It is often difficult to transform the metal binding into signals in a general way without interfering with the binding. In contrast, since it is relatively easy to modify DNA with a reporter group, the reporter can be placed further away from the binding site and the signal transduction can be realized based on the melting temperature differences before and after metal binding. The third advantage of DNA-based sensors is the straightforward nature of transforming metal ion recognition into different signal outputs and achieving efficient signal amplification for more sensitive detection without sacrificing metal ion selectivity.23,34–40,42–61,192,193,279–299 As shown in Figure 2, by introducing fluorophores, chromophores, nanomaterials, electrochemical tags, and Raman tags into the same design strategy, DNA-based sensors for metal ions based on fluorescence, colorimetry, electrochemistry, and surface Raman enhancement have been developed. In addition, many DNA-related enzymatic reactions and DNA-functionalized nanomaterials have been successfully incorporated into DNA-based sensors for signal amplification to achieve more sensitive detection of metal ions.
The fourth advantage of DNAzyme-based metal sensors is a general method to tune the dynamic range of detection.108,121,144 This feature is especially important for sensing and imaging metal ions because every metal ion has a threshold level above which it is considered toxic. More importantly, this threshold level varies depending on the sample matrix or location where the metal ion resides. For example, the threshold for Pb2+ in soil is 400 ppm, but much lower in drinking water (75 nM, defined by the US EPA). Even for the same matrix, the threshold can be different. For example, the defined Pb2+ level for paint on walls is 1.0 mg/dL, but lower for paint on toys (100 ppm) because Pb2+ in toys poses more danger to children. Therefore, it is not enough to have sensitive and selective sensors for metal ions; the sensors must also possess tunable dynamic ranges. DNAzymes can fulfill this requirement.
Unlike sensors for diamagnetic metal ions such as Ca2+,300,301 Zn2+,302–304 Cu+,305,306 Hg2+,307–309 and Pb2+,310,311 designing and synthesizing sensitive and selective sensors for paramagnetic metal ions, such as iron, remains a significant challenge. Even though fluorescent and chemiluminescent sensors based on various chelating agents have been reported for Co2+,312–316 Cu2+,317–335 Fe2+,336–338 and Fe3+,339–342 many of them are based on quenching of fluorescence due to the paramagnetic metal ions’ intrinsic fluorescence quenching properties, which is generally undesirable for analytical purposes because of the small dynamic range and potential false positives caused by non-specific quenching in real samples. Other problems with current sensors include the requirement for oxidizing reagents such as hydrogen peroxide, and poor selectivity. Therefore, the fifth advantage of DNA-based sensors is the ease of rational design to circumvent the quenching effect of paramagnetic ions by spatially separating the metal recognition part from the fluorescent signaling moiety, so that they are independent of each other. For example, our previously reported metal ion sensing platform based on DNAzyme catalytic beacons spatially separated the two elements (fluorophore/quencher and metal ion binding site) by rigid double-stranded DNA, resulting in fluorescent “turn-on” sensors, not only for diamagnetic metal ions such as Pb2+78,87,89 and UO22+74 but also for paramagnetic Cu2+,93 with high sensitivity and selectivity.
Finally, DNA is biocompatible and biodegradable, and is not recombinant. Thus it is environmentally benign. Under physiological conditions, DNA is nearly 1,000-fold more stable to hydrolysis than proteins/antibodies and nearly 100,000-fold more stable than RNA.343 The well-defined globular structure of catalytic DNAs are also not easily recognized by endo- or exonucleases, and thus are more resistant to nuclease attack than single- or even double-stranded DNA/RNA.344 When folded, the compact globular catalytic DNAs are also less likely to bind other biomolecules in cells than single- or double-stranded DNA/RNA. In addition, unlike proteins or antibodies, most DNAzymes can be denatured and renatured many times without losing binding ability or activity. They can be stored under rather harsh, denaturing conditions and can be used when the correct conditions are restored. Therefore, DNA has a much longer shelf-life than many other biomolecules and is thus more suitable for field studies. Finally, DNA is adaptable to fiber optic and microarray technology, 345–347 which is important for onsite or remote sensing of multiple metal ions simultaneously.
6.2 Future directions
One important task in this field is the identification of new DNAzymes or DNA structures that can recognize more metal ions. Currently, DNA-based sensors are excellent in the detection of only a series of metal ions including K+, Mg2+, Zn2+, Pb2+, Cu2+, Co2+, Mn2+, Hg2+, Ag+, and UO22+. However, for other important metal ions such as Fe2+, Fe3+, Ni2+, Cr3+, Cd2+, there are very few DNA sequences found to show high specificity and affinity toward them. To address this challenge, more in-depth investigation is required to improve the classic DNA selection or discovery techniques. In addition, modified DNA bases and backbones with functional groups capable of metal binding are promising candidates for incorporation into DNA sequences to obtain DNAzymes that can recognize the metal ions that may be difficult for natural DNAs.
While a number of DNAzymes have been obtained specific for different metal ions, fundamental understanding of structural features responsible for the remarkable selectivity is still lacking. Biochemical studies have identified conserved sequences for the metal binding and catalytic activities.20,80,91,96,348–350 Biophysical studies, such as FRET and smFRET studies have suggested certain DNAzymes use the “lock-and-key” mode of metal binding and catalysis for the most active metal ions, similar to protein enzymes.79,82,92,221,351,352 However, due to difficulty in obtaining three dimensional structures of these DNAzymes, the exact 3D structural features remain to be elucidated.
Another challenge is multiplexed detection of different metal ions simultaneously. Although a few studies using quantum dots and microarrays have demonstrated great promise, the number of metal ion species that can be analyzed in one test is still limited. Not only are new DNA-based sensors needed for other metal ions, but a buffer condition compatible for the detection of many metal ions is also demanded. For the former, it can be anticipated that new DNA sequences for more metal ions will be identified through selection or discovery in the near future. For the latter, it is highly recommended that the selection or discovery of new DNA-based sensors for metal ions should be carried out under the same conditions (including buffer pH, ionic strength, and temperature) as that for known sensors, to ensure all the sensors can be used in one solution for multiple metal ions without compromising the performance of any sensor.
As the public demand for monitoring hazardous metal ions quantitatively at the point of interest increases, a large market of portable sensors for metal ions is emerging and is expected to grow rapidly. Although achievements have been made in metal ion detection using some commercial devices for the public, such as glucose meters and computer CD drives, there is still a need to make these methods of detection more user-friendly for public usage.
Finally, while DNA and DNAzyme sensors for detection of metal ions in the environment have been relatively well developed, including commercially available products,353 there are relatively fewer reports of using the DNA and DNAzymes as sensing or imaging agents for metal for detecting metal ions in living cells and in vivo. Recent reports of DNAzyme-based imaging agents for detection uranyl in cells278 and DNAzyme-based MRI contrast agents354 are encouraging. Such sensors and imaging agents will provide more exciting opportunities for scientists to uncover the roles of metal ions in biological systems.
Acknowledgments
The Lu group research mentioned in this review has been supported by the U.S. National Institutes of Health (Grant ES016865), by the Office of Science (BER), the U.S. Department of Energy (DE-FG02-08ER64568), and National Science Foundation (CTS-0120978, CMMI 0749028 and DMR-0117792). The authors thank Ms. Li Huey Tan for helping prepare the graphics in this review.
References
- 1.Tsien RY. In: Fluorescenct Chemosensors for Ion and Molecule Recognization. 538. Czarnik AW, editor. American Chemical Society; Washington, DC: 1993. pp. 130–146. [Google Scholar]
- 2.Czarnik AW. Acc Chem Res. 1994;27:302–308. [Google Scholar]
- 3.de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE. Chem Rev. 1997;97:1515–1566. doi: 10.1021/cr960386p. [DOI] [PubMed] [Google Scholar]
- 4.Prodi L, Bolletta F, Montalti M, Zaccheroni N. Coord Chem Rev. 2000;205:59–83. [Google Scholar]
- 5.Domaille DW, Que EL, Chang CJ. Nature Chem Biol. 2008;4:168–175. doi: 10.1038/nchembio.69. [DOI] [PubMed] [Google Scholar]
- 6.Nolan EM, Lippard SJ. Chem Rev. 2008;108:3443–3480. doi: 10.1021/cr068000q. [DOI] [PubMed] [Google Scholar]
- 7.Que EL, Domaille DW, Chang CJ. Chem Rev. 2008;108:1517–1549. doi: 10.1021/cr078203u. [DOI] [PubMed] [Google Scholar]
- 8.Chen PR, He C. Curr Opinion Chem Biol. 2008;12:214–221. doi: 10.1016/j.cbpa.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Nolan EM, Lippard SJ. Acc Chem Res. 2009;42:193–203. doi: 10.1021/ar8001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McRae R, Bagchi P, Sumalekshmy S, Fahrni CJ. Chem Rev. 2009;109:4780–4827. doi: 10.1021/cr900223a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kikuchi K. Chem Soc Rev. 2009;39:2048–2053. doi: 10.1039/b819316a. [DOI] [PubMed] [Google Scholar]
- 12.Tomat E, Lippard SJ. Curr Opinion Chem Biol. 2010;14:225–230. doi: 10.1016/j.cbpa.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer AE, Qin Y, Park JG, McCombs JE. Trends Biotechnol. 2011;29:144–152. doi: 10.1016/j.tibtech.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Checa SK, Zurbriggen MD, Soncini FC. Curr Opin Biotech. 2012;23:766–772. doi: 10.1016/j.copbio.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Sigel RKO, Sigel H. Acc Chem Res. 2010;43:974–984. doi: 10.1021/ar900197y. [DOI] [PubMed] [Google Scholar]
- 16.Sabat M, Lippert B. Metal Ions in Biological Systems. 1996;33:143–176. [PubMed] [Google Scholar]
- 17.Navarro JAR, Lippert B. Coord Chem Rev. 1999;185–6:653–667. [Google Scholar]
- 18.Lippert B. Coord Chem Rev. 2000;200:487–516. [Google Scholar]
- 19.Al-Sogair FM, Operschall BP, Sigel A, Sigel H, Schnabl J, Sigel RKO. Chem Rev. 2011;111:4964–5003. doi: 10.1021/cr100415s. [DOI] [PubMed] [Google Scholar]
- 20.Breaker RR, Joyce GF. Chem & Biol. 1994;1:223–229. doi: 10.1016/1074-5521(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 21.Cuenoud B, Szostak JW. Nature. 1995;375:611–614. doi: 10.1038/375611a0. [DOI] [PubMed] [Google Scholar]
- 22.Soukup GA, Breaker RR. Curr Opin Struct Biol. 2000;10:318–325. doi: 10.1016/s0959-440x(00)00090-7. [DOI] [PubMed] [Google Scholar]
- 23.Lu Y. Chem Eur J. 2002;8:4589–4596. [PubMed] [Google Scholar]
- 24.Freisinger E, Sigel RKO. Coord Chem Rev. 2007;251:1834–1851. [Google Scholar]
- 25.Lan T, Lu Y. Met Ions Life Sci. 2012;10:217–248. doi: 10.1007/978-94-007-2172-2_8. [DOI] [PubMed] [Google Scholar]
- 26.Sen D, Gilbert W. Nature. 1990;344:410–414. doi: 10.1038/344410a0. [DOI] [PubMed] [Google Scholar]
- 27.Smirnov I, Shafer RH. J Mol Biol. 2000;296:1–5. doi: 10.1006/jmbi.1999.3441. [DOI] [PubMed] [Google Scholar]
- 28.Monchaud D, Yang P, Lacroix L, Teulade-Fichou MP, Mergny JL. Angew Chemie Inter Ed. 2008;47:4858–4861. doi: 10.1002/anie.200800468. [DOI] [PubMed] [Google Scholar]
- 29.Qin HX, Ren JT, Wang JH, Wang EK. Chem Commun. 2010;46:7385–7387. doi: 10.1039/c0cc01695k. [DOI] [PubMed] [Google Scholar]
- 30.Zhou XH, Kong DM, Shen HX. Anal Chem. 2010;82:789–793. doi: 10.1021/ac902421u. [DOI] [PubMed] [Google Scholar]
- 31.Johannsen S, Megger N, Bohme D, Sigel RKO, Muller J. Nat Chem. 2010;2:229–234. doi: 10.1038/nchem.512. [DOI] [PubMed] [Google Scholar]
- 32.Ono A, Torigoe H, Tanaka Y, Okamoto I. Chem Soc Rev. 2011;40:5855–5866. doi: 10.1039/c1cs15149e. [DOI] [PubMed] [Google Scholar]
- 33.Scharf P, Muller J. Chempluschem. 2013;78:20–34. [Google Scholar]
- 34.Lu Y, Liu JW, Li J, Bruesehoff PJ, Pavot CMB, Brown AK. Biosens Bioelectron. 2003;18:529–540. doi: 10.1016/s0956-5663(03)00013-7. [DOI] [PubMed] [Google Scholar]
- 35.Nutiu R, Mei S, Liu ZJ, Li YF. Pure Appl Chem. 2004;76:1547–1561. [Google Scholar]
- 36.Liu JW, Lu Y. J Fluoresc. 2004;14:343–354. doi: 10.1023/b:jofl.0000031816.06134.d3. [DOI] [PubMed] [Google Scholar]
- 37.Navani NK, Li YF. Curr Opin Chem Biol. 2006;10:272–281. doi: 10.1016/j.cbpa.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Lu Y, Liu JW. Curr Opin Biotechnol. 2006;17:580–588. doi: 10.1016/j.copbio.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Sato K, Hosokawa K, Maeda M. Anal Sci. 2007;23:17–20. doi: 10.2116/analsci.23.17. [DOI] [PubMed] [Google Scholar]
- 40.Lu Y, Liu JW. Acc Chem Res. 2007;40:315–323. doi: 10.1021/ar600053g. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Lu Y. Functional Nucleic Acids for Analytical Applications. Springer; 2008. [Google Scholar]
- 42.Palchetti I, Mascini M. Analyst. 2008;133:846–854. doi: 10.1039/b802920m. [DOI] [PubMed] [Google Scholar]
- 43.Willner I, Shlyahovsky B, Zayats M, Willner B. Chem Soc Rev. 2008;37:1153–1165. doi: 10.1039/b718428j. [DOI] [PubMed] [Google Scholar]
- 44.Knecht MR, Sethi M. Anal Bioanal Chem. 2009;394:33–46. doi: 10.1007/s00216-008-2594-7. [DOI] [PubMed] [Google Scholar]
- 45.Schlosser K, Li YF. Chem Biol. 2009;16:311–322. doi: 10.1016/j.chembiol.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Wang H, Yang RH, Yang L, Tan WH. ACS Nano. 2009;3:2451–2460. doi: 10.1021/nn9006303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu Y, Liu JW. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:35–46. doi: 10.1002/wnan.21. [DOI] [PubMed] [Google Scholar]
- 48.Wang ZD, Lu Y. J Mater Chem. 2009;19:1788–1798. doi: 10.1039/B813939C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu JW, Cao ZH, Lu Y. Chem Rev. 2009;109:1948–1998. doi: 10.1021/cr030183i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li D, Song SP, Fan CH. Acc Chem Res. 2010;43:631–641. doi: 10.1021/ar900245u. [DOI] [PubMed] [Google Scholar]
- 51.Teller C, Willner I. Curr Opin Biotechnol. 2010;21:376–391. doi: 10.1016/j.copbio.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 52.de la Escosura-Muniz A, Medina M, Merkoci A. In: Nucleic Acid Biosensors for Environmental Pollution Monitoring. Mascini M, Palchetti I, editors. 2011. pp. 141–164. [DOI] [PubMed] [Google Scholar]
- 53.Kosman J, Juskowiak B. Anal Chim Acta. 2011;707:7–17. doi: 10.1016/j.aca.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 54.Ma DL, Chan DSH, Man BYW, Leung CH. Chem Asian J. 2011;6:986–1003. doi: 10.1002/asia.201000870. [DOI] [PubMed] [Google Scholar]
- 55.Xu J, Cai LL, Kong DM, Shen HX. Anal Lett. 2011;44:2582–2592. [Google Scholar]
- 56.Nagraj N, Lu Y. In: Nucleic Acid Biosensors for Environmental Pollution Monitoring. Mascini M, Palchetti I, editors. 2011. pp. 82–98. [DOI] [PubMed] [Google Scholar]
- 57.Zhang XB, Kong RM, Lu Y. In: Annual Review of Analytical Chemistry. Cooks RG, Yeung ES, editors. Vol. 4. 2011. pp. 105–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee J, Lin L, Li Y. In: Advanced Fluorescence Reporters in Chemistry and Biology Iii: Applications in Sensing and Imaging. Demchenko AP, editor. Vol. 10. 2011. pp. 201–221. [Google Scholar]
- 59.Yin XB. TRAC-Trend Anal Chem. 2012;33:81–94. [Google Scholar]
- 60.Ali MM, Aguirre SD, Mok WWK, Li Y. Methods in Molecular Biology. 2012;848:395–418. doi: 10.1007/978-1-61779-545-9_25. [DOI] [PubMed] [Google Scholar]
- 61.Tram K, Kanda P, Li Y. J Nucleic Acids. 2012;2012:958683–958683. doi: 10.1155/2012/958683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee JH, Wang Z, Lu Y. In: Molecular Biological Technologies for Ocean Sensing. Tiquia-Arashiro SM, editor. Springer; New York: 2012. [Google Scholar]
- 63.Li L, Lu Y. In: DNA Nanotechnology. Fan C, editor. Springer; Berlin Heidelberg: 2013. pp. 277–305. [Google Scholar]
- 64.Ellington AD, Szostak JW. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 65.Robertson DL, Joyce GF. Nature. 1990;344:467–468. doi: 10.1038/344467a0. [DOI] [PubMed] [Google Scholar]
- 66.Tuerk C, Gold L. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 67.Bruesehoff PJ, Li J, Angustine AJ, Lu Y. Comb Chem High Throughput Screen. 2002;5:327–335. doi: 10.2174/1386207023330264. [DOI] [PubMed] [Google Scholar]
- 68.Ihms HE, Lu Y. Methods Mol Biol. 2012;848:297–316. doi: 10.1007/978-1-61779-545-9_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Breaker RR, Joyce GF. Chemistry & Biology. 1995;2:655–660. doi: 10.1016/1074-5521(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 70.Santoro SW, Joyce GF. Proc Natl Acad Sci U S A. 1997;94:4262–4266. doi: 10.1073/pnas.94.9.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li J, Zheng WC, Kwon AH, Lu Y. Nucleic Acids Res. 2000;28:481–488. doi: 10.1093/nar/28.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Santoro SW, Joyce GF, Sakthivel K, Gramatikova S, Barbas CF., III J Am Chem Soc. 2000;122:2433–2439. doi: 10.1021/ja993688s. [DOI] [PubMed] [Google Scholar]
- 73.Carmi N, Shultz LA, Breaker RR. Chemistry & Biology. 1996;3:1039–1046. doi: 10.1016/s1074-5521(96)90170-2. [DOI] [PubMed] [Google Scholar]
- 74.Liu JW, Brown AK, Meng XL, Cropek DM, Istok JD, Watson DB, Lu Y. Proc Natl Acad Sci U S A. 2007;104:2056–2061. doi: 10.1073/pnas.0607875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hollenstein M, Hipolito C, Lam C, Dietrich D, Perrin DM. Angew Chem, Int Ed. 2008;47:4346–4350. doi: 10.1002/anie.200800960. [DOI] [PubMed] [Google Scholar]
- 76.Nelson KE, Ihms HE, Mazumdar D, Bruesehoff PJ, Lu Y. ChemBioChem. 2012;13:381–391. doi: 10.1002/cbic.201100724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chandra M, Sachdeva A, Silverman SK. Nature Chemical Biology. 2009;5:718–720. doi: 10.1038/nchembio.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li J, Lu Y. J Am Chem Soc. 2000;122:10466–10467. [Google Scholar]
- 79.Liu JW, Lu Y. J Am Chem Soc. 2002;124:15208–15216. doi: 10.1021/ja027647z. [DOI] [PubMed] [Google Scholar]
- 80.Brown AK, Li J, Pavot CMB, Lu Y. Biochemistry. 2003;42:7152–7161. doi: 10.1021/bi027332w. [DOI] [PubMed] [Google Scholar]
- 81.Kim HK, Liu JW, Li J, Nagraj N, Li MX, Pavot CMB, Lu Y. J Am Chem Soc. 2007;129:6896–6902. doi: 10.1021/ja0712625. [DOI] [PubMed] [Google Scholar]
- 82.Kim HK, Rasnik I, Liu JW, Ha TJ, Lu Y. Nat Chem Biol. 2007;3:763–768. doi: 10.1038/nchembio.2007.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim HK, Li J, Nagraj N, Lu Y. Chem Eur J. 2008;14:8696–8703. doi: 10.1002/chem.200701789. [DOI] [PubMed] [Google Scholar]
- 84.Mazumdar D, Nagraj N, Kim HK, Meng XL, Brown AK, Sun Q, Li W, Lu Y. J Am Chem Soc. 2009;131:5506–5515. doi: 10.1021/ja8082939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tyagi S, Kramer FR. Nat Biotechnol. 1996;14:303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]
- 86.Liu JW, Lu Y. Anal Chem. 2004;76:1627–1632. doi: 10.1021/ac0351769. [DOI] [PubMed] [Google Scholar]
- 87.Liu JW, Lu Y. Anal Chem. 2003;75:6666–6672. doi: 10.1021/ac034924r. [DOI] [PubMed] [Google Scholar]
- 88.Nagraj N, Liu JW, Sterling S, Wu J, Lu Y. Chem Commun. 2009:4103–4105. doi: 10.1039/b903059j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lan T, Furuya K, Lu Y. Chem Commun. 2010;46:3896–3898. doi: 10.1039/b926910j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang XB, Wang ZD, Xing H, Xiang Y, Lu Y. Anal Chem. 2010;82:5005–5011. doi: 10.1021/ac1009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brown AK, Liu JW, He Y, Lu Y. Chembiochem. 2009;10:486–492. doi: 10.1002/cbic.200800632. [DOI] [PubMed] [Google Scholar]
- 92.He Y, Lu Y. Chem Eur J. 2011;17:13732–13742. doi: 10.1002/chem.201100352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu JW, Lu Y. J Am Chem Soc. 2007;129:9838–9839. doi: 10.1021/ja0717358. [DOI] [PubMed] [Google Scholar]
- 94.Mei SHJ, Liu ZJ, Brennan JD, Li YF. J Am Chem Soc. 2003;125:412–420. doi: 10.1021/ja0281232. [DOI] [PubMed] [Google Scholar]
- 95.Liu ZJ, Mei SHJ, Brennan JD, Li YF. J Am Chem Soc. 2003;125:7539–7545. doi: 10.1021/ja035208+. [DOI] [PubMed] [Google Scholar]
- 96.Shen YT, Brennan JD, Li YF. Biochemistry. 2005;44:12066–12076. doi: 10.1021/bi050746f. [DOI] [PubMed] [Google Scholar]
- 97.Chiuman W, Li YF. Nucleic Acids Res. 2007;35:401–405. doi: 10.1093/nar/gkl1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ali MM, Aguirre SD, Lazim H, Li Y. Angew Chemie Inter Ed. 2011;50:3751–3754. doi: 10.1002/anie.201100477. [DOI] [PubMed] [Google Scholar]
- 99.Thomas JM, Ting R, Perrin DM. Org Biomol Chem. 2004;2:307–312. doi: 10.1039/b310154a. [DOI] [PubMed] [Google Scholar]
- 100.Rupcich N, Chiuman W, Nutiu R, Mei S, Flora KK, Li YF, Brennan JD. J Am Chem Soc. 2006;128:780–790. doi: 10.1021/ja053336n. [DOI] [PubMed] [Google Scholar]
- 101.Chiuman W, Li YF. PLoS ONE. 2007;2:e1224. doi: 10.1371/journal.pone.0001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang F, Orbach R, Willner I. Chem Eur J. 2012;18:16030–16036. doi: 10.1002/chem.201201479. [DOI] [PubMed] [Google Scholar]
- 103.Wang H, Kim YM, Liu HP, Zhu Z, Bamrungsap S, Tan WH. J Am Chem Soc. 2009;131:8221–8226. doi: 10.1021/ja901132y. [DOI] [PubMed] [Google Scholar]
- 104.Yim TJ, Liu JW, Lu Y, Kane RS, Dordick JS. J Am Chem Soc. 2005;127:12200–12201. doi: 10.1021/ja0541581. [DOI] [PubMed] [Google Scholar]
- 105.Kim JH, Han SH, Chung BH. Biosens Bioelectron. 2011;26:2125–2129. doi: 10.1016/j.bios.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 106.Liu M, Zhao HM, Chen S, Yu HT, Zhang YB, Quan X. Biosens Bioelectron. 2011;26:4111–4116. doi: 10.1016/j.bios.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 107.Wang L, Jin Y, Deng J, Chen GZ. Analyst. 2011;136:5169–5174. doi: 10.1039/c1an15783c. [DOI] [PubMed] [Google Scholar]
- 108.Wen YQ, Peng C, Li D, Zhuo L, He SJ, Wang LH, Huang Q, Xu QH, Fan CH. Chem Commun. 2011;47:6278–6280. doi: 10.1039/c1cc11486g. [DOI] [PubMed] [Google Scholar]
- 109.Yao JJ, Li JS, Owens J, Zhong WW. Analyst. 2011;136:764–768. doi: 10.1039/c0an00709a. [DOI] [PubMed] [Google Scholar]
- 110.Zhao XH, Kong RM, Zhang XB, Meng HM, Liu WN, Tan WH, Shen GL, Yu RQ. Anal Chem. 2011;83:5062–5066. doi: 10.1021/ac200843x. [DOI] [PubMed] [Google Scholar]
- 111.Yin BC, Zuo P, Huo H, Zhong XH, Ye BC. Anal Biochem. 2010;401:47–52. doi: 10.1016/j.ab.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 112.Yu Y, Liu Y, Zhen SJ, Huang CZ. Chem Commun. 2013;49:1942–1944. doi: 10.1039/c3cc38129c. [DOI] [PubMed] [Google Scholar]
- 113.Wu CS, Oo MKK, Fan XD. ACS Nano. 2010;4:5897–5904. doi: 10.1021/nn1021988. [DOI] [PubMed] [Google Scholar]
- 114.Swearingen CB, Wernette DP, Cropek DM, Lu Y, Sweedler JV, Bohn PW. Anal Chem. 2005;77:442–448. doi: 10.1021/ac0401016. [DOI] [PubMed] [Google Scholar]
- 115.Wernette DP, Swearingen CB, Cropek DM, Lu Y, Sweedler JV, Bohn PW. Analyst. 2006;131:41–47. doi: 10.1039/b510071b. [DOI] [PubMed] [Google Scholar]
- 116.Dalavoy TS, Wernette DP, Gong MJ, Sweedler JV, Lu Y, Flachsbart BR, Shannon MA, Bohn PW, Cropek DM. Lab Chip. 2008;8:786–793. doi: 10.1039/b718624j. [DOI] [PubMed] [Google Scholar]
- 117.Zuo P, Yin BC, Ye BC. Biosens Bioelectron. 2009;25:935–939. doi: 10.1016/j.bios.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 118.Liu MY, Lou XH, Du J, Guan M, Wang J, Ding XF, Zhao JL. Analyst. 2012;137:70–72. doi: 10.1039/c1an15633k. [DOI] [PubMed] [Google Scholar]
- 119.Nie DD, Wu HY, Zheng QS, Guo LQ, Ye PR, Hao YL, Li YN, Fu FF, Guo YH. Chem Commun. 2012;48:1150–1152. doi: 10.1039/c2cc16635f. [DOI] [PubMed] [Google Scholar]
- 120.Shen YT, Mackey G, Rupcich N, Gloster D, Chiuman W, Li YF, Brennan JD. Anal Chem. 2007;79:3494–3503. doi: 10.1021/ac070235u. [DOI] [PubMed] [Google Scholar]
- 121.Xiang Y, Tong AJ, Lu Y. J Am Chem Soc. 2009;131:15352–15357. doi: 10.1021/ja905854a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang FL, Wu Z, Lu YX, Wang J, Jiang JH, Yu RQ. Anal Biochem. 2010;405:168–173. doi: 10.1016/j.ab.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 123.Xiang Y, Wang ZD, Xing H, Wong NY, Lu Y. Anal Chem. 2010;82:4122–4129. doi: 10.1021/ac100244h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu M, Zhao HM, Chen S, Yu HT, Zhang YB, Quan X. Chem Commun. 2011;47:7749–7751. doi: 10.1039/c1cc12006a. [DOI] [PubMed] [Google Scholar]
- 125.Zhang LB, Han BY, Li T, Wang EK. Chem Commun. 2011;47:3099–3101. doi: 10.1039/c0cc04523c. [DOI] [PubMed] [Google Scholar]
- 126.Chen X, Guan HL, He ZK, Zhou XD, Hu JM. Anal Method. 2012;4:1619–1622. [Google Scholar]
- 127.Song PS, Xiang Y, Xing H, Zhou ZJ, Tong AJ, Lu Y. Anal Chem. 2012;84:2916–2922. doi: 10.1021/ac203488p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang LL, Zhang YY, Wei MJ, Yi YH, Li HT, Yao SZ. New J Chem. 2013;37:1252–1257. [Google Scholar]
- 129.Yoshimoto K, Nishizawa S, Minagawa M, Teramae N. J Am Chem Soc. 2003;125:8982–8983. doi: 10.1021/ja029786m. [DOI] [PubMed] [Google Scholar]
- 130.Sankaran NB, Nishizawa S, Seino T, Yoshimoto K, Teramae N. Angew Chem, Int Ed. 2006;45:1563–1568. doi: 10.1002/anie.200502979. [DOI] [PubMed] [Google Scholar]
- 131.Ihara T, Uemura A, Futamura A, Shimizu M, Baba N, Nishizawa S, Teramae N, Jyo A. J Am Chem Soc. 2009;131:1386–1387. doi: 10.1021/ja809023n. [DOI] [PubMed] [Google Scholar]
- 132.Li M, Sato Y, Nishizawa S, Seino T, Nakamura K, Teramae N. J Am Chem Soc. 2009;131:2448–2449. doi: 10.1021/ja8095625. [DOI] [PubMed] [Google Scholar]
- 133.Li N, Mei L, Xiang Y, Tong A, Nishizawa S, Teramae N. Anal Chim Acta. 2007;597:97–102. doi: 10.1016/j.aca.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 134.Xu Z, Morita K, Sato Y, Dai Q, Nishizawa S, Teramae N. Chem Commun. 2009:6445–6447. doi: 10.1039/b908345f. [DOI] [PubMed] [Google Scholar]
- 135.Xu Z, Sato Y, Nishizawa S, Teramae N. Chem Eur J. 2009;15:10375–10378. doi: 10.1002/chem.200901226. [DOI] [PubMed] [Google Scholar]
- 136.Liu JW, Lu Y. J Am Chem Soc. 2003;125:6642–6643. doi: 10.1021/ja034775u. [DOI] [PubMed] [Google Scholar]
- 137.Liu JW, Lu Y. J Am Chem Soc. 2004;126:12298–12305. doi: 10.1021/ja046628h. [DOI] [PubMed] [Google Scholar]
- 138.Liu JW, Lu Y. Chem Mater. 2004;16:3231–3238. [Google Scholar]
- 139.Liu JW, Lu Y. J Am Chem Soc. 2005;127:12677–12683. doi: 10.1021/ja053567u. [DOI] [PubMed] [Google Scholar]
- 140.Liu JW, Lu Y. Org Biomol Chem. 2006;4:3435–3441. doi: 10.1039/b605799c. [DOI] [PubMed] [Google Scholar]
- 141.Liu JW, Lu Y. Chem Commun. 2007:4872–4874. doi: 10.1039/b712421j. [DOI] [PubMed] [Google Scholar]
- 142.Liu JW, Lu Y. Angew Chem, Int Ed. 2007;46:7587–7590. doi: 10.1002/anie.200702006. [DOI] [PubMed] [Google Scholar]
- 143.Lee JH, Wang ZD, Liu JW, Lu Y. J Am Chem Soc. 2008;130:14217–14226. doi: 10.1021/ja803607z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang ZD, Lee JH, Lu Y. Adv Mater. 2008;20:3263–3267. [Google Scholar]
- 145.Wei H, Li BL, Li J, Dong SJ, Wang EK. Nanotechnology. 2008:19. doi: 10.1088/0957-4484/19/9/095501. [DOI] [PubMed] [Google Scholar]
- 146.Zhao WA, Lam JCF, Chiuman W, Brook MA, Li YF. Small. 2008;4:810–816. doi: 10.1002/smll.200700757. [DOI] [PubMed] [Google Scholar]
- 147.Bi S, Yan YM, Hao SY, Zhang SS. Angew Chem, Int Ed. 2010;49:4438–4442. doi: 10.1002/anie.201000840. [DOI] [PubMed] [Google Scholar]
- 148.Wang Y, Yang F, Yang XR. Nanotechnology. 2010:21. doi: 10.1088/0957-4484/21/20/205502. [DOI] [PubMed] [Google Scholar]
- 149.Lin HX, Zou Y, Huang YS, Chen J, Zhang WY, Zhuang ZX, Jenkins G, Yang CJ. Chem Commun. 2011;47:9312–9314. doi: 10.1039/c1cc12290h. [DOI] [PubMed] [Google Scholar]
- 150.Miao XM, Ling LS, Shuai XT. Chem Commun. 2011;47:4192–4194. doi: 10.1039/c0cc05344a. [DOI] [PubMed] [Google Scholar]
- 151.Luo YH, Zhang Y, Xu LL, Wang LS, Wen GQ, Liang AH, Jiang ZL. Analyst. 2012;137:1866–1871. doi: 10.1039/c2an00039c. [DOI] [PubMed] [Google Scholar]
- 152.Miao XM, Ling LS, Cheng D, Shuai XT. Analyst. 2012;137:3064–3069. doi: 10.1039/c2an35217f. [DOI] [PubMed] [Google Scholar]
- 153.Miao XM, Ling LS, Shuai XT. Anal Biochem. 2012;421:582–586. doi: 10.1016/j.ab.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 154.Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. Nature. 1996;382:607–609. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- 155.Alivisatos AP, Johnsson KP, Peng XG, Wilson TE, Loweth CJ, Bruchez MP, Schultz PG. Nature. 1996;382:609–611. doi: 10.1038/382609a0. [DOI] [PubMed] [Google Scholar]
- 156.Liu JW, Mazumdar D, Lu Y. Angew Chemie Inter Ed. 2006;45:7955–7959. doi: 10.1002/anie.200603106. [DOI] [PubMed] [Google Scholar]
- 157.Mazumdar D, Liu JW, Lu G, Zhou JZ, Lu Y. Chem Commun. 2010;46:1416–1418. doi: 10.1039/b917772h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fang ZY, Huang J, Lie PC, Xiao Z, Ouyang CY, Wu Q, Wu YX, Liu GD, Zeng LW. Chem Commun. 2010;46:9043–9045. doi: 10.1039/c0cc02782k. [DOI] [PubMed] [Google Scholar]
- 159.Chen JH, Zhou XM, Zeng LW. Chem Commun. 2013;49:984–986. doi: 10.1039/c2cc37598b. [DOI] [PubMed] [Google Scholar]
- 160.Li YF, Sen D. Nat Struct Biol. 1996;3:743–747. doi: 10.1038/nsb0996-743. [DOI] [PubMed] [Google Scholar]
- 161.Travascio P, Li YF, Sen D. Chemistry & Biology. 1998;5:505–517. doi: 10.1016/s1074-5521(98)90006-0. [DOI] [PubMed] [Google Scholar]
- 162.Sen D, Poon LCH. Crit Rev Biochem Mol. 2011;46:478–492. doi: 10.3109/10409238.2011.618220. [DOI] [PubMed] [Google Scholar]
- 163.Kong DM. Progress in Chemistry. 2011;23:2119–2131. [Google Scholar]
- 164.Elbaz J, Shlyahovsky B, Willner I. Chem Commun. 2008:1569–1571. doi: 10.1039/b716774a. [DOI] [PubMed] [Google Scholar]
- 165.Moshe M, Elbaz J, Willner I. Nano Lett. 2009;9:1196–1200. doi: 10.1021/nl803887y. [DOI] [PubMed] [Google Scholar]
- 166.Yin BC, Ye BC, Tan WH, Wang H, Xie CC. J Am Chem Soc. 2009;131:14624–14625. doi: 10.1021/ja9062426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhu X, Gao XY, Liu QD, Lin ZY, Qiu B, Chen GN. Chem Commun. 2011;47:7437–7439. doi: 10.1039/c1cc11349f. [DOI] [PubMed] [Google Scholar]
- 168.Zhang Q, Cai Y, Li H, Kong DM, Shen HX. Biosens Bioelectron. 2012;38:331–336. doi: 10.1016/j.bios.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 169.Xiao Y, Rowe AA, Plaxco KW. J Am Chem Soc. 2007;129:262–263. doi: 10.1021/ja067278x. [DOI] [PubMed] [Google Scholar]
- 170.Shen L, Chen Z, Li YH, He SL, Xie SB, Xu XD, Liang ZW, Meng X, Li Q, Zhu ZW, Li MX, Le XC, Shao YH. Anal Chem. 2008;80:6323–6328. doi: 10.1021/ac800601y. [DOI] [PubMed] [Google Scholar]
- 171.Zhu X, Lin ZY, Chen LF, Qiu B, Chen GA. Chem Commun. 2009:6050–6052. doi: 10.1039/b911191c. [DOI] [PubMed] [Google Scholar]
- 172.Li LD, Luo L, Mu XJ, Sun TY, Guo L. Anal Method. 2010;2:627–630. [Google Scholar]
- 173.Yang XR, Xu J, Tang XM, Liu HX, Tian DB. Chem Commun. 2010;46:3107–3109. doi: 10.1039/c002137g. [DOI] [PubMed] [Google Scholar]
- 174.Chen ZB, Li LD, Mu XJ, Zhao HT, Guo L. Talanta. 2011;85:730–735. doi: 10.1016/j.talanta.2011.04.056. [DOI] [PubMed] [Google Scholar]
- 175.Ma F, Sun B, Qi HL, Zhang HG, Gao QA, Zhang CX. Anal Chim Acta. 2011;683:234–241. doi: 10.1016/j.aca.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 176.Wang YL, Irudayaraj J. Chem Commun. 2011;47:4394–4396. doi: 10.1039/c0cc04140h. [DOI] [PubMed] [Google Scholar]
- 177.Zhang HX, Jiang BY, Xiang Y, Su J, Chai YQ, Yuan R. Biosens Bioelectron. 2011;28:135–138. doi: 10.1016/j.bios.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 178.Gao XY, Huang HM, Niu SY, Ye HZ, Lin ZY, Qiu B, Chen GN. Anal Method. 2012;4:947–952. [Google Scholar]
- 179.Pelossof G, Tel-Vered R, Willner I. Anal Chem. 2012;84:3703–3709. doi: 10.1021/ac3002269. [DOI] [PubMed] [Google Scholar]
- 180.Ziolkowski R, Gorski L, Oszwaldowski S, Malinowska E. Anal Bioanal Chem. 2012;402:2259–2266. doi: 10.1007/s00216-011-5510-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ocana C, Malashikhina N, del Valle M, Pavlov V. Analyst. 2013;138:1995–1999. doi: 10.1039/c3an36778a. [DOI] [PubMed] [Google Scholar]
- 182.Wen YQ, Li FBY, Dong XC, Zhang J, Xiong QH, Chen P. Adv Healthcare Mater. 2013;2:271–274. doi: 10.1002/adhm.201200220. [DOI] [PubMed] [Google Scholar]
- 183.Zhang M, Ge L, Ge SG, Yan M, Yu JH, Huang JD, Liu S. Biosens Bioelectron. 2013;41:544–550. doi: 10.1016/j.bios.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 184.Zhuang JY, Fu LB, Xu MD, Zhou Q, Chen GN, Tang DP. Biosens Bioelectron. 2013;45:52–57. doi: 10.1016/j.bios.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 185.Ono A, Togashi H. Angewandte Chemie-International Edition. 2004;43:4300–4302. doi: 10.1002/anie.200454172. [DOI] [PubMed] [Google Scholar]
- 186.Okamoto I, Iwamoto K, Watanabe Y, Miyake Y, Ono A. Angew Chemie Inter Ed. 2009;48:1648–1651. doi: 10.1002/anie.200804952. [DOI] [PubMed] [Google Scholar]
- 187.Ono A, Cao S, Togashi H, Tashiro M, Fujimoto T, Machinami T, Oda S, Miyake Y, Okamoto I, Tanaka Y. Chem Commun. 2008:4825–4827. doi: 10.1039/b808686a. [DOI] [PubMed] [Google Scholar]
- 188.Megger DA, Muller J. Nucleosides Nucleotides Nucleic Acids. 2010;29:27–38. doi: 10.1080/15257770903451579. [DOI] [PubMed] [Google Scholar]
- 189.Megger DA, Guerra CF, Hoffmann J, Brutschy B, Bickelhaupt FM, Muller J. Chem Eur J. 2011;17:6533–6544. doi: 10.1002/chem.201002944. [DOI] [PubMed] [Google Scholar]
- 190.Megger N, Johannsen S, Muller J, Sigel RKO. Chem Biodiversity. 2012;9:2050–2063. doi: 10.1002/cbdv.201100437. [DOI] [PubMed] [Google Scholar]
- 191.Kaul C, Muller M, Wagner M, Schneider S, Carell T. Nat Chem. 2011;3:794–800. doi: 10.1038/nchem.1117. [DOI] [PubMed] [Google Scholar]
- 192.Li BL, Dong SJ, Wang EK. Chem Asian J. 2010;5:1262–1272. doi: 10.1002/asia.200900660. [DOI] [PubMed] [Google Scholar]
- 193.Vinkenborg JL, Karnowski N, Famulok M. Nat Chem Biol. 2011;7:519–527. doi: 10.1038/nchembio.609. [DOI] [PubMed] [Google Scholar]
- 194.Sundquist WI, Klug A. Nature. 1989;342:825–829. doi: 10.1038/342825a0. [DOI] [PubMed] [Google Scholar]
- 195.Ueyama H, Takagi M, Takenaka S. J Am Chem Soc. 2002;124:14286–14287. doi: 10.1021/ja026892f. [DOI] [PubMed] [Google Scholar]
- 196.Majhi PR, Shafer RH. Biopolymers. 2006;82:558–569. doi: 10.1002/bip.20507. [DOI] [PubMed] [Google Scholar]
- 197.Li T, Wang E, Dong S. Anal Chem. 2010;82:1515–1520. doi: 10.1021/ac902638v. [DOI] [PubMed] [Google Scholar]
- 198.Lee JS, Han MS, Mirkin CA. Angew Chemie Inter Ed. 2007;46:4093–4096. doi: 10.1002/anie.200700269. [DOI] [PubMed] [Google Scholar]
- 199.Xue X, Wang F, Liu X. J Am Chem Soc. 2008;130:3244–3245. doi: 10.1021/ja076716c. [DOI] [PubMed] [Google Scholar]
- 200.Wang ZD, Lee JH, Lu Y. Chem Commun. 2008:6005–6007. doi: 10.1039/b812755g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Li T, Dong SJ, Wang E. Anal Chem. 2009;81:2144–2149. doi: 10.1021/ac900188y. [DOI] [PubMed] [Google Scholar]
- 202.Li T, Li BL, Wang EK, Dong SJ. Chem Commun. 2009:3551–3553. doi: 10.1039/b903993g. [DOI] [PubMed] [Google Scholar]
- 203.Zhang D, Deng MG, Xu L, Zhou YY, Yuwen J, Zhou X. Chem Eur J. 2009;15:8117–8120. doi: 10.1002/chem.200901268. [DOI] [PubMed] [Google Scholar]
- 204.Kong DM, Wu J, Wang N, Yang W, Shen HX. Talanta. 2009;80:459–465. doi: 10.1016/j.talanta.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 205.Li L, Li BX, Qi YY, Jin Y. Anal Bioanal Chem. 2009;393:2051–2057. doi: 10.1007/s00216-009-2640-0. [DOI] [PubMed] [Google Scholar]
- 206.Lu N, Shao CY, Deng ZX. Analyst. 2009;134:1822–1825. doi: 10.1039/b908018j. [DOI] [PubMed] [Google Scholar]
- 207.Xu XW, Wang J, Jiao K, Yang XR. Biosens Bioelectron. 2009;24:3153–3158. doi: 10.1016/j.bios.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 208.Shimron S, Elbaz J, Henning A, Willner I. Chem Commun. 2010;46:3250–3252. doi: 10.1039/b926003j. [DOI] [PubMed] [Google Scholar]
- 209.Kong DM, Wang N, Guo XX, Shen HX. Analyst. 2010;135:545–549. doi: 10.1039/b924014d. [DOI] [PubMed] [Google Scholar]
- 210.Wu DH, Zhang Q, Chu X, Wang HB, Shen GL, Yu RQ. Biosens Bioelectron. 2010;25:1025–1031. doi: 10.1016/j.bios.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 211.Freeman R, Liu XQ, Willner I. J Am Chem Soc. 2011;133:11597–11604. doi: 10.1021/ja202639m. [DOI] [PubMed] [Google Scholar]
- 212.Jia SM, Liu XF, Li P, Kong DM, Shen HX. Biosens Bioelectron. 2011;27:148–152. doi: 10.1016/j.bios.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 213.Kang T, Yoo SM, Yoon I, Lee S, Choo J, Lee SY, Kim B. Chem Eur J. 2011;17:2211–2214. doi: 10.1002/chem.201001663. [DOI] [PubMed] [Google Scholar]
- 214.Lin YW, Liu CW, Chang HT. Talanta. 2011;84:324–329. doi: 10.1016/j.talanta.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 215.Lin ZZ, Li XH, Kraatz HB. Anal Chem. 2011;83:6896–6901. doi: 10.1021/ac2014096. [DOI] [PubMed] [Google Scholar]
- 216.Long F, Gao C, Shi HC, He M, Zhu AN, Klibanov AM, Gu AZ. Biosens Bioelectron. 2011;26:4018–4023. doi: 10.1016/j.bios.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 217.Pelossof G, Tel-Vered R, Liu XQ, Willner I. Chem Eur J. 2011;17:8904–8912. doi: 10.1002/chem.201100601. [DOI] [PubMed] [Google Scholar]
- 218.Torabi SF, Lu Y. Faraday Discuss. 2011;149:125–135. doi: 10.1039/c005404f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Du J, Liu MY, Lou XH, Zhao T, Wang Z, Xue Y, Zhao JL, Xu YS. Anal Chem. 2012;84:8060–8066. doi: 10.1021/ac301954j. [DOI] [PubMed] [Google Scholar]
- 220.Helwa Y, Dave N, Froidevaux R, Samadi A, Liu JW. ACS Appl Mater Interfaces. 2012;4:2228–2233. doi: 10.1021/am300241j. [DOI] [PubMed] [Google Scholar]
- 221.Kiy MM, Jacobi ZE, Liu JW. Chem Eur J. 2012;18:1202–1208. doi: 10.1002/chem.201102515. [DOI] [PubMed] [Google Scholar]
- 222.Qi L, Zhao YX, Yuan H, Bai K, Zhao Y, Chen F, Dong YH, Wu YY. Analyst. 2012;137:2799–2805. doi: 10.1039/c2an35437c. [DOI] [PubMed] [Google Scholar]
- 223.Shi L, Liang G, Li XH, Liu XH. Anal Method. 2012;4:1036–1040. [Google Scholar]
- 224.Wang L, Xu M, Han L, Zhou M, Zhu CZ, Dong SJ. Anal Chem. 2012;84:7301–7307. doi: 10.1021/ac300521d. [DOI] [PubMed] [Google Scholar]
- 225.Bi S, Ji B, Zhang ZP, Zhu JJ. Chem Sci. 2013;4:1858–1863. [Google Scholar]
- 226.Chung CH, Kim JH, Jung J, Chung BH. Biosens Bioelectron. 2013;41:827–832. doi: 10.1016/j.bios.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 227.Hoang CV, Oyama M, Saito O, Aono M, Nagao T. Sci Rep. 2013:3. doi: 10.1038/srep01175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Li M, Zhou XJ, Ding WQ, Guo SW, Wu NQ. Biosens Bioelectron. 2013;41:889–893. doi: 10.1016/j.bios.2012.09.060. [DOI] [PubMed] [Google Scholar]
- 229.Sharon E, Liu XQ, Freeman R, Yehezkeli O, Willner I. Electroanalysis. 2013;25:851–856. [Google Scholar]
- 230.Zhang ZP, Yin JA, Wu ZY, Yu RQ. Anal Chim Acta. 2013;762:47–53. doi: 10.1016/j.aca.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 231.Li BL, Du Y, Dong SJ. Anal Chim Acta. 2009;644:78–82. doi: 10.1016/j.aca.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 232.Li T, Shi LL, Wang EK, Dong SJ. Chem Eur J. 2009;15:3347–3350. doi: 10.1002/chem.200900056. [DOI] [PubMed] [Google Scholar]
- 233.Wang YX, Li JS, Wang H, Jin JY, Liu JH, Wang KM, Tan WH, Yang RH. Anal Chem. 2010;82:6607–6612. doi: 10.1021/ac101114w. [DOI] [PubMed] [Google Scholar]
- 234.Wu CK, Xiong C, Wang LJ, Lan CC, Ling LS. Analyst. 2010;135:2682–2687. doi: 10.1039/c0an00201a. [DOI] [PubMed] [Google Scholar]
- 235.Zhou XH, Kong DM, Shen HX. Anal Chim Acta. 2010;678:124–127. doi: 10.1016/j.aca.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 236.Tang CX, Bu NN, He XW, Yin XB. Chem Commun. 2011;47:12304–12306. doi: 10.1039/c1cc15323d. [DOI] [PubMed] [Google Scholar]
- 237.Wang FZ, Wu YG, Zhan SS, He L, Zhi WT, Zhou XX, Zhou P. Aust J Chem. 2013;66:113–118. [Google Scholar]
- 238.Kuzuya A, Sakai Y, Yamazaki T, Xu Y, Komiyama M. Nat Commun. 2011:2. doi: 10.1038/ncomms1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Nagatoishi S, Nojima T, Juskowiak B, Takenaka S. Angew Chem, Int Ed. 2005;44:5067–5070. doi: 10.1002/anie.200501506. [DOI] [PubMed] [Google Scholar]
- 240.Nagatoishi S, Nojima T, Galezowska E, Juskowiak B, Takenaka S. ChemBioChem. 2006;7:1730–1737. doi: 10.1002/cbic.200600179. [DOI] [PubMed] [Google Scholar]
- 241.Huang C-C, Chang H-T. Chem Commun. 2008:1461–1463. doi: 10.1039/b718752a. [DOI] [PubMed] [Google Scholar]
- 242.Li T, Wang EK, Dong SJ. Anal Chem. 2010;82:7576–7580. doi: 10.1021/ac1019446. [DOI] [PubMed] [Google Scholar]
- 243.Fan XY, Li HT, Zhao J, Lin FB, Zhang LL, Zhang YY, Yao SZ. Talanta. 2012;89:57–62. doi: 10.1016/j.talanta.2011.11.056. [DOI] [PubMed] [Google Scholar]
- 244.He F, Tang YL, Wang S, Li YL, Zhu DB. J Am Chem Soc. 2005;127:12343–12346. doi: 10.1021/ja051507i. [DOI] [PubMed] [Google Scholar]
- 245.Kim B, Jung IH, Kang M, Shim HK, Woo HY. J Am Chem Soc. 2012;134:3133–3138. doi: 10.1021/ja210360v. [DOI] [PubMed] [Google Scholar]
- 246.Wang L, Liu X, Hu X, Song S, Fan C. Chem Commun. 2006:3780–3782. doi: 10.1039/b607448k. [DOI] [PubMed] [Google Scholar]
- 247.Yang X, Li T, Li BL, Wang EK. Analyst. 2010;135:71–75. doi: 10.1039/b913036e. [DOI] [PubMed] [Google Scholar]
- 248.Zhang YF, Li BX. Biosens Bioelectron. 2011;27:137–140. doi: 10.1016/j.bios.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 249.Sun HJ, Li XH, Li YC, Fan LZ, Kraatz HB. Analyst. 2013;138:856–862. doi: 10.1039/c2an36564b. [DOI] [PubMed] [Google Scholar]
- 250.Radi A-E, O’Sullivan CK. Chem Commun. 2006:3432–3434. doi: 10.1039/b606804a. [DOI] [PubMed] [Google Scholar]
- 251.Wu ZS, Chen CR, Shen GL, Yu RQ. Biomaterials. 2008;29:2689–2696. doi: 10.1016/j.biomaterials.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 252.Li T, Dong SJ, Wang EK. J Am Chem Soc. 2010;132:13156–13157. doi: 10.1021/ja105849m. [DOI] [PubMed] [Google Scholar]
- 253.Mo ZH, Gao YM, Wen ZY, Fan YP. Chem J Chinese U. 2010;31:2181–2183. [Google Scholar]
- 254.Wang Y, Wang JA, Yang F, Yang XR. Microchim Acta. 2010;171:195–201. [Google Scholar]
- 255.Chen GZ, Jin Y, Wang WH, Zhao YN. Gold Bull. 2012;45:137–143. [Google Scholar]
- 256.Li CL, Liu KT, Lin YW, Chang HT. Anal Chem. 2011;83:225–230. doi: 10.1021/ac1028787. [DOI] [PubMed] [Google Scholar]
- 257.Guo LQ, Nie DD, Qiu CY, Zheng QS, Wu HY, Ye PR, Hao YL, Fu FF, Chen GN. Biosens Bioelectron. 2012;35:123–127. doi: 10.1016/j.bios.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 258.Jacobi ZE, Li L, Liu JW. Analyst. 2012;137:704–709. doi: 10.1039/c2an15754c. [DOI] [PubMed] [Google Scholar]
- 259.Li CL, Huang CC, Chen WH, Chiang CK, Chang HT. Analyst. 2012;137:5222–5228. doi: 10.1039/c2an35599j. [DOI] [PubMed] [Google Scholar]
- 260.He HZ, Leung KH, Yang H, Chan DSH, Leung CH, Zhou J, Bourdoncle A, Mergny JL, Ma DL. Biosens Bioelectron. 2013;41:871–874. doi: 10.1016/j.bios.2012.08.060. [DOI] [PubMed] [Google Scholar]
- 261.Li M, Zhou XJ, Guo SW, Wu NQ. Biosens Bioelectron. 2013;43:69–74. doi: 10.1016/j.bios.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 262.Zhao Y, Zhang Q, Wang WH, Jin Y. Biosens Bioelectron. 2013;43:231–236. doi: 10.1016/j.bios.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 263.Lu Y, Li X, Wang GK, Tang W. Biosens Bioelectron. 2013;39:231–235. doi: 10.1016/j.bios.2012.07.045. [DOI] [PubMed] [Google Scholar]
- 264.Lin ZZ, Chen Y, Li XH, Fang WH. Analyst. 2011;136:2367–2372. doi: 10.1039/c1an15080d. [DOI] [PubMed] [Google Scholar]
- 265.Li F, Yang LM, Chen MQ, Li P, Tang B. Analyst. 2013;138:461–466. doi: 10.1039/c2an36227a. [DOI] [PubMed] [Google Scholar]
- 266.Jiang ZL, Fan YY, Liang AH, Wen GQ, Liu QY, Li TS. Plasmonics. 2010;5:375–381. [Google Scholar]
- 267.Liang AH, Zhang J, Wen GQ, Liu QY, Li TS, Jiang ZL. Chem J Chinese U. 2010;31:2366–2371. [Google Scholar]
- 268.Zhang LB, Zhu JB, Ai J, Zhou ZX, Jia XF, Wang EK. Biosens Bioelectron. 2013;39:268–273. doi: 10.1016/j.bios.2012.07.058. [DOI] [PubMed] [Google Scholar]
- 269.Kong DM, Cai LL, Shen HX. Analyst. 2010;135:1253–1258. doi: 10.1039/b925168e. [DOI] [PubMed] [Google Scholar]
- 270.Guo JH, Kong DM, Shen HX. Biosens Bioelectron. 2010;26:327–332. doi: 10.1016/j.bios.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 271.Liu L, Liu WT, Hong TT, Weng XC, Zhai QQ, Zhou X. Anal Method. 2012;4:1935–1939. [Google Scholar]
- 272.Lu YJ, Ma N, Li YJ, Lin ZY, Qiu B, Chen GN, Wong KY. Sensor Actuat B-Chem. 2012;173:295–299. [Google Scholar]
- 273.Wang HL, Ou LML, Suo YR, Yu HZ. Anal Chem. 2011;83:1557–1563. doi: 10.1021/ac103177w. [DOI] [PubMed] [Google Scholar]
- 274.Xiang Y, Lu Y. Nat Chem. 2011;3:697–703. doi: 10.1038/nchem.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Xiang Y, Lu Y. Chem Commun. 2013;49:585–587. doi: 10.1039/c2cc37156a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Su J, Xu J, Chen Y, Xiang Y, Yuan R, Chai YQ. Biosens Bioelectron. 2013;45:219–222. doi: 10.1016/j.bios.2013.01.069. [DOI] [PubMed] [Google Scholar]
- 277.Xu XY, Daniel WL, Wei W, Mirkin CA. Small. 2010;6:623–626. doi: 10.1002/smll.200901691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Wu PW, Hwang KV, Lan T, Lu Y. J Am Chem Soc. 2013;135:5254–5257. doi: 10.1021/ja400150v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Silverman SK. Nucleic Acids Res. 2005;33:6151–6163. doi: 10.1093/nar/gki930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Vannela R, Adriaens P. Crit Rev Environ Sci Technol. 2006;36:375–403. [Google Scholar]
- 281.Balamurugan S, Obubuafo A, Soper SA, Spivak DA. Anal Bioanal Chem. 2008;390:1009–1021. doi: 10.1007/s00216-007-1587-2. [DOI] [PubMed] [Google Scholar]
- 282.Li YL, Guo L, Zhang ZY, Tang JJ, Xie JW. Sci China, Ser B. 2008;51:193–204. [Google Scholar]
- 283.Rusling JF, Hvastkovs EG, Hull DO, Schenkman JB. Chem Commun. 2008:141–154. doi: 10.1039/b709121b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Singh RP, Oh BK, Koo KK, Jyoung JY, Jeong S, Choi JW. Biochip J. 2008;2:223–234. [Google Scholar]
- 285.Chiu TC, Huang CC. Sensors. 2009;9:10356–10388. doi: 10.3390/s91210356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Guo SJ, Dong SJ. TRAC-Trend Anal Chem. 2009;28:96–109. [Google Scholar]
- 287.Sefah K, Phillips JA, Xiong XL, Meng L, Van Simaeys D, Chen H, Martin J, Tan WH. Analyst. 2009;134:1765–1775. doi: 10.1039/b905609m. [DOI] [PubMed] [Google Scholar]
- 288.Wang GQ, Wang YQ, Chen LX, Choo J. Biosens Bioelectron. 2010;25:1859–1868. doi: 10.1016/j.bios.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 289.Sassolas A, Blum LJ, Leca-Bouvier BD. Analyst. 2011;136:257–274. doi: 10.1039/c0an00281j. [DOI] [PubMed] [Google Scholar]
- 290.Freeman R, Willner B, Willner I. In: Functional Nanoparticles for Bioanalysis, Nanomedicine, and Bioelectronic Devices. Hepel M, Zhong CJ, editors. Vol. 1. Vol. 1112. 2012. pp. 1–31. [Google Scholar]
- 291.Ihara T, Kitamura Y. J Photoch Photobio C. 2012;13:148–167. [Google Scholar]
- 292.Ma DL, Ma VPY, Chan DSH, Leung KH, He HZ, Leung CH. Coord Chem Rev. 2012;256:3087–3113. [Google Scholar]
- 293.Du Y, Li BL, Wang EK. Acc Chem Res. 2013;46:203–213. doi: 10.1021/ar300011g. [DOI] [PubMed] [Google Scholar]
- 294.Yu HZ, Li YC, Ou LML. Acc Chem Res. 2013;46:258–268. doi: 10.1021/ar300104b. [DOI] [PubMed] [Google Scholar]
- 295.Lu Y, Li J, Bruesehoff PJ, Pavot CMB, Gao L, Brown AK, Nelson KE, Augustine AJ. J Inorg Biochem. 2001;86:67–67. [Google Scholar]
- 296.Lu Y, Brown AK, Liu JW, Li J, Bruesehoff PJ. J Inorg Biochem. 2003;96:182–182. [Google Scholar]
- 297.Lu Y, Liu JW, Bruesehoff PJ, Brown AK, Nelson KE, Kim HK, Wernette DP. J Inorg Biochem. 2003;96:30–30. [Google Scholar]
- 298.Zhao WA, Ali MM, Brook MA, Li YF. Angew Chemie Inter Ed. 2008;47:6330–6337. doi: 10.1002/anie.200705982. [DOI] [PubMed] [Google Scholar]
- 299.Lau PS, Li Y. Curr Org Chem. 2011;15:557–575. [Google Scholar]
- 300.Adams SR, LevRam V, Tsien RY. Chem & Biol. 1997;4:867–878. doi: 10.1016/s1074-5521(97)90119-8. [DOI] [PubMed] [Google Scholar]
- 301.Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 302.Hirano T, Kikuchi K, Urano Y, Higuchi T, Nagano T. J Am Chem Soc. 2000;122:12399–12400. [Google Scholar]
- 303.Walkup GK, Burdette SC, Lippard SJ, Tsien RY. J Am Chem Soc. 2000;122:5644–5645. doi: 10.1021/ja010059l. [DOI] [PubMed] [Google Scholar]
- 304.Burdette SC, Walkup GK, Spingler B, Tsien RY, Lippard SJ. J Am Chem Soc. 2001;123:7831–7841. doi: 10.1021/ja010059l. [DOI] [PubMed] [Google Scholar]
- 305.Yang LC, McRae R, Henary MM, Patel R, Lai B, Vogt S, Fahrni CJ. Proc Natl Acad Sci USA. 2005;102:11179–11184. doi: 10.1073/pnas.0406547102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Zeng L, Miller EW, Pralle A, Isacoff EY, Chang CJ. J Am Chem Soc. 2006;128:10–11. doi: 10.1021/ja055064u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Chan WH, Yang RH, Wang KM. Anal Chim Acta. 2001;444:261–269. [Google Scholar]
- 308.Chen P, He CA. J Am Chem Soc. 2004;126:728–729. doi: 10.1021/ja0383975. [DOI] [PubMed] [Google Scholar]
- 309.Yoon S, Albers AE, Wong AP, Chang CJ. J Am Chem Soc. 2005;127:16030–16031. doi: 10.1021/ja0557987. [DOI] [PubMed] [Google Scholar]
- 310.Deo S, Godwin HA. J Am Chem Soc. 2000;122:174–175. [Google Scholar]
- 311.Chen P, Greenberg B, Taghavi S, Romano C, van der Lelie D, He CA. Angew Chemie Inter Ed. 2005;44:2715–2719. doi: 10.1002/anie.200462443. [DOI] [PubMed] [Google Scholar]
- 312.Mori I, Takasaki K, Fujita Y, Matsuo T. Talanta. 1998;47:631–637. doi: 10.1016/s0039-9140(98)00118-0. [DOI] [PubMed] [Google Scholar]
- 313.Zeng ZT, Jewsbury RA. Analyst. 1998;123:2845–2850. [Google Scholar]
- 314.Ma QL, Cao QE, Zhao YK, Wu SQ, Hu ZD, Xu QH. Food Chem. 2000;71:123–127. [Google Scholar]
- 315.Monteil-Rivera F, Dumonceau J. Anal Bioanal Chem. 2002;374:1105–1112. doi: 10.1007/s00216-002-1580-8. [DOI] [PubMed] [Google Scholar]
- 316.Li CY, Zhang XB, Jin Z, Han R, Shen GL, Yu RQ. Anal Chim Acta. 2006;580:143–148. doi: 10.1016/j.aca.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 317.Sasaki DY, Shnek DR, Pack DW, Arnold FH. Angew Chem Int Ed. 1995;34:905–907. [Google Scholar]
- 318.Dujols V, Ford F, Czarnik AW. J Am Chem Soc. 1997;119:7386–7387. [Google Scholar]
- 319.Torrado A, Walkup GK, Imperiali B. J Am Chem Soc. 1998;120:609–610. [Google Scholar]
- 320.Grandini P, Mancin F, Tecilla P, Scrimin P, Tonellato U. Angew Chemie Inter Ed. 1999;38:3061–3064. doi: 10.1002/(sici)1521-3773(19991018)38:20<3061::aid-anie3061>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 321.Klein G, Kaufmann D, Schurch S, Reymond JL. Chem Commun. 2001:561–562. [Google Scholar]
- 322.Yang JS, Lin CS, Hwang CY. Org Lett. 2001;3:889–892. doi: 10.1021/ol015524y. [DOI] [PubMed] [Google Scholar]
- 323.Zheng YJ, Huo Q, Kele P, Andreopoulos FM, Pham SM, Leblanc RM. Org Lett. 2001;3:3277–3280. doi: 10.1021/ol0101638. [DOI] [PubMed] [Google Scholar]
- 324.Kaur S, Kumar S. Chem Commun. 2002:2840–2841. doi: 10.1039/b209053h. [DOI] [PubMed] [Google Scholar]
- 325.Boiocchi M, Fabbrizzi L, Licchelli M, Sacchi D, Vazquez M, Zampa C. Chem Commun. 2003:1812–1813. doi: 10.1039/b305456j. [DOI] [PubMed] [Google Scholar]
- 326.Roy BC, Chandra B, Hromas D, Mallik S. Org Lett. 2003;5:11–14. doi: 10.1021/ol026891s. [DOI] [PubMed] [Google Scholar]
- 327.Zheng Y, Orbulescu J, Ji X, Andreopoulos FM, Pham SM, Leblanc RM. J Am Chem Soc. 2003;125:2680–2686. doi: 10.1021/ja0293610. [DOI] [PubMed] [Google Scholar]
- 328.Zheng YJ, Cao XH, Orbulescu J, Konka V, Andreopoulos FM, Pham SM, Leblanc RM. Anal Chem. 2003;75:1706–1712. doi: 10.1021/ac026285a. [DOI] [PubMed] [Google Scholar]
- 329.Wu QY, Anslyn EV. J Am Chem Soc. 2004;126:14682–14683. doi: 10.1021/ja0401038. [DOI] [PubMed] [Google Scholar]
- 330.Royzen M, Dai ZH, Canary JW. J Am Chem Soc. 2005;127:1612–1613. doi: 10.1021/ja0431051. [DOI] [PubMed] [Google Scholar]
- 331.Xu ZC, Xiao Y, Qian XH, Cui JN, Cui DW. Org Lett. 2005;7:889–892. doi: 10.1021/ol0473445. [DOI] [PubMed] [Google Scholar]
- 332.Kim SH, Kim JS, Park SM, Chang SK. Org Lett. 2006;8:371–374. doi: 10.1021/ol052282j. [DOI] [PubMed] [Google Scholar]
- 333.Martinez R, Zapata F, Caballero A, Espinosa A, Tarraga A, Molina P. Org Lett. 2006;8:3235–3238. doi: 10.1021/ol0610791. [DOI] [PubMed] [Google Scholar]
- 334.Wen ZC, Yang R, He H, Jiang YB. Chem Commun. 2006:106–108. doi: 10.1039/b510546c. [DOI] [PubMed] [Google Scholar]
- 335.Xiang Y, Tong AJ, Jin PY, Ju Y. Org Lett. 2006;8:2863–2866. doi: 10.1021/ol0610340. [DOI] [PubMed] [Google Scholar]
- 336.Bowie AR, Achterberg EP, Sedwick PN, Ussher S, Worsfold PJ. Environ Sci Technol. 2002;36:4600–4607. doi: 10.1021/es020045v. [DOI] [PubMed] [Google Scholar]
- 337.Chen JL, Zhuo SJ, Wu YQ, Fang F, Li L, Zhu CQ. Spectrochim Acta A. 2006;63:438–443. doi: 10.1016/j.saa.2005.04.057. [DOI] [PubMed] [Google Scholar]
- 338.Zhang M, Gao YH, Li MY, Yu MX, Li FY, Li L, Zhu MW, Zhang JP, Yi T, Huang CH. Tetrahedron Lett. 2007;48:3709–3712. [Google Scholar]
- 339.Wolf C, Mei XF, Rokadia HK. Tetrahedron Lett. 2004;45:7867–7871. [Google Scholar]
- 340.Xiang Y, Tong AJ. Org Lett. 2006;8:1549–1552. doi: 10.1021/ol060001h. [DOI] [PubMed] [Google Scholar]
- 341.Zhang XB, Cheng G, Zhang WJ, Shen GL, Yu RQ. Talanta. 2007;71:171–177. doi: 10.1016/j.talanta.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 342.Grabchev I, Chovelon JM, Petkov C. Spectrochim Acta A. 2008;69:100–104. doi: 10.1016/j.saa.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 343.Breaker RR. Nat Biotechnol. 1999;17:422–423. doi: 10.1038/8588. [DOI] [PubMed] [Google Scholar]
- 344.Chow CS, Bogdan FM. Chem Rev. 1997;97:1489–1513. doi: 10.1021/cr960415w. [DOI] [PubMed] [Google Scholar]
- 345.Ferguson JA, Steemers FJ, Walt DR. Anal Chem. 2000;72:5618–5624. doi: 10.1021/ac0008284. [DOI] [PubMed] [Google Scholar]
- 346.Walt DR. Science. 2000;287:451–452. doi: 10.1126/science.287.5452.451. [DOI] [PubMed] [Google Scholar]
- 347.Taylor LC, Walt DR. Anal Biochem. 2000;278:132–142. doi: 10.1006/abio.1999.4440. [DOI] [PubMed] [Google Scholar]
- 348.Liu Y, Sen D. J Mol Biol. 2008;381:845–859. doi: 10.1016/j.jmb.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 349.Sekhon GS, Sen D. Biochemistry. 2010;49:9072–9077. doi: 10.1021/bi1013547. [DOI] [PubMed] [Google Scholar]
- 350.Wang B, Cao LQ, Chiuman W, Li YF, Xi Z. Biochemistry. 2010;49:7553–7562. doi: 10.1021/bi100304b. [DOI] [PubMed] [Google Scholar]
- 351.Lee NK, Koh HR, Han KY, Kim SK. J Am Chem Soc. 2007;129:15526–15534. doi: 10.1021/ja0725145. [DOI] [PubMed] [Google Scholar]
- 352.Jung J, Han KY, Koh HR, Lee J, Choi YM, Kim C, Kim SK. J Phys Chem B. 2012;116:3007–3012. doi: 10.1021/jp2117196. [DOI] [PubMed] [Google Scholar]
- 353.http://www.andalyze.com.
- 354.Xu WC, Xing H, Lu Y. Analyst. 2013;138:6266–6269. doi: 10.1039/c3an01182h. [DOI] [PMC free article] [PubMed] [Google Scholar]