Abstract
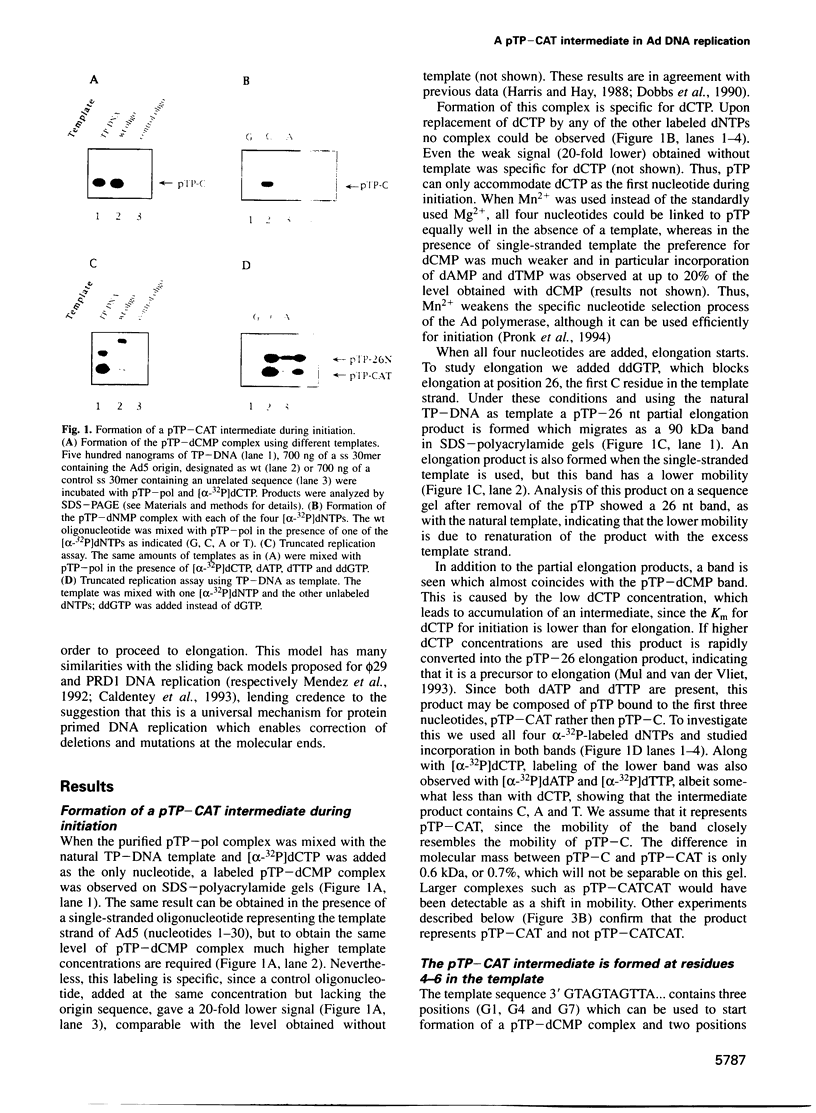
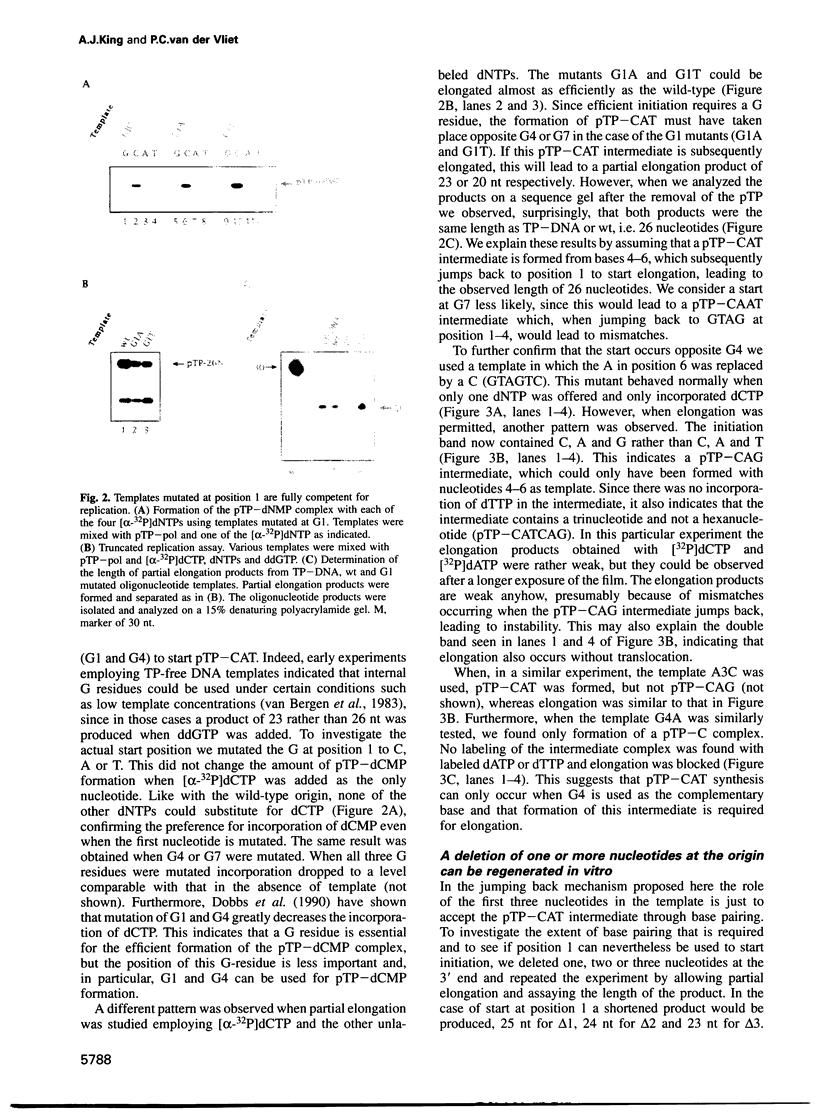
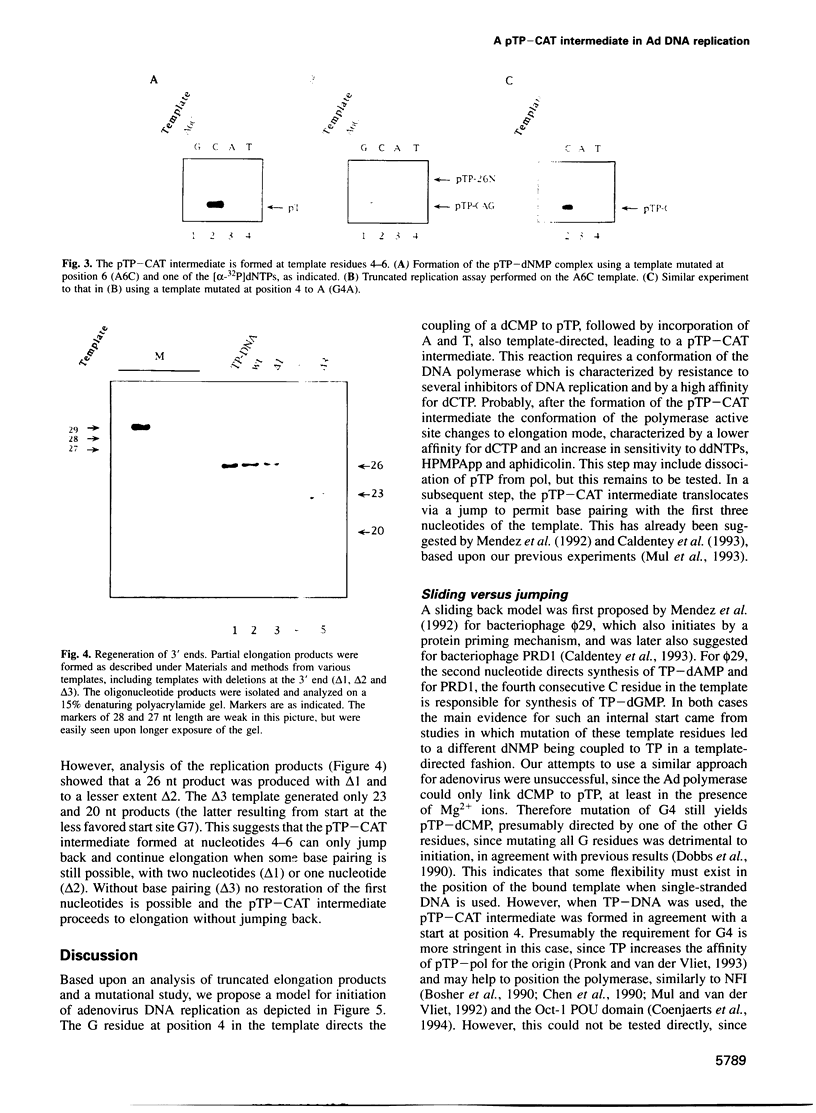
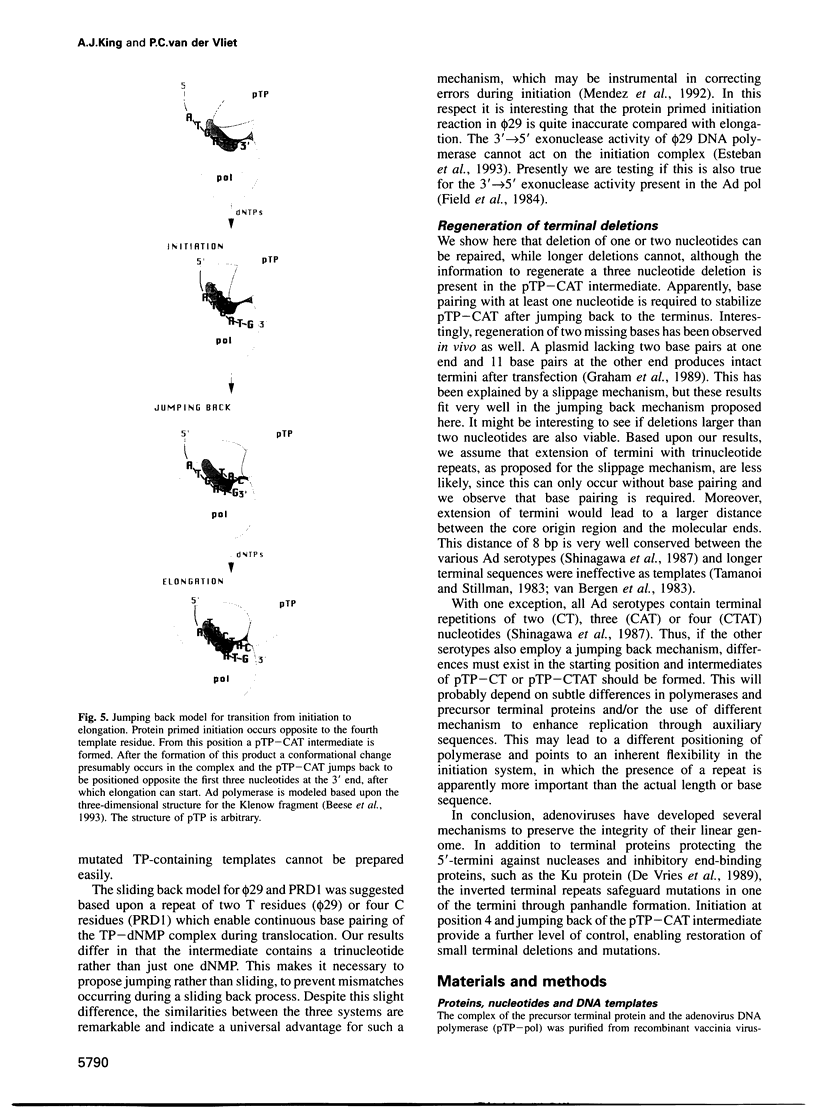
The adenovirus type 5 origin sequence starts with 3' GTAGTA. Initiation of replication occurs by a protein priming mechanism in which the viral precursor terminal protein (pTP) is covalently linked to the first nucleotide of the nascent chain, a dCMP residue. This suggests that a pTP-dCMP (pTP-C) complex functions as an initiation intermediate. Employing a reconstituted replication system and both synthetic oligonucleotides and the natural TP-DNA as templates, we show that pTP-CAT rather than pTP-C is an intermediate in initiation. By replicating oligonucleotide templates mutated at different positions and analyzing the product lengths, we observed that the GTA at positions 4-6, rather than 1-3, are used as a template for pTP-CAT formation. Moreover, deletions of one or two nucleotides at the molecular ends were regenerated upon in vitro replication. Our results support a model in which the pTP-CAT intermediate, synthesized opposite to positions 4-6, jumps back to position 1 of the template to start elongation. In order to permit elongation, some base pairing between pTP-CAT and template residues 1-3 is required. This jumping-back mechanism ensures the integrity of terminal sequences during replication of the linear genome.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beese L. S., Derbyshire V., Steitz T. A. Structure of DNA polymerase I Klenow fragment bound to duplex DNA. Science. 1993 Apr 16;260(5106):352–355. doi: 10.1126/science.8469987. [DOI] [PubMed] [Google Scholar]
- Bosher J., Robinson E. C., Hay R. T. Interactions between the adenovirus type 2 DNA polymerase and the DNA binding domain of nuclear factor I. New Biol. 1990 Dec;2(12):1083–1090. [PubMed] [Google Scholar]
- Caldentey J., Blanco L., Bamford D. H., Salas M. In vitro replication of bacteriophage PRD1 DNA. Characterization of the protein-primed initiation site. Nucleic Acids Res. 1993 Aug 11;21(16):3725–3730. doi: 10.1093/nar/21.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J. Animal virus DNA replication. Annu Rev Biochem. 1989;58:671–717. doi: 10.1146/annurev.bi.58.070189.003323. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Feb;76(2):655–659. doi: 10.1073/pnas.76.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Mermod N., Horwitz M. S. Protein-protein interactions between adenovirus DNA polymerase and nuclear factor I mediate formation of the DNA replication preinitiation complex. J Biol Chem. 1990 Oct 25;265(30):18634–18642. [PubMed] [Google Scholar]
- Coenjaerts F. E., van Oosterhout J. A., van der Vliet P. C. The Oct-1 POU domain stimulates adenovirus DNA replication by a direct interaction between the viral precursor terminal protein-DNA polymerase complex and the POU homeodomain. EMBO J. 1994 Nov 15;13(22):5401–5409. doi: 10.1002/j.1460-2075.1994.tb06875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs L., Zhao L. J., Sripad G., Padmanabhan R. Mutational analysis of single-stranded DNA templates active in the in vitro initiation assay for adenovirus DNA replication. Virology. 1990 Sep;178(1):43–51. doi: 10.1016/0042-6822(90)90377-4. [DOI] [PubMed] [Google Scholar]
- Enomoto T., Lichy J. H., Ikeda J. E., Hurwitz J. Adenovirus DNA replication in vitro: purification of the terminal protein in a functional form. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6779–6783. doi: 10.1073/pnas.78.11.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban J. A., Salas M., Blanco L. Fidelity of phi 29 DNA polymerase. Comparison between protein-primed initiation and DNA polymerization. J Biol Chem. 1993 Feb 5;268(4):2719–2726. [PubMed] [Google Scholar]
- Field J., Gronostajski R. M., Hurwitz J. Properties of the adenovirus DNA polymerase. J Biol Chem. 1984 Aug 10;259(15):9487–9495. [PubMed] [Google Scholar]
- Graham F. L., Rudy J., Brinkley P. Infectious circular DNA of human adenovirus type 5: regeneration of viral DNA termini from molecules lacking terminal sequences. EMBO J. 1989 Jul;8(7):2077–2085. doi: 10.1002/j.1460-2075.1989.tb03616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenheimer R. A., Stillman B. W., Nagata K., Tamanoi F., Hurwitz J. DNA sequences required for the in vitro replication of adenovirus DNA. Proc Natl Acad Sci U S A. 1984 May;81(10):3069–3073. doi: 10.1073/pnas.81.10.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. P., Hay R. T. DNA sequences required for the initiation of adenovirus type 4 DNA replication in vitro. J Mol Biol. 1988 May 5;201(1):57–67. doi: 10.1016/0022-2836(88)90438-x. [DOI] [PubMed] [Google Scholar]
- Hay R. T. Origin of adenovirus DNA replication. Role of the nuclear factor I binding site in vivo. J Mol Biol. 1985 Nov 5;186(1):129–136. doi: 10.1016/0022-2836(85)90263-3. [DOI] [PubMed] [Google Scholar]
- Hay R. T., Russell W. C. Recognition mechanisms in the synthesis of animal virus DNA. Biochem J. 1989 Feb 15;258(1):3–16. doi: 10.1042/bj2580003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny M. K., Hurwitz J. Initiation of adenovirus DNA replication. II. Structural requirements using synthetic oligonucleotide adenovirus templates. J Biol Chem. 1988 Jul 15;263(20):9809–9817. [PubMed] [Google Scholar]
- Leegwater P. A., van Driel W., van der Vliet P. C. Recognition site of nuclear factor I, a sequence-specific DNA-binding protein from HeLa cells that stimulates adenovirus DNA replication. EMBO J. 1985 Jun;4(6):1515–1521. doi: 10.1002/j.1460-2075.1985.tb03811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mul Y. M., Van der Vliet P. C. Nuclear factor I enhances adenovirus DNA replication by increasing the stability of a preinitiation complex. EMBO J. 1992 Feb;11(2):751–760. doi: 10.1002/j.1460-2075.1992.tb05108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mul Y. M., Verrijzer C. P., van der Vliet P. C. Transcription factors NFI and NFIII/oct-1 function independently, employing different mechanisms to enhance adenovirus DNA replication. J Virol. 1990 Nov;64(11):5510–5518. doi: 10.1128/jvi.64.11.5510-5518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mul Y. M., van Miltenburg R. T., De Clercq E., van der Vliet P. C. Mechanism of inhibition of adenovirus DNA replication by the acyclic nucleoside triphosphate analogue (S)-HPMPApp: influence of the adenovirus DNA binding protein. Nucleic Acids Res. 1989 Nov 25;17(22):8917–8929. doi: 10.1093/nar/17.22.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mul Y. M., van der Vliet P. C. The adenovirus DNA binding protein effects the kinetics of DNA replication by a mechanism distinct from NFI or Oct-1. Nucleic Acids Res. 1993 Feb 11;21(3):641–647. doi: 10.1093/nar/21.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez J., Blanco L., Esteban J. A., Bernad A., Salas M. Initiation of phi 29 DNA replication occurs at the second 3' nucleotide of the linear template: a sliding-back mechanism for protein-primed DNA replication. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9579–9583. doi: 10.1073/pnas.89.20.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Enomoto T., Lichy J. H., Hurwitz J. Adenovirus DNA replication in vitro: identification of a host factor that stimulates synthesis of the preterminal protein-dCMP complex. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6438–6442. doi: 10.1073/pnas.79.21.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Hurwitz J. Adenovirus DNA replication in vitro: synthesis of full-length DNA with purified proteins. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4266–4270. doi: 10.1073/pnas.80.14.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano R., Zhao L. J., Padmanabhan R. Overproduction of adenovirus DNA polymerase and preterminal protein in HeLa cells. Gene. 1991 Sep 15;105(2):173–178. doi: 10.1016/0378-1119(91)90148-5. [DOI] [PubMed] [Google Scholar]
- Pronk R., Van Driel W., Van der Vliet P. C. Replication of adenovirus DNA in vitro is ATP-independent. FEBS Lett. 1994 Jan 3;337(1):33–38. doi: 10.1016/0014-5793(94)80624-1. [DOI] [PubMed] [Google Scholar]
- Pronk R., van der Vliet P. C. The adenovirus terminal protein influences binding of replication proteins and changes the origin structure. Nucleic Acids Res. 1993 May 25;21(10):2293–2300. doi: 10.1093/nar/21.10.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruijn G. J., van Driel W., van der Vliet P. C. Nuclear factor III, a novel sequence-specific DNA-binding protein from HeLa cells stimulating adenovirus DNA replication. Nature. 1986 Aug 14;322(6080):656–659. doi: 10.1038/322656a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P. J., Kelly T. J. Purification of nuclear factor I by DNA recognition site affinity chromatography. J Biol Chem. 1986 Jan 25;261(3):1398–1408. [PubMed] [Google Scholar]
- Rosenfeld P. J., O'Neill E. A., Wides R. J., Kelly T. J. Sequence-specific interactions between cellular DNA-binding proteins and the adenovirus origin of DNA replication. Mol Cell Biol. 1987 Feb;7(2):875–886. doi: 10.1128/mcb.7.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas M. Protein-priming of DNA replication. Annu Rev Biochem. 1991;60:39–71. doi: 10.1146/annurev.bi.60.070191.000351. [DOI] [PubMed] [Google Scholar]
- Shinagawa M., Iida Y., Matsuda A., Tsukiyama T., Sato G. Phylogenetic relationships between adenoviruses as inferred from nucleotide sequences of inverted terminal repeats. Gene. 1987;55(1):85–93. doi: 10.1016/0378-1119(87)90251-4. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Tamanoi F. Adenoviral DNA replication: DNA sequences and enzymes required for initiation in vitro. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):741–750. doi: 10.1101/sqb.1983.047.01.085. [DOI] [PubMed] [Google Scholar]
- Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu Rev Cell Biol. 1989;5:197–245. doi: 10.1146/annurev.cb.05.110189.001213. [DOI] [PubMed] [Google Scholar]
- Sussenbach J. S., van der Vliet P. C. The mechanism of adenovirus DNA replication and the characterization of replication proteins. Curr Top Microbiol Immunol. 1984;109:53–73. doi: 10.1007/978-3-642-69460-8_2. [DOI] [PubMed] [Google Scholar]
- Tamanoi F., Stillman B. W. Initiation of adenovirus DNA replication in vitro requires a specific DNA sequence. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6446–6450. doi: 10.1073/pnas.80.21.6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrijzer C. P., Kal A. J., Van der Vliet P. C. The DNA binding domain (POU domain) of transcription factor oct-1 suffices for stimulation of DNA replication. EMBO J. 1990 Jun;9(6):1883–1888. doi: 10.1002/j.1460-2075.1990.tb08314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries E., van Driel W., Bergsma W. G., Arnberg A. C., van der Vliet P. C. HeLa nuclear protein recognizing DNA termini and translocating on DNA forming a regular DNA-multimeric protein complex. J Mol Biol. 1989 Jul 5;208(1):65–78. doi: 10.1016/0022-2836(89)90088-0. [DOI] [PubMed] [Google Scholar]
- van Bergen B. G., van der Ley P. A., van Driel W., van Mansfeld A. D., van der Vliet P. C. Replication of origin containing adenovirus DNA fragments that do not carry the terminal protein. Nucleic Acids Res. 1983 Apr 11;11(7):1975–1989. doi: 10.1093/nar/11.7.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]