Abstract
Biobanks raise challenges for developing ethically sound and practicable consent policies. Biobanks comprised of dried bloodspots (DBS) left over from newborn screening, maintained for long-term storage, and potential secondary research applications are no exception. Michigan has been a leader in transforming its DBS collection, marketing its biobank of de-identified samples for health research use. The Michigan BioTrust for Health includes approximately 4 million unconsented retrospective samples collected as early as 1984 and prospective samples added since the fall of 2010 with blanket parental consent. We engaged Michigan citizens to ascertain public attitudes, knowledge, and beliefs about the BioTrust and informed consent. A convenience sampling of 393 participants from communities around the state of Michigan (oversampling for minority populations) participated in meetings addressing newborn screening, the BioTrust and informed consent, yielding quantitative and qualitative survey and discussion data. Participants affirmed the principle of voluntary informed participation in research and advocated for greater public awareness of the existence of the BioTrust. Most expressed support for the use of DBS for research and a desire for greater involvement in granting permission for research use. Opinions varied as to which specific research uses were acceptable. Participants indicated a desire for greater engagement, public awareness, and more active decision making on the part of biobank participants and parents. Diversity of opinion over which research areas were deemed acceptable problematizes the blanket consent model that currently applies to the BioTrust’s prospective DBS collection and that could become the new norm for research using de-identified data under proposed changes to the Common Rule.
Electronic supplementary material
The online version of this article (doi:10.1007/s12687-013-0162-0) contains supplementary material, which is available to authorized users.
Keywords: Biobank, Public health, Informed consent, Newborn screening, Community engagement
Background
Formally marketing residual dried newborn screening bloodspots (DBS) for health research, Michigan’s BioTrust for Health is one of the largest biobanks in the US. Run by the Michigan Department of Community Health (MDCH), the BioTrust offers a promising collection of biospecimens whose size, unbiased sampling, and linkability to public health data make it a “goldmine” (Couzin-Frankel 2009) for public health assessment and a potential key to important health questions. The BioTrust’s nonprofit organization, the Michigan Neonatal Biobank, provides health researchers access to double de-identified bloodspots, contingent on scientific review, and IRB approval. The biobank comprises a retrospective (“legacy”) collection of more than 4 million Guthrie cards stored from virtually all babies born in Michigan between July 1984 and April 2010—before consent mechanisms were put in place—along with a prospective collection of DBS added to the biobank since its formal inception in fall 2010, and included in the research pool only with a written consent.
A third of US states retain DBS leftover from newborn screening programs for long-term storage and potential secondary research applications (Olney et al. 2006); policies for these large population “biobanks” (in effect if not in name), operated by state departments of health, vary widely (Health Resources and Services Administration 2011; Lewis et al. 2011). While informed consent is an ethical cornerstone of human subjects research, biobanks present challenges for developing ethically sound, practicable consent policies, since in many cases, specific research projects are unknown, and risks and benefits cannot be clearly articulated. In the case of retrospective collections, consent waivers addressing practicability concerns open the door for biobanks to operate without general public awareness and to store biospecimens for research without informed consent from sample “donors” (Clayton 2005).
Two cases involving the DBS collections from state Newborn Screening Programs in Texas and Minnesota were making their way through the courts and the press at the time when this research was conceived and carried out. Lawsuits brought by parents in both states brought the practice of retention and secondary research use of DBS into the spotlight. In Texas, ∼4.5 million stored specimens were destroyed in 2010 as part of the settlement the state reached with plaintiffs (Beleno vs. Texas Dept. of State Health Services). In (Bearder vs. Minnesota) the State Supreme Court ruling determined that research, including quality assurance, quality control, and quality improvement testing, require written individual consent from the donors.
National debate regarding research on de-identified biospecimens has also been fueled by recent proposals for changes to the Common Rule coming out of the 2011 ANPRM, including the requirement that researchers obtain “written consent for research use of biospecimens, even those that have been stripped of identifiers.” Furthermore, “consent could be obtained using a standard short form by which a person could provide open-ended consent for most research uses of a variety of biospecimens (such as all clinical specimens that might be collected at a particular hospital)” (HHS.gov 2013). These changes would apply only to biospecimens collected after the law went into effect.
Given the legal and ethical uncertainties that surround biobanking and the potential for public mistrust on this issue, researchers have, in recent years, increasingly sought a better understanding of public attitudes about the secondary use of DBS for research and informed consent. Turning to communities of stakeholders to consider what appropriate policies, measures, and limits should be enacted in this complex arena, researchers are beginning to discern (and offer policy makers) a richer conception of where the public stands on issues such as informed consent, privacy, and altruism (Botkin et al. 2012; Murphy et al. 2009; Rothwell et al. 2010; Simon et al. 2011).
Engagement and research on the Michigan BioTrust’s legacy collection is particularly significant because it provides an opportunity to educate and survey stakeholders about a program that, like other retrospective biobanks, exists without widespread awareness (State of the State Survey-60 2011; State of the State Survey-63 2012). Since these DBS were collected before their formal inclusion in a secondary research pool, parents were never asked or educated on the front end about the storage or research use of their children’s DBS. A waiver of consent from the MDCH IRB, developed in due consideration of grounds set by the Common Rule (e.g., the impracticability of conducting research without such a waiver), released MDCH of the obligation to re-contact retrospective biobank participants1 and parents, although the IRB stipulated that annual renewals of the consent waiver would depend on MDCH making a good faith effort to educate the public about the BioTrust, its retrospective collection, and the right of the public to opt out (Mongoven and McGee 2012). Under the BioTrust’s consent model, parents and adult biobank participants can, on their own initiative, contact MDCH to request their DBS be destroyed or removed from the research pool.
Tarini et al. (2010) represented one of the first attempts to broadly canvas public attitudes about the use of DBS for research with respect to parental permission in Michigan. The findings were fairly stark; 76.2 % of parents would be either very or somewhat willing to allow their children’s DBS used for research, while that number dropped precipitously to 28.2 % in the absence of permission. A handful of additional studies in Michigan represented first efforts to survey knowledge, attitudes, and beliefs about the BioTrust among the public, but sample sizes and/or depth of engagement in these cases were limited. This paper describes a series of ten community meetings that the University of Michigan’s Life Sciences and Society Program (LSS) held throughout the state to collect a wider array of data on citizens’ knowledge, attitudes, and beliefs about the BioTrust than had previously been available.
Among our key research questions, we wanted to know: Were participants inclined to support the existing approach to consent established by the BioTrust? What were the key informational/educational needs of Michigan residents and communities with regard to this program? What were the most salient hopes and concerns regarding the BioTrust in these communities? What procedures, policies, and DBS research uses would the public we engaged favor and oppose?
Although not representative of the state of Michigan, our snapshot of community perspectives captured a wide breadth of attitudes and beliefs that raise important issues and points for further study in the era of growing use and development of large-scale biobanks.
Methods
We conducted ten community meetings across the state of Michigan between May 2009 and October 2010. The purpose of the meetings was both to engage these publics on the issue of the BioTrust and to collect feedback/data on attitudes and beliefs about the BioTrust. The sites were chosen to establish a broad, not representative, sampling of the state’s population and geographic, economic, and political diversity. We deliberately oversampled underserved communities so as not to miss key concerns that could arise from any group.
For each location, we partnered with a community organization (Table 1) that served as a key collaborator throughout the project. A primary task the community groups took on was recruiting participants to attend the events. LSS staff provided support as needed to aid this process (e.g., producing recruitment posters). This approach had the benefit of enabling face-to-face recruitment from a familiar or trusted community organizer, which we believe was instrumental in helping us approximate our recruitment goal of 400 participants.
Table 1.
Community partner organizations
| Location | Date | Community partner | Number of participants |
|---|---|---|---|
| Flint | 5/4/2010 | Community-based organization partners (CBOP) | 44 |
| Flint | 5/11/2010 | Community-based organization partners (CBOP) | 45 |
| Grand Rapids | 8/21/2010 | The Asian Center | 39 |
| Dearborn | 9/28/2010 | Arab Community Center for Economic and Social Services (ACCESS) | 43 |
| Detroit | 10/12/2010 | Friends of Parkside | 49 |
| Jackson | 10/2/2010 | Alliance health | 39 |
| Petoskey | 3/22/2011 | InterTribal Council of Michigan | 22 |
| Grand Rapids | 4/8/2011 | The Asian Center | 41 |
| Detroit | 5/5/2011 | Latino family services | 43 |
| Ann Arbor | 10/5/2011 | University of Michigan campus-wide recruitment | 27 |
Additionally, community groups coordinated meeting logistics (facilities and hosting) and suggested participants to serve as table discussion facilitators. Each organization was asked to recommend five individuals from among their recruitment pool to serve as table facilitators during the community meetings. (On average, each table was made up of one facilitator and seven participants.) Considerations such as the anticipated literacy levels of the groups, potential need for translation, and personality factors guided the selection of these facilitators. Facilitators met with one of the presenters during the meal (just prior to the meeting) in order to go over the nature of the role, receive guidance on facilitating dialogue, and have their questions answered. Appendix 1 presents the workbook activities and the instructions for facilitators that were printed in the facilitator workbooks (modified versions of the participant workbooks). In general, these facilitators were asked to help move the small table discussions along, encourage a civil and open atmosphere, and then share these small group conversations with the larger meeting. In this sense, they were not considered to be fulfilling a facilitator’s role as in a traditional deliberative democracy context, which typically involves extensive training and more formal criterion for participation in this role.
All meetings were co-facilitated by the principle investigator (a geneticist and professor of Epidemiology) and the assistant director of the LSS Program (an expert in group dialogue facilitation). The presentation was preceded by introductions by the host community organization personnel and an overview of the meetings’ goals and processes, including reading and signing informed consent documents, orientation to the agenda, materials and methods of data capturing, and the process for receiving the subject incentive ($50) at the end of the meeting. In addition to the consent documents, each participant received a packet containing a participant workbook, a clicker (“i>clicker”) device, and a human subjects payment form.
After viewing a brief introductory film about the BioTrust produced by LSS, the co-facilitators, with the aid of PowerPoint slides and six laminated tabletop learning aids (e.g., definitions and key terms and Michigan’s Guthrie card), guided the participants through the three main content sections as follows: newborn screening, the BioTrust, and informed consent. The presentation of material was interspersed at key moments with clicker questions, workbook activities, table talk, and larger group discussion/feedback sessions. Workbooks included a demographic survey (completed at the outset), exercises, and questions administered throughout the meeting, as well as an exit survey to gather feedback and final impressions. Workbook exercises were used to help focus the discussion, and we did not necessarily expect them to be answered fully by all participants, rather respondents were asked to answer to the best of their ability. As such, not all exercises in the workbook yielded data that warranted inclusion in this article. Several questions were conducted by clicker at early phases of the meeting and then repeated in the final exit survey in the workbook, enabling us to assess the extent to which opinions may have shifted during the meetings.
Each of the community meetings followed the same pattern, with minor variation emerging in each community depending on the curiosities, concerns, and areas that the participants found most worthy of deeper discussion. All communities received basic information about the process and purpose of newborn screening, the development and purpose of Michigan’s biobank, actual and possible DBS research uses, and the policies and regulations that govern the BioTrust. Participants further learned about the BioTrust’s consent policies for its legacy and prospective collections and about the vetting of applications for DBS use by the MDCH IRB and a scientific review board. The presentation also explained the process of double de-identification of samples and noted the linkability of DBS to existing public health data sources.
Two of the meetings, in Dearborn and one Detroit event, required simultaneous translation of the presentation, in Arabic and Spanish, respectively. Workbook and consent form translations were available in Spanish, Arabic, Chinese, Japanese, and Korean. A list of relevant scientific terms and concepts was also translated into these languages for use by participants as needed.
In total, 393 individuals participated. On average, 39 community members participated at each meeting (range 22 to 49) in eight different mid- to large-sized cities across Michigan. Six meetings were held in major urban areas of the state—two each in Detroit, Flint, and Grand Rapids. Workbooks were collected from all 393 participants; 355 were completed in part or in full in English. Twelve workbooks were filled out, in part or in full, in Spanish; 25 were filled out, in part or in full, in Arabic. Workbook comments from these participants were translated into English for inclusion in our dataset.
Data collection instruments
Three modes of data collection were used during the community meetings. First, responses to survey questions posed during the presentation were tallied in real-time using a live clicker multiple-choice response system. Second, participants completed exercises and surveys in workbooks before, during, and after the presentation. The activities included quantifiable demographic, opinion, and evaluation questions as well as extensive long answer qualitative comment fields. Third, group discussions were captured in real-time using flip charts and/or laptop computers set up to project these comments and questions, as well as points of agreement and disagreement. All survey questions and workbook exercises were reviewed by a researcher with expertise in the areas of public health and genetics, and previous experience holding town hall meetings on comparable issues, who provided feedback through multiple iterations of the materials.
Analytic approach
All data were recorded in an Excel spreadsheet for analysis. Survey questions were imported into Stata12.0 to generate descriptive statistics and evaluate demographic characteristics of our participants as well as their responses to the survey questions specified in Fig. 1 and Table 2. Locations reflect the sites of our community partners; as such the data from both Flint meetings and both Grand Rapids meetings were combined in the analysis.
Fig. 1.
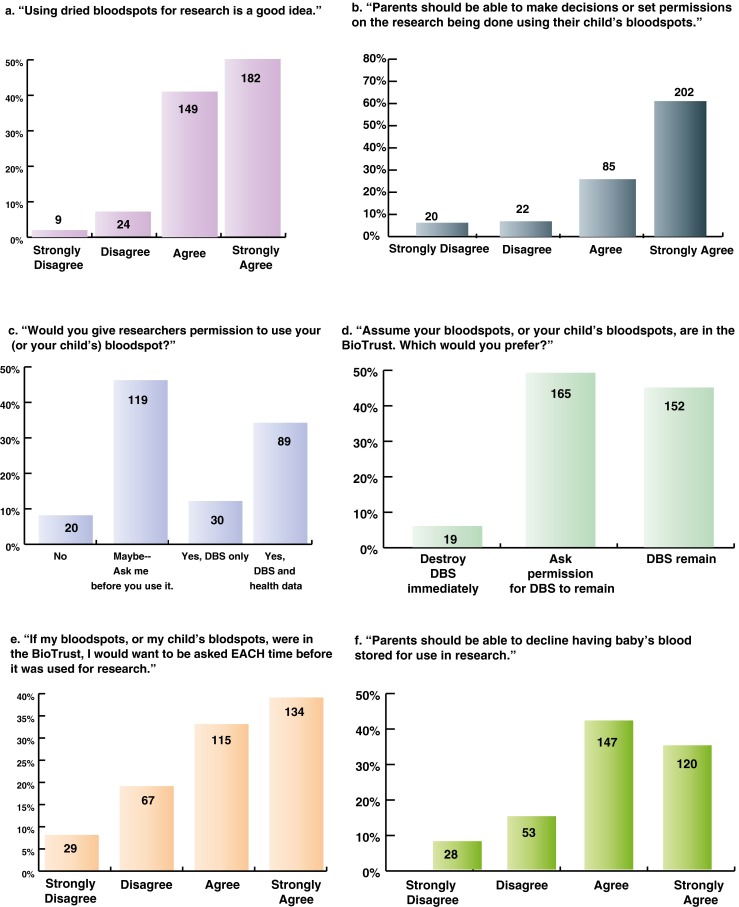
Consent preferences. Overall, 91 % of community members agreed that using DBS for research is a good idea, while 87 % felt that “parents should be able to make decisions or set permissions on the research being done using their child’s bloodspots.” Forty-six percent of participants said they would give researchers permission to use their or their child’s DBS with or without health data, while 20 % said they would not, and 46 % responded “maybe: ask me before you use it.” A minority of participants (6 %) said they would want to have their or their child’s DBS destroyed if they were in the BioTrust; 45 % said they would want them to remain, and 49 % said they would want to be asked permission for them to remain. Seventy-two percent of participants said they would want to be asked each time their or their child’s DBS would be used for research. Twenty-three percent of participants indicated they felt that parents should not be able to “decline having baby’s blood stored for use in research”
Table 2.
Responses to six survey questions regarding consent preferences by demographic characteristics
| Demographic categories | Questions | Mid–Meeting | Exit survey | ||||||
|---|---|---|---|---|---|---|---|---|---|
| "Using DBS for research is a good idea." (iClicker) | "Parents should be able to make decisions or set permissions on research done using their child’s bloodspots." (iClicker) | "Would you give researchers permission to use your (or your child’s) bloodspot?" (Workbook exercise) | "Parents should be able to decline having their baby’s blood stored for use in research." | "If my bloodspots, or my child’s bloodspots, were in the BioTrust,I would want to be asked each time before it was used for research." | "Assume your bloodspots, or your child’s bloodspots, are in the BioTrust. Which would you prefer?" | ||||
| % Agree | % Agree | No or Maybe | Yes DBS only or DBS & Health data | % Agree | % Agree | Ask my permission for DBS to remain | DBS remain in Biotrust | ||
| Location | Flint 1 & 2 | 72.7a, b, c, d, e | 95.0 | 80.0 | 20.0 | 84.1 | 89.9a, b | 74.6a | 25.4a |
| Grand Rapids 1 & 2 | 100a | 83.3 | 52.6 | 47.4 | 85.3 | 67.6 | 43.5 | 56.5 | |
| Dearborn | 100b | 86.8 | 45.8 | 54.2 | 59.4 | 58.1 | 33.3 | 66.7 | |
| Detroit 1 | 90.9 | 95.4 | 66.7 | 33.3 | 91.5a | 84.8 | 70.7 | 29.3 | |
| Jackson | 94.9c | 84.6 | 38.2 | 61.8 | 59.5 | 51.4a | 37.8 | 62.2 | |
| Petoskey | 85.0 | 90.5 | 57.1 | 42.9 | 95.2 | 57.1 | 68.4 | 31.6 | |
| Detroit 2 | 95.1d | 90.5 | 62.5 | 37.5 | 53.9a | 89.7 | 45.2 | 54.8 | |
| Ann Arbor | 96.3e | 70.4 | 29.6 | 70.4 | 71.4 | 50.0b | 29.6a | 70.4a | |
| Age | <25 | 91.8 | 87.2 | 56.6 | 43.4 | 83.3 | 72.6 | 57.7 | 42.3 |
| 26–35 | 95.9 | 91.3 | 51.2 | 48.8 | 72.0 | 72.0 | 51.1 | 48.9 | |
| 36–45 | 95.9 | 80.6 | 48.3 | 51.7 | 70.8 | 63.9 | 41.4 | 58.6 | |
| 46–55 | 95.2 | 89.7 | 54.6 | 45.5 | 76.3 | 66.7 | 42.9 | 57.1 | |
| >56 | 88.4 | 85.7 | 56.3 | 43.8 | 73.4 | 79.4 | 56.9 | 43.1 | |
| Race/Ethnicity | African–American | 84.2a, b, c, d | 92.5 | 69.1 | 30.9 | 84.7a | 85.3a, b | 69.9a | 30.1a |
| Arab/Arab–American | 100a | 86.8 | 45.8 | 54.2 | 59.4 | 58.1 | 33.3 | 66.7 | |
| Asian/Pacific Islander | 100b | 86.5 | 50.0 | 50.0 | 85.92b | 68.6 | 44.6 | 55.4 | |
| Hispanic | 97.5c | 87.8 | 55.0 | 45.0 | 52.6a, b | 84.2a | 39.4 | 60.6 | |
| Native American | 90.0 | 90.9 | 71.4 | 28.6 | 90.9 | 72.7 | 80.0 | 20.0 | |
| White, not Hispanic | 96.6d | 80.7 | 33.3 | 66.7 | 67.2 | 47.5b | 33.9a | 66.1a | |
| Other | 80.0 | 66.7 | 77.8 | 22.2 | 81.8 | 63.6 | 55.6 | 44.4 | |
| Education | High school/less | 93.7 | 92.1 | 58.1 | 41.9 | 67.4 | 79.3 | 51.3 | 48.7 |
| Some/full college | 90.2 | 86.5 | 56.9 | 43.1 | 79.9 | 67.3 | 54.0 | 46.0 | |
| Graduate | 98.6 | 80.7 | 40.0 | 60.0 | 78.3 | 68.1 | 45.3 | 54.7 | |
| Gender | Male | 91.0 | 83.3 | 41.5a | 58.5a | 72.3 | 65.1 | 48.0 | 52.0 |
| Female | 91.8 | 88.3 | 57.8a | 42.3a | 76.9 | 73.9 | 53.4 | 46.6 | |
| Overall | 90.9 | 87.2 | 53.9 | 46.1 | 76.1 | 72.2 | 52.1 | 47.9 | |
a, b, c, d, e Pairwise significant differences (alpha = .05; testwise alpha = .05/[J(J–1)/2]
To evaluate differences in responses by sex, race/ethnicity, education, age, and meeting site, we used one-way ANOVA methods to test the null hypothesis that in at least one group the mean response was different. In cases where the difference was determined to be statistically significant (p < 0.05), we used the Bonferroni method to conduct pairwise comparisons of group means.
Workbook comments and discussion comments and questions were coded and analyzed thematically by three analysts. Using a codebook developed by the research team to capture all themes of public commentary on the BioTrust across multiple community engagement types, we reviewed and coded data to consider the breadth of issues that participants raised about public health biobanking and the frequency of particular attitudes and beliefs expressed in written comments and in discussion. Two individuals coded each comment. In instances of disagreement, the whole group discussed the comment until a consensus was achieved. Using Freelon’s ReCal3 (“Reliability Calculator for three or more coders”) online tool, average pairwise percent agreement among the coders was determined to be 95.04 % (Krippendorff’s alpha score = 0.7) (Freelon 2010).
Results
We present our results in the following four main sections: participant demographics, public awareness, consent, and “What would I be consenting to?” The latter three sections correspond with three key findings (1) that the participants called for greater awareness of Michigan’s biobank, (2) that many wanted greater involvement in permission-giving than the status quo consent policies require, and (3) that variability in research uses supported by community members problematizes the blanket consent model. In Appendix 2, we provide direct quotes from participant workbooks and discussions, organized around an exhaustive list of major themes; in this paper, we focus on the themes of awareness and consent.
I. Participant demographics
Forty percent of the participants were 35 years old or younger; 21 % were 36–45 years old, and 39 % were 45 and older. Participants were 75 % female. Most participants (80 %) had a high school or college diploma. Our overall sample represented diverse minority populations; only 16 % of participants identified as non-Hispanic white, 32 % African–American, 19 % Asian/Pacific islander, 11 % Arab American, 11 % Hispanic, and 3 % Native American. Due to the geographical distribution of minority populations in the state of Michigan as well as the services and mission of the community partners who hosted our events, many meetings were homogeneous with respect to race/ethnicity. Given the high correlation between location and race/ethnicity, we cannot isolate the independent effects of each. On the night of the meetings, more than 95 % of participants across all meetings self-reported to be in fair to excellent health, indicating that participation was not biased toward those seeking help or insight into a personal health problem. (See Table 3 for demographic summary statistics).
Table 3.
Demographics of study participants (n = 393)
| Characteristic | n | % |
|---|---|---|
| Gender | ||
| Male | 93 | 25 |
| Female | 286 | 75 |
| Age | ||
| < 25 | 91 | 25 |
| 26–35 | 53 | 15 |
| 36–45 | 77 | 21 |
| 46–55 | 65 | 18 |
| > 56 | 76 | 21 |
| Race/ethnicity | ||
| Arab or Arab–American | 43 | 12 |
| African–American | 124 | 34 |
| Asian or Pacific Islander | 76 | 21 |
| Hispanic | 42 | 11 |
| Native American | 12 | 3 |
| White (not Hispanic) | 59 | 16 |
| Mixed Race/Other/NR | 11 | 3 |
| Education level | ||
| < 12 years | 108 | 30 |
| 12–16 years | 180 | 50 |
| > 16 years | 72 | 20 |
| Blood donor? | ||
| Yes | 112 | 34 |
| No/not sure | 253 | 66 |
| Does participant or their child have DBS in the biobank? | ||
| Yes | 190 | 64 |
| Not Sure | 30 | 10 |
| No | 77 | 26 |
| Subjective personal health rating | ||
| Excellent | 47 | 12.9 |
| Very good | 162 | 44.6 |
| Fair | 137 | 37.7 |
| Not so hot | 16 | 4.4 |
| Poor | 1 | 0.3 |
II. Public awareness
Prior knowledge
Prior knowledge about informed consent, newborn screening, and the BioTrust, which was ascertained by clicker, was highly variable across communities. Overall, 36 % of respondents (n = 141/393) indicated that they had heard of newborn screening, ranging from 5 % at Dearborn (n = 2/40) to 76 % in Detroit (n = 31/41). Almost 23 of 25 (88 %) of participants in Ann Arbor had said they had heard of informed consent, compared to 37 % (17 of 45) at a Detroit meeting. An unexpected 22 %, (ranging from 4 % (1 of 28) in Ann Arbor to 48 % at a meeting held in Flint (19 of 39)), said they had heard of the BioTrust, but we came to realize some respondents were indicating prior knowledge based on awareness that came about from the recruitment process for the community meetings.2
Many community meeting participants were concerned that Michigan residents are generally not aware of the existence of the BioTrust; 50 workbook comments spoke to the need for greater awareness of the BioTrust among the public and/or the need to educate the public about it. As one Grand Rapids participant wrote in a workbook “Massive number(s) of people have to be educated about it.” (Grand Rapids, Asian–American, M); (Appendix 2-I).
In a workbook survey question gauging “initial feelings about the BioTrust,” 145 attendees indicated that they “wished they had known (about the BioTrust) sooner.” In written workbook comments, 14 noted the fact that they had personally never heard of the BioTrust or asked why they had never been informed. Respondents from Flint, for example, asked in their workbooks, “If this is great, why are you just telling us about this?” (Flint, n/a, F) and “Why weren’t we educated?” (Flint, African–American, F)
A demand for broader public awareness
Individual workbook comments advanced a variety of reasons for communicating with the public about the BioTrust, as follows: To maintain awareness of potential or actual DBS research or non-research uses and goals, to receive personally relevant health information, to maintain parental oversight of the child’s interests, to inform biobank participants when they reach adulthood, to make the public aware that they have the option to withdraw, to foster trust among the public, to guide ongoing decision making for continued participation or withdrawal from the BioTrust, and to achieve public awareness of the BioTrust’s successes and research findings.
In seven instances, workbook comments cited lack of communication or transparency as a potential cause for mistrust. As an Ann Arbor participant put it, “Communication is key. If you’re not staying informative, people will assume you’re hiding something.” (Ann Arbor, White, M) A few participants invoked the “right to know” about biobank participation, e.g., “People have the right to know,” (Flint, White, F); “I believe the hospital should have the mother’s consent, and let the mother know what’s going on because she has a right to know!” (Detroit, Hispanic, F); or “I have a right to know how my blood is used for research.” (Ann Arbor, White, M); (Appendix 2-II).
Some workbook comments addressed the question of how and when information about the BioTrust would ideally be communicated. Several referred specifically to the need for information to be clear and accessible in multiple languages (Appendix 2-I). A few suggested periodic or ongoing communications with the public, such as notification of participants whose blood samples have been used, return of research results, or annual communications about how DBS in the biobank collection have been utilized (Appendix 2-IX).
Recommendations for public education included workshops for parents or expecting parents, outreach from community organizations, and surveying public opinion through deliberative democratic processes. Several suggested using social networking, including Facebook, Twitter, and MySpace. Some suggested large-scale awareness campaigns. “This is 2010—we can all discuss the same commercials from the Superbowl,” said one participant in Ann Arbor. “If they wanted people to know, why not do a mass media campaign (Yahoo news, TV ads…)?”
In Ann Arbor, two participants expressed skepticism about the value of public awareness in workbook comments. For example, one participant wrote: “Do not let people know what is going on. Government decides what to do based on the benefit of everyone…” (Hispanic, M) Such comments were only recorded at this meeting. Comments recorded during the Ann Arbor discussion also speculated that sharing information about the BioTrust could result in public backlash. “Perhaps they don’t want an eruption of public hysteria,” said one participant, and another noted “people can’t sue if they don’t know that it exists.”
III. Consent
Consent options
We asked six questions around the theme of consent preferences and beliefs; Fig. 1 shows the results. We found that overall, 91 % felt that using the bloodspots for research was a good idea. We also found that asking permission and allowing participants to opt out were highly valued; 87 % felt that parents should be able to set permissions for DBS research uses, and 76 % responded that parents should be able to decline having their baby’s DBS used for research. When asked whether they would give permission to researchers to use DBS, 46 % said yes; the remaining 54 % responded no or maybe. Data collected from both workbooks and discussions supports the quantitative data, indicating strong support for the use of DBS for research uses. However, participants generally opposed the storage of bloodspots for research use in the absence of explicit consent from informed participants (Fig. 1). A full 72 % said they would want to be asked each time their or their child’s DBS was used for research, while a few indicated in workbooks that they would not want to be bothered with ongoing contact.
Recommendations about consent policies collected from discussions and workbook comments, including incentivizing decision making, gradually destroying legacy bloodspots, determining consent requirements for research on a case-by-case basis, and re-contacting participants for periodic consent renewals, are presented in Appendix 2-II.
Community members were prompted to make suggestions about how consent models could be improved and operationalized. Participants noted that parents need time to make a thoughtful decision. Five addressed the timing of the informed consent process for the new DBS, specifically suggesting that parents be informed prior to childbirth. One wrote, “(My greatest hope for this program is) more education about this issue for mothers and expecting mothers prior to them giving birth!” (Grand Rapids, Asian–American, F). Individual participants suggested using the infrastructures of low-income housing, the ballot, or the Secretary of State (which handles motor vehicles in Michigan) to inform the public or obtain consent. Other individual suggestions included adding consent options to state tax forms or using a tax rebate to incentivize decision making.
In our analysis of responses to survey questions about consent (Table 2), we found few significant differences in responses to these questions when evaluated by demographic group. There were no statistically significant differences by age or education. Men and women answered the questions similarly, except for the question of whether you would give permission for research use. For this question, men were more likely than women to say yes (59 vs. 42 %, p = 0.024).
We found that most significant differences fell along the lines of location and race/ethnicity. For example, we found that despite majority agreement across all groups, participants in Flint (27.3 %) were more likely to disagree with the statement “using bloodspots for research is a good idea” than participants in Grand Rapids (0 %), Dearborn (0 %), Detroit (second meeting) (4.9 %), Jackson (5.1 %), and Ann Arbor (3.7 %) (p < 0.001). We similarly found that African–Americans (15.8 %) were more likely to disagree that using bloodspots for research was a good idea than Arab–Americans (0 %), Asian/Pacific islanders (0 %), and non-Hispanic whites (3.4 %) (p < 0.001). Group differences by location and race/ethnicity were also detected in responses to the questions of whether parents should be able to decline having the baby’s DBS used for research, whether parents would like to be asked each time, and whether bloodspots should remain in the BioTrust with or without permission. In general, Flint participants and African–Americans expressed greater skepticism and a greater desire to be asked permission as compared to other groups.
Feelings about informed consent
After a brief introduction to the BioTrust initiative, participants completed a multiple choice workbook exercise designed to capture their initial feelings about the BioTrust. In this exercise, 184 participants selected “positive,” compared to 15 who selected “negative.” Most respondents made multiple selections; in addition to “positive,” the most frequently selected options were “eager to learn more” (n = 234), “curious” (n = 219), and “hopeful” (n = 154). The least-selected options were “negative,” “angry” (n = 15), “fearful” (n = 51), and “neutral” (n = 63). Notably, however, 123 selected “suspicious.”
Twenty-six workbook comments suggested feelings of suspicion, mistrust, or powerlessness (Appendix 2-X). “I feel like they are doing or were doing something shady; they better find a way to contact me and get my consent that should have been asked for in the first place,” wrote a Flint participant (n/a, F). In Ann Arbor, a participant referred to the BioTrust as “1984-esque,” (White, M), and workbook comments included “I’m not sure what to think. I’m not harmed/being hurt by it, but it’s also suspicious that neither me nor my parents ever knew they had my blood and what they were doing with it,” (White, F) and “It’s interesting that the name is BioTrust—if people are not given adequate information about this bloodspot reserve or are not made aware of its existence—how can they trust it? It is like it was named to evoke a positive feeling from someone the moment they hear about it…” (Ann Arbor, White, F). In this vein, participants in Detroit listed as their greatest concerns: “They are researching information about me without me knowing” (Detroit, African–American, F), and “There are going to be problems when people really find out what they are doing without consent.” (Detroit, African–American, F)
Comments conveyed a range of emotions (Appendix 2-XII) that bore on attitudes about consent, including excitement about health research, skepticism toward government, concerns about racial and cultural discrimination (Appendix 2-XI), and parental protectiveness.
In the exit survey, participants shared their greatest hopes and concerns about the BioTrust (Appendix 1, p.11). Participants expressed optimism and hope for the research potential of the initiative and, specifically in 140 instances, its ability to improve health, prevent, or find cures to diseases. Apart from the issue of informed consent (31 comments), concerns centered on privacy and security of data (11 comments), worries that the data would “get into the wrong hands” (9 comments), and fears about the misuse of DBS and data (69 comments), including non-research uses (7 comments), insurance discrimination (8 comments), cloning (8 comments), warfare uses (1 comment), and uses that would oppress disempowered groups (15 comments) (Appendix 2-III-VII).
Limited support for the status quo
A minority of participants supported the inclusion of DBS in the BioTrust without the explicit consent of parents or participants. Almost a quarter of respondents—81 of 348—indicated in one exercise that parents should not be able to decline having their baby’s bloodspot used for research (Fig. 1F). Eight participants wrote workbook comments explaining this position, citing benefits for the common good, the advantage of maintaining an unbiased, complete dataset, and practical considerations. One opined that new parents would not understand the risks and benefits of participation. Some agreed that obtaining consent for the 4 million retrospective bloodspots would be impracticable. “It is a huge undertaking,” said an Ann Arbor participant in the discussion. “That’s so many people to track down. If we can’t get a full response for the Census, why would they be successful with this?” For some respondents, the need to preserve potential public health outcomes serving the “common good” outweighed the principle of individual choice.
In discussions and comments about its consent policies, very few participant comments (<10) explicitly supported the status quo policies such as the consent waiver for legacy spots or blanket consent forms for the prospective collection.
IV. What would I be consenting to?
Participants asked questions in meeting discussions and workbooks seeking the basic information that would typically be included in informed consent materials, such as the scope of allowable DBS use or the risks and benefits of participation. They held varying positions on what health research uses should be acceptable, and raised concerns about potential consequences, intended or not, of storing and using DBS for indefinite and undefined future uses. During a discussion in Detroit, a community member asked, “What if they use the bloodspots and health data for something else, besides what we are consenting to?” In their questions, participants wondered about specific types of research that could be conducted using the bloodspots, about the ethical accountability of the organization and about its commitment to informing the public about its research and operation. The questions raised reflected a strong desire among community groups to understand the details of the BioTrust’s policies, governance, and oversight. As one Detroit participant commented during the discussion, “We want to know exactly what we’re consenting to.” Additionally, community members asked for details about identifiability, security, governance, and research assessment (Appendix 2, III and V). They asked how long bloodspots are kept, whether they expire, and whether they are kept after death of the donor.
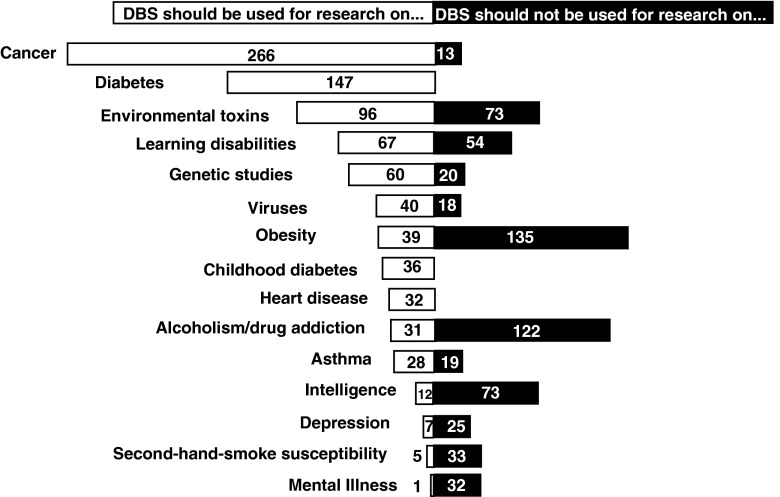
Support or critique of the BioTrust initiative seemed tightly linked to its specific endeavors. In discussing the scope of health research that could or should be done using the bloodspots, there was a high degree of variability in responses. On the one hand, most agreed that some chronic diseases should be a priority for permissible research. In a workbook activity, participants were asked to cite three health research uses each that they favored and opposed (Appendix 1, p. 6). Considered in aggregate, the top five priorities for our participants were cancer (n = 266), diabetes (n = 183), environmental toxins (n = 95), learning disabilities (n = 67), and genetic studies (n = 60). However, several issues that were most important for some were deemed unacceptable by others. The top five uses that participants said they would restrict were obesity (n = 134), alcoholism/drug addiction (n = 122), environmental toxins (n = 73), intelligence (n = 73), and learning disabilities (n = 54) (Fig. 2).
Fig. 2.
Accepted and not accepted research uses for Michigan BioTrust DBS. The top ten accepted uses cited by community meeting participants in descending order were cancer, diabetes, environmental toxins, learning disabilities, genetic studies, viruses, obesity, childhood diabetes, heart disease, and alcohol and drug addiction. The top ten not accepted uses cited were obesity, alcohol and drug addiction, intelligence, environmental toxins, learning disabilities, secondary smoke susceptibility, mental illness, depression, genetic studies, and asthma
Discussion
Although participants were not asked to weigh the relative value of informing versus consenting biobank participants, comments suggested that notifying participants and parents about the storage of bloodspots for research use was at least as important as asking permission. The breadth of issues that participants raised (see Appendix 2) indicates that permission, while a central issue for many, is but one of several areas that stakeholders would be eager to understand in greater detail.
Our participants’ clear demand for education suggests a challenge to the paternalistic culture of public health that sometimes informs decision making on this issue. The possibilities that the public might not care about Michigan’s biobank or that a mass education effort could be unnecessary, given the broad support for biobank goals or benevolent stewardship of the biobank, were both belied by the demands for more information we found in our meetings. Limited resources are a challenge for implementing broad public awareness efforts, but communication technologies can be a tremendous asset for this effort. Social media channels can be used to cost-effectively target and engage adult research participants on this relatively obscure topic (Platt et al. 2013).
The lack of public awareness about the BioTrust poses ethical questions about the meaningfulness of the available “opt-out” option for the legacy DBS (Petrini 2010; Shickle 2006). It could also become a liability for the BioTrust if it leads to public mistrust or the kind of backlash that occurred in Texas and Minnesota. Key themes raised by participants such as a perceived lack of transparency and the lag time between the creation of the BioTrust and the time when they learned about the program highlighted the potential for mistrust, whereas greater efforts to inform the Michigan public about the BioTrust were seen as opportunities to build goodwill and support for the program. Our findings suggest that fostering trust may be a key component to the future success of the BioTrust and similar initiatives.
A fundamental tension in the attitude of community participants was consistent with the findings of the state and national biobank surveys. The majority of the public supports the use of biobanked samples for research—but not without permission (Tarini et al. 2010; Botkin et al. 2012). But participants expressed divergent attitudes and a variety of ideas about which consent models should be implemented by Michigan’s BioTrust.
It is not surprising that the participants’ attitudes about biobanking ethics and policies were wide ranging; consensus eludes experts as well when it comes to the ethical complexities of consent. Some experts, for example, suggest a blanket consent approach is an appropriate way to satisfy ethical obligations due to biobank donors under certain conditions (Petrini 2010), while others have argued that informed consent fails to “fit” in biobanking, and that a strict policy of anonymization of samples should be pursued in its place (Caplan 2009). This latter option would come at a cost to the scientific promise of public health biobanks, whose linkability to public health data make them so potentially valuable.
The most significant findings of our study around the issue of informed consent included the following: (1) participants strongly supported an “ask each time” model; notably, this finding is inconsistent with the results of two recent studies exploring public attitudes toward consent in biobanking, both indicating that a broad one-time opt-in is preferred by the public in general (Simon et al. 2011; Wendler 2006), (2) participants expressed dissatisfaction with the “opt-out” model, which currently applies to the BioTrust’s legacy collection, and (3) participants’ varying attitudes about which research uses are acceptable problematizes the blanket consent model that applies to the prospective collection.
The latter finding was notable because it showed greater contrast among research categories deemed acceptable for the DBS in the BioTrust than had been previously ascertained. The largest survey to date (n = 3,082) on public preferences for DBS research uses in Michigan was conducted in the 2008 Michigan Behavioral Risk Factor Survey, where 72.3 % favored research intended to benefit the health of Michigan residents in general, and support for three other queried categories of research—on childhood diseases, on adult diseases, and on harmful substances—was relatively consistent (ranging from 84 to 86.8 %). While these data suggest broad and unvaried support for DBS research use across generic health categories, our data indicate significant diversity of opinion. Within the broad category of “adult diseases” are potential research uses that many community meeting participants deemed unacceptable, including some, like obesity, that public health researchers might consider to be low-risk and uncontroversial. One policy recommendation suggested by this finding is for blanket consent documents to state that potential research uses of biospecimens might not align with the individuals’ values or views about research priorities.
These three findings are significant in part because they counterpose national and global trends in biobanking policy. The recent proposals for changes to the Common Rule coming out of the 2011 ANPRM would make federal requirements consistent with the current BioTrust practice of obtaining broad consent at the time of collection of the biospecimen. Notably, the rule change would neither apply to the BioTrust’s legacy collection of DBS nor to existing collections in biobanks nationwide. The summer 2013 issue of the Journal of Law, Medicine and Ethics highlights challenges associated with implementing the proposed changes to the Common Rule, including implications for certificates of confidentiality (Williams and Wolf 2013) and IRB reforms (Lidz and Garverich 2013). Our study informs this unfolding discussion, since popular attitudes toward the BioTrust may well presage those that will arise in future cases of research involving broad consent and de-identified biospecimens. Participants expressed divergent hopes and concerns about Michigan’s biobank and were inconsistent in their support for specific secondary research uses, raising the question of whether blanket or broad consent forms can adequately inform decision makers and respect diverse values.
While the formal incorporation of a DBS biobank is unique to Michigan, the results of this study have translatable implications for research biobanks generally and for state DBS collections that may be utilized for broader research, now or in the future; the creation of the Newborn Screening Translational Research Network is an indicator that these data-rich sources will likely become more available for health research. Despite its uniqueness, the BioTrust encompasses several notable factors that may make it more or less comparable to other programs, including the following: its inclusion of double de-identified biospecimens and linkable health data, the derivation of samples in the biobank from a non-research purpose, the opt-out policy that applies to its retrospective biobank collection, and its operation in the context of low awareness among the public. Lessons from this study may also apply to other contexts, as large population biobanks are harbingers of the ethical issues that will continue to arise in this new era of integrated health information technology.
Broader implications of biobanking policy will be seen with the growing trend of implementing learning health systems, which exponentially increase the opportunities to study large populations by linking data and biological specimens within and across institutional boundaries (Friedman et al. 2010). Public acceptance of the linkage of large population cohorts of data and biospecimens will depend on a public that is aware, trusting, and engaged. The results of our meetings indicate that people are not generally aware of Michigan’s large population biobank and may be skeptical because they are not informed but still supportive of the research goals when they are asked and educated about the program. Good policy is usually based on good evidence of both the risks and benefits associated with the policy. Public engagement research can develop an evidentiary base on how the public perceives both the risks of involvement and expectations for the distribution of benefits. This data can be a guide for policy makers and supports appropriate allocation of resources to address education and communication needs.
Opinions expressed by our study participants about informed consent diverged significantly from practices and policies that are widely implemented. Policy makers should consider not only the consent models that were favored and disfavored among the communities that we sampled but also the attitudes and concerns underlying those preferences. For example, given the strong support indicated for an “ask each time” consent model, policymakers in favor of the blanket consent model might consider how they can better convince the public of the benefits of that policy. Whether or not the “ask each time” model itself is feasible, our findings indicate a desire on the part of the public to be more informed and engaged about how, and by whom, their biospecimens and health information are used. Given the great potential of large population biobanks for health research, policy makers, as stewards of samples and data, should explore partnership models with the public to strengthen two-way communication and determine policies that could be more broadly accepted among institutions, researchers, participants, and citizens (Kaye et al. 2012).
Limitations and future research
Michigan is unique in that it is the only state that has an organization formally established to manage the research uses of its newborn screening bloodspots. Our sampling, while broad, was not representative of the state’s demographics; meetings were held primarily in urban areas and did not extend as far north as Michigan’s Upper Peninsula, and as we oversampled minority communities to better understand their perspectives, we cannot generalize our findings to the state or the nation as a whole. Recruitment of men was a challenge for some community partners; the attendees of our community meetings were overwhelmingly female (2:1). We do note, however, that subsequent survey data indicate that there is no significant difference between male and female attitudes on this subject (State of the State Survey-60 2011; State of the State Survey-63 2012). The Ann Arbor recruitment was unique in that it was comprised of undergraduate and graduate students, though age and education level were not statistically significant factors in survey responses. The Michigan-only sample might also challenge extrapolation of findings to other states and contexts. Overall, the power of the study findings is limited by the inability to evaluate significant differences between groups given the multiple variables and relatively small numbers of matched individuals within and across each group. Some participants did not complete the workbooks in full, though we have no reason to suspect, those who did not complete all survey questions were significantly different from those who did.
We also note that future engagement efforts that would raise awareness on DBS biobanking should take into account a persistent source of confusion surrounding this issue, namely the conflation of newborn screening and DBS research. In spite of concerted efforts to separate them, some confusion among our participants remained. Distinguishing the BioTrust and DBS research generally from newborn screening is particularly important, since conflation of the two programs could create confusion or mistrust about NBS. Participants were overwhelmingly enthusiastic about newborn screening, and the possibility that a backlash against DBS research could redound on NBS programs is a genuine concern, one that has been noted in other public engagement research (Rothwell et al. 2012). A further common misunderstanding revealed in participant comments was the expectation that by participating in BioTrust research, biobank participants and parents might receive personally relevant health information (i.e., return of individual health results), which is not the case.
Future research could further investigate the public’s understanding and acceptance of health information being used for public health, health care, and research. As data becomes more fluid across these institutional and disciplinary arenas, policies still hinge on what might be considered “primary uses.” Public health data, for example, is often exempt from informed consent requirements, if its intended use is public health practice; the distinction between “research” and “practice” is often fuzzy.
While our study did not find significant differences between respondent attitudes across many demographic characteristics, there is some indication that marginalized populations are more skeptical about the benefits of biobank research. Further studies addressing the attitudes and beliefs among these groups should explore the specific factors contributing to this mistrust and potential mechanisms for addressing inequity.
The diversity of expectations we found in our study about the potential research uses of the DBS challenges the meaning and legitimacy of broad consent. This result bears further investigation and deeper follow-up than was afforded by our instruments. Specifically, our workbook exercise on this issue asked participants to indicate, among several candidate health conditions, which they would deem to be their top three and bottom three priorities. While we were able to get a sense of their view of research priorities, this approach did not allow us to fully capture the degree of sentiment and weighting of approved and disapproved research in one direction or another, which could be a very helpful dimension of such preference rankings to understand (Appendix 1, p.6).
Ultimately, the development and testing of specific consent models, novel tools for informing individuals and curating consent preferences for use of legacy biobanks, are needed to clarify the extent to which such systems are able to satisfy the theoretical preferences voiced by individuals and communities in research such as we present here. Furthermore, the move to testing such participant centric initiatives (PCIs) will help determine what actual steps individuals will be willing and able to take to meet the demand for greater involvement in research. Recent efforts in this arena are already yielding insights on the feasibility of this approach (Kaye et al. 2012; Terry and Terry 2011; Terry et al. 2013).
Finally, the desire for more information and greater awareness we found in this study suggests that development of communications best practices in biobanking could inform biobank governance generally.
Conclusion
We found significant support for the use of DBS for secondary research, and yet we also found dissent among the public we queried from the status quo consent policies that apply to the legacy and prospective collections of Michigan’s public health biobank. The surveyed group did not view a public health biobank of de-identified samples as an exception to the general principle that informed consent should be required for research use. We found less concurrence of opinion on how consent should be gathered and under what conditions. We also saw variability in research uses that community members deemed acceptable and unacceptable, a finding that raises questions about the appropriateness of blanket consent for biobank participation. Communities also called for large-scale education about Michigan’s public health biobank, and noted that lack of transparency could create a climate of mistrust for an initiative whose major goal—the use of DBS for health research—was broadly supported. The consent preferences of those we surveyed, along with the hopes and concerns about the BioTrust initiative that underlay them, has implications for biobanks and DBS collections nationally, especially those with comparable features.
Electronic supplementary material
(PDF 108 kb)
(PDF 398 kb)
Acknowledgments
This work was funded by an ARRA challenge grant issued through the National Human Genome Research Institute (5RC1HG005439-02). The authors gratefully acknowledge the community partner organizations, without whom this work would not have been possible, as follows: Community-based organization partners (Flint), The Asian Center (Grand Rapids), Arab Community Center for Economic and Social Services (Dearborn), Friends of Parkside (Detroit), Alliance Health (Jackson), InterTribal Council of Michigan, and Latino Family Services (Detroit). We would like to thank the participants who came to the community meetings for their time and input on this issue. We acknowledge the contributions of Nicole Fisher and Jamie Liebert who provided outstanding research assistance throughout the project. We also thank our colleagues at Michigan State University, Ann Mongoven, Meta Kreiner, and Andrea Sexton, with whom we developed the qualitative codebook used in this analysis. In addition, the authors acknowledge the work of Joan Scott, who served in an advisory capacity during the development and implementation of the community meetings. Additional support for this work was provided by a grant from the Eunice Kennedy Shriver National Institute for Child Health and Human Development (1R01HD067264).
Compliance with ethics
This study was conducted with oversight by the University of Michigan IRB and in compliance with current laws in the United States of America. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all participants for being included in the study.
Footnotes
N.B. The choice of how to refer to individuals who have DBS in the retrospective collection of the BioTrust is somewhat problematic. The term “subject” is controversial given that, according to the Common Rule definition of research using de-identified DBS, these individuals are specifically not research subjects. “Donors” is also problematic as the term typically connotes an intention on the part of the individual to have their samples be in the research pool. We have opted for the term “biobank participants,” though this is somewhat unsatisfactory, given how few people are aware of their “participation.”
In two subsequent state-wide polls conducted through Michigan State University’s Institute for Public Policy and Social Research, the percentage of Michiganders responding affirmatively to the question of whether they had ever heard or read about the BioTrust was 6 % in 2011 and 7 % in 2012 (State of the State Survey-60 2011; State of the State Survey-63, 2012).
References
- Botkin JR, Rothwell E, Anderson R, Stark L, Goldenberg A, Lewis M, Wong B. Public attitudes regarding the use of residual newborn screening specimens for research. Pediatrics. 2012;129(2):231–238. doi: 10.1542/peds.2011-0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan A. The ethics of research biobanking. Springer: Dordrecht; London; 2009. [Google Scholar]
- Clayton EW. Informed consent and biobanks. J Law Med Ethics. 2005;33(1):15–21. doi: 10.1111/j.1748-720X.2005.tb00206.x. [DOI] [PubMed] [Google Scholar]
- Couzin-Frankel J. Science gold mine, ethical minefield. Science. 2009;324(5924):166–168. doi: 10.1126/science.324.5924.166. [DOI] [PubMed] [Google Scholar]
- Freelon D. ReCal/Intercoder reliability calculation as a web service. Int J Internet Sci. 2010;5(1):20–33. [Google Scholar]
- Friedman CP, Wong AK, Blumenthal D. Achieving a nationwide learning health system. Sci Transl Med. 2010;2(57):7. doi: 10.1126/scitranslmed.3001456. [DOI] [PubMed] [Google Scholar]
- Health Resources and Services Administration 2011 Considerations and Recommendations for National Guidance Regarding the Retention and Use of Residual Dried Blood Spot Specimens After Newborn Screening. (2011). (Briefing Paper). Retrieved from: http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/recommendations/correspondence/briefingdriedblood.pdf
- HHS.gov (2013) "Regulatory Changes in ANPRM." http://www.hhs.gov/ohrp/humansubjects/anprmchangetable.html accessed 3 April 2013
- Kaye J, Curren L, Anderson N, Edwards K, Fullerton SM, Kanellopoulou N, Lund D, et al. From patients to partners: participant-centric initiatives in biomedical research. Nat Rev Genet. 2012;13(5):371–376. doi: 10.1038/nrg3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M, Goldenberg A, Anderson R, Rothwell E, Botkin J. State laws regarding the retention and use of residual newborn screening blood samples. Pediatrics. 2011;127(4):703–712. doi: 10.1542/peds.2010-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidz C, Garverich S. What the ANPRM missed: additional needs for IRB reform. J Law Med Ethics. 2013;41(2):390–396. doi: 10.1111/jlme.12050. [DOI] [PubMed] [Google Scholar]
- Mongoven A, McGee H (2012) IRB Review and Public Health Biobanking: A Case Study of the Michigan BioTrust for Health. IRB: Ethics & Human Research [PubMed]
- Murphy J, Scott J, Kaufman D, Geller G, LeRoy L, Hudson K. Public perspectives on informed consent for biobanking. Am J Public Health. 2009;99(12):2128–2134. doi: 10.2105/AJPH.2008.157099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney RS, Moore CA, Ojodu JA, Lindegren ML, Hannon WH. Storage and use of residual dried blood spots from state newborn screening programs. J Pediatr. 2006;148(5):618–622. doi: 10.1016/j.jpeds.2005.12.053. [DOI] [PubMed] [Google Scholar]
- Petrini C. "Broad" consent, exceptions to consent, and the question of using biological samples for research purposes different from the initial collection purpose. Soc Sci Med. 2010;70:217–220. doi: 10.1016/j.socscimed.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Platt J, Platt T, Thiel D, Kardia SLR (2013) ‘Born in Michigan? You’re in the Biobank’: Engaging Population Biobank Participants through Facebook Advertisements. Public Health Genomics 16(4). doi:10.1159/000351451 [DOI] [PMC free article] [PubMed]
- Rothwell E, Anderson R, Botkin J. Policy issues and stakeholder concerns regarding the storage and use of residual newborn dried blood samples for research. Policy Polit Nurs Pract. 2010;11(1):5–12. doi: 10.1177/1527154410365563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell E, Anderson R, Goldenberg A, Lewis MH, Stark L, Burbank M, Botkin JR. Assessing public attitudes on the retention and use of residual newborn screening blood samples: a focus group study. Soc Sci Med. 2012;74(8):1305–1309. doi: 10.1016/j.socscimed.2011.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shickle D (2006) The Consent Problem within DNA Biobanks. Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences 37(3): 503–519. doi:10.1016/j.shpsc.2006.06.007 [DOI] [PubMed]
- Simon CM, L’Heureux J, Murray JC, Winokur P, Weiner G, Newbury E, Zimmerman B. Active choice but not too active: public perspectives on biobank consent models. Genet Med. 2011;13(9):821–831. doi: 10.1097/GIM.0b013e31821d2f88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- State of the State Survey-60 (2011) Retrieved from MSU Institute for Public Policy and Social Research: http://www.ippsr.msu.edu/SOSS
- State of the State Survey-63 (2012) Retrieved from MSU Institute for Public Policy and Social Research: http://www.ippsr.msu.edu/SOSS
- Tarini BA, Goldenberg A, Singer D, Clark SJ, Butchart A, Davis MM. Not without my permission: parents’ willingness to permit use of newborn screening samples for research. Public Health Genomics. 2010;13(3):125–130. doi: 10.1159/000228724. [DOI] [PubMed] [Google Scholar]
- Terry SF, Shelton R, Biggers G, Baker D, Edwards K (2013) The haystack is made of needles Genetic testing and molecular biomarkers 17(3):175–177. doi:10.1089/gtmb.2012.1542 [DOI] [PubMed]
- Terry SF, Terry PF, (2011) Power to the people: Participant ownership of clinical trial data. Sci Transl Med 3(69):69cm3 [DOI] [PubMed]
- Wendler D. One-time general consent for research on biological samples. BMJ. 2006;332(7540):544–547. doi: 10.1136/bmj.332.7540.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BA, Wolf LE. Biobanking, consent, and certificates of confidentiality: does the ANPRM muddy the water? J Law Med Ethics. 2013;41(2):440–453. doi: 10.1111/jlme.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 108 kb)
(PDF 398 kb)