Abstract
The objective of this study was to assess attitudes and opinions of women declining the offer of cystic fibrosis (CF) carrier screening through a population-based programme in Victoria, Australia. Between December 2009 and May 2011, women declining an offer of CF carrier screening were invited to participate in a questionnaire-based study. Recruitment was at two private obstetric ultrasound clinics and two private obstetric practices in Melbourne. Of the participants (n = 54), the majority were well educated (76%), aged 30–34 years (54%), with a household income of >AUD$100,000 (76%). Compared to those who accepted screening (reported in a previous study) (Ioannou et al., Public Health Genomics 13:449–56, 2010), knowledge levels were significantly lower in participants declining screening (t = 3.32, p < 0.01). The main reasons for declining screening were having no family history of CF (58%) and not considering a termination of pregnancy for CF (53%). Providers and consumers should be informed that most children born with autosomal-recessive conditions such as CF have no family history of the condition.
Keywords: Cystic fibrosis, Carrier screening, Population screening, Attitudes
Introduction
Cystic fibrosis (CF) is the most common, severe autosomal recessive disease in Caucasians, with about 1 in 2,500 live births affected and a carrier frequency of 1 in 25 (Southern et al. 2007). CF is characterised by chronic suppurative lung disease and pancreatic exocrine insufficiency, with a life expectancy of 30–40 years (Rowe et al. 2005). There is currently no cure for CF. Treatment involves time-consuming daily therapies including chest physiotherapy, antibiotics, pancreatic enzymes, a controlled diet and lung transplantation for some (O'Sullivan and Freedman 2009).
CF results from mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. To date, more than 1,800 alterations have been identified with the most common mutation being p.F508del, which accounts for approximately 70% of all mutations in individuals from Northern Europe (CFTR Mutation Database 2008). Since the discovery of the gene in 1989, screening for carriers of CF has been possible.
CF carrier screening involves testing healthy, unaffected individuals or couples to determine if they are heterozygous carriers of specific CFTR mutations that could be inherited by their children. Screening offers couples, where both partners are carriers, reproductive choices regarding the birth of a child with CF. There are two approaches to carrier identification: cascade testing and population screening. Cascade testing is the testing of individuals who have a family history of CF and are therefore at increased risk of being a carrier. This method of testing is widely accepted and utilised worldwide. However, it has been shown that more than 95% of carriers have no family history of CF (Boulton et al. 1996). Population screening aims to educate and test as many individuals as possible regardless of whether or not they have a family history of the disease.
In 1999, the National Institutes of Health recommended that CF carrier screening should be offered to all couples planning a pregnancy and seeking prenatal care (Grody et al. 2001). Subsequently, the American College of Obstetricians and Gynecologists and the American College of Medical Genetics released statements supporting the availability of population carrier screening for CF, recommending that all pregnant women and couples planning a pregnancy should be offered CF screening (Grody et al. 2001). In Australia, similar recommendations were made with the Human Genetics Society of Australasia stating that all pregnant couples and couples planning a pregnancy should be made aware of the availability of carrier screening for CF (Human Genetics Society of Australasia 2011).
In the state of Victoria, Australia, a population-based CF carrier screening programme was implemented in 2006. The programme offers screening to women and couples before or during the early stages of pregnancy via private obstetricians and general practitioners. It is currently a fee-for-service programme with each test costing AUD$220. During the first 3 years of the programme (2006–2008), 3,200 individuals were screened, all partners of carriers were screened and carrier couples used the information received to make reproductive decisions (Massie et al. 2009). In 2008, we conducted a study exploring the attitudes and outcomes of individuals who accepted CF carrier screening, excluding carrier couples, through this programme (Ioannou et al. 2010). The programme was found to be adequate with relatively high knowledge retention and recall of carrier status. There was no difference in the level of anxiety between carriers and non-carriers and many carriers passed on information about the risk of carrier status to other family members (Ioannou et al. 2010).
The aim of this study was to explore the attitudes of pregnant women who declined an offer of CF carrier screening and compare these to the attitudes of individuals who accepted CF carrier screening from our previous study (Ioannou et al. 2010).
Materials and methods
The CF carrier screening programme
The CF carrier screening programme in Victoria, Australia, is conducted by Genetic Health Services Victoria (GHSV), and screening is offered to women and couples before or during the early stages of pregnancy by private obstetricians and general practitioners. Pre-test advice and information on CF and screening is provided by the offering doctor, information brochure and the programme website (www.cfscreening.com.au).
The brochure outlines the clinical features of CF and the risk of an individual from the general population being a carrier, and having a baby with CF. It explains that there is a test to determine carrier status and when is the most beneficial time to have carrier screening. It also provides details on the interpretation of results including residual risk (meaning those with a negative result do not carry one of the common CFTR mutations tested, and their risk of being a carrier is greatly reduced but not eliminated) as well as the implications of being identified as a carrier couple.
The test screens for 12 of the most common CFTR mutations and is conducted using a check brush swab at a cost of AUD$220, with no government or health insurance rebate.
Participants
Participants were recruited using two different methods:
Women were approached in the waiting room of two private obstetric ultrasound clinics in Melbourne, Victoria. Those who had received an offer of CF carrier screening and declined the offer were invited to participate in the study.
Two obstetricians who have private practices and offered CF carrier screening to their patients through the GHSV programme invited patients to participate in the study upon declining a direct offer of screening.
Questionnaire
The purpose-designed questionnaire assessed the following domains: demographic variables, knowledge of CF and CF carrier screening, reasons for declining screening, satisfaction with the decision to decline screening, evaluation of carrier screening information provided and attitude towards CF carrier screening. The knowledge questions and factors influencing the decision to decline screening were sourced from the questionnaire used in the previous study of people who accepted the offer of carrier screening, to allow for comparison (Ioannou et al. 2010). The questionnaire can be viewed at http://www.mcri.edu.au/cfscreening.
The questionnaires were completed anonymously, either in the waiting room and handed to the researcher or returned via a reply paid envelope. As details of the people who were given questionnaires to return by post were not recorded, further contact was not possible if the questionnaire was not returned.
There were 15 statements regarding knowledge about CF and carrier screening requiring one of three responses: true, false or unsure. Answers were scored as being correct or incorrect, with unsure being scored as incorrect. The total knowledge score for each participant was calculated as the sum of correct responses.
The decisional scale used in the questionnaire was the validated Decision Regret Scale (Brehaut et al. 2003).
Comparison group
The results of the current study were compared to a previous study involving 47 carriers and 65 non-carriers, exploring the characteristics of individuals who chose to have CF carrier screening and their attitudes towards carrier screening (Ioannou et al. 2010).
Analysis
Data analysis was conducted using SPSS (Windows, version 17.0; SPSS Inc., Chicago, IL, USA). Preliminary descriptive analysis generated frequency data to elicit the description of participants.
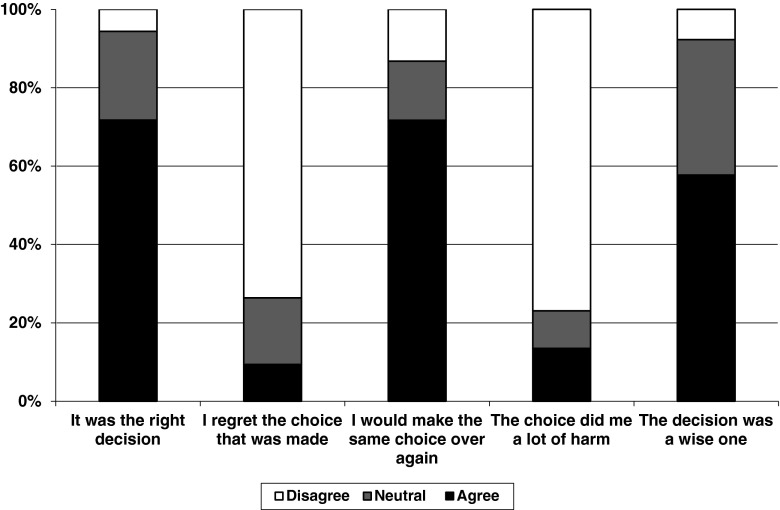
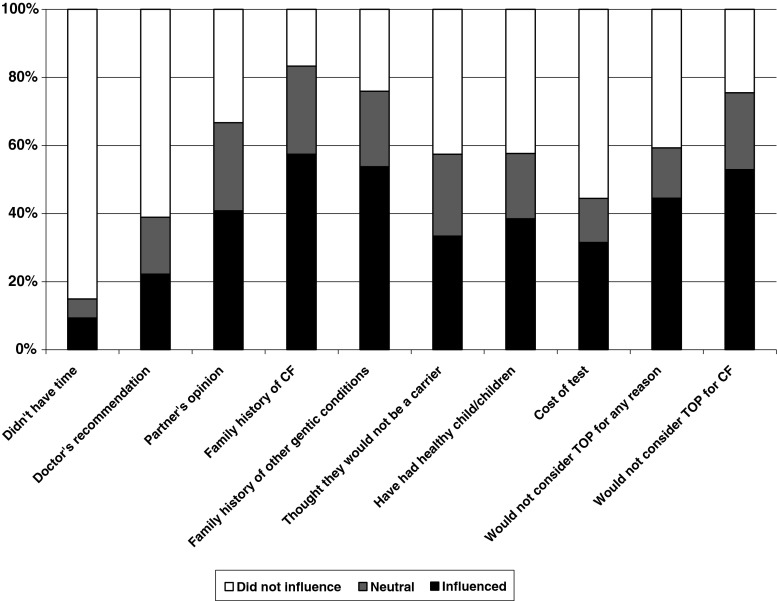
Factors influencing the decision to decline screening were measured on 5-point Likert scales. For analysis, points ‘1’ and ‘2’ were combined to form the category ‘did not influence’, the middle point ‘3’ remained neutral, while points ‘4’ and ‘5’ were combined to form the category ‘influenced’. Satisfaction with the decision to decline screening was assessed according to the level of agreement with five statements (listed in Fig. 4), using 5-point Likert scale responses. For analysis, points ‘1’ and ‘2’ were combined to form the category ‘agree’, the middle point ‘3’ remained neutral, while points ‘4’ and ‘5’ were combined to form the category ‘disagree’.
Fig. 4.
Satisfaction with decision to decline CF carrier screening
The data from the current study of participants who declined screening were compared with data from the previous study evaluating the attitudes and outcomes for individuals who had accepted screening (Ioannou et al. 2010). Analysis of categorical variables was undertaken using χ2 analysis, and, for continuous variables, differences in means between groups were assessed using t tests. A p < 0.05 was considered statistically significant.
Ethics committee approval
This study was approved by the Human Research Ethics Committee of the Department of Human Services, Victoria, Australia (HREC 15/05).
Results
Response
Between December 2009 and May 2011, a total of 308 women were approached in the waiting room of two private obstetric ultrasound clinics, of whom only 69 (22%) had been offered CF carrier screening. Of these women, 33 declined the offer of screening and were invited to participate in the study. Twenty-nine completed questionnaires were received, giving a response rate of 88%.
In addition, 25 completed questionnaires were received, over a period of 5 months, recruited by the participating obstetricians. No response rate was able to be recorded for this method of recruiting as the number of women provided with the questionnaire was not reported. Overall, 54 completed questionnaires were received and used in the analysis.
Demographic variables
The demographic features of those who declined screening and those who accepted screening are presented in Table 1. All participants were female with 29 (54%) aged between 30 and 34 years, 41 (76%) having a university degree and 40 (76%) having an annual household income of more than AUD$100,000 per annum. All participants had a partner and were pregnant at the time of receiving the offer of CF carrier screening.
Table 1.
Demographics of participants who were offered screening through the GHSV CF carrier screening programme
| Demographic | Categories | Number of participants (%) | ||
|---|---|---|---|---|
| Accepted (n = 112) | Declined (n = 54) | Significance (χ²) | ||
| Gender | Male | 3 (2.7) | 0 (0.0) | 1.47, p = 0.22 |
| Female | 109 (97.3) | 54 (100.0) | ||
| Age (in years) | 25–29 | 9 (8.2) | 5 (9.3) | 12.16 (df = 3), p = 0.01* |
| 30–34 | 32 (29.1) | 29 (53.7) | ||
| 35–39 | 54 (49.1) | 19 (35.2) | ||
| 40+ | 14 (12.7) | 1 (1.9) | ||
| Highest completed level of education | Secondary/trade/apprenticeship | 10 (9.3) | 3 (5.7) | 0.89 (df = 3), p = 0.83 |
| College certificate or diploma | 20 (18.7) | 9 (16.7) | ||
| University degree | 75 (70.1) | 41 (75.9) | ||
| Other | 2 (1.9) | 1 (1.9) | ||
| Occupation | Managerial | 30 (28.3) | 17 (32.1) | 2.43 (df = 4), p = 0.66 |
| Professional | 46 (43.4) | 22 (41.5) | ||
| Office duties | 13 (12.3) | 4 (7.5) | ||
| Skilled/trades | 16 (15.1) | 8 (15.1) | ||
| Unskilled | 1 (0.9) | 2 (3.8) | ||
| Household income (in AUD$1000s) | <60 | 7 (9.6) | 1 (1.9) | 2.14 (df = 3), p = 0.54 |
| 61–80 | 10 (9.6) | 4 (7.5) | ||
| 81–100 | 14 (13.5) | 8 (15.1) | ||
| >100 | 70 (67.3) | 40 (75.5) | ||
| Ethnicity | Australian | 62 (56.9) | 23 (43.4) | 7.37 (df = 3), p = 0.06 |
| North European | 28 (25.7) | 11 (20.8) | ||
| South European | 13 (11.9) | 15 (28.3) | ||
| Other | 6 (5.5) | 4 (7.5) | ||
| Affinity with a religion | Yes | 45 (41.3) | 28 (51.9) | 1.51, p = 0.22 |
| No | 63 (58.7) | 26 (48.1) | ||
| Partner at time of testing | Yes | 107 (98.2) | 54 (100.0) | 1.00, p = 0.32 |
| No | 2 (1.8) | 0 (0.0) | ||
| Pregnant at time of testing | Yes | 90 (82.6) | 54 (100.0) | 10.65, p = 0.00* |
| No | 19 (17.4) | 0 (0.0) | ||
| Number of children at time of testing | 0 | 31 (29.0) | 16 (29.6) | 0.28 (df = 3), p = 0.96 |
| 1 | 50 (46.7) | 26 (48.1) | ||
| 2 | 20 (18.7) | 10 (18.5) | ||
| 3 or more | 6 (5.6) | 2 (3.7) | ||
*p < 0.05 for comparison of proportions in current versus previous study using χ 2 test
Those who declined the offer of screening were significantly younger in age, with the majority in the 30–34 age group, compared to those who accepted the offer of screening, where the majority were in the 35+ age group (χ2 = 12, p < 0.01, df = 3).
Knowledge about cystic fibrosis and screening
Participants were asked to select a response to 15 knowledge statements regarding CF and carrier screening. Twenty-five (47%) of the participants selected the correct response to 10 or more of these knowledge statements. There were five knowledge statements for which <50% of participants selected the correct response. These were the following: (1) CF affects more males than females (false); (2) couples who have a child with CF usually have a family history of this condition (false); (3) CF genetic test can identify all CF carriers (false); (4) if only one partner is identified as a carrier, there is still a small chance of having a child with CF (true); and (5) if no gene change is found the person cannot be a carrier of CF (false). With the exception of knowledge statement (4), <50% of participants who accepted screening selected the correct response for these statements as well (Ioannou et al. 2010).
Knowledge of CF and CF carrier screening was significantly lower in those who declined screening compared to those who accepted screening (t = 3.32, p < 0.01; Fig. 1).
Fig. 1.
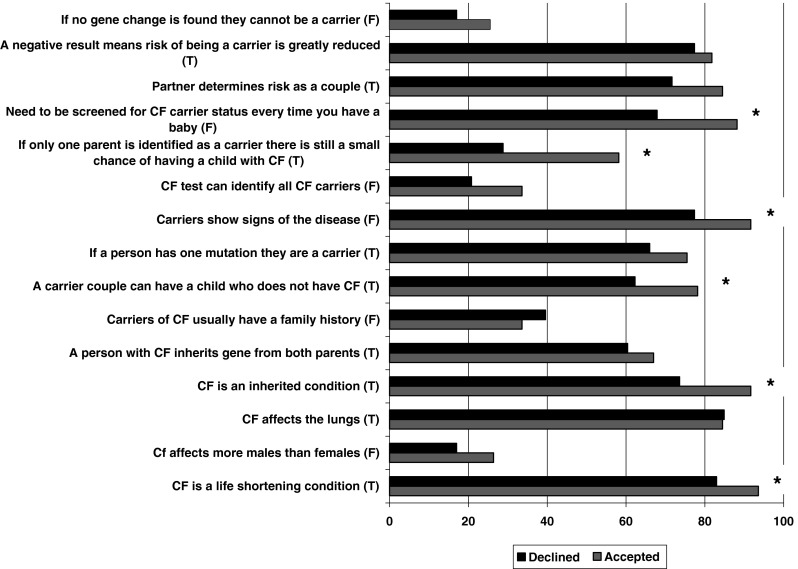
Comparison of CF and CF screening knowledge of participants who declined and accepted screening. *p < 0.05 for comparison of percent correct in current versus previous study using χ 2 test
Factors influencing the decision to decline screening
Participants were asked to rate factors that might have influenced their decision to decline CF carrier screening on a Likert scale. The factors most commonly rated as influencing the decision to decline screening were having no family history of CF and having no family history of other genetic conditions, chosen by 31 (58%) and 29 (54%) participants, respectively. Believing that they would not consider a termination of pregnancy for CF was identified as an influential factor for 24 (45%) participants. Thirty-three (61%) and 46 (84%) participants, respectively, stated that their doctor’s recommendation or lack of time did not influence their decision to decline screening (Fig. 2).
Fig. 2.
Influence of various factors in the decision to decline CF carrier screening
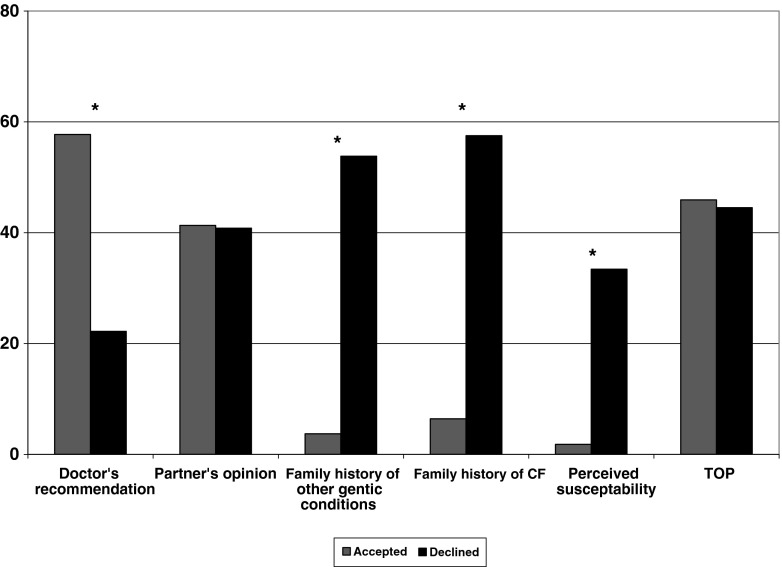
Three factors were considered to be influential in the decision by a significantly greater proportion of those who declined screening than those who accepted screening. These were (1) family history of CF (χ2 = 83, p < 0.01, df = 2), (2) family history of other genetic conditions (χ2 = 79, p < 0.01, df = 2) and (3) perceived susceptibility of being a carrier of CF (χ2 = 43, p < 0.01, df = 2). Doctor’s recommendation was an influencing factor more often amongst those who had screening than those who declined screening (χ2 = 18, p < 0.01, df = 2; Fig. 3).
Fig. 3.
Comparison of factors that influenced the decision to decline or accept CF carrier screening. *p < 0.05 for comparison of proportions in current versus previous study using χ 2 test
Twenty (37%) participants who declined screening believed that a reasonable price to pay for CF carrier testing is between AUD$50 and AUD$100, and 17 (32%) thought the test should be free. Only nine (16.7%) participants indicated that over AUD$100 is a reasonable price to pay.
Satisfaction with decision to decline screening
Participants were asked to rate their feelings in regards to their decision not to have CF carrier screening (Fig. 4). Thirty-eight (72%) participants felt they had made the right decision, 30 (58%) felt their decision was a wise one and 38 (72%) stated they would make the same choice if they had to do it over again. However, seven (14%) participants felt that their decision did them a lot of harm, and five (9%) regretted the choice that they made.
Pre-test Information
Forty-one (76%) participants believed they had enough information to make the decision to decline screening, with 32 (60%) stating that they received the bulk of their information from their doctor followed by the brochure (32%). Forty (80%) participants were satisfied with the information provided with only 11 (20%) seeking further information—the main source of further information was family and friends (55%) followed by their doctor (36%). None of the participants viewed the GHSV CF carrier screening programme website.
Attitude towards CF carrier screening
Thirteen (24%) participants wished to be offered testing at another time—of these, 72% stated that they would have liked to be offered testing before pregnancy. Fifty-one (95%) participants believe that CF carrier screening should be available to those who wish to have it, with two participants being unsure and one stating that it should not be.
Discussion
This study explored the reasons why pregnant women chose to decline an offer of population-based CF carrier screening, and compared these to the factors that influenced women to accept screening as determined in a previous study (Ioannou et al. 2010). All participants in this study were women who were pregnant and had a partner at the time of receiving an offer for CF carrier screening. The most common reason for declining screening was a lack of family history of CF or other genetic conditions. Most participants were satisfied with the information provided; however, 24% of participants wished that they had been offered screening at another time, and 95% of participants believe CF carrier screening should be available to those who wish to have it.
The majority of participants were well educated with higher than average income, reflecting the private health setting in which screening is currently offered in Victoria, Australia. Women who declined screening were significantly younger than those who accepted screening (Ioannou et al. 2010). Other studies have found that women who have had previous healthy children are less likely to accept the offer of screening (Fries et al. 2005). A study conducted in Western Australia found that individuals without children were 50% more likely to have screening than those with children (Honnor et al. 2000). In our study, we found no difference in the number of children between those who declined screening and those who accepted it, although having previous healthy children was chosen as an influencing factor by a significantly greater proportion of participants who declined screening than of those who accepted it.
Participants who declined screening had significantly less knowledge in relation to CF and CF screening than acceptors. This effect is not due to the difference in time interval between being offered screening and completing the questionnaire, with those who declined screening completing the questionnaire a maximum of 4 weeks after receiving an offer of screening compared to those who accepted screening with the majority completing the questionnaire more than 12 months after being tested (Ioannou et al. 2010).
Studies have shown that knowledge level prior to participating in screening is low, with individuals being unaware that CF: is an inherited condition, affects the lungs, majority of carriers have no family history and carriers do not show symptoms of the disease (Decruyenaere et al. 1992; Magnay et al. 1992; Williamson et al. 1989; Grody et al. 1997; Hill et al. 1995). Lack of knowledge appears to be a significant factor in the decision to decline screening, with the main reason for declining screening being the lack of a family history of CF or family history of other genetic conditions. However, the majority of carriers of CF and children born with CF in fact have no known family history of the condition (Boulton et al. 1996). A study from Canada had similar findings, with participants who declined screening stating that the main reason for declining screening was having no family history of CF (O'Connor and Cappelli 1999).
The current programme is inequitable with the test only being offered in the private health system to those willing to pay. Reports from other screening programmes suggest that the cost of screening is a significant factor in the decision whether to have screening, yet this did not appear to be a major factor in our study (Durfy et al. 1994; Barlow-Stewart et al. 2003). Although the majority of participants felt that a reasonable price to pay for the test would be between AUD$50–100, the cost of the test was not stated as an influencing factor in the decision to have screening by two thirds of participants. This is most likely due to the setting in which screening is currently offered, with women in the private health system, on average, having a significantly higher household income than those in the public health system (Australian Bureau of Statistics 2012). Nevertheless, it could be reasonably anticipated that if screening were free, then uptake would be higher.
While education about CF and carrier status and equity of access are important factors likely to increase the uptake of CF carrier screening, many pregnant women will still choose not to have screening even if they are well informed and the test is free, as many would not consider a termination of pregnancy in the event of a CF diagnosis. While there was no significant difference in affiliation with religion between acceptors and decliners, not considering a termination of pregnancy for CF was an influencing factor in the decision to decline screening for a large proportion of participants. Similarly, another study found that non-pregnant women often cited that abortion and religious beliefs are important factors in the decision whether or not to have screening (Clayton et al. 1996). In a number of studies that explored the reasons for declining CF carrier screening, all found that a main reason for declining screening was not intending to terminate a pregnancy (Cuckle et al. 1996; Levenkron et al. 1997; Livingstone et al. 1993; Loader et al. 1996; Mennie et al. 1992).
Of the participants who wished to be offered testing at another time, the majority would have liked to receive an offer of screening prior to pregnancy. While preconception screening is believed to be the best time to offer screening, as identification of carrier couples preconceptionally provides the most reproductive options as well as giving couples more time to make reproductive decisions compared to prenatal screening, it has been associated with lower uptake than prenatal screening (Ioannou et al. 2010). This is due to lack of interest at this life stage, lack of preconception health care setting in which to offer screening and a large number of unplanned pregnancies (Poppelaars et al. 2003).
Health professionals are often the gatekeepers of screening, and their attitudes, opinions and knowledge regarding screening are significant in the effectiveness of offering population-based screening for CF carrier status. A recent study by Stark et al. (2013) showed that barriers identified by Australian obstetricians in regard to routinely offering genetic (not just CF) carrier screening were: time constraints, costs and availability of supporting services. A lack of knowledge and experience in regard to CF and genetic screening has also been indicated as a barrier, with health professionals not feeling confident in their ability to provide screening to their patients, which may contribute to women’s reasons for declining screening.
There are a few limitations to this study. The screening programme is currently only offered in the private health sector, and not every obstetrician informs his or her patients of the availability of CF carrier screening. Participants were all sourced from relatively few obstetricians, with approximately half of the participants being recruited from a single obstetric clinic. This has implications for the results, as there would be limited variability in the way information about the test was provided to participants. In addition, there is a lack of information on the consistency of pre-test counselling with the offer of screening by obstetricians ranging from an oral discussion and direct offer to including the screening programme brochure in a pre-pregnancy information pack with no direct offer of screening. The response rate for recruitment at the ultrasound clinics was 88%, which is very high for this type of study, and is likely to be representative of the women who declined screening in this programme, as the majority of private obstetricians offering CF carrier screening refer their patients to these clinics.
In conclusion, the main factor affecting uptake of CF screening is lack of knowledge regarding the inheritance patterns of recessive genetic conditions. In order to increase uptake of CF carrier screening and facilitate informed decision making, the programme needs to focus more on informing and educating both providers and consumers and ensuring equity of access by offering the test in the public as well as the private health sector, at a lower cost.
Acknowledgements
MBD is a National Health and Medical Research Council Practitioner Fellow. SL is funded by a National Health and Medical Research Council Capacity Building Grant in Population Health. This study was supported by the Victorian Government's Operational Infrastructure Support Program. We thank Drs Andrew Ngu, Lionel Steinberg and Chester Yeoh for assistance with subject recruitment.
Conflict of interest
There is no conflict of interest for any of the authors on this paper.
Abbreviations
- CF
Cystic fibrosis
- CFTR
Cystic fibrosis transmembrane conductance regulator
- GHSV
Genetic Health Services Victoria
References
- Australian Bureau of Statistics. (2012) Household income and income distribution Australia 2009–2010. AusStats2011, 6523.0. http://www.ausstats.abs.gov.au/ausstats/subscriber.nsf/0/DBE855896D8CA36DCA2578FB0018533C/$File/65230_2009-10.pdf. Accessed 2 Jul 2012
- Barlow-Stewart K, Burnett L, Proos A, et al. A genetic screening programme for Tay–Sachs disease and cystic fibrosis for Australian Jewish high school students. J Med Genet. 2003;40:e45. doi: 10.1136/jmg.40.4.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton M, Cummings C, Williamson R. The views of general practitioners on community carrier screening for cystic fibrosis. Br J Gen Pract. 1996;46:299–301. [PMC free article] [PubMed] [Google Scholar]
- Brehaut JC, O’Connor AM, Wood TJ. Variation of a decision regret scale. Med Decis Making. 2003;23:281–292. doi: 10.1177/0272989X03256005. [DOI] [PubMed] [Google Scholar]
- CFTR Mutation Database (2008) www.genet.sickkids.on.ca/cftr/StatisticsPage.html. Accessed 16 Oct 2008
- Clayton EW, Hannig VL, Pfotenhauer JP, Parker RA, Campbell PW, 3rd, Phillips JA., 3rd Lack of interest by nonpregnant couples in population-based cystic fibrosis carrier screening. Am J Hum Genet. 1996;58:617–627. [PMC free article] [PubMed] [Google Scholar]
- Cuckle H, Quirke P, Sehmi I, et al. Antenatal screening for cystic fibrosis. Br J Obstet Gynaecol. 1996;103:795–799. doi: 10.1111/j.1471-0528.1996.tb09876.x. [DOI] [PubMed] [Google Scholar]
- Decruyenaere M, Evers-Kiebooms G, Denayer L, Van den Berghe H. Cystic fibrosis: community knowledge and attitudes towards carrier screening and prenatal diagnosis. Clin Genet. 1992;41:189–196. doi: 10.1111/j.1399-0004.1992.tb03661.x. [DOI] [PubMed] [Google Scholar]
- Durfy SJ, Page A, Eng B, Chang PL, Waye JS. Attitudes of high school students toward carrier screening and prenatal diagnosis of cystic fibrosis. J Genet Couns. 1994;3:141–155. doi: 10.1007/BF01423176. [DOI] [PubMed] [Google Scholar]
- Fries MH, Bashford M, Nunes M. Implementing prenatal screening for cystic fibrosis in routine obstetric practice. Am J Obstet Gynecol. 2005;192:527–534. doi: 10.1016/j.ajog.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Grody WW, Cutting GR, Klinger KW, Richards SC, Watson MS, Desnick RJ. Laboratory standards and guidelines for populationbased cystic fibrosis carrier screening. Genet Med. 2001;3:149–154. doi: 10.1097/00125817-200103000-00010. [DOI] [PubMed] [Google Scholar]
- Grody WW, Dunkel-Schetter C, Tatsugawa ZH, et al. PCR-based screening for cystic fibrosis carrier mutations in an ethnically diverse pregnant population. Am J Hum Genet. 1997;60:935–947. [PMC free article] [PubMed] [Google Scholar]
- Hill R, Stanisstreet M, Boyes E, O'Sullivan H. Public lacks knowledge about genetic testing and gene therapy. BMJ. 1995;311:1370. doi: 10.1136/bmj.311.7016.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honnor M, Zubrick SR, Walpole I, Bower C, Goldblatt J. Population screening for cystic fibrosis in Western Australia: community response. Am J Med Genet. 2000;93:198–204. doi: 10.1002/1096-8628(20000731)93:3<198::AID-AJMG7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Human Genetics Society of Australasia (2011) Cystic fibrosis population screening position paper. Position Paper 2010, PS02. https://www.hgsa.org.au/website/wpcontent/uploads/2010/06/2010PS02-CYSTIC-FIBROSIS-POPULATION-SCREENING1.pdf. Accessed 1 Sept 2011
- Ioannou L, Massie J, Collins V, McClaren B, Delatycki M. Population-based genetic screening for cystic fibrosis: attitudes and outcomes. Publ Health Genomics. 2010;13:449–456. doi: 10.1159/000276544. [DOI] [PubMed] [Google Scholar]
- Levenkron JC, Loader S, Rowley PT. Carrier screening for cystic fibrosis: test acceptance and one year follow-up. Am J Med Genet. 1997;73:378–386. doi: 10.1002/(SICI)1096-8628(19971231)73:4<378::AID-AJMG2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Livingstone J, Axton RA, Mennie M, Gilfillan A, Brock DJ. A preliminary trial of couple screening for cystic fibrosis: designing an appropriate information leaflet. Clin Genet. 1993;43:57–62. doi: 10.1111/j.1399-0004.1993.tb04427.x. [DOI] [PubMed] [Google Scholar]
- Loader S, Caldwell P, Kozyra A, et al. Cystic fibrosis carrier population screening in the primary care setting. Am J Hum Genet. 1996;59:234–247. [PMC free article] [PubMed] [Google Scholar]
- Magnay D, Wilson O, el Hait S, Balhamar M, Burn J. Carrier testing for cystic fibrosis: knowledge and attitudes within a local community. J. R. Coll. Physicians Lond. 1992;26:69–70. [PMC free article] [PubMed] [Google Scholar]
- Massie J, Petrou V, Forbes R, et al. Population-based carrier screening for cystic fibrosis in Victoria: the first three years experience. Aust N Z J Obstet Gynaecol. 2009;49:484–489. doi: 10.1111/j.1479-828X.2009.01045.x. [DOI] [PubMed] [Google Scholar]
- Mennie ME, Gilfillan A, Compton M, et al. Prenatal screening for cystic fibrosis. Lancet. 1992;340:214–216. doi: 10.1016/0140-6736(92)90476-J. [DOI] [PubMed] [Google Scholar]
- O'Connor BV, Cappelli M. Health beliefs and the intent to use predictive genetic testing for cystic fibrosis carrier status. Psychol Health Med. 1999;4:157–169. doi: 10.1080/135485099106298. [DOI] [Google Scholar]
- O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009;373:1891–1904. doi: 10.1016/S0140-6736(09)60327-5. [DOI] [PubMed] [Google Scholar]
- Poppelaars FAM, et al. Possibilities and barriers in the implementation of a preconceptional screening programme for cystic fibrosis carriers: a focus group study. Public Health. 2003;117:396–403. doi: 10.1016/S0033-3506(03)00136-7. [DOI] [PubMed] [Google Scholar]
- Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. New Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- Southern KW, Munck A, Pollitt R, et al. A survey of newborn screening for cystic fibrosis in Europe. J Cyst Fibros. 2007;6:57–65. doi: 10.1016/j.jcf.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Stark Z, Massie J, McClaren BJ et al. (2013) Current practice and attitudes of Australian obstetricians toward population-based carrier screening for inherited conditions. Twin Res Hum Genet 16(2):601–607 [DOI] [PubMed]
- Williamson R, Allison ME, Bentley TJ, et al. Community attitudes to cystic fibrosis carrier testing in England: a pilot study. Prenat Diagn. 1989;9:727–734. doi: 10.1002/pd.1970091008. [DOI] [PubMed] [Google Scholar]