Abstract
Regeneration of large bone losses has been achieved with limited success due to either donor site complications in autogenous bone graft or lack of an ideal biomaterial in the case of allografts. Magnesium calcium phosphate-gelatin sponges were prepared with different concentrations of MCP; namely 0, 50 and 90 wt%. Eight mm defects were drilled in the calvaria of 48 male Fischer 344 rats. MCP-gelatin scaffolds containing or without bone morphogenetic protein were placed at the defect site. Animals were sacrificed at 3 and 12 weeks, post-operatively, with evaluation of bone regeneration by using micro CT and histological examinations. Results showed that the combination of BMP-2 and gelatin sponges could provide a slow release system that significantly enhanced bone regeneration at both 3 and 12 weeks in comparison with the non BMP-2-containing 90 wt% MCP-gelatin sponges. The combination of 50 wt% MCP-gelatin sponges and BMP-2 showed significant bone formation at 3 weeks in comparison with the non BMP-2 containing gelatin sponges, indicating that the addition of MCP to the gelatin scaffold had a synergistic advantage in combination with BMP-2. This novel scaffold has shown adequate porosity to allow cell growth, amenability for sterilization, biocompatibility and biodegradability with the ability to provide a slow release system for BMP-2 to enhance bone regeneration.
Keywords: Bone regeneration, Magnesium calcium phosphate, Gelatin, In vivo, Bone morphogenetic protein-2, Rat, Calvarial defect, MCP-gelatin scaffold
Introduction
Efficacious bone regeneration could revolutionize the clinical management of many bone and musculoskeletal disorders. Bone has the unique M ability to regenerate and continuously remodel itself throughout life. However, clinical situations arise when bone is unable to heal itself, as with segmental bone loss, fracture non-union, and failed spinal fusion. This leads to significant morbidity and mortality. Recent attempts to improve bone healing have met with limited success, fueling the development of improved techniques [1]. Although the autogenous bone graft has been considered the gold standard, it has been associated with many limitations such as increased operative time, limited availability and significant morbidity related to blood loss, wound complications, local sensory loss and, most importantly, chronic pain [2]. To overcome these limitations, different organic and inorganic biomaterials have been proposed such as ceramics, poly lactic acid, poly glycolic acid and gelatin; nevertheless, no ideal material has yet been identified.
Gelatin is one of the materials that has been used extensively in the biomedical field. It is a biodegradable polymer which can be easily chemically modified and is used in pharmaceutical, medical and food applications [3]. Magnesium is the fourth most abundant cation in the human body and is naturally found in bone [4]. It is closely involved in the osteogenic differentiation of cells and bone formation.
Bone morphogenetic protein-2 is one of the most promising cytokines for treating bone disorders; many animal studies on osteoinduction have been performed [5–7]. It has been used clinically in reconstructive surgery for bony defects and augmentation [8]. The clinical application of BMP requires a suitable delivery system for local, defined, and controllable bone formation [9].
Recently, a novel biodegradable scaffold composed of gelatin and magnesium calcium phosphate has been developed by our team. It has shown promising physical, mechanical and biological properties in vitro [10].
To assess clinical use, different concentrations of MCP- gelatin complex, combined with BMP-2, are used to regenerate bone. Large bone defects were created in the skulls because it was assumed that this would reflect patients with severe bone loss, such as in the case of accident or tumor resection. Moreover, MCP-gelatin complex containing BMP-2 sponges were implanted into large rat skull defects. Bone regeneration is evaluated using radiological and histological methods, 3 and 12 weeks afterwards.
Materials and Methods
Materials
Gelatin samples with an isoelectric point (IEP) of 9.0 were kindly supplied by Nitta Gelatin Co., Osaka, Japan. Calcium dihydrogen phosphate, magnesium oxide and calcium chloride were obtained from Nacalai Tesque Ltd., Osaka, Japan. All materials were used without further purification.
Preparation of gelatin sponges incorporating MCP
Gelatin sponges containing different amounts of MCP were prepared according to the method stated by Hussain et al. [10]. Briefly, calcium dihydrogen phosphate and magnesium oxide at a molar ratio of 2:1 were mixed with 3 wt% of gelatin aqueous solution at different weight percentages of 0, 50, and 90 wt%. The mixed solution was agitated at 5,000 rpm for 3 min by using a homogenizer (ED-12, Nihonseiki Co., Tokyo, Japan). The resulting foamy solution was cast into a polypropylene dish of 138–138 cm2 and 5 mm depth and then immediately frozen at −80 °C. Then, the sponges were freeze-dried and dehydrothermally cross-linked at 140 °C for 96 h. Gelatin sponges with or without MCP incorporation were cut into cylinders of 8 mm diameter and 1.25 ± 0.25 mm using a biopsy punch and scalpel (Kai industries co. Ltd., Seki, Japan). Specimens were sterilized with ethylene oxide gas.
SEM of the Sponges
The inner structure of sponges was viewed in a scanning electron microscope (SEM, S2380N, HITACHI, Japan) after sputter coating with gold/palladium. The mean pore diameters of the sponges were calculated by using ImageJ Version 1.43, Wayne Rasband, National institute of Health. The image scale bar was used to ensure standardization of calculations.
Animal Surgery, Euthanasia, and Implant Retrieval
12 h before the operation, 2.5 μg of BMP-2 [11] were added to gelatin sponges containing different concentrations of MCP, namely 0, 50 and 90 wt% of MCP, and they are incubated for about 12 h at 4 °C to allow for complete adsorption of the BMP-2 into the gelatin sponges. As a control, other gelatin sponges similar to those used for BMP-2 were incubated with 1X phosphate buffered saline solution (PBS, pH 7.4) under the same conditions and for the same period.
Twelve-week-old male Fischer 344 rats were purchased from Japan SLC, Inc. (Shizouka, Japan) and kept under pathogen-free conditions in our animal facility. The animal experiments were conducted in accordance with the guidelines of the Kyoto University Animal Research Committee. Forty-eight rats were divided into 3 groups, 16 rats per group, with each group representing a concentration of MCP.
Rats were anesthetized by intra-peritoneal injection of sodium pentobarbital (50 mg/kg of body weight). The hair on the head was shaved with electric clippers. The skin was disinfected with ethyl alcohol 70 %, and a mid-longitudinal 15 mm skin incision was made on the dorsal surface of the cranium. Care was taken to ensure that the periosteum was completely cleared from the surface of the cranial bone by scraping. A trephine bur was used to create a circular 8 mm diameter defect in the rat cranium. The full thickness of the cranial bone was removed and scaffolds were immediately placed in the defect.
The skin over the defect was closed with (4/0) nylon sutures. After 3, and 12 weeks, the animals were euthanized with carbon monoxide, and the implants with the surrounding native bone were retrieved. The retrieved specimens were fixed with 10 % neutral buffered formalin (pH 7.4).
Micro-Computed Tomography Imaging—Animal Specimens
The harvested cranial bone defects were imaged using high resolution micro-computed tomography (micro-CT, SMX-100CT SHIMADZU, Kyoto, Japan). Each specimen was fixed vertically by the sample holder and placed in the micro-CT specimen chamber. The scanner voltage was set at 34 kV and current at 30 mA with no aluminum filter in place. Serial coronally oriented tomograms were reconstructed from the raw images. The number of slices was set at 25 slices. High resolution 3D images were constructed using the VGStudio MAX Reconstruction software package (Volume Graphics GmbH, Heidelberg, Germany). The bone regenerated at the 8 mm defect was scored according to the criteria listed in Table 1.
Table 1.
Scoring guide for extent of bony bridging and union obtained using maximum intensity projections of microcomputed tomography datasets
| Healing score | Calvarial bone regenerated |
|---|---|
| 0 | No bone regenerated |
| 1 | Bone spicules |
| 2 | = or < than 25 % of the bone regenerated |
| 3 | = or < than 50 % of the bone regenerated |
| 4 | = or < than 75 % of the bone regenerated |
| 5 | More than 75 % of the bone regenerated |
Histological Processing
After micro CT scanning, the implants were decalcified via incubation in a solution (10 % EDTA and 4 % phosphate buffered formalin at a 7:4 ratio). After decalcification, the specimens were dehydrated in a graded series of ethanol solutions (70–100 %) followed by xylene, and embedded in paraffin. Histological sections 10 μm thick were prepared from the middle of the sample using a microtome (Leica RM2165, Wetzlar, Germany). Three sections per sample were stained with hematoxylin and eosin (H&E) and viewed using an OLYMPUS AX80 (OLYMPUS CO., Ltd., Tokyo, Japan).
Statistical Analysis
The bone regeneration scores at the defect site obtained from the 3D images were analyzed. Analysis of variance was used to identify if there were significant differences among groups (p < 0.05). The Tukey-Kramer multiple comparison test was then conducted to identify the specific groups that showed statistically significant differences. Graph pad prism 5 software (Graph pad software, CA., USA) was used to conduct the statistical analysis.
Results
Physical Appearance of the Sponges
All sponges showed a similar basic structure in terms of porosity and pore connectivity. The sponges revealed different size pores (Fig. 1). The mean pore diameter of the gelatin sponges of 0, 50 and 90 wt% of MCP were 153.0 ± 60.72, 139.0 ± 37.1 and 143.0 ± 48.2, respectively. No significant difference was observed in pore diameter among the groups.
Fig. 1.
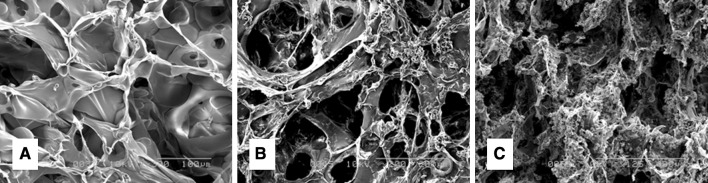
Scanning electron micrographs of gelatin sponges incorporating 0 (A), 50 (B) and 90 wt% of MCP (C)
Bone Regeneration Evaluation
All animals had an uneventful recovery. No infection occurred during the healing.
The effect of the scaffolds with or without BMP-2 on bone formation was evaluated with an 8 mm cranial size defect over 3 and 12 weeks periods.
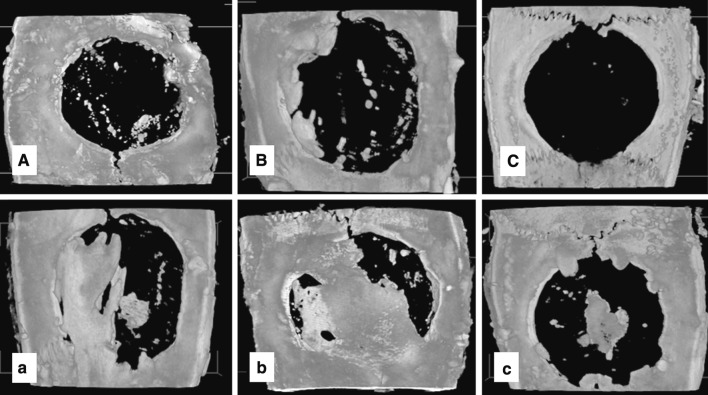
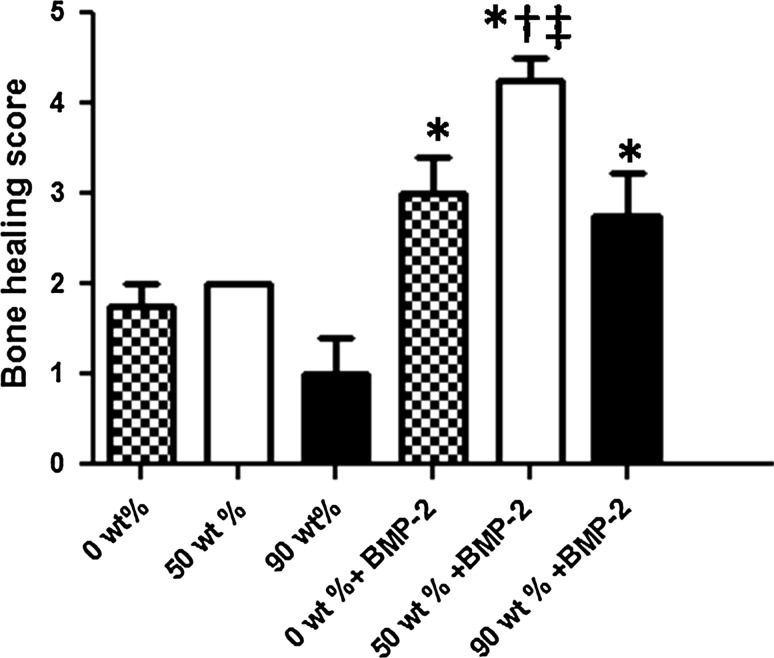
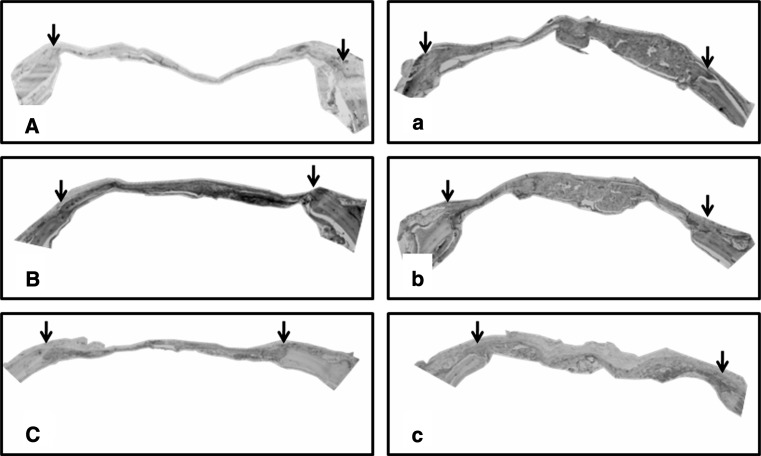
At the 3 week period, the combination of 50 wt% MCP-containing gelatin sponges and BMP-2 showed significant bone regeneration in comparison with the 0, 50 and 90 wt% MCP-containing gelatin sponges without BMP-2 incorporation. On the other hand, the 0 and 90 wt% MCP-gelatin sponges containing BMP-2 showed significance in bone formation in comparison with the non BMP-2-containing 90 wt% MCP-gelatin sponges (Figs. 2, 3).
Fig. 2.
Representative images of maximum intensity projections of rat cranial defects obtained by micro CT at 3 weeks post-operatively. Group (A), (B) and (C), represents cranial defects implanted with 0, 50 and 90 wt% MCP gelatin sponges, without BMP-2, respectively. Group (a), (b) and (c), represents cranial defects implanted with 0, 50 and 90 wt% MCP gelatin sponges containing BMP-2, respectively
Fig. 3.
Average bone union score within the defect as measured from maximum intensity projections obtained by micro CT at 3 weeks. The results are expressed as mean ± SD. *, p < 0.05; Significant against 90 wt% gelatin sponges. †, p < 0.05; Significant against 0 wt% gelatin sponges. ‡, p < 0.05; Significant against 50 wt% gelatin sponges
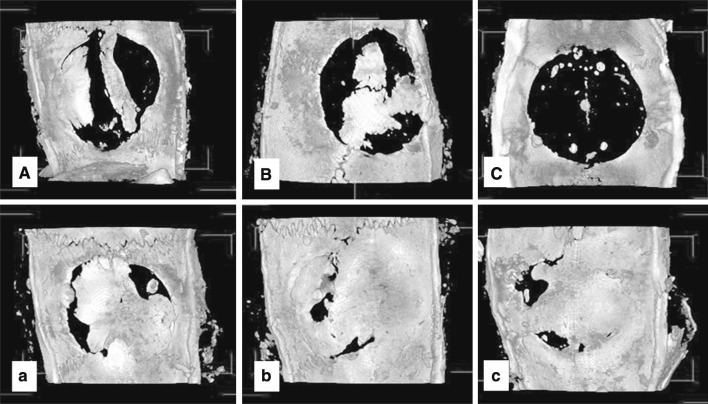
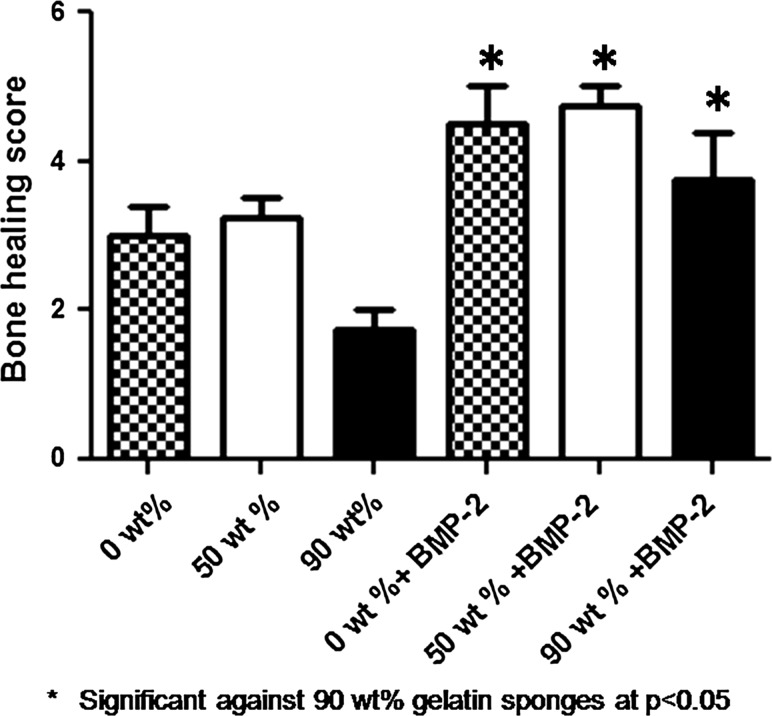
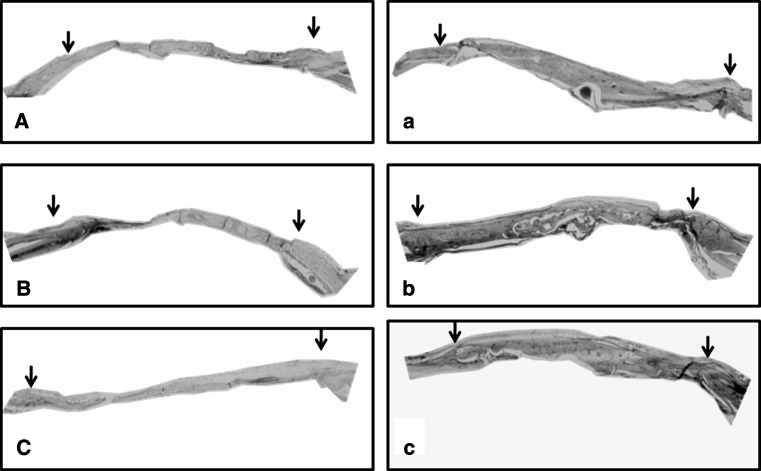
At 12 weeks, the calvarial defects implanted with BMP-2-containing gelatin sponges showed significant bone formation in comparison with the calvarial defects implanted with the non-BMP-2-containing 90 wt% MCP gelatin sponges (Figs. 4, 5).
Fig. 4.
Representative images of maximum intensity projections of rat cranial defects obtained by micro CT at 12 weeks post-operatively. Group (A), (B) and (C) represents cranial defects implanted with 0, 50 and 90 wt% MCP gelatin sponges, without BMP-2, respectively. Group (a), (b) and (c), represents cranial defects implanted with 0, 50 and 90 wt% MCP gelatin sponges containing BMP-2, respectively
Fig. 5.
Average bone union score within the defect as measured from maximum intensity projections obtained by micro CT at 12 weeks. The results are expressed as mean ± SD. *, p < 0.05; Significant against 90 wt% gelatin sponges
Histological Evaluation
Histological analysis was performed on the samples to further investigate the tissue response to the implanted sponges.
At 3 weeks, all groups showed signs of inflammation which was characterized by the presence of inflammatory cells at the sponge site (Fig. 6). The combination of BMP-2 and 0 or 50 wt% gelatin sponges resulted in more bone regeneration in comparison with the 90 wt% gelatin sponges. The gelatin sponges without BMP-2 did not show bone formation at the defect site (Fig. 7).
Fig. 6.
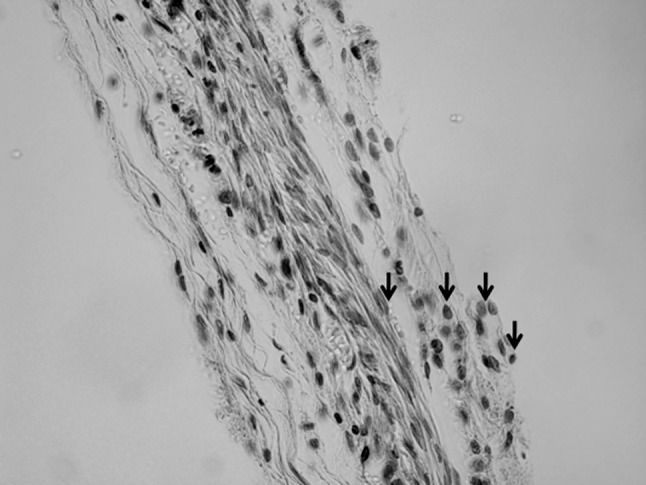
Representative histological sections of sample stained with hematoxylin and eosin 3 weeks post-operatively at ×40 magnification. Black arrows refer to the inflammatory cells
Fig. 7.
Representative histological sections of samples stained with hematoxylin and eosin 3 weeks post-operatively at ×1.25 magnification. Group (A), (B) and (C), represents cranial defects implanted with 0, 50 and 90 wt% MCP gelatin sponges, without BMP-2, respectively. Group (a), (b) and (c), represents cranial defects implanted with 0, 50 and 90 wt% MCP gelatin sponges containing BMP-2, respectively. Black arrows refer to the borders of the defect site
At 12 weeks, calvarial defects implanted with BMP-2-containing gelatin sponges showed almost complete bone regeneration in comparison with those implanted with the non BMP-2-containing gelatin sponges, which showed little bone formation (Fig. 8).
Fig. 8.
Representative histological sections of samples stained with hematoxylin and eosin 12 weeks post-operatively at ×1.25 magnification. Group (A), (B) and (C), represents cranial defects implanted with 0, 50 and 90 wt% MCP gelatin sponges, without BMP-2, respectively. Group (a), (b) and (c), represents cranial defects implanted with 0, 50 and 90 wt% MCP gelatin sponges containing BMP-2, respectively. Black arrows refer to the border to the defect site
Discussion
The use of magnesium calcium phosphate with gelatin has shown promising results in vitro. In this study, we tried to clarify the effect of the MCP concentration in the gelatin sponge on the osteoinductive ability of BMP-2. Furthermore, to our knowledge this is the first study to investigate the relation between MCP concentrations in gelatin sponges on the BMP-2 action in terms of bone formation in vivo.
The addition of MCP to the gelatin during the sponge preparation phase resulted in a uniform distribution of the mineral throughout the scaffold, with no precipitation of the mineral away from the gelatin. Additionally, the mineral did not affect the pore size diameter of the gelatin scaffold, as there was no significant difference in pore size diameter among groups (Fig. 1).
The main role of the delivery system for BMP-2 is to retain the factor at the site for a prolonged period of time [12]. At 3 weeks, (Figs. 2, 3, 7), the 0 wt% MCP-gelatin sponges containing BMP-2 showed a significant difference in terms of bone formation in comparison with the non BMP-2-containing 90 wt% MCP-gelatin sponges. This proves that the cross linked gelatin sponges have the ability to slowly release BMP-2 in a manner that induces bone regeneration in vivo [13, 14]. Moreover, the combination of 50 wt% MCP-gelatin sponges and BMP-2 showed significant bone regeneration both on Micro CT and histological slides in comparison with all non BMP-2 containing gelatin sponges. This synergistic combination between MCP and BMP-2 can be explained by the fact that magnesium at low concentration enhances endothelial cell migration, proliferation and differentiation [15]. These endothelial cells are important in new blood vessels formation, which in turn is important for providing essential nutrients to newly forming bone cells. Additionally, magnesium has been shown to improve the osteogenic differentiation of cells, and indirectly, bone formation [16, 17]. Moreover, our previous results showed that 50 wt% MCP gelatin sponges have improved mesenchymal cellular proliferation and alkaline phosphatase activity in comparison with the 0 wt% gelatin sponges and improved osteocalcin activity in comparison with the 90 wt% MCP gelatin sponges. In other words, the 50 wt% gelatin sponges showed the best characteristics to enhance the early and late osteogenic differentiation of mesenchymal stem cells [10].
At 12 weeks, (Figs. 4, 5, 8), all the BMP-2-containing gelatin sponges showed excellent bone regeneration in comparison with the non BMP-2-containing gelatin sponges. This proves the ability of the prepared sponges to slowly release the growth factor in a manner that enhances bone regeneration. Another advantage of the prepared scaffolds is biodegradability; the gelatin scaffolds were completely biodegraded at the end of the healing period with no gelatin or MCP remnants, as shown by the histological slides (Fig. 8).
The significant difference in terms of bone regeneration observed at 3 and 12 weeks between the BMP-2-containing gelatin sponges and the non BMP-2-containing 90 wt% gelatin sponges reflects the inhibitory effects of high concentration MCP on bone regeneration. This could be due to the fact that a high magnesium concentration increases the expression of the anticalcification protein (osteopontin), which is known as an active inducer of decalcification [18], and up regulation of the calcification inhibitor matrix Gla protein [19].
This study supports previous in vitro work regarding the effect of serial concentrations of MCP-containing gelatin sponges on the cellular proliferation and osteogenic differentiation of mesenchymal stem cells, and provides in vivo evidence of the ability of prepared gelatin sponges to slowly release BMP-2 in a manner that enhances bone regeneration; the addition of 50 wt% MCP to the gelatin sponges had a significant positive effect on bone regeneration when BMP-2 protein was used in combination.
Conclusion
It can be concluded that the prepared scaffold has the ability to slowly release BMP-2 as has been noticed through bone regeneration. Moreover, the addition of 50 wt% of magnesium calcium phosphate to the gelatin sponge, in the presence of BMP-2, has synergistically enhanced bone regeneration.
Contributor Information
Ahmed Hussain, Phone: +81-75-751-3401, FAX: +81-75-761-9732, Email: frontierscientist@gmail.com.
Yasuhiko Tabata, Phone: +81-75-751-4128, FAX: +81-75-751-4646.
References
- 1.Luo J, Sun MH, Kang Q, Peng Y, Jiang W, Luu HH, Luo Q, Park JY, Li Y, Haydon RC, He TC. Gene therapy for bone regeneration. Curr Gene Ther. 2005;5:167–179. doi: 10.2174/1566523053544218. [DOI] [PubMed] [Google Scholar]
- 2.Kurz LT, Garfin SR, Booth JR. Harvesting autogenous iliac bone grafts: a review of complications and techniques. Spine. 1989;14:1324–1331. doi: 10.1097/00007632-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Kawai K, Suzuki S, Tabata Y, Ikada Y, Nishimura Y. Accelerated tissue regeneration through incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis. Biomaterials. 2000;21:489–499. doi: 10.1016/S0142-9612(99)00207-0. [DOI] [PubMed] [Google Scholar]
- 4.Straiger MP, Pietak AM, Huadmai J, Kas G. Magnesium and its alloys as orthopedic biomaterials: review. Biomaterials. 2006;27:1728. doi: 10.1016/j.biomaterials.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Fujimura K, Bessho K, Kusumoto K, Ogawa Y, Iizuka T. Experimental studies on bone inducing activity of composites of atelopeptide type I collagen as a carrier for ectopic osteoinduction by rhBMP-2. Biochem Biophys Res Commun. 1995;208:316–322. doi: 10.1006/bbrc.1995.1340. [DOI] [PubMed] [Google Scholar]
- 6.Okubo Y, Bessho K, Fujimura K, Kusumoto K, Ogawa Y, Tani Y, Iizuka T. Comparative study of intramuscular and intraskeletal osteogenesis by recombinant human bone morphogenetic protein-2. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:34–38. doi: 10.1016/S1079-2104(99)70291-X. [DOI] [PubMed] [Google Scholar]
- 7.Okubo Y, Bessho K, Fujimura K, Kusumoto K, Ogawa Y, Iizuka T. Effect of elcatonin on osteoinduction by recombinant human bone morphogenetic protein-2. Biochem Biophys Res Commun. 2000;269:317–321. doi: 10.1006/bbrc.2000.2294. [DOI] [PubMed] [Google Scholar]
- 8.Okubo Y, Kusumoto K, Bessho K. Accelerators of osteogenesis by recombinant human bone morphogenetic protein-2. Drug Target Insights. 2007;2:55–60. [PMC free article] [PubMed] [Google Scholar]
- 9.Omura S, Mizuki N, Kawabe R, Ota S, Kobayashi S, Fujita K. A carrier for clinical use of recombinant human BMP-2: dehydrothermally cross-linked composite of fibrillar and denatured atelocollagen sponge. Res Dev. 1998;2:129–134. doi: 10.1016/s0901-5027(98)80312-3. [DOI] [PubMed] [Google Scholar]
- 10.Hussain A, Bessho K, Takahashi K, Tabata Y. Magnesium calcium phosphate as a novel component enhances mechanical/physical properties of gelatin scaffold and osteogenic differentiation of bone marrow mesenchymal stem cells. Tissue Eng Part A. 2012;18:768–774. doi: 10.1089/ten.tea.2011.0310. [DOI] [PubMed] [Google Scholar]
- 11.Bessho K, Konishi Y, Kaihara S, Fujimura K, Okubo Y, Iizuka T. Bone induction by escherichia coli-derived recombinant human bone morphogenetic protein-2 compared with Chinese Hamster ovary cell-derived recombinant human bone morphogenetic protein-2. Br J Oral Max Surg. 2000;38:645–649. doi: 10.1054/bjom.2000.0533. [DOI] [PubMed] [Google Scholar]
- 12.Li RH, Wozney JM. Delivering on the promise of bone morphogenetic proteins. Trends Biotechnol. 2001;7:255–265. doi: 10.1016/S0167-7799(01)01665-1. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto M, Ikada Y, Tabata Y. Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials. 2003;24:4375–4383. doi: 10.1016/S0142-9612(03)00337-5. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto M, Tabata Y, Ikada Y. Ectopic bone formation induced by biodegradable hydrogels incorporating bone morphogenetic protein. J Biomater Sci Polym Ed. 1998;5:439–458. doi: 10.1163/156856298X00550. [DOI] [PubMed] [Google Scholar]
- 15.Banai S, Haggroth L, Epstein SE, Casscells W. Influence of extracellular magnesium on capillary endothelial cell proliferation and migration. Cir Res. 1990;67:645–650. doi: 10.1161/01.RES.67.3.645. [DOI] [PubMed] [Google Scholar]
- 16.Webster TJ, Ergun C, Doremus RH, Bizios R. Hydroxylapatite with substituted magnesium, zinc, cadmium, and yttrium. II. Mechanisms of osteoblast adhesion. J Biomed Mater Res. 2002;59:312. doi: 10.1002/jbm.1247. [DOI] [PubMed] [Google Scholar]
- 17.Yamasaki Y, Yoshida Y, Okazaki M, Shimazu A, Kubo T, Akagawa Y, Uchida T. Action of FGMGCO3 Ap collagen composite in promoting bone formation. Biomaterials. 2003;24:4913. doi: 10.1016/S0142-9612(03)00414-9. [DOI] [PubMed] [Google Scholar]
- 18.Cho HJ, Cho HJ, Kim HS. Osteopontin: a multifunctional protein at the crossroads of inflammation, atherosclerosis, and vascular calcification. Curr Atheroscler Rep. 2009;11:206–213. doi: 10.1007/s11883-009-0032-8. [DOI] [PubMed] [Google Scholar]
- 19.Montezano AC, Zimmerman D, Yusuf H, Burger D, Chignalia AZ, Wadhera V, Van leeuwen FN, Touyz RM. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension. 2010;56:453–462. doi: 10.1161/HYPERTENSIONAHA.110.152058. [DOI] [PubMed] [Google Scholar]