Abstract
This paper aim to assess the anatomical spaces of head and neck region and causative microorganisms responsible for infections, evaluate the resistance of antibiotics used in treatment and compare the findings with previously reported microbial flora in the orofacial infection. Forty-two patients were recorded. All underwent surgical incision and drainage, received antibiotics cover, and had culture and sensitivity test performed for gram positive and gram negative aerobes. There were 33 male (78.57 %) and 9 female (21.42 %). Out of the 42 patients 28 (66.66 %) presented with single space involvement. The submandibular space was the most frequent location for single space abscess (28.12 %). Fourteen patients presented with multiple space involvement, with a total of 64 spaces being involved. Forty microorganisms were isolated. There were 28 aerobes and 10 anaerobes. Two fungi were also identified. The most common bacteria isolated were Staphylococcus aureus, Klebsiella, Escherichia coli, Peptostreptococcus. The key issue here, which needs to be remembered, is that antibiotics alone cannot resolve odontogenic infection satisfactorily. Quick recovery of patients results with proper basic management comprising of early drainage/decompression which is equally important.
Keywords: Microbial flora, Orofacial abscess, Space infection, Antibiotic susceptibility
Introduction
Humans are subject to various infections; infections in the orofacial region are known to be commonly from dental origin. Odontogenic infections are most frequently occurring infectious processes known to both antiquity and present day health practice [1, 2].
What is considered to be the indigenous maxillofacial flora begins during birth with the acquisition of the flora of the birth canal and then changes from infancy to adulthood as a result of the specific anatomic region, environmental influences and host factors. The changes in the microbiology of infections of odontogenic etiology rest in the changes in nomenclature and in our ability to isolate organisms.
Fortunately, a vast majority of these abscesses can be successfully treated by incision of any soft tissue swelling together with extraction or root canal therapy of the affected tooth (Piecuch) [3]. However, when drainage cannot be achieved or the patient shows signs of systemic involvement, then antibiotic therapy is indicated (Guralnick) [4]. There are increasing number of immuno-compromised patients who require antibiotic therapy for opportunistic infections.
In 1928, Sir Alexander Fleming observed that colonies of bacterium Staphylococcus aureus could be destroyed by the mold Penicillium notatum. The routine use of penicillin did not begin until the 1940s, when Howard Florey and Ernst Chain developed a powdery form of the antibiotic. The discovery of penicillin significantly changed the management of odontogenic infections.
Just after drug companies began mass-producing penicillin in 1943, antibiotic-resistant microorganisms began to develop. The increased prevalence of antibiotic resistance is an outcome of evolution [5].
To combat penicillin resistance, synthetic antibiotics have subsequently been synthesized; Penicillin still remains the empirical drug of choice for odontogenic infections because of its effectiveness, minimal side effects, low cost, patient tolerability, and ready availability.
The purpose of this study was to assess the anatomical spaces and causative microorganisms responsible for deep fascial space head and neck infections, to evaluate the resistance of antibiotics used in treatment of these infections and compare the findings with previously reported microbial flora in the oral and maxillofacial infection.
Patients and Methods
In the present study 42 patients having odontogenic infection over a period of 6 months were evaluated in Department of Oral and Maxillofacial Surgery at Sharad Pawar Dental College and Jawaharlal Nehru Medical College, Sawangi (Meghe) Wardha. The specimens were obtained by aspirating abscess using sterile 18/22-gauge needle and 5 ml syringe either intraorally or extraorally. After aspirating, a drop of specimen was immediately inoculated in sterile Robertson cooked meat broth (RCM) for isolation of anaerobic organisms. The inoculated RCM was incubated at 37 °C for 48 h, following which subcultures were made on solid media. A filter paper disc containing metronidazole (5 μg) was put in between primary and secondary line of streaking. The plate was incubated anaerobically in McIntosh field jar at 37 °C for 48 h.
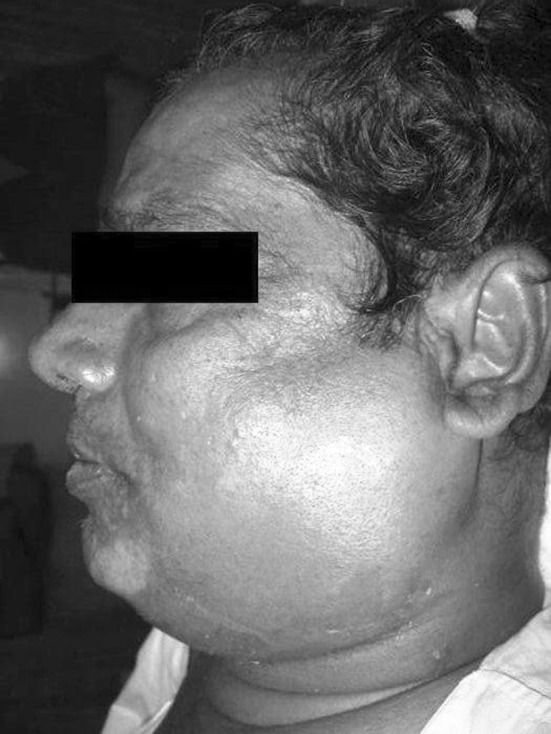
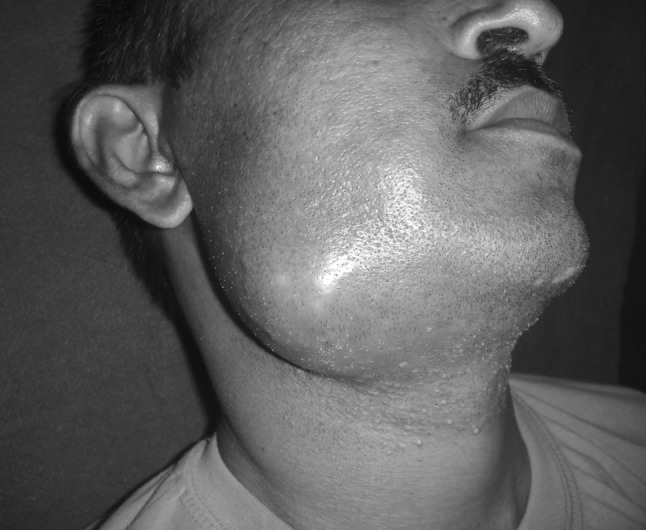
Rest of the specimen was collected in sterile plain bulb for isolation of aerobic organism. The specimens for aerobic culture were inoculated on to blood agar (BA), (Fig. 1) McConkey agar (MA) and nutrient broth and incubated aerobically at 37 °C for 18–24 h (Fig. 2). An antibiotic sensitivity test for the aerobic isolates was performed on Muller Hinton agar by Kirby-Bauer disc diffusion method. The media used were BA with gentamicin, brain heart infusion (BHI) agar and bacteroides bile esculin (BBE) agar. A smear for gram staining was also prepared. All the patients in this study underwent surgical incision and drainage. Patients characteristics reviewed were gender, age, fascial space (s) involved (Figs. 3, 4, 5, 6), bacteria identified, and antibiotic resistance from culture and sensitivity for aerobes.
Fig. 1.
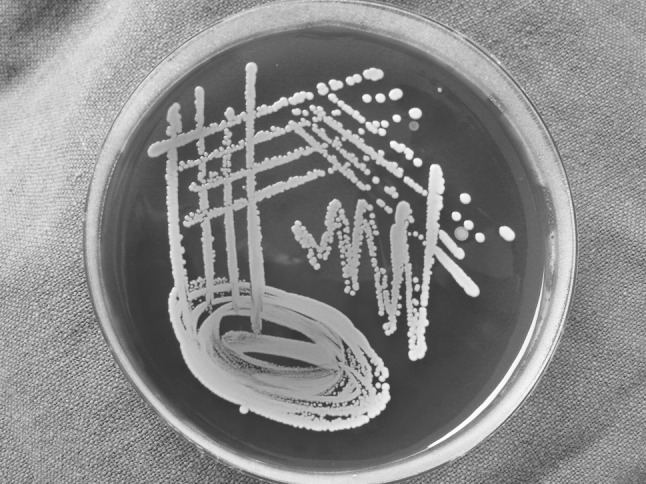
Staphylococcus aureus in blood agar
Fig. 2.
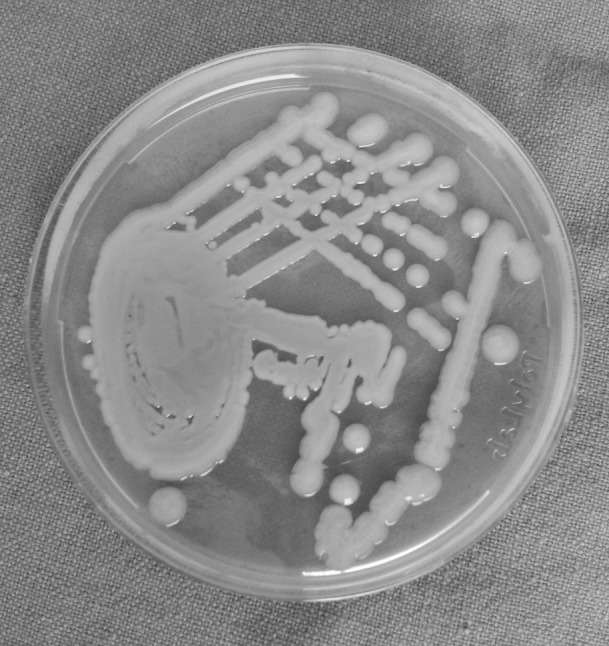
Klebsiella on mc conkey
Fig. 3.
Buccal space infection
Fig. 4.
Submandibular space infection
Fig. 5.
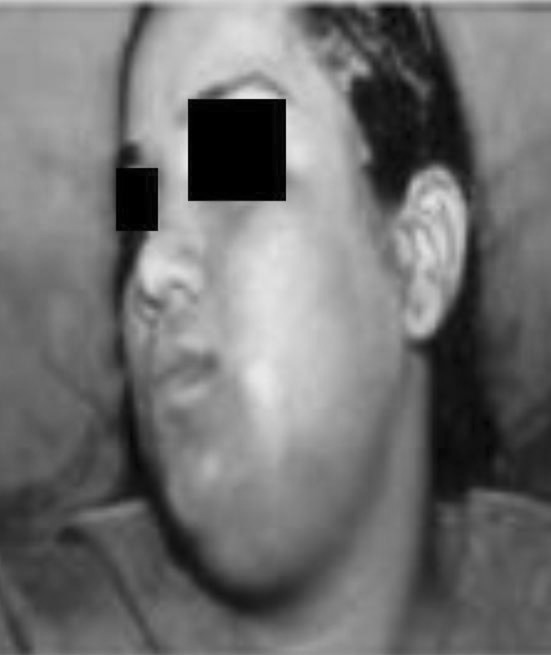
Submental and submandibular space infection
Fig. 6.
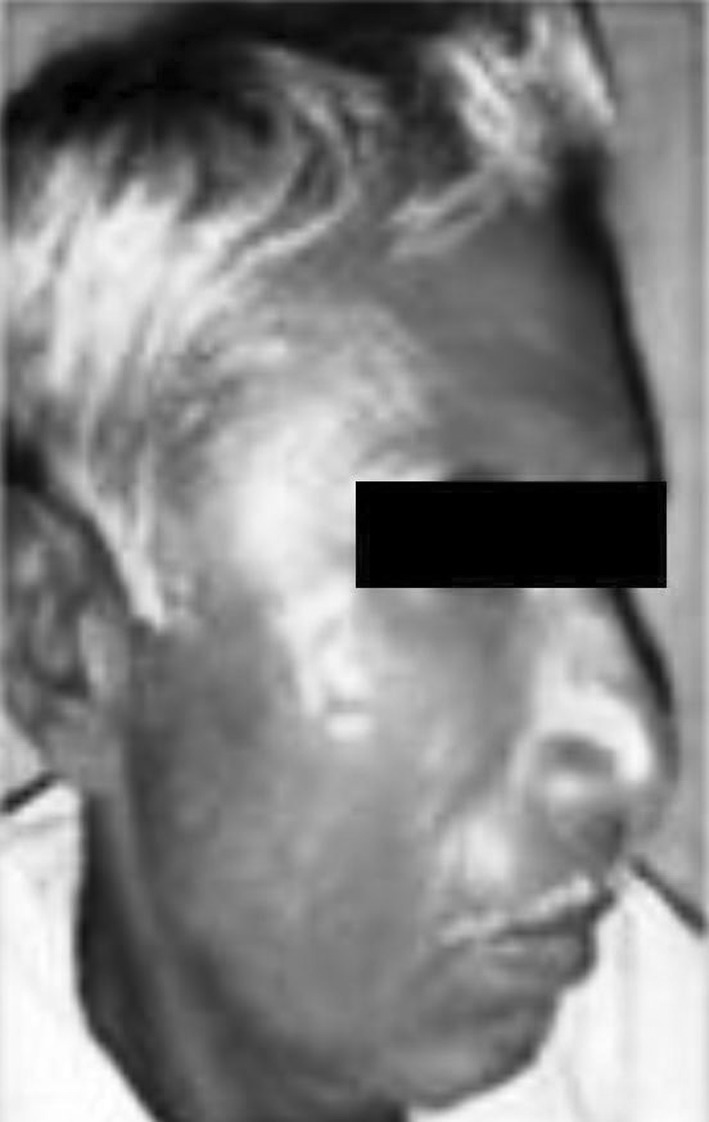
Temporal space infection
Results
There were 33 (78.57 %) males and 9 (21.43 %) females ranging in age of 13–75 years, with the mean age of 38.04 years. In 42 cases, 64 spaces were involved (single, multiple) Single space involvement was seen in 28 patients (66.66 %) (Table 1) the submandibular space was the most frequent location for a single-space abscess in 18 (28.12 %), followed by the buccal space in 14 (21.87 %), canine space in 9 (14.06 %) and submental space in 9 (14.06 %).
Table 1.
Single space abscess
| Spaces | Number out of 28 | % |
|---|---|---|
| Submandibular | 8 | 28.57 |
| Buccal | 6 | 21.42 |
| Canine | 5 | 17.85 |
| Submental | 2 | 7.14 |
| Masseteric | 1 | 3.57 |
| Pterygomandibular | 2 | 7.14 |
| Dentoalveolar | 3 | 10.71 |
| Periapical | 1 | 3.57 |
| Total | 28 | 100.00 |
In 42 patients, 36 multiple spaces were involved, (Table 2), instance of submandibular space involvement was 10 (27.77 %), submental space 7 (19.44 %), and buccal space 8 (22.22 %).
Table 2.
Multiple space
| Space | Number | % |
|---|---|---|
| Submandibular | 10 | 27.77 |
| Submental | 7 | 19.44 |
| Buccal | 8 | 22.22 |
| Canine | 4 | 11.11 |
| Sublingual | 3 | 8.33 |
| Mediastinal space | 1 | 2.77 |
| Submasseteric | 3 | 8.33 |
| Total | 36 | 100.00 |
The present study has microbiological isolates ranging from aerobes, anaerobes and mixture of aerobes and anaerobes. In addition we also isolated fungi in 2 cases (5 %). Of the total 40 isolates, 28 (70 %) were aerobes, 18 were gram-positive aerobes (64 %) and 10 were gram-negative aerobes (36 %). This difference in the gram positive and the gram-negative aerobes was significant. Out of the 40 isolates, 10 were anaerobes (25 %).
The predominantly isolated microorganism was S. aureus isolated in 7 patients (17.50 %). This high rate isolate of staphylococci, Streptococcus group A was isolated in 2 patients (5 %), Streptococcus mutans in 2 patients (5 %), and Streptococcus milleri in 2 patients (5 %). Enterococcus faecalis was found in 1 patient (2.5 %) and Staphylococcus sp. in 4 patients (10 %) (Table 3).
Table 3.
Predominantly isolated microorganism
| No. | Microorganisms Isolated | Instances | % |
|---|---|---|---|
| 1. | Staphylococci sp. | 04 | 10.00 |
| 2. | Staph aureus | 07 | 17.50 |
| 3. | Klebsiella sp. | 04 | 10.00 |
| 4. | Escherichia coli | 04 | 10.00 |
| 5. | Streptococcus mutans | 02 | 5.00 |
| 6. | Pseudomonas aeuroginosa | 02 | 5.00 |
| 7. | Enterococcus faecalis | 01 | 2.50 |
| 8. | Streptococcus group.A | 02 | 5.00 |
| 9. | Peptostreptococcus sp. | 04 | 10.00 |
| 10. | Bacteriodes corrodens | 01 | 2.5 |
| 11. | Fusobacterium nucleatum | 01 | 2.50 |
| 12. | Bacteriodes fragilis | 02 | 5.00 |
| 13. | Bacteriodes melaninogenicus | 02 | 5.00 |
| 14. | Candida albicans | 02 | 5.00 |
| 15. | Streptococcus milleri | 02 | 5.00 |
| Total | 40 | 100.0 | |
The gram-negative aerobes were isolated in 10 patients (25 %) and the percentage distribution of the gram-negative aerobes was Klebsiella in 4 isolates (10 %), Escherichia coli in 4 isolates (10 %) and Pseudomonas aeuroginosa in 2 isolates (5 %) (Table 3).
The predominant anaerobic flora isolated was Peptostreptococcus in 4 (10 %), Bacteroides melaninogenicus in 2 (5 %), Bacteriodes fragilis in 2 (5 %), Bacteroides corrodens in 1 (2.5 %) and Fusobacterium nucleatum in 1 (2.5 %) (Table 3).
The gram-negative aerobes were isolated in 10 patients (25 %) and the percentage distribution of the gram-negative aerobes was Klebsiella in 4 isolates (10 %), E. coli in 4 isolates (10 %) and P. aeuroginosa in 2 isolates (5 %). (Table 3).
Candida albicans, was isolated in two cases (5 %), as a super infection out of which one was diabetic and both the patients were on longstanding antibiotic therapy.
The resistance to penicillin by the gram-positive aerobes was noted in (38.88 %) of the isolates in our study, of which, S. aureus was resistant in 5 instances (71.42 %) to penicillin and erythromycin, whereas 100 % susceptibility was noted to Gentamicin, Ciprofloxacin, and Cephotaxime. Few isolates of staphylococcus are susceptible to penicillin. Streptococci viridans showed resistances with 1 isolate (25 %) and was susceptible with 3 isolates (75 %) to penicillin as well as to Erythromycin and Gentamicin, whereas 100 % susceptibility was noted to Ciprofloxacin and Cephotaxime. Cephotaxime along with ciprofloxacillin was effective against the entire gram-positive microorganism isolated in this study. This finding indicates that the above two antibiotics were effective in managing odontogenic infections caused by gram-positive organisms.
In this study, the antibiotic susceptibility of the gram-negative microorganisms was seen predominantly with Amikacin. E. coli and Klebsiella were found 100 % susceptible to Amikacin, whereas pseudomonas was 100 % resistant to Amikacin, but was sensitive to Cephotaxime, Cefuroxime and Ciprofloxacin.
Discussion
Head and neck infections of odontogenic origins are routinely treated. Untreated or rapidly spreading odontogenic infections can be potentially life threatening secondary to airway compromise or septicemia.
The mean age of the patients reported was 38.04 years, with the youngest patient being 13-years old and the oldest 75-years old. This mean age of 38.04 years in this study is consistent with the other studies reported in the literature [6].
Of the 42 patients, presented with the clinical finding of pain and swelling (100 %); pain ranged from mild to very severe. This correlated to the study by Bridgeman et al. [7] who they reported a prevalence of 100 % for pain and 98 % for swelling as a clinical presentation.
Of the 42 patients, 30 patients (71.42 %) presented with fever, in contrast with the findings of Bridgeman et al., who reported 50 % patients reporting with fever in his study. Generally patients with acute bacterial infections may present with fever, and elevation of body temperature above normal (about 37 °C). It was also seen that patients do not have fever or pyrexia even when there is an obvious swelling due to bacterial infection.
Trismus, a common feature of odontogenic infection, was seen in 14 patients (33.33 %) in the present study, which is in contrast to the study of Bridgeman et al. [7] where 46 % of trismus was seen in his study. This clinical sign is commonly seen when the infection involves the masticatory spaces.
Of the 42 patients, 28 patients (66.66 %) reported with single space involvement whereas, 14 patients (33.34 %) reported with multiple space involvement. This difference in the involvement of spaces is significant and in contradiction to the study by Rega et al. [8] who reported the incidence of 61.2 %, for involvement of multiple spaces, which, he attributed to the latency in presentation of the patient to the treating facility.
Staphylococci are frequently associated with abscess formation. These microorganisms produce coagulase, an enzyme that is deposited which can cause fibrin deposition in citrated or oxalated blood. Streptococci are associated more often with cellulites, which produce enzymes such as streptokinase (fibrinolysin), hylouronidaze, and streptodornase. These enzymes break down fibrin and connective tissue ground substance, and lyse cellular debris, thus facilitating rapid spread of bacterial invaders. Although there are barriers, these are violated by the end products of the microorganisms and guide the infection to spread into deeper planes.
An odontogenic infection spreads to fascial spaces because the anatomy of the fascial planes of the head and neck is such that it has an ineffective barrier to the spread of infection [9], and plays a vital role in the clinical localization of an abscess. The involvement of the facial planes by cellulitis, aids in the surgical drainage.
Of the 28 patients with single space involvement, submandibular space was involved in 8 patients (28.57 %), buccal space in 6 patients (21.42 %) and, canine space in 5 patients (17.85 %). This finding correlates with the other studies reported in the literature [10, 11].
In the multiple space involved patients, there was higher incidence for the involvement of the submandibular space (27.77 %). As compared to the incidence for the involvement of buccal space (22.22 %), submenta (19.44 %), canine (11.11 %), submassetric (8.33 %), sublingual (8.33 %) and mediastinal space (2.77 %). This is contradictory to the incidence documented in the literature as far as involvement of the lateral pharyngeal space is concerned [12].
The microbiological isolates ranged from aerobes, anaerobes and mixture of aerobes, and anaerobes [13]. In addition we also isolated fungi in 2 cases (5 %). Of the total 40 isolates, 28 (70 %) were aerobes, of which 18 were gram-positive aerobes (64 %) and 10 were gram-negative aerobes (36 %). This difference in the gram positive and the gram-negative aerobes was significant. Whereas, out of the 40 isolates, 10 isolates were anaerobes (25 %). There was a significant difference in the incidence of aerobes and anaerobes. However, in the present study, in 36 % of patients gram-negative microorganisms were isolated which warrants a critical attention towards the rational use of antibiotics effective against gram-negative organisms.
In the present study, predominantly isolated microorganism was S. aureus which was isolated in 7 patients (17.50 %). This high rate isolate of staphylococci may be due to contamination of cultures from the skin as most of the infections were drained by extra oral approach. This contradicts the other studies carried out which show the predominance of Streptococcus viridans species [5]. The isolation of S. aureus has clinical significance, as the resistant strains are known to occur which do not respond to routine antibiotic therapy [14]. The gram-negative aerobes were isolated in 10 patients (25 %) and the percentage distribution of the gram-negative aerobes was Klebsiella in 4 isolates (10 %), E. coli in 4 isolates (10 %) and Pseudomonas aeuroginosa in 2 isolates (5 %). Goldberg [15], Sabiston and Gold [16] reported the isolation of gram-negative bacilli like Klebsiella, Chromobacterium violaceus, E. coli.
Candida albicans, was isolated in two cases (5 %).The occurrence of candida in pus was consistent with the literature reported by Mcmanners et al. [17].
The resistance to penicillin by the gram-positive aerobes was noted in (38.88 %) of the isolates in our study; Very few isolates of staphylococcus are now susceptible to penicillin [18].
Streptococci viridans showed resistance with 1 isolate (25 %) and was susceptible with 3 isolates (75 %) to penicillin as well as to Erythromycin and Gentamicin, whereas 100 % susceptibility was noted to Ciprofloxacin, Cephotaxime. These correlates with the finding of Kuriyama et al. [19]. Streptococci viridans, have a susceptibility rate of 77 % to penicillin as well as erythromycin and 100 % to cefepime.
In the present study, the antibiotic susceptibility of the gram-negative microorganisms was seen predominantly with Amikacin. E. coli and Klebsiella were found 100 % susceptible to Amikacin, whereas pseudomonas was 100 % resistant to Amikacin, which was sensitive to Cephotaxime, Cefuroxime and Ciprofloxacin.
The basic beta lactum antibiotics are key antibiotics to be started for treating odontogenic infections as these infections are predominantly of gram-positive aerobes [20]. Metronidazole or Tinidazole should not be just started because anaerobic microorganisms are normal flora of oral cavity; they should be started purely on the clinical presentation in the form of chronic abscess, massive cellulitis, presence of crepitus (gas), evidence of tissue necrosis, sloughing, presence of thick foul smelling pus as evident from the present study. Gram-negative organisms also play major role in maxillofacial infections.
The key issue here, which needs to be remembered, is that antibiotic alone cannot resolve odontogenic infection satisfactorily. Quick recovery of patients results with proper basic management comprising of early drainage/decompression which is equally important.
Therefore with odontogenic infections it is always appropriate to always begin with the empiric antibiotic regimen with correlation to clinical presentation thinking of the most likely suspected microorganisms involved in the infections, which are usually the normal flora of the region, without forgetting the importance of early surgical intervention to reduce morbidity and complications.
Contributor Information
Inderdeep Singh Walia, Phone: +91-9878500030, Email: waliainderdeep@yahoo.com.
Abhilasha O. Yadav, Email: drabhilasha@yahoo.com
References
- 1.Huang TT, Tseng FY, Yeh TH, Hsu CJ, Chen YS. Factors affecting the bacteriology of deep neck infection: A retrospective study of 128 patients. Acta Otolaryngol. 2006;126:396–401. doi: 10.1080/00016480500395195. [DOI] [PubMed] [Google Scholar]
- 2.Parhiscar A, Har-El G. Deep neck abscess: A retrospective review of 210 cases. Ann Otol Rhinol Laryngol. 2001;110:1051–1054. doi: 10.1177/000348940111001111. [DOI] [PubMed] [Google Scholar]
- 3.Piecuch JE. Odontogenic infections. Dent Clin North Am. 1982;26:129–145. [PubMed] [Google Scholar]
- 4.Guralnick W. Odontogenic infections. Br Dent J. 1984;156:440–447. doi: 10.1038/sj.bdj.4805396. [DOI] [PubMed] [Google Scholar]
- 5.Hawkey PM. Mechanisms of resistance to antibiotics. Intensive Care Med. 2000;26:S9. doi: 10.1007/s001340051112. [DOI] [PubMed] [Google Scholar]
- 6.Hunt DE, King TJ, Fuller GE. Antibiotic susceptibility of bacteria isolated from oral infections. J Oral Surg. 1989;47:327. [PubMed] [Google Scholar]
- 7.Bridgeman A, Wiesenfeld D, Hellyar A, Sheldon W. Major maxillofacial infections. An evaluation of 107 cases. Aust Dent J. 1995;40:281–288. doi: 10.1111/j.1834-7819.1995.tb04814.x. [DOI] [PubMed] [Google Scholar]
- 8.Rega AJ, Aziz SR, Ziccardi VB. Microbiology and antibiotic sensitivity of head and neck space infections of odontogenic origin. J Oral Maxillofac Surg. 2006;64:1377–1380. doi: 10.1016/j.joms.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Grodinsky M, Holyoke MA. The fasciae and fascial spaces of the head, neck, and adjacent regions. Am J Anat. 1938;63:367–408. doi: 10.1002/aja.1000630303. [DOI] [Google Scholar]
- 10.Konow V, Nord LCE, Nordenram A. Anaerobic bacteria in dentoalveolar infections. Int J Oral Surg. 1981;10:313–322. doi: 10.1016/S0300-9785(81)80027-0. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald JB, Socransky SS, Gibbons RJ. Aspects of the pathogenesis of mixed anaerobic infections of mucous membranes. J Dent Res. 1963;42:529–544. doi: 10.1177/00220345630420016301. [DOI] [Google Scholar]
- 12.Storoe W, Haug RH, Lillich TT. The changing face of odontogenic infections. J Oral Maxillofacial Surg. 2001;59:739. doi: 10.1053/joms.2001.24285. [DOI] [PubMed] [Google Scholar]
- 13.Gill Y, Scully C. Orofacial odontogenic infections: review of microbiology and current treatment. Oral Surg Oral Med Oral Pathol. 1990;70(2):155–158. doi: 10.1016/0030-4220(90)90109-6. [DOI] [PubMed] [Google Scholar]
- 14.Sims W. The clinical bacteriology of purulent oral infections. Br J Oral Surg. 1974;12:1–12. doi: 10.1016/0007-117X(74)90055-9. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg MH. The changing biological nature of acute dental infection. J Am Dent Assoc. 1970;80:1048–1051. doi: 10.14219/jada.archive.1970.0244. [DOI] [PubMed] [Google Scholar]
- 16.Sabiston CB, Gold WA. Anaerobic bacteria in oral infections. Oral Surg Oral Med Oral Pathol. 1974;38:187–192. doi: 10.1016/0030-4220(74)90054-1. [DOI] [PubMed] [Google Scholar]
- 17.McManner J, Samaranayake LP. Suppurative oral candidiasis: review of literature and report of case. Int J Oral Maxillofac Surg. 1990;19:257–259. doi: 10.1016/S0901-5027(05)80413-8. [DOI] [PubMed] [Google Scholar]
- 18.Barker KF. Antibiotic resistance: a current perspective. Br J Clin Pharmacol. 1999;48:109. doi: 10.1046/j.1365-2125.1999.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuriyama T, Karasawa T, Nakagawa K, et al. Bacteriologicfeatures and antimicrobial susceptibility in isolates from orofacial odontogenic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:600. doi: 10.1067/moe.2000.109639. [DOI] [PubMed] [Google Scholar]
- 20.Kuriyama T, Nakagawa K, Karasawa T, Saiki Y, Yamamoto E, Nakamura S. Past administration of beta-lactam antibiotics and increase in the emergence of beta-lactamase-producing bacteria in patients with orofacial odontogenic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:186–192. doi: 10.1067/moe.2000.102040. [DOI] [PubMed] [Google Scholar]


