Abstract
Several genes critical to the enzymatic regulation of melanin production in mammals have recently been cloned and mapped to the albino, brown and slaty loci in mice. All three genes encode proteins with similar structures and features, but with distinct catalytic capacities; the functions of two of those gene products have previously been identified. The albino locus encodes tyrosinase, an enzyme with three distinct melanogenic functions, while the slaty locus encodes tyrosinase-related protein 2 (TRP2), an enzyme with a single specific, but distinct, function as DOPAchrome tautomerase. Although the brown locus, encoding TRP1, was actually the first member of the tyrosinase gene family to be cloned, its catalytic function (which results in the production of black rather than brown melanin) has been in general dispute. In this study we have used two different techniques (expression of TRP1 in transfected fibroblasts and immunoaffinity purification of TRP1 from melanocytes) to examine the enzymatic function(s) of TRP1. The data demonstrate that the specific melanogenic function of TRP1 is the oxidation of 5,6-dihydroxyindole-2-carboxylic acid (DHICA) to a carboxylated indole-quinone at a down-stream point in the melanin biosynthetic pathway. This enzyme activity appears to be essential to the further metabolism of DHICA to a high molecular weight pigmented biopolymer.
Full text
PDF


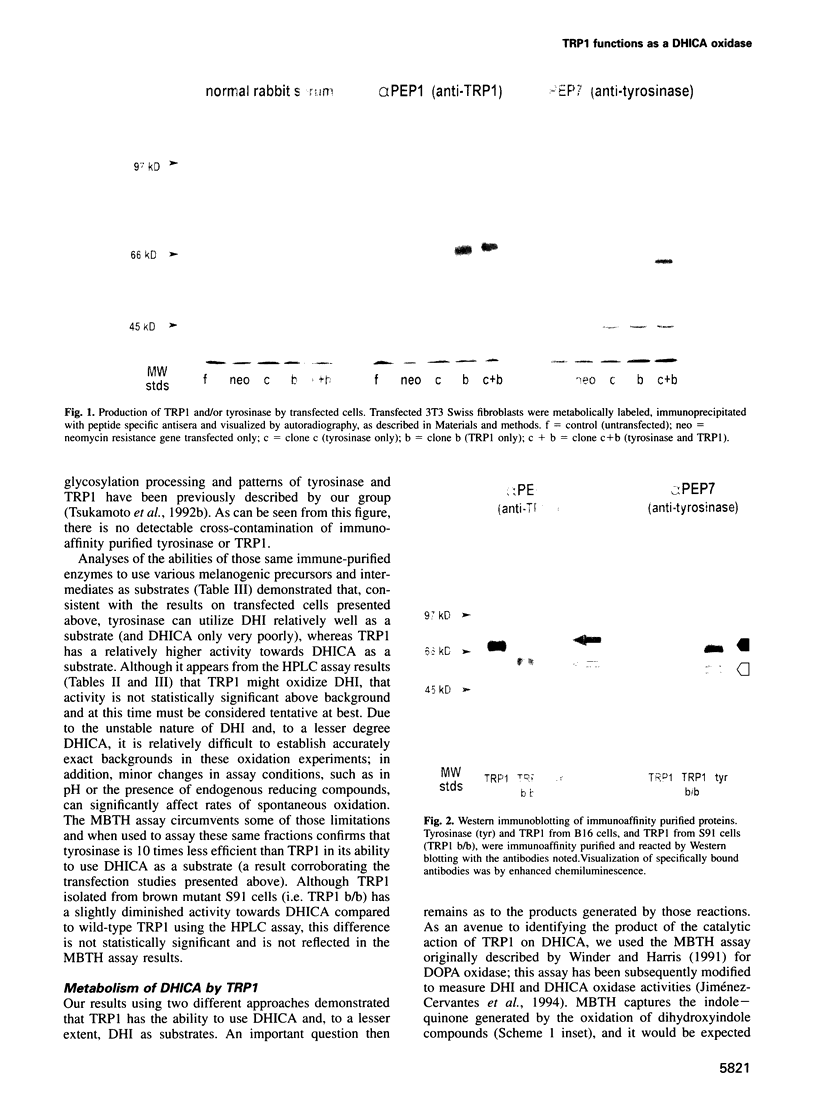
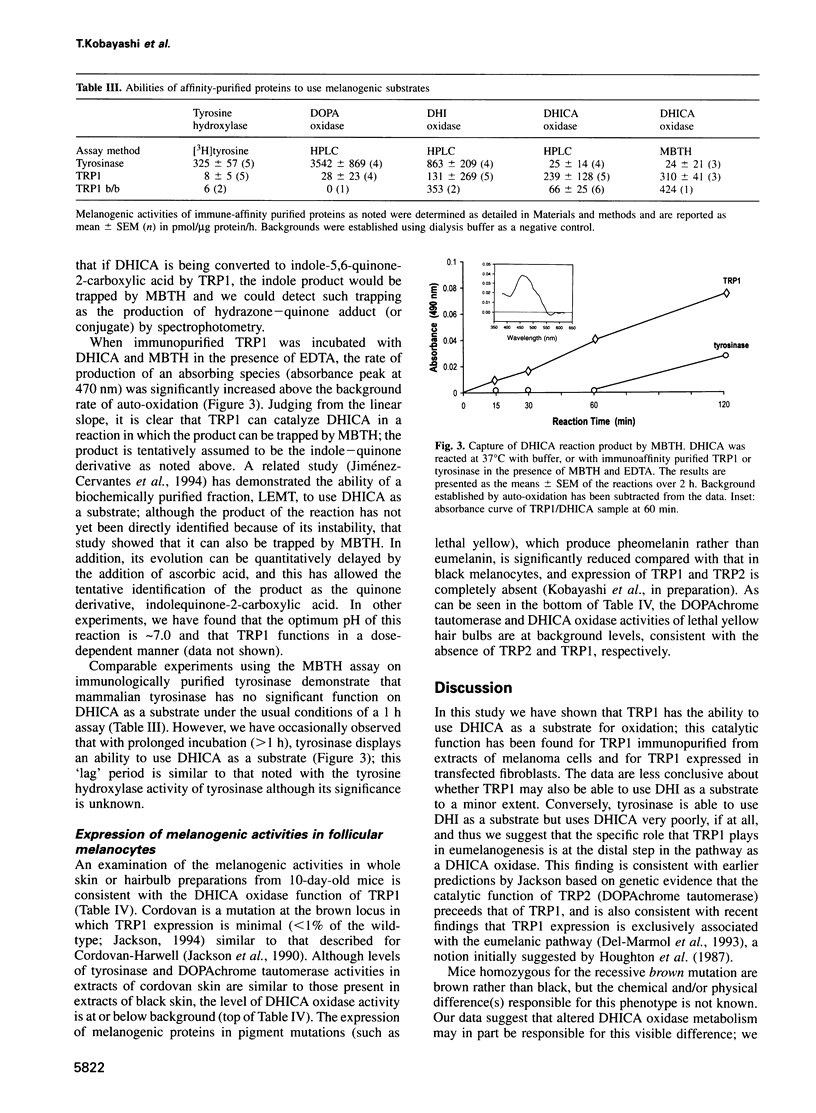



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aroca P., Garcia-Borron J. C., Solano F., Lozano J. A. Regulation of mammalian melanogenesis. I: Partial purification and characterization of a dopachrome converting factor: dopachrome tautomerase. Biochim Biophys Acta. 1990 Sep 14;1035(3):266–275. doi: 10.1016/0304-4165(90)90088-e. [DOI] [PubMed] [Google Scholar]
- Aroca P., Martinez-Liarte J. H., Solano F., García-Borrón J. C., Lozano J. A. The action of glycosylases on dopachrome (2-carboxy-2,3-dihydroindole-5,6-quinone) tautomerase. Biochem J. 1992 May 15;284(Pt 1):109–113. doi: 10.1042/bj2840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroca P., Urabe K., Kobayashi T., Tsukamoto K., Hearing V. J. Melanin biosynthesis patterns following hormonal stimulation. J Biol Chem. 1993 Dec 5;268(34):25650–25655. [PubMed] [Google Scholar]
- Barber J. I., Townsend D., Olds D. P., King R. A. Dopachrome oxidoreductase: a new enzyme in the pigment pathway. J Invest Dermatol. 1984 Aug;83(2):145–149. doi: 10.1111/1523-1747.ep12263381. [DOI] [PubMed] [Google Scholar]
- Bennett D. C., Cooper P. J., Dexter T. J., Devlin L. M., Heasman J., Nester B. Cloned mouse melanocyte lines carrying the germline mutations albino and brown: complementation in culture. Development. 1989 Feb;105(2):379–385. doi: 10.1242/dev.105.2.379. [DOI] [PubMed] [Google Scholar]
- Bennett D. C., Cooper P. J., Hart I. R. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int J Cancer. 1987 Mar 15;39(3):414–418. doi: 10.1002/ijc.2910390324. [DOI] [PubMed] [Google Scholar]
- Brilliant M. H. The mouse pink-eyed dilution locus: a model for aspects of Prader-Willi syndrome, Angelman syndrome, and a form of hypomelanosis of Ito. Mamm Genome. 1992;3(4):187–191. doi: 10.1007/BF00355717. [DOI] [PubMed] [Google Scholar]
- Gardner J. M., Nakatsu Y., Gondo Y., Lee S., Lyon M. F., King R. A., Brilliant M. H. The mouse pink-eyed dilution gene: association with human Prader-Willi and Angelman syndromes. Science. 1992 Aug 21;257(5073):1121–1124. doi: 10.1126/science.257.5073.1121. [DOI] [PubMed] [Google Scholar]
- Halaban R., Moellmann G. Murine and human b locus pigmentation genes encode a glycoprotein (gp75) with catalase activity. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4809–4813. doi: 10.1073/pnas.87.12.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta S., Mishima Y., Ichihashi M., Ito S. Melanin monomers within coated vesicles and premelanosomes in melanin synthesizing cells. J Invest Dermatol. 1988 Aug;91(2):181–184. doi: 10.1111/1523-1747.ep12464454. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Jr Mammalian monophenol monooxygenase (tyrosinase): purification, properties, and reactions catalyzed. Methods Enzymol. 1987;142:154–165. doi: 10.1016/s0076-6879(87)42024-7. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Korner A. M., Pawelek J. M. New regulators of melanogenesis are associated with purified tyrosinase isozymes. J Invest Dermatol. 1982 Jul;79(1):16–18. doi: 10.1111/1523-1747.ep12510422. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Tsukamoto K. Enzymatic control of pigmentation in mammals. FASEB J. 1991 Nov;5(14):2902–2909. [PubMed] [Google Scholar]
- Hearing V. J., Tsukamoto K., Urabe K., Kameyama K., Montague P. M., Jackson I. J. Functional properties of cloned melanogenic proteins. Pigment Cell Res. 1992 Nov;5(5 Pt 2):264–270. doi: 10.1111/j.1600-0749.1992.tb00547.x. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Vieira W. D., Law L. W. Malignant melanoma: cross-reacting (common) tumor rejection antigens. Int J Cancer. 1985 Mar 15;35(3):403–409. doi: 10.1002/ijc.2910350317. [DOI] [PubMed] [Google Scholar]
- Houghton A. N., Real F. X., Davis L. J., Cordon-Cardo C., Old L. J. Phenotypic heterogeneity of melanoma. Relation to the differentiation program of melanoma cells. J Exp Med. 1987 Mar 1;165(3):812–829. doi: 10.1084/jem.165.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Wakamatsu K. Melanin chemistry and melanin precursors in melanoma. J Invest Dermatol. 1989 May;92(5 Suppl):261S–265S. doi: 10.1111/1523-1747.ep13076587. [DOI] [PubMed] [Google Scholar]
- Jackson I. J. A cDNA encoding tyrosinase-related protein maps to the brown locus in mouse. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4392–4396. doi: 10.1073/pnas.85.12.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson I. J., Chambers D. M., Tsukamoto K., Copeland N. G., Gilbert D. J., Jenkins N. A., Hearing V. A second tyrosinase-related protein, TRP-2, maps to and is mutated at the mouse slaty locus. EMBO J. 1992 Feb;11(2):527–535. doi: 10.1002/j.1460-2075.1992.tb05083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson I. J., Chambers D., Rinchik E. M., Bennett D. C. Characterization of TRP-1 mRNA levels in dominant and recessive mutations at the mouse brown (b) locus. Genetics. 1990 Oct;126(2):451–459. doi: 10.1093/genetics/126.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson I. J. Molecular and developmental genetics of mouse coat color. Annu Rev Genet. 1994;28:189–217. doi: 10.1146/annurev.ge.28.120194.001201. [DOI] [PubMed] [Google Scholar]
- Jiménez-Cervantes C., Solano F., Kobayashi T., Urabe K., Hearing V. J., Lozano J. A., García-Borrón J. C. A new enzymatic function in the melanogenic pathway. The 5,6-dihydroxyindole-2-carboxylic acid oxidase activity of tyrosinase-related protein-1 (TRP1). J Biol Chem. 1994 Jul 8;269(27):17993–18000. [PubMed] [Google Scholar]
- Jiménez M., Kameyama K., Maloy W. L., Tomita Y., Hearing V. J. Mammalian tyrosinase: biosynthesis, processing, and modulation by melanocyte-stimulating hormone. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3830–3834. doi: 10.1073/pnas.85.11.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez M., Maloy W. L., Hearing V. J. Specific identification of an authentic clone for mammalian tyrosinase. J Biol Chem. 1989 Feb 25;264(6):3397–3403. [PubMed] [Google Scholar]
- Jiménez M., Tsukamoto K., Hearing V. J. Tyrosinases from two different loci are expressed by normal and by transformed melanocytes. J Biol Chem. 1991 Jan 15;266(2):1147–1156. [PubMed] [Google Scholar]
- Kobayashi T., Urabe K., Winder A., Tsukamoto K., Brewington T., Imokawa G., Potterf B., Hearing V. J. DHICA oxidase activity of TRP1 and interactions with other melanogenic enzymes. Pigment Cell Res. 1994 Aug;7(4):227–234. doi: 10.1111/j.1600-0749.1994.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Kwon B. S., Chintamaneni C., Kozak C. A., Copeland N. G., Gilbert D. J., Jenkins N., Barton D., Francke U., Kobayashi Y., Kim K. K. A melanocyte-specific gene, Pmel 17, maps near the silver coat color locus on mouse chromosome 10 and is in a syntenic region on human chromosome 12. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9228–9232. doi: 10.1073/pnas.88.20.9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B. S., Halaban R., Kim G. S., Usack L., Pomerantz S., Haq A. K. A melanocyte-specific complementary DNA clone whose expression is inducible by melanotropin and isobutylmethyl xanthine. Mol Biol Med. 1987 Dec;4(6):339–355. [PubMed] [Google Scholar]
- Körner A. M., Pawelek J. Dopachrome conversion: a possible control point in melanin biosynthesis. J Invest Dermatol. 1980 Aug;75(2):192–195. doi: 10.1111/1523-1747.ep12522650. [DOI] [PubMed] [Google Scholar]
- Körner A., Pawelek J. Mammalian tyrosinase catalyzes three reactions in the biosynthesis of melanin. Science. 1982 Sep 17;217(4565):1163–1165. doi: 10.1126/science.6810464. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mishima Y. A post melanosomal era: control of melanogenesis and melanoma growth. Pigment Cell Res. 1992;Suppl 2:3–16. doi: 10.1111/j.1600-0749.1990.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Orlow S. J., Boissy R. E., Moran D. J., Pifko-Hirst S. Subcellular distribution of tyrosinase and tyrosinase-related protein-1: implications for melanosomal biogenesis. J Invest Dermatol. 1993 Jan;100(1):55–64. doi: 10.1111/1523-1747.ep12354138. [DOI] [PubMed] [Google Scholar]
- Orlow S. J., Zhou B. K., Chakraborty A. K., Drucker M., Pifko-Hirst S., Pawelek J. M. High-molecular-weight forms of tyrosinase and the tyrosinase-related proteins: evidence for a melanogenic complex. J Invest Dermatol. 1994 Aug;103(2):196–201. doi: 10.1111/1523-1747.ep12392743. [DOI] [PubMed] [Google Scholar]
- Palumbo A., d'Ischia M., Misuraca G., Prota G. Effect of metal ions on the rearrangement of dopachrome. Biochim Biophys Acta. 1987 Aug 13;925(2):203–209. doi: 10.1016/0304-4165(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Pawelek J. M. After dopachrome? Pigment Cell Res. 1991 Mar;4(2):53–62. doi: 10.1111/j.1600-0749.1991.tb00315.x. [DOI] [PubMed] [Google Scholar]
- Pawelek J. M. Dopachrome conversion factor functions as an isomerase. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1328–1333. doi: 10.1016/0006-291x(90)91011-g. [DOI] [PubMed] [Google Scholar]
- Rinchik E. M., Bultman S. J., Horsthemke B., Lee S. T., Strunk K. M., Spritz R. A., Avidano K. M., Jong M. T., Nicholls R. D. A gene for the mouse pink-eyed dilution locus and for human type II oculocutaneous albinism. Nature. 1993 Jan 7;361(6407):72–76. doi: 10.1038/361072a0. [DOI] [PubMed] [Google Scholar]
- Shibahara S., Tomita Y., Sakakura T., Nager C., Chaudhuri B., Müller R. Cloning and expression of cDNA encoding mouse tyrosinase. Nucleic Acids Res. 1986 Mar 25;14(6):2413–2427. doi: 10.1093/nar/14.6.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T., Prota G., Mishima Y. Non-melanosomal regulatory factors in melanogenesis. J Invest Dermatol. 1993 Mar;100(3):274S–280S. [PubMed] [Google Scholar]
- Tripathi R. K., Hearing V. J., Urabe K., Aroca P., Spritz R. A. Mutational mapping of the catalytic activities of human tyrosinase. J Biol Chem. 1992 Nov 25;267(33):23707–23712. [PubMed] [Google Scholar]
- Tsukamoto K., Jackson I. J., Urabe K., Montague P. M., Hearing V. J. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J. 1992 Feb;11(2):519–526. doi: 10.1002/j.1460-2075.1992.tb05082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto K., Palumbo A., D'Ischia M., Hearing V. J., Prota G. 5,6-Dihydroxyindole-2-carboxylic acid is incorporated in mammalian melanin. Biochem J. 1992 Sep 1;286(Pt 2):491–495. doi: 10.1042/bj2860491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urabe K., Aroca P., Hearing V. J. From gene to protein: determination of melanin synthesis. Pigment Cell Res. 1993 Aug;6(4 Pt 1):186–192. doi: 10.1111/j.1600-0749.1993.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Wilczek A., Mishima Y. Regulatory factors for polymerization of melanin monomers within coated vesicles and premelanosomes in melanoma cells. Melanoma Res. 1993 Aug;3(4):255–262. [PubMed] [Google Scholar]
- Winder A. J. Expression of a mouse tyrosinase cDNA in 3T3 Swiss mouse fibroblasts. Biochem Biophys Res Commun. 1991 Jul 31;178(2):739–745. doi: 10.1016/0006-291x(91)90170-c. [DOI] [PubMed] [Google Scholar]
- Winder A. J., Harris H. New assays for the tyrosine hydroxylase and dopa oxidase activities of tyrosinase. Eur J Biochem. 1991 Jun 1;198(2):317–326. doi: 10.1111/j.1432-1033.1991.tb16018.x. [DOI] [PubMed] [Google Scholar]
- Winder A. J., Wittbjer A., Rosengren E., Rorsman H. Fibroblasts expressing mouse c locus tyrosinase produce an authentic enzyme and synthesize phaeomelanin. J Cell Sci. 1993 Feb;104(Pt 2):467–475. doi: 10.1242/jcs.104.2.467. [DOI] [PubMed] [Google Scholar]
- Winder A. J., Wittbjer A., Rosengren E., Rorsman H. The mouse brown (b) locus protein has dopachrome tautomerase activity and is located in lysosomes in transfected fibroblasts. J Cell Sci. 1993 Sep;106(Pt 1):153–166. doi: 10.1242/jcs.106.1.153. [DOI] [PubMed] [Google Scholar]
- d'Ischia M., Napolitano A., Prota G. Peroxidase as an alternative to tyrosinase in the oxidative polymerization of 5,6-dihydroxyindoles to melanin(s). Biochim Biophys Acta. 1991 Mar 4;1073(2):423–430. doi: 10.1016/0304-4165(91)90152-7. [DOI] [PubMed] [Google Scholar]
- del Marmol V., Ito S., Jackson I., Vachtenheim J., Berr P., Ghanem G., Morandini R., Wakamatsu K., Huez G. TRP-1 expression correlates with eumelanogenesis in human pigment cells in culture. FEBS Lett. 1993 Aug 2;327(3):307–310. doi: 10.1016/0014-5793(93)81010-w. [DOI] [PubMed] [Google Scholar]