Abstract
Objective : The aim of study was to investigate the distribution of the integrons in Escherichia coli (E. coli) isolates, and analyze the possible relationship between the antimicrobial resistance profiles and the integrons.
Methods : The antimicrobial profiles of 376 E. coli strains were analysed by disk diffusion test. The integron genes and variable regions were detected by PCR. Some amplicons were sequenced to determine the gene cassettes style.
Results : Of 376 isolates, 223 isolates (59.3%) were confirmed as ESBL-EC. Comparison to ESBL-negative E. coli, the high rates of resistance to the third and fourth generation of cephalosporins, penicillins and amikacin were found in ESBL-EC. Only class 1 was integron detected in the isolates, and the prevalence of it was 66.5%. It was commonly found in ESBL-EC (77.6%, 173/223), which was higher than that of ESBL-negative E. coli (50.3%, 77/153) (p<0.001). Six different genes cassettes were detected in this study and were classified into three groups: dfr17-aadA5, dfrA12-aadA2 and aacA4-CmlA1. Additionally, more than one gene array harboured in 13.9% isolates of ESBL-EC, while in 9.1% isolates of ESBL-negative E.coli.
Conclusion : The high incidence of ESBL-EC with resistance to multiple antibiotics were detected in the isolates from Blood stream infection (BSI). More resistant gene cassettes in ESBL-EC may partially underlie the high resistance to amikacin, while no relation exists between the high incidence of ESBL-EC and classes 1~ 3 integrons in this region.
Key Words: Blood stream infection, ESBL-EC, Integron, Gene cassette.
INTRODUCTION
Bloodstream infection (BSI) is associated with major morbidity and mortality, and Escherichia coli (E.coli) is one of the most comment microorganisms isolated from this infection.1 In recent years, the detection rate of extended spectrum β-lactamases (ESBL) in enterobacteriaceae bacteria, specially Escherichia coli (E. coli) is increasing.2 It is well known that ESBL-producing organisms are resistant to multiple unrelated antibiotics.3 As a result, multiple drug-resistant clinical infections of E. coli are now common. Integrons are widespread genetic elements responsible for dissemination of antibiotic resistance among Gram-negative bacteria, being commonly found in plasmids and/or transposons.4 Recently integrons have become an important drug-resistance mechanism of E. coli.5 ESBL-producing E.coli (ESBL-EC) carrying integrons will have more drug resistance genes and resistance to many other drugs.
Knowledge of the characterization of integrons among E. coli isolated will contribute to assess the phenotypic and genetic characterization of antimicrobial profiles of the organism in BSI. In this study, we investigated classes 1, 2, and 3 integrons associated integrases genes in E.coli isolates from blood to evaluate integron characterization and typing of this organism.
RESULTS
A total of 376 E. coli isolates were taken during the study period. Of these, 223 isolates (59.3%) were comfirmed as ESBL-EC. None of isolates were all susceptible to antimicrobial agents, and the phenotypic characterization of antimicrobial profiles are shown in Table-II. Among the isolates, the high rate of resistance to levofloxacin, ciprofloxacin and piperacillin were 59.8%, 64.9% and 84.8% respectively, with the low rate of resistance to imipenem and meropenem (0.5% and 0%). Comparison to ESBL-negative E. coli, the high rates of resistance to the third and fourth generation of cephalosporins, penicillins and amikacin were observed in ESBL-EC.
Table-II.
Antimicrobial resistance for 376 E. coli isolated from BSI (%).
| Antimicrobials* | AMZ | AK | SCF | CIP | CRO | FOX | IMP | LEV | MEM | PRL | CTX | CAZ | TZP | FEP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESBL-EC (223) | 26.5 | 52.0 | 12.6 | 78.0 | 100 | 26.0 | 0.9 | 78.0 | 0 | 100 | 100 | 60.0 | 26.0 | 60.5 |
| ESBL-negative E. coli | 13.1 | 3.9 | 0 | 45.8 | 12.4 | 8.5 | 0 | 33.3 | 0 | 62.7 | 16.3 | 3.9 | 0 | 0 |
| Total | 21.0 | 32.4 | 7.4 | 64.9 | 64.4 | 18.9 | 0.5 | 59.8 | 0 | 84.8 | 66.0 | 37.2 | 15.4 | 35.9 |
*Abbreviations in the table: Amoxicillin–clavulanic acid (AMZ); Amikacin (AK); Cefoperazone/sulbactam (SCF); Ciprofloxacin (CIP); Ceftriaxone (CRO); Cefoxitin (FOX); Imipenem (IMP); Levofloxacin (LEV); Meropenem (MEM); Piperacillin (PRL); Cefotaxime (CTX); Ceftazidime(CAZ); Piperacillin-Tazobactam (TZP); Cefepime (FEP)
The Prevalence of classes 1, 2 and 3 integrons status in E. coli: The prevalence of classe 1 integron in E. coli isolates was 66.5% (250/376). It was commonly found in ESBL-EC (77.6%, 173/223), which was higher than that of ESBL-negative E. coli (50.3%, 77/153) (p<0.001). No amplification products were obtained from any of these isolates when the primers specific for intI2 or intI3 were used.
Classe 1 integron was detected on genomic and plasmid DNA in 155 isolates (77%, 155/201) of ESBL-EC, but it was detected on genomic or plasmid DNA in 9 isolates respectively. While Classe 1 integron was haboured on genomic and plasmid DNA in 29 isolates (37.7%, 29/77) of ESBL-negative E. coli, most of others (30 isolates) was haboured on plasmid DNA.
The Characteristic of Class 1 Integron in Isolates: Each gene-cassette region in class 1 integrons was amplified to identify characteristic of gene cassette in the isolates. 164 ESBL-EC isolates (94.8%, 164/173) of carrying class 1 integron contained resistant gene cassette, and the other 9 isolates were detected no resistant gene cassette. For ESBL-negative E. coli, 56 isolates (72.7%, 56/77) were detected resistant gene cassette.
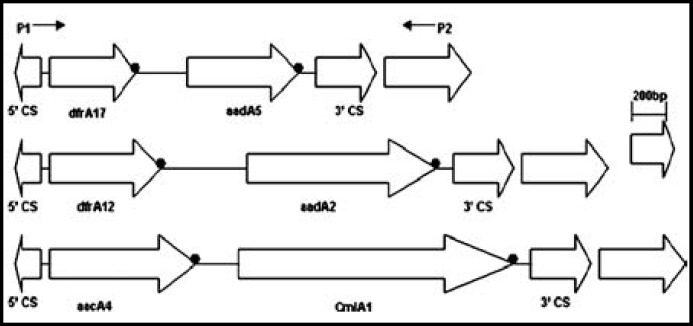
All these gene cassettes detected were divided into 6 different ones: dfrA17, aadA5, dfrA12, aadA2, aacA4 and CmlA1. The proteins encoded by gene cassette may contribute to the resistance of bacteria isolates to trimethoprim (dfr17 and dfrA12), aminoglycosides (aadA5, aadA2, and aacA4), and chloramphenicol (CmlA1).
The integrons were classified into three groups according to the length of amplicons (Fig.1). The dfr17-aadA5 array was the most prevalent in the isolates which lengths most were 1,600bp and the other two arrays were dfrA12-aadA2, and aacA4-CmlA1 which lengths were 2,000bp and 2,400bp, respectively. More than one gene array harboured in 13.9% (24/173) isolates of ESBL-EC, while in 9.1% (7/77) isolates of ESBL-negative E.coli.
Fig.1.
Schematic representation of the genetic elements of Class 1 Integron in E coli isolated from BSI.
DISCUSSION
Despite the great advances in medical science in the past century, BSI remains a growing public health concern worldwide. E. coli is one of the most common pathogens for this kind infection. Over the past few years, a significant increase in the number of ESBL-EC-associated BSI is being reported in several parts of the world.1,8 In our study, about 60% E. coli isolates from BSI were ESBL-EC, which is higher than other reports (7.3% in Hong Kong, 1.5–16.7% in Taiwan).9,10
Table-I.
Oligonucleotide primers used in the PCR assay
| Primer | Nucleotide sequence (5’-3’) | PCR target | Expected size (bp) |
|---|---|---|---|
| IntI1F | ACGAGCGCAAGGTTTCGGT | ||
| IntI1R | GAAAGGTCTGGTCATACATG | Class 1 integrase gene | 565 |
| IntI2F | GTGCAACGCATTTTGCAGG | ||
| IntI2R | CAACGGAGTCATGCAGATG | Class 2 integrase gene | 403 |
| IntI3F | CATTTGTGTTGTGGACGGC | ||
| IntI3R | GACAGATACGTGTTTGGCAA | Class 3 integrase gene | 717 |
| 5'-CS | GGCATCCAAGCAGCAAG | ||
| 3'-CS | AAGCAGACTTGACCTGAT | Variable region of integrons | Uncertain |
The high rates of resistance to levofloxacin and ciprofloxacin were found in the study, while low rates of resistance to imipenem and meropenem existed in vitro. Carbapenems use has been associated with the low risk of death in cases of serious infections caused by these pathogens.11,12 Thus, these antimicrobials are first line therapy in patients with serious infections. Additionally, the resistance to amikacin was significantly higher in ESBL-EC than in ESBL-negative E. coli except to the third and fourth generation of cephalosporins and penicillins.
Integrons had come under observation in 1989 by Stokes for the first time13, which could carry more than forty resistance genes. The resistant gene could spread to other bacteria through the integrons, so it is very important to monitor E. coli strains isolates from BSI.
We screened the three classes integrons associated integrases genes by PCR, and found only class 1 integron gene was haboured in E. coli isolated from BSI. The prevalence of class 1 integron in E. coli isolates was in accordance with other’s reports14, while the prevalence was higher in ESBL-EC than in ESBL-negative E.coli. Additionally, the prevalence of class 1 integron on genomic and plasmid DNA was higher in ESBL-EC. We found six different gene cassettes in class 1 integron and three arrays in the study. These results also showed that the characterization of integrons in bacteria obviously had regional difference.15,16 Although the same resistant gene cassettes and arrays were found on ESBL-EC and ESBL-negative E.coli, the carrying rate of gene cassettes in ESBL-EC was higher than that in ESBL-negative E.coli.
In conclusion, the high incidence of ESBL-EC with resistance to multiple antibiotics were detected in the isolates from BSI. Comparision to ESBL-negative E.coli, more resistant gene cassettes in ESBL-EC may partially underlie the high resistance to amikacin, while no relation exists between the high incidence of ESBL-EC and classes 1~3 integrons in this region.
ACKNOWLEDGMENTS
This work was supported by the Key Technologies R & D Program of Weihai (Grant No. 2011-2-88-1) and the National Science and Technology Major Project of China (2013ZX10004217-003-002).
Disclosure of interest: The authors declare that they have no conflicts of interest concerning this article.
Authors contribution:
Ming-yi WANG: Designed the study and did statistical analysis
Lu-Ming Li, Xiao-Yan Yuan, Hong-Jun Wang: Contributed in data collection and analysis
Qin Li, YA-Mei Zhu: Helped in drafting and revising the manuscript.
References
- 1.Cornejo-Juárez P, Pérez-Jiménez C, Silva-Sánchez J. Molecular analysis and risk factors for Escherichia coli producing extended-spectrum β-lactamase bloodstream infection in hematological malignancies. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035780. doi: 10.1371/journal.pone.0035780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danel F, Page MGP, Livermore DM. Class D b-lactamases. In: Bonomo RA, Tolmasky ME, editors. Enzyme-Mediated Resistance to Antibiotics. Washington, DC: ASM Press; 2007. pp. 163–194. [Google Scholar]
- 3.Pitout JDD, Laupland KB. Extended-spectrum beta-lactamaseproducing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8:159–166. doi: 10.1016/S1473-3099(08)70041-0. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 4.Guérin E, Jové T, Tabesse A. High-level gene cassette transcription prevents integrase expression in class 1 integrons. J Bacteriol. 2011;193(20):5675–5682. doi: 10.1128/JB.05246-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gebreyes WA, Altier C. Molecular characterization of multidrug-resistant Salmonella enterica subsp. Enteric serovar Typhimurium isolates from swine. J Clin Microbiol. 2002;40(8):2813–2822. doi: 10.1128/JCM.40.8.2813-2822.2002. doi: 10.1128/ JCM.40.8.2813-2822.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wayne, Pa CLSI, authors. Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute. 2012 [Google Scholar]
- 7.Xu H, Su Z, Wang S. Four novel resistance integron gene-cassette occurrences in bacterial isolates from Zhenjiang, China. Curr Microbiol. 2009;59(2):113–117. doi: 10.1007/s00284-009-9405-z. [DOI] [PubMed] [Google Scholar]
- 8.Gudiol C, Calatayud L, Garcia-Vidal C. Bacteraemia due to extended-spectrum beta-lactamase-producing Escherichia coli (ESBL-EC) in cancer patients: clinical features, risk factors, molecular epidemiology and outcome. J Antimicrob Chemother. 2010;65(2):333–341. doi: 10.1093/jac/dkp411. doi: 10.1093/jac/dkp411. [DOI] [PubMed] [Google Scholar]
- 9.Yu WL, Chuang YCH, Rasmussen JW. Extended-spectrum betalactamases in Taiwan: epidemiology, detection, treatment and infection control. J Microbiol Immunol Infect. 2006;39:264–277. [PubMed] [Google Scholar]
- 10.Ho PL, Poon WW, Loke SL. Community emergence of CTX-M type extended-spectrum beta-lactamases among urinary Escherichia coli from women. J Antimicrob Chemother. 2007;60(1):140–144. doi: 10.1093/jac/dkm144. doi: 10.1093/jac/dkm144. [DOI] [PubMed] [Google Scholar]
- 11.Hyle EP, Lipworth AD, Zaoutis TE. Impact of inadequate initial antimicrobial therapy on mortality in infections due to extended-spectrum beta-lactamase-producing enterobacteriaceae: variability by site of infection. Arch Intern Med. 2005;165(12):1375–1380. doi: 10.1001/archinte.165.12.1375. doi:10.1001/archinte.165.12.1375. [DOI] [PubMed] [Google Scholar]
- 12.Peña C, Gudiol C, Tubau F. Risk-factors for acquisition of extended-spectrum beta-lactamase-producing Escherichia coli among hospitalised patients. Clin Microbiol Infect. 2006;12(3):279–284. doi: 10.1111/j.1469-0691.2005.01358.x. doi:10.1111/j.1469-0691.2005.01358.x. [DOI] [PubMed] [Google Scholar]
- 13.Stokes HW, Hall RM. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol Microbiol. 1989;3(12):1669–1683. doi: 10.1111/j.1365-2958.1989.tb00153.x. doi:10.1111/j. 1365-2958.1989.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 14.Van Essen-Zandbergen A, Smith H, Veldman K. Occurrence and characteristics of class 1, 2 and 3 integrons in Escherichia coli, Salmonella and Campylobacter spp. in the Netherlands. J Antimicrob Chemother. 2007;59(4):746–750. doi: 10.1093/jac/dkl549. doi: 10.1093/jac/dkl549. [DOI] [PubMed] [Google Scholar]
- 15.Sun C, Su Z, Zhou C. Complex class 1 integron containing bla (CTX-M-1) genes isolated from Escherichia coli: a potentially novel resistant gene-capturing tool kit. Curr Microbiol. 2012;64(3):265–270. doi: 10.1007/s00284-011-0062-7. doi: 10.1007/s00284-011-0062-7. [DOI] [PubMed] [Google Scholar]
- 16.Van Meervenne E, Boon N, Verstraete K. Integron characterization and typing of Shiga toxin-producing Escherichia coli isolates in Belgium. J Med Microbiol. 2013;62(Pt 5):712–9. doi: 10.1099/jmm.0.048934-0. doi: 10.1099/jmm.0.048934-0. [DOI] [PubMed] [Google Scholar]