Abstract
Examining seasonal mortality patterns can yield insights into the drivers of mortality and thus potential selection pressures acting on individuals in different environments. We compiled adult and juvenile mortality data from nine wild non-human primate taxa to investigate the role of seasonality in patterns of mortality and address the following questions: Is mortality highly seasonal across species? Does greater environmental seasonality lead to more seasonal mortality patterns? If mortality is seasonal, is it higher during wet seasons or during periods of food scarcity? and Do folivores show less seasonal mortality than frugivores? We found seasonal mortality patterns in five of nine taxa, and mortality was more often tied to wet seasons than food-scarce periods, a relationship that may be driven by disease. Controlling for phylogeny, we found a positive relationship between the degree of environmental seasonality and mortality, with folivores exhibiting more seasonal mortality than frugivores. These results suggest that mortality patterns are influenced both by diet and degree of environmental seasonality. Applied to a wider array of taxa, analyses of seasonal mortality patterns may aid understanding of life-history evolution and selection pressures acting across a broad spectrum of environments and spatial and temporal scales.
Keywords: Climate change, fitness, natural selection, paleontology, phylogenetic comparative analysis, seasonality
Determining the selection pressures that act on individuals across different environments is crucial to understanding evolutionary processes. Throughout their evolutionary history, primates have inhabited a range of environments in dramatically different ecosystems (Reed and Fleagle 1995; Fleagle 1999). The varying selection pressures acting across these environments have played a central role in speciation (Coyne and Orr 2004) and led to a tremendous diversity of primate behaviors, diets, and morphologies (Fleagle 1984, 1999; Groves 2001; Strier 2007). The extent of environmental seasonality often varies across habitats and this variation has been widely implicated as a driver of life-history evolution and speciation (Boyce 1979; Wright 1999; Brockman and van Schaik 2005; Martin et al. 2009). An analysis of mortality patterns may provide insight into selection pressures acting in different environments, as well as aid in the interpretation of dietary strategies inferred from the fossil record. Primates are well suited for investigating the role of seasonality on mortality patterns and its implications for selection pressures, because their phylogeny is well resolved, facilitating comparative analyses. In addition, groups are followed over long periods so that conclusive, rather than inferred, mortality data are available. We examined mortality data in nine wild non-human primate taxa across three continents to understand how seasonal variation in rainfall and resource availability may act as selection pressures by influencing mortality.
Many factors contribute to primate mortality, including disease (Alexander 1974; Dunbar 1980; Milton 1996; Walsh et al. 2005; Kühl et al. 2008; Williams et al. 2008), predation (Cheney et al. 1981; Cheney and Wrangham 1987; Isbell 1994; Karpanty and Wright 2007; Teelen 2008; Irwin et al. 2009), injury during both interspecific and intraspecific interactions (van Schaik and Janson 2000; Cheney et al. 2006;Williams et al. 2008), and starvation (Dittus 1977, 1980; Dunbar 1980; Hamilton 1985). Resource availability for both primates and their predators, or other environmental variables, such as rainfall and temperature, may influence the importance of these different sources of mortality (Dunbar 1980; Richard 1985). Although the literature includes numerous reports of the causes of mortality, mortality patterns are rarely analyzed over broad spatial or temporal scales and have not been compared acrossmultiple species. If seasonal patterns inmortality are detectable, these could yield insight into the timing and drivers of selection pressures in primate populations (Richard 1985).
We expect causes of mortality to differ in the degree of seasonality they impose on mortality patterns, and for the relative contribution of these causes of mortality to overall mortality patterns to differ across species. For instance, primate habitats vary in the amount and seasonality of rainfall, whichmay lead to seasonal differences in resource availability (Janson and Verdolin 2005). There may be an increase in mortality during periods of resource scarcity related to increased competition for food, resulting in starvation in extreme cases (Dittus 1977, 1980; Hamilton 1985). In addition, it has been argued that leaves are a superabundant and evenly distributed resource over which primates will not have to compete (Isbell 1991; but see; Sterck and Steenbeek 1997; Snaith and Chapman 2005). During times of resource scarcity, foliage is likely more abundant and available than fruits (Wrangham 1980; Snaith and Chapman 2005), which may result in differences in seasonal mortality between folivores and frugivores.
Disease patterns are also commonly seasonal and may have important impacts on the behavior and life history of primates (Dunbar 1980; Nunn and Altizer 2006). Many vector-borne diseases increase in prevalence with increasing temperature and rainfall, as these affect arthropod vector distribution and abundance, parasite development, and parasite transmission rates (Harvell et al. 2002). Thus, elevated mortality during rainy seasons may be related to increased disease risk (Dunbar 1980; Milton 1996). Alternatively, seasonality of resources caused by a dry season may induce dietary stress as resource availability declines, depressing the immune system, increasing susceptibility to disease (Nelson 2004; Chapman et al. 2006), and potentially confounding the ultimate causes of mortality (i.e., starvation vs. disease). Such season-specific patterns in resource availability and disease susceptibility suggest that species living in more seasonal environments will exhibit more seasonal mortality patterns.
An analysis of seasonal mortality patterns can also aid in the interpretation of fossil assemblages. Vertebrate taphonomists are concerned with the ways in which the accumulation and preservation of fossils reflect palaeoenvironments (Behrensmeyer 1980; Behrensmeyer and Kidwell 1985; Alemseged 2003), the composition and abundance of source faunas and floras (Behrensmeyer et al. 2000), and even social behaviors (Barnosky 1985; Berger et al. 2001; Mihlbachler 2003). The relationship between fossil assemblages and seasonality is a matter of major concern (Kurten 1983; Rogers 1990; Lyman 1994; Lubinski and O’Brien 2001), especially with regard to interpreting dental microwear, which is used to infer diet. Short-term (e.g., seasonal) variations in diet can result in differences in microwear fabrics, a phenomenon amply documented in extant species (Teaford and Oyen 1989; Teaford and Glander 1991, 1996; Merceron et al. 2010). As a result of this turnover, microwear will fossilize information pertaining to items consumed just before an individual’s death (the so-called “Last Supper Effect”; Grine 1986). If primates typically die during the food-scarce period when their diets consist mainly of fallback foods (i.e., resources that are critical for a population’s survival and that may present significant structural obstacles to comminution, but that are not preferred food items; Marshall et al. 2009), then the dental microwear preserved in the fossil record would be expected to over-represent those fallback regimes.
For many species, it is unknown whether seasonal patterns in mortality exist, primarily because of the scarcity of long-term mortality data. However, a number of studies support the hypothesis that mortality in primates is linked to environmental variables. For example, howler monkey (Alouatta palliata) mortality on Barro Colorado Island, Panama, is highly seasonal (Otis et al. 1981) and most deaths occur in the rainy season. This corresponds with the seasonality of botfly parasitism, rather than changes in diet (Milton 1996). Similarly, among gelada baboons (Theropithecus gelada), mortality was highest during the rainy season, rather than the period of food scarcity (Dunbar 1980). Mortality is high in Milne Edwards’ sifakas (Propithecus edwardsi) at Ranomafana National Park in the drier winter months with fruit scarcity (Irwin et al. 2009), and is higher during years with major cyclones and a longer dry season than years with more evenly distributed rainfall (Dunham et al. 2008, 2010). Chacma baboons (Papio hamadryas ursinus) at the Moremi Game Reserve in Botswana have elevated mortality during the flooding season, predominantly due to predation (Cheney et al. 2004), whereas a population in the Namib Desert in Namibia had elevated mortality during severe droughts that resulted in malnutrition and starvation (Hamilton 1985).
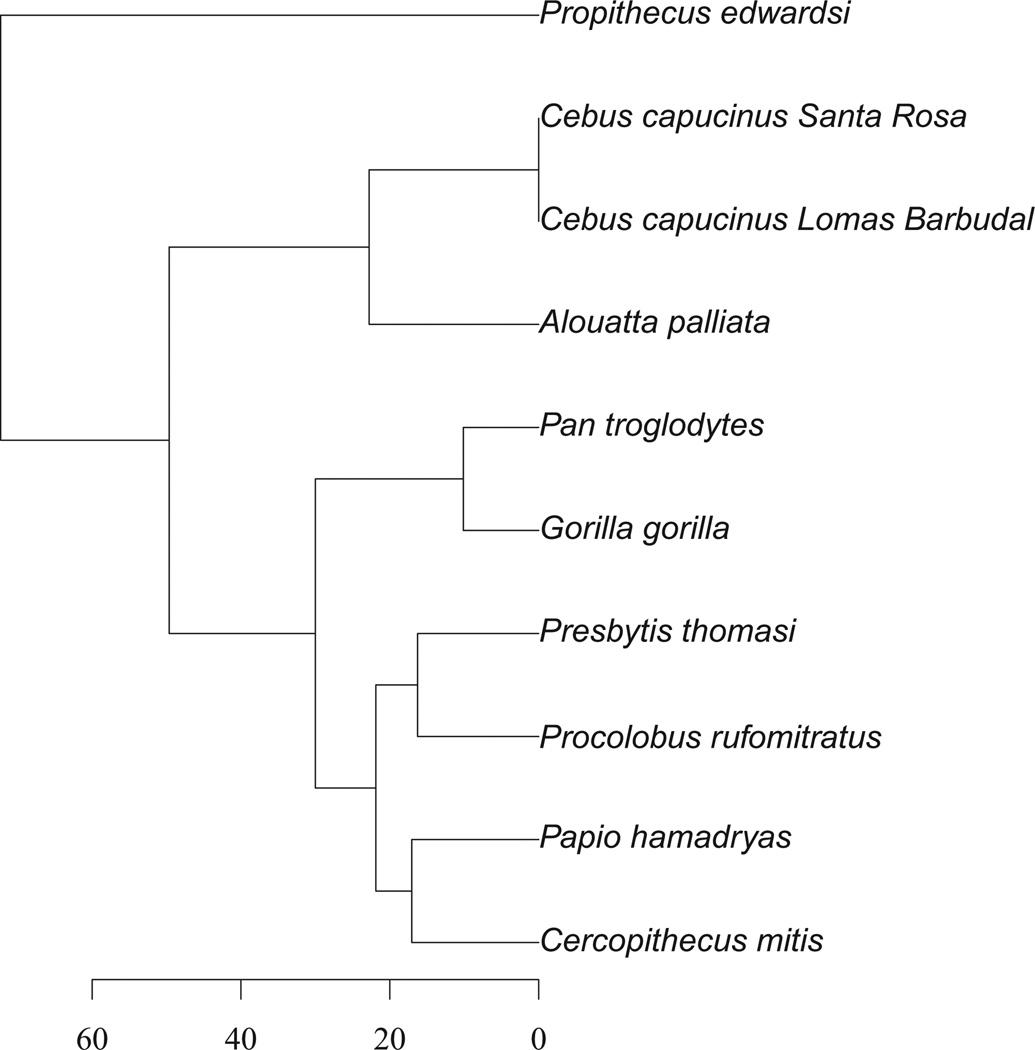
We sought to understand mortality patterns in a wide range of non-human primates to provide a comparative basis for understanding the variety and timing of selection pressures in different environments. To our knowledge, a comparison of mortality patterns across primate species and their interaction with seasonal resource scarcity and other environmental factors has never been performed. One reason is that there are few mortality data available to address the question. When data on primate mortality are available, they are typically presented in a way that does not facilitate examining seasonal patterns (e.g., life tables or survival studies: Pochron et al. 2004; Isbell et al. 2009; Cords and Chowdhury 2010; Bronikowski et al. 2011). Here, we compile and examine an extensive dataset on adult and juvenile mortality of nine wild non-human primate taxa representing diverse ecological conditions and spanning a broad temporal scale and range of the primate phylogeny (Fig. 1). These include primates from all major radiations, including one strepsirrhine (P. edwardsi), three New World monkeys (one A. palliata population and two Cebus capucinus populations), two cercopithecines (Cercopithecus mitis and P. hamadryas), one Asian colobine (Presbytis thomasi), one African colobine (Procolobus rufomitratus), and two African apes (Gorilla gorilla and Pan troglodytes). Diet varies across taxa, and our dataset includes five frugivorous and five folivorous populations (categorical diet classification follows that used by Nunn and van Schaik 2001). Rainfall seasonality also varies across sites, from marked seasonality to more evenly distributed rainfall throughout the year. We investigate five nonmutually exclusive hypotheses regarding the mortality patterns of primates: (1) mortality is highly seasonal across species, (2) mortality is higher during wet seasons than dry seasons, (3) mortality is higher during periods of fallback food consumption or food scarcity, (4) greater environmental seasonality corresponds with more seasonal mortality patterns, and (5) folivores have less seasonal mortality than frugivores.
Figure 1.
Phylogeny of the primate populations included in this analysis. Modified from a consensus tree (see text) from the 10kTrees project (Arnold et al. 2010), with the scale in millions of years.
Methods
DATA COLLECTION
We present mortality data from seven study sites and three published studies (Milton 1996; Watts 1998; Cheney et al. 2004), for a total of 10 wild primate populations. Ecological conditions vary across sites, with rainfall ranging from 430 to 3200 mm per year. Rainfall has a highly seasonal distribution at some sites, but not others (Table 1).
Table 1.
Details regarding study species, sites, sample sizes, and environmental variables.
| Species | Study site | Diet |
N deaths (years of study) |
Annual rainfall (mm) |
Rainfall seasonality (r-statistic) |
Wet season |
Food- scarce months(s) |
|---|---|---|---|---|---|---|---|
| Alouatta palliata | BCI, Panama | Fol | 179 (7) | 2600 | 0.320 | May–Nov1 | May–Dec |
| Cebus capucinus | Lomas Barbudal, Costa Rica | Fr | 31 (11) | 1600 | 0.536 | May–Nov2 | May |
| Cebus capucinus | Santa Rosa, Costa Rica | Fr | 20 (15) | 1820 | 0.540 | May–Dec3 | Oct–Mar |
| Cercopithecus mitis | Kakamega, Kenya | Fr | 46 (13) | 2000 | 0.198 | Mar–Nov4,11 | Mar–July and Nov |
| Gorilla gorilla | Karisoke, Rwanda | Fol | 38 (25) | 1810 | 0.115 | Mar–May and Sep–Dec5 | Feb and Jun–Oct |
| Pan troglodytes | Gombe, Tanzania | Fr | 112 (50) | 1500 | 0.456 | Nov–Apr6 | Weight loss: May–Nov Fruit scarce: Apr–May |
| Papio hamadryas | Moremi Game Reserve, Botswana | Fr | 38 (11) | 430 | 0.775 | Nov–Mar7,12 Flooding: Jul–Sep | Oct–Jun |
| Presbytis thomasi | Gunung Leuser, Indonesia | Fol | 42 (15) | 3230 | 0.088 | Oct–May8,11 | Fruit scarce: Sept–June Young leaf scarce: Apr–Oct |
| Procolobus rufomitratus | Kibale, Uganda | Fol | 11 (10) | 1700 | 0.122 | Sep–Nov and Mar–April9 | Jan–Feb and May–Aug |
| Propithecus edwardsi | Ranomafana, Madagascar | Fol | 23 (24) | 2970 | 0.262 | Nov–Mar10,11 | Apr–Oct |
Fr indicates predominantly frugivorous species whereas Fol indicates predominantly folivorous species (see text for details). Sources for annual rainfall, rainfall seasonality, and wet season distinction:
(Fedigan and Jack (2001); Campos and Fedigan (2009); L. Fedigan, unpubl. data
M. Cords, unpubl. data; Ekernas and Cords (2007)
Goodall (1986); Janson and Verdolin (2005); rainfall data recorded at Kasakela Station 942909 by the Directorate of Meteorology, Tanzania from 1989 to 1997, available via Jim Moore’s Gombe Climatological Data webpage: http://weber.ucsd.edu/~jmoore/apesites/Gombe/GombeClimate.html#Rain
The distinction between the wet and dry season is difficult to make and there is high interannual variability in this environment.
We could test only the period Oct–Mar because mortality data were binned in three-month intervals (Cheney et al. 2004).
The dataset includes only animals that were found dead (i.e., a carcass was found), or whose disappearance could confidently be registered as a death because at least one of three criteria was met: (1) an individual disappeared from the population that was a member of the nondispersing sex, (2) a juvenile disappeared before reaching the minimum known dispersal age, or (3) an individual was clearly ill or seriously wounded prior to disappearance. We included only those deaths that could be assigned to a given month of the year, using death dates with an error not exceeding ±15 days, with the midpoint between the minimum and maximum date of death used in the analysis (with the exception of one dataset available from the literature: see below). Sampling effort for the study periods we included was generally equally distributed over all months of the year. Many species exhibit seasonal reproduction (Janson and Verdolin 2005) and elevated infant mortality immediately following birth (Altmann et al. 1977; Dunbar 1988; Wright 1995; Cheney et al. 2004; Isbell et al. 2009; Jin et al. 2009), so we focused on adult and juvenile mortality. Identification of infants differs among populations, and is described below.
Each of our species was either frugivorous or folivorous based on the food category that dominated feeding time (following: Nunn and van Schaik 2001). For all species, we defined a food-scarce period. Where possible, we used site-specific plant phenology data to define this period. In other cases, we used feeding or weight loss data and observations from researchers working with the species at the site (Table 1). In addition, we delineated wet and dry seasons using long-term rainfall records combined with literature descriptions (Table 1).We describe specific details on the methods at each site below.
STUDY SITES AND SPECIES: DIRECT OBSERVATIONS
White-faced capuchins (C. capucinus)
S. Perry and L. Fedigan collected mortality data on C. capucinus from populations at two sites in northwestern Costa Rica: Lomas Barbudal and Santa Rosa National Park (site, population, and methods described in Perry 2009 and Fedigan and Jack 2001, respectively). At Lomas Barbudal, year-round observations (i.e., monthly) occurred during 11 years (1992, 2001–2010). Many trees fruit in the dry season in this forest and the only time the monkeys seemed short of food was in May, which we defined as the food-scarce period (S. Perry, pers. obs.).Among the C. capucinus at Lomas Barbudal, some of the causes of mortality among juveniles and adults were anthropogenic (e.g., poaching, car accidents, and electrocution), but these deaths were not included in this analysis.
For C. capucinus at Santa Rosa National Park, we defined the food-scarce period using phenology records of common food plants (data and analysis available in Carnegie et al. 2011). Reliable recording of death/disappearance dates began in 1988, but we included only the 15 years in which there were no gaps in data collection. For both populations of C. capucinus, we excluded individuals younger than one year of age, the age at which individuals are no longer riding dorsally and after which suckling is infrequent (Fedigan et al. 2008).
Blue monkeys (C. mitis)
M. Cords collected mortality data in C. mitis from 1997 to 2009 in Kakamega Forest, Kenya (site, population, and methods described in Cords 1987; Pazol and Cords 2005; Ekernas and Cords 2007; Cords and Chowdhury 2010).We identified periods of fruit scarcity based on five years of phenological monitoring combined with vegetation surveys that contributed information about the abundance and size of different food trees (M. Cords, unpubl. data). We excluded young infants, defined here as individuals younger than one year old, the age of the youngest orphan to have survived independently (M. Cords, unpubl. data).
Chimpanzees (P. troglodytes)
J. Goodall, A. Pusey, and colleagues collected mortality data on P. troglodytes from 1960 to 2010, from two communities in Gombe National Park, Tanzania (for a description of the site see Wrangham 1977; Goodall 1986; for a description of the database see Strier et al. 2010). Phenology data were not available, so we identified the period of food scarcity as (1) months during which average female body mass decreased (Pusey et al. 2005), and (2) months in which fruits were observed to be less available and contributed less to the overall diet (Wrangham 1977; Goodall 1986; Pusey et al. 2005). We excluded individuals younger than two years because the period of high mortality associated with infancy in P. troglodytes drops off after reaching this age (Goodall 1986).
Thomas langurs (P. thomasi)
S. Wich, E. Sterck, and others collected monthly mortality data for P. thomasi from 1987 to 2001 at for the Ketambe research site in Gunung Leuser National Park, Sumatra, Indonesia (site, population, and methods described in Wich et al. 2007). Presbytis thomasi are generally folivorous (Steenbeek and van Schaik 2001), but young leaf production does not always display predictable seasonal patterns in this ecosystem (van Schaik 1986). In addition, P. thomasi prefer fruits when they are available (Steenbeek and van Schaik 2001). Fruit production peaks in July and August, which we assigned as the period of food abundance (van Schaik 1986). In addition, we tested whether there was decreased mortality in the period when young leaf flushes were most common (Nov–Mar: van Schaik 1986). We excluded infants younger than 14 months, the age at which individuals can survive on their own (Sterck et al. 2005).
Red colobus (P. rufomitratus)
C. Chapman, L. Chapman, and colleagues collected data on P. rufomitratus mortality at Kibale National Park, Uganda between 2005 and 2011 (site, population, and methods described in Chapman and Lambert 2000; Chapman et al. 2007). We included two additional deaths reported for the same population during observations between 1970 and 1974 (Struhsaker 1975). To assess the food-scarce period, we used phenology data of P. rufomitratus foods collected between January 2007 and September 2010 to determine food availability (C. A. Chapman and L. J. Chapman, unpubl. data). We excluded infants from the analysis, defined here as those individuals less than half the size of an adult female (Struhsaker 1975).
Milne-Edwards’ sifakas (P. edwardsi)
P. Wright collected mortality data for P. edwardsi at Ranomafana National Park, Madagascar, between 1986 and 2009 (study site, population, and methods described in Wright 1995).Young leaves and fruits are more abundant during the wet season, so we defined this as the period of food abundance (Wright 1999). For P. edwardsi, we excluded infants, defined here as individuals that are not weaned (occurs at one year of age: King et al. 2011).
STUDY SITES AND SPECIES: LITERATURE DATA
Howler monkey (A. palliata)
We obtained data on A. palliata mortality from surveys for cadavers on Barro Colorado Island (BCI), Panama, conducted between 1986 and 1993 (study site, population, and methods described in Milton 1996). A total of 179 adult and juvenile A. palliata deaths were recorded (Milton 1996). Although this species is predominantly folivorous, fruits constitute a substantial (>40%) part of its diet when they are available (Milton 1979), and flower and fruit production peaks in the late dry season (Jan–Apr; Zimmerman et al. 2007). We therefore regarded this period as the season of food abundance. For A. palliata, we excluded infants, defined by Milton (1996) as being younger than one year of age.
Mountain gorilla (G. gorilla)
We obtained G. gorilla mortality data from the Karisoke Research Centre, in the Parc National des Volcans, Rwanda, and from long-term Karisoke records on G. gorilla demography and morbidity (site, population, and methods described in Watts 1998). Data collection began in 1967 andwas continuous until 1991. Although the G. gorilla diet is fairly uniform throughout the year, these apes preferentially eat bamboo when new shoots are available; thus, we used the period of bamboo consumption as the time of food abundance (Watts 1998). For G. gorilla, births are nonseasonal and reported infant mortality rates are low (Watts 1991; 1998). We excluded all cases of infanticide, but published data include other cases of infant mortality (Watts 1998).
Chacma baboon (P. hamadryas ursinus)
We obtained P. hamadryas mortality data between 1992 and 2002 from the Moremi Game Reserve, Botswana (study site, population, andmethods described in Cheney et al. 2004). Dates of death for five individuals who died from illness were not available, so we excluded these individuals from the analysis (Cheney et al. 2004). Unlike the previous studies, in which the date of death could be confidently ascribed to a particular month, we had to partition P. hamadryas data into four three-month bins (Jan–Mar, Apr–June, July–Sept, and Oct–Dec: Cheney et al. 2004). The Moremi Game Reserve is a highly seasonal environment that undergoes yearly flooding after the rainy season, so we also tested whether mortality was higher during this period. Although plant phenology data are not available from this site, Cheney et al. (2004) describe food as being more plentiful following high rainfall, specifically in the months following July and August. For P. hamadryas, infants were defined by Cheney et al. (2004) as being younger than 12 months, thus we excluded these death dates from our analysis.
DATA ANALYSIS
We used circular statistics to test for seasonality in primate mortality because they permit analysis of data on a continuous axis and do not require any arbitrary decision about how to divide the year into repeating units (Batschelet 1981; Jammalamadaka and Sengupta 2001; Janson and Verdolin 2005). To illustrate the value of circular statistics, consider the following extreme example where, in a hypothetical species, 30 deaths occurred in December, and another 30 occurred in February. If one uses a linear framework and takes the standard division of a year from January–December and attempts to calculate the mean month of death for this species, one will get July. By contrast, if one divides the year based on an American academic calendar starting the year in September, one would obtain a mean month of death of January. Similarly, disparate results would result for most other descriptive statistics (e.g., variance and the coefficient of variation) and for many different statistical tests (e.g., Kolmogorov–Smirnov test). The mean vector length (r) obtained from circular statistical analyses is well suited as an index of seasonality, and the r-statistic is robust to differences in sample size facilitating the comparison of seasonality measures between species (Janson and Verdolin 2005). For mortality, when deaths are spread evenly across months (not seasonal), r is close to zero, whereas when deaths all occur at exactly the same time of year (extremely seasonal), r is one. We conducted Rayleigh tests to test a null hypothesis of a random distribution of primate deaths across the 12 months of a year (i.e., whether there is statistical evidence of directedness or seasonality to mortality). To test for bimodal distributions using the r-statistic, we doubled the angles calculated for each month (except for P. hamadryas for which data were in three month bins, which precluded the use of this test; Janson and Verdolin 2005). Because of uncertainty in death dates, we grouped data by month and used a correction factor for grouping (c = 1.0115, except for P. hamadryas for which data were in three month bins and c = 1.1107) when calculating r-statistics (Batschelet 1981).
To compare mortality between wet and dry seasons, or between food-scarce and food-plentiful periods, we conducted G-tests on mortality grouped by month, with expected values related to the number of months that were wet versus dry, or in which food was scarce versus abundant (McDonald 2009). We used R 2.13.1 for all statistical analyses (R Core Development Team 2011).
To account for nonindependence of datapoints related to the shared evolutionary history of the species included in this analysis, we used phylogenetic generalized least squares (PGLS) regression (Felsenstein 1988; Rohlf 2001). PGLS uses an estimate of phylogenetic correlation, Pagel’s λ, that varies between zero (indicating phylogenetic independence) and one (indicating species’ traits covary in direct proportion to the degree of shared evolutionary history) and controls for this phylogenetic nonindependence of species values in a generalized least squares framework (Freckleton et al. 2002). We used the R packages “ape” and “caper” to conduct PGLS regressions and for all phylogenetic analyses (Paradis et al. 2004; Orme et al. 2011).We used the consensus tree of the taxa of interest from the 10kTrees project (Arnold et al. 2010). For those species or subspecies for which genetic data were not available, we used a well-established sister taxon that excluded all other species or subspecies in the analysis (e.g., G. g. gorilla was used in place of G. g. beringei). To incorporate the two populations of C. capucinus, we added a short branch of 10,000 years to the tip of the C. capucinus lineage (Fig. 1; Martins et al. 2002).
To test for a relationship between the degree of seasonality of the environment and the degree of seasonality of mortality, we conducted a bivariate PGLS regression of the r-statistic of rainfall on the r-statistic of mortality. In addition, because of the predicted impact of diet on the relationship between seasonality of environments and mortality patterns, we conducted a multiple PGLS regression of the r-statistic of rainfall and diet on the r-statistic of mortality. We treated diet as a dichotomous categorical variable (i.e., species were categorized as either frugivorous or folivorous; Table 1).
Results
Hypothesis 1: Mortality is highly seasonal across species.
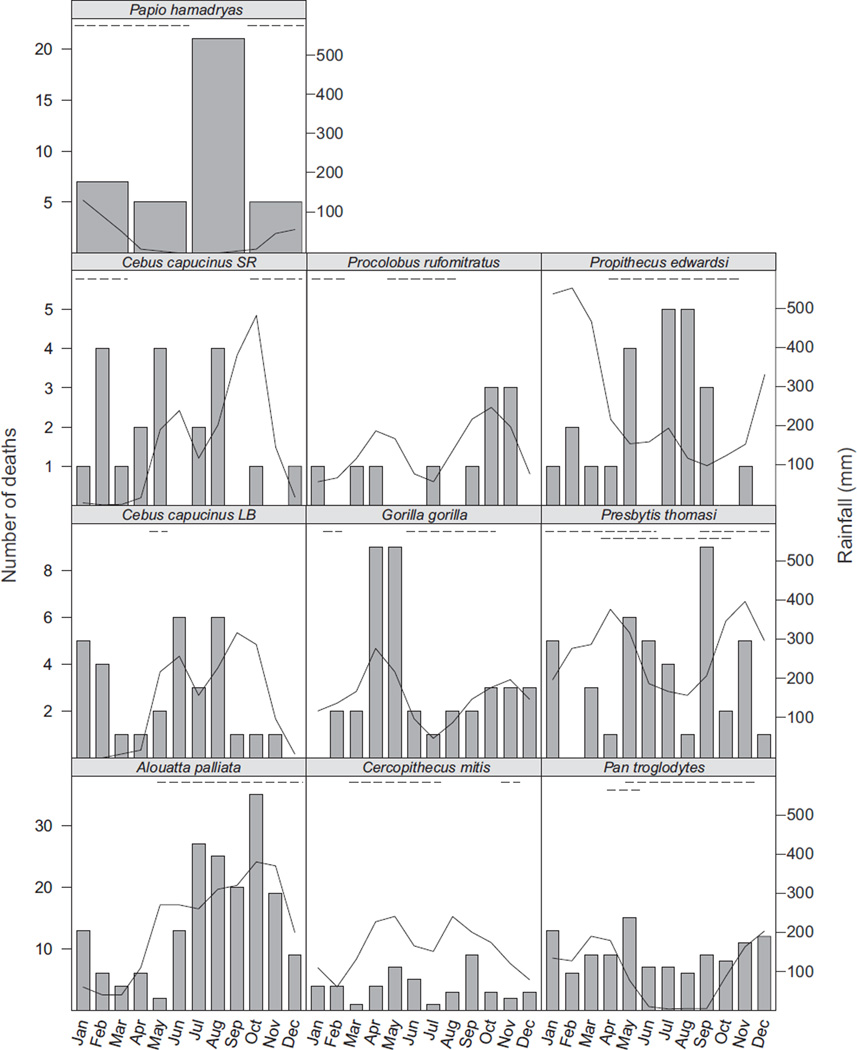
Mortality was seasonal in A. palliata, G. gorilla, P. hamadryas, and P. edwardsi (Table 2). Procolobus rufomitratus had the highest r-statistic of mortality and although this r-statistic was not significant, the results of the G-tests further suggest mortality is seasonal in this species (Table 2). We did not detect significant seasonal mortality in P. troglodytes, C. mitis, P. thomasi, or C. capucinus (Table 2). Procolobus rufomitratus had the highest r-statistic of mortality (r = 0.445) and P. troglodytes had the least seasonal mortality. The two populations of C. capucinus had similar r-statistics of mortality (Lomas Barbudal r = 0.198, Santa Rosa r = 0.258) and very similar patterns of mortality (Fig. 2), including a four-month stretch (Sept–Dec) in which only five of 51 deaths occurred (Lomas Barbudal: three of 31 deaths, Santa Rosa: two of 20 deaths). Propithecus edwardsi had a high r-statistic of mortality (r = 0.382) as did A. palliata (r = 0.413), P. hamadryas (r = 0.409), and G. gorilla (r = 0.297), whereas C. mitis (r = 0.154) and P. thomasi (r = 0.189) exhibited much lower seasonal mortality patterns (Table 2).
Table 2.
By-species results for tests of seasonality in mortality patterns.
| Deaths in food-scarce period | Deaths in wet season | ||||||
|---|---|---|---|---|---|---|---|
| (N deaths) | Mortality seasonality (r-statistic) |
Deaths observed (%) |
Deaths expected (%) |
G- test results |
Deaths observed (%) |
Deaths expected (%) |
G-test results (G) |
| Alouatta palliata (179) | 0.413*** | 84 | 67 | 26.77*** | 79 | 58 | 33.46*** |
| Cebus capucinus Lomas Barbudal (31) | 0.198 | 7 | 8 | ns | 65 | 58 | 0.50 ns |
| Cebus capucinus Santa Rosa (20) | 0.258 | 40 | 50 | 0.81 ns | 60 | 67 | 0.39 ns |
| Cercopithecus mitis (46) | 0.096 | 44 | 50 | 0.78 ns | 76 | 75 | 0.03 ns |
| Gorilla gorilla (38) | 0.297* | 321 | 50 | 5.28*1 | 82 | 58 | 9.37** |
| Pan troglodytes (112) | 0.077 | WL:56 FS:21 |
58 | 0.20 ns 1.71 ns |
54 | 50 | 0.57 ns |
| Papio hamadryas (38) | 0.409** | 451 | 75 | 15.75***1 | WS:322 FL:55 |
50 |
5.28*2 15.75*** |
| Presbytis thomasi (42) | 0.189 | FS:88 LS:67 |
83 58 |
0.75 ns 1.23 ns |
55 | 67 | 2.56 ns |
| Procolobus rufomitratus (11) | 0.445 | 181 | 50.0 | 4.82*1 | 82 | 42 | 7.48** |
| Propithecus edwardsi (23) | 0.400* | 78 | 58 | 4.07* | 22b | 42 | 4.08*2 |
WL indicates period of weight loss, FS indicates period of fruit scarcity, and LS indicates period of young leaf scarcity. WS indicates wet season whereas FL indicates period of flooding (see text for details). Bold values indicate significant results with ***P < 0.001, **P < 0.01, and *P < 0.05. Nonsignificant results are indicated with ns.
Fewer deaths during food-scarce period than expected.
Mortality was higher than expected in the dry period.
Figure 2.
The number of deaths in each month for the 10 populations included in the current study. Bars indicate number of deaths with the axis on the left. The solid line indicates the monthly rainfall in mm, with the axis on the right. The food-scarce period is indicated by the dashed line at the top of each population’s graph, and the population is indicated in the header above the graph. For Presbytis thomasi, the topmost dashed line indicates the period of fruit scarcity, whereas the lower indicates the period of young leaf scarcity. For Pan troglodytes, the topmost dashed line indicates the period of weight loss, whereas the lower line indicates the period of fruit scarcity.
We detected evidence for a bimodal pattern to mortality, with an increase in the r-statistic after doubling the angles associated with each month (see methods), for the C. capucinus population at Lomas Barbudal (r = 0.380, P = 0.009) and G. gorilla (r = 0.438, P = 0.0005). Although there was an increase in the r-statistic for P. troglodytes (r = 0.120, P = 0.191) when testing for bimodality, the difference from the unimodal r-statistic was minimal and the distribution of deaths was still not significantly seasonal. In the case of G. gorilla, the bimodal pattern to mortality may be linked with the bimodality of rainfall. For the population of C. capucinus at Lomas Barbudal, the bimodal pattern to mortality does not appear to be related to rainfall or food availability (Fig. 2 and the following two sections).
Hypothesis 2: Mortality is higher during wet seasons than dry seasons.
Three of the 10 populations (P. rufomitratus, A. palliata, G. gorilla) had elevated mortality during the wet season. Propithecus edwardsi had elevated mortality during the dry season, and P. hamadryas had elevated mortality during the annual period of flooding (Fig. 2; Table 2). Four of the five folivorous species had seasonal mortality (three in the wet season and one in the dry season), whereas only one of the five frugivorous populations had seasonal mortality (during the period of flooding).
Hypothesis 3: Mortality is higher during periods of fallback food consumption or food scarcity.
Two species (A. palliata and P. edwardsi) had elevated mortality during the food-scarce period, whereas three species (P. hamadryas, P. rufomitratus, and G. gorilla) had elevated mortality during the food-abundant period. Both species with elevated mortality during the food-scarce period also exhibited an effect of rainfall on mortality (Fig. 2, Table 2).
Hypotheses 4 and 5: Greater environmental seasonality leads to more seasonal mortality patterns, and folivores have less seasonal mortality than frugivores.
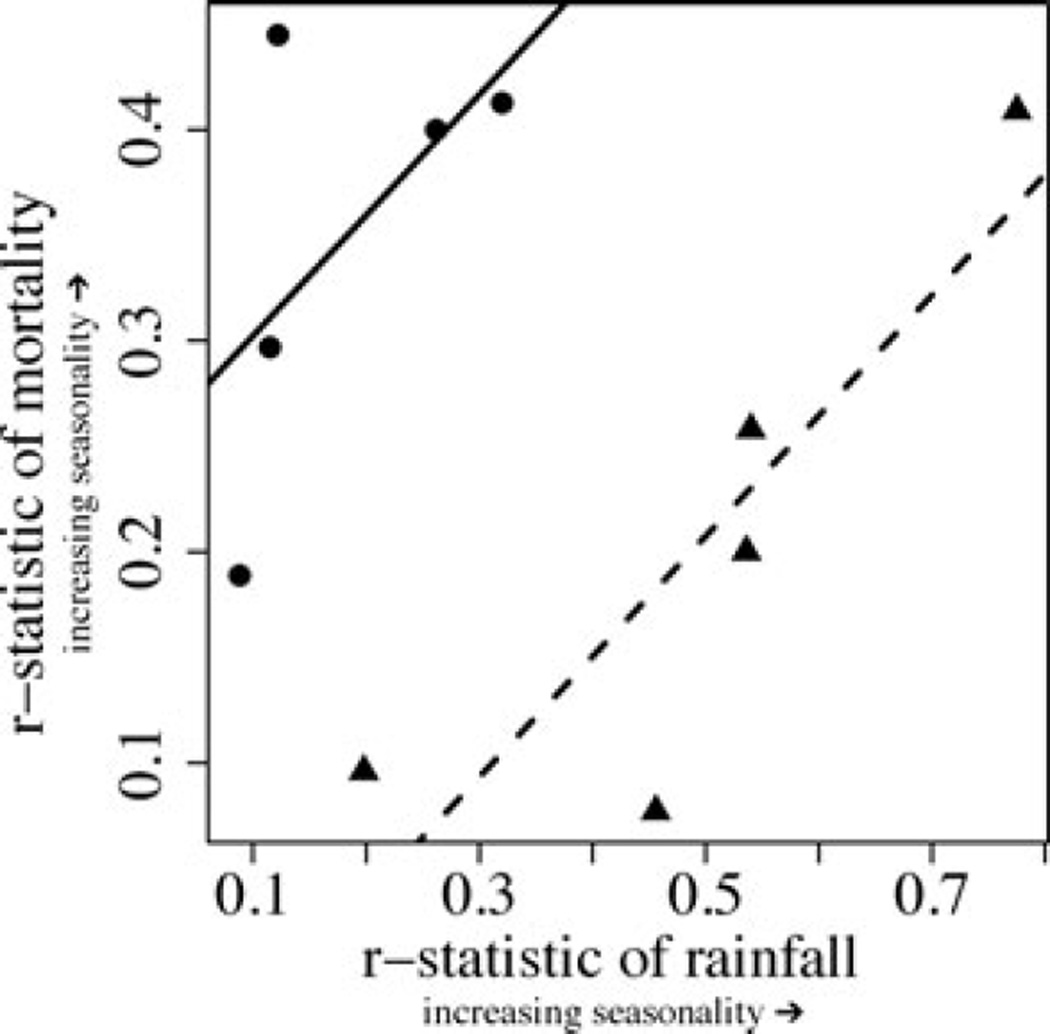
There was no significant relationship between the degree of rainfall seasonality (the r-statistic of rainfall) and the degree of seasonality of mortality (the r-statistic of mortality) when diet was not included as factor in the model (R2 = 0.0007, F[2,8] = 0.005, P = 0.995, λ < 0.001). When diet was included in the model, the overall model was significant (R2 = 0.636, F[3,7] = 8.880, P = 0.009, λ < 0.001), as were both factors (diet: T = 4.210, P = 0.004; r-statistic rainfall: T = 3.223, P = 0.015). Folivores exhibited more seasonal mortality than frugivores, and greater seasonality of environments was associated with higher seasonal mortality in both frugivores and folivores (Fig. 3).
Figure 3.
The relationship between the seasonality of mortality (the r-statistic of mortality) and the seasonality of the environment (the r-statistic of rainfall) for the 10 primate taxa in the dataset (see Methods for details). Circles represent folivorous species and triangles represent frugivorous species. The lines represent the results of the multiple PGLS regression of the r-statistic of rainfall and diet on the r-statistic of mortality (dashed lines: frugivorous species, solid line: folivorous species).
Discussion
Nearly half of the primate species examined exhibited seasonal mortality, although none of the r-statistics approached the seasonality observed for other aspects of life-history traits (e.g., P. edwardsi has an r-statistic for births of 0.887, and the highest r-statistic of mortality in the current study was 0.445; Janson and Verdolin 2005). Even for the most seasonal patterns of mortality, deaths still occurred throughout the year. The occurrence of deaths throughout the year likely reflects the multiple causes of mortality such as predation, disease, and injury, which may act independently and be differentially influenced by environmental seasonality.
Where it occurred, seasonal mortality was tied to rainfall patterns. We found only limited support for the hypothesis that mortality is higher during periods of food scarcity. Contrary to our prediction, three of the five species that demonstrated seasonal mortality in relation to diet actually had elevated mortality during the food-abundant period. Also contrary to our prediction, seasonal mortality patterns appeared to be more common in folivorous than frugivorous species.
In addition, we found that mortality was more seasonal in more seasonal environments when diet (i.e., frugivore vs. folivore) was included in the model explaining variance in mortality patterns (Fig. 3). The PGLS analysis showed that folivores exhibited more seasonal mortality than frugivores at any given degree of rainfall seasonality. Interestingly, the small values of Pagel’s λ estimated in the PGLS regressions suggest that there is not a strong phylogenetic component to this relationship. Either this relationship is ecologically based, or small sample sizes may have limited our ability to detect a significant phylogenetic signal. Our dataset did not include folivores in environments that were as seasonal as the environments occupied by the frugivores, although there was considerable overlap in the seasonality of environments of the species in both dietary categories. The apparent pattern of folivores exhibiting more seasonal mortality patterns than frugivores is perhaps more striking, given that the folivorous species tended to come from less seasonal environments than the frugivorous species. The different relationships between the seasonality of the environment and seasonality of mortality exhibited by frugivores and folivores is intriguing and suggests that dietary strategy is tied to the selection pressures exerted by seasonality in different environments.
IMPLICATIONS FOR SELECTION AND ECOLOGY
The pattern of increased mortality in the wet season observed for some of the populations may result from seasonal exposure to diseases. Increased moisture has been associated with increased parasitism both within (Huffman et al. 1997; Chapman et al. 2010b; van Dijk et al. 2010) and between species (Nunn and Altizer 2006: pp. 92–95).Analysis of human diseases has revealed a strong association between parasite diversity and latitude, which is driven in large part by climatic variables, including temperature and precipitation (Guernier et al. 2004). Similarly, a comparative analysis across primates demonstrated that white blood cell counts, a measure of immune function, exhibit a strong positive correlation with rainfall (Semple et al. 2002). The findings of Semple et al. (2002) suggest that disease exerts a strong selective force on populations, and that the nature of this selection is mediated by ambient rainfall. Even if the direct cause of death were predation, animals suffering the ill effects of disease would likely be most vulnerable (Penteriani et al. 2008; Genovart et al. 2010).
In P. edwardsi and P. hamadryas, the majority of deaths are caused by predation and both species exhibit strongly seasonal mortality (Wright 1998; Cheney et al. 2004; Irwin et al. 2009; Wright et al. 2009). Among P. troglodytes, G. gorilla, and A. palliata, the majority of deaths have been attributed to disease and parasitism, with G. gorilla and A. palliata exhibiting strongly seasonal mortality (Milton 1996; Watts 1998; Williams et al. 2008). Given that 58% of the P. troglodytes deaths of known causes in the Kasekela community have been attributed to disease, and other studies have shown seasonal (albeit conflicting) patterns in disease and parasitism (Goodall 1983; Gillespie et al. 2010; Lonsdorf et al. 2011), it is perhaps surprising that we detected no seasonal mortality in chimpanzees. This is especially intriguing given the large size of our study sample. Several factors may contribute to the lack of seasonal mortality observed. First, the Gombe chimpanzees have recently been shown to have a high prevalence of SIVcpz (Keele et al. 2009). If SIV-induced mortality is nonseasonal but important, it might drive the lack of seasonal mortality observed in this population. However, deaths in humans with acquired immunodeficiency syndrome (AIDS) are often seasonal, possibly because of susceptibility to other diseases that are seasonal (Lin and Nichol 2001). Second, the Gombe chimpanzee communities were initially provisioned and occasionally medicated throughout the study; both of these interventions may have contributed to the lack of seasonality, although the data encompass a lengthy period after provisioning ceased (Pusey et al. 2008). Third, the fission–fusion societies of chimpanzees, the use of medicinal plants, and other behavioral, physiological, or social adaptations of chimpanzees might reduce disease risk during the rainy season (Huffman and Wrangham 1994). Lastly, it is possible that different sources of mortality vary in their seasonality and in when they exert their greatest effect, combining to create a nonseasonal pattern to mortality. For example, the chimpanzees at Gombe hunt more often in the fruit-abundant dry periods (Stanford et al. 1994). This hunting could increase the probability of acquiring diseases from their preferred colobus prey during the dry season, whereas wet season mortality may be high due to heightened risk of parasitism (Gillespie et al. 2010). Further studies on the seasonality of specific causes of death may help explain the observed patterns.
Contrary to expectation, we found that folivores exhibitmore seasonal mortality than frugivores. Although early studies suggested that folivores may not be resource limited because leaves are abundant, recent studies suggest that folivores exhibit within-group competition over resources and are in fact resource-limited (Koenig 2000; Snaith and Chapman 2005; Snaith and Chapman 2007; Borries et al. 2008; Harris et al. 2010). In addition, young leaf flushes are often seasonal events and all folivores in the current study, with the exception of G. gorilla, exhibited some degree of seasonal frugivory. Leaf-eating primates also ingest larger volumes of food than frugivores, leading them to ingest more parasites whose infectious stages contaminate leaf materials. Comparative studies have indicated that the degree of folivory is positively correlated with diversity of helminth species across primate species (Nunn et al. 2003; Vitone et al. 2004). Thus, the higher seasonal mortality apparent in folivores may result from a combination of seasonal abundance of pathogens combined with a greater exposure to pathogens owing to diet. Frugivores may also better adapted physiologically, behaviorally, or socially to energy fluctuations in the environment because of the seasonal availability of many fruits (Jones 2011); these adaptations may render them less prone to seasonal mortality. The different mortality patterns associated with alternative dietary strategies suggest differential selection pressures are acting on species with different diets. The varying selection pressures exerted by seasonality on frugivores and folivores may be a mechanism driving diversification of dietary strategies among primates.
The available data forced us to use rather broad categorical variables for assessing food availability and environmental seasonality. Although it would likely be informative, we were unable to evaluate interannual variation in the current analysis. In addition, we were unable to assess the degree of food scarcity across sites and how variation in food scarcity relative to the animals’ requirements influences the degree of seasonal mortality. Future studies should incorporate these factors as continuous variables measured on a finer scale, which will increase statistical power and allow for tests of the interaction between factors. Due to limitations of our data, we were also unable to incorporate infant mortality into the current analysis. However, age-structured models suggest that human fitness may be particularly sensitive to changes in prereproductive survival probabilities (Jones 2009), and understanding infant mortality patterns represents an important area of future research. Although we have done our best to control for variation in sampling methods between species and sites, the slow life history of primates means that sample sizes are generally small. Further, long-term studies will be useful in confirming these results and clarifying the particular causes of the seasonal mortality patterns documented here.
The seasonal nature of mortality in some primates suggests that climate change may contribute to changing mortality patterns (Dunham et al. 2008, 2010), potentially altering selection pressures. Increases in seasonality and extreme weather events are predicted with climate change (Frich et al. 2002; Tebaldi et al. 2006; Kharin et al. 2007), especially in the tropical environments that many primates inhabit (IPCC 2001). One-third of primate species are currently threatened with extinction (IUCN 2010) and changes in plant phenology patterns along with shifts in disease dynamics associated with climate change have already been documented for many plant and animal species (Parmesan and Yohe 2003; Parmesan 2006). Increases in primate mortality and changes to mortality patterns are also expected (Pedersen et al. 2007). However, climate change may also reduce certain vector-borne diseases in some tropical areas (e.g., Paaijmans et al. 2009); understanding the interaction between climate change and disease dynamics presents a promising area of future research. Identifying the regions where changing seasonality of climate will coincide with and potentially amplify mortality patterns may provide a means of setting conservation priorities.
IMPLICATIONS FOR PALEONTOLOGY
We found limited support for the idea that microwear patterns in the fossil record will over-represent periods of food scarcity. The primates we examined did not die preferentially during periods of food scarcity, and several species actually exhibited elevated mortality when food was abundant. In addition, despite the “Last Supper Effect” (Grine 1986), the distribution of mortality across seasons, even when there was a seasonal pattern, suggests that microwear studies incorporating teeth from many individuals may capture the entire diet of a species. Chimpanzees are perhaps the best model for the diet of our hominin ancestors, and yet the chimpanzees of Gombe demonstrated the least seasonal mortality of all species, further suggesting that microwear will not over-represent a given period of the year in the hominin fossil record.
Taphonomic processes play a role in fossil preservation and fossil assemblages may therefore not represent the same seasonal pattern as the animals that die. Fossils may be more likely to be preserved when animals die in a particular season of the year (e.g., in the rainy season or during periods of flooding), which would influence the types of microwear patterns preserved in the fossil record. Our findings suggest that these effects may be more universally important than seasonal mortality in influencing the fossil record and its interpretation.
SUMMARY
We found that primate mortality was seasonal in approximately half the primate species examined, and it was more often tied to seasonal patterns of rainfall than seasonal patterns of food availability. These results suggest that selection pressures on some populations may be strongest during the wet season. We also found that more seasonal environments were associated with increased seasonal mortality and that the nature of this relationship is influenced by dietary strategy, with folivores exhibiting more seasonal mortality patterns than frugivores. Climate change may exacerbate the observed effects and patterns reported here. Application of these methods to a broader array of vertebrate taxawould further elucidate the role of seasonality in mortality patterns.
Supplementary Material
ACKNOWLEDGMENTS
We thank C. Borries, M. Campenni, J. Herrera, C. Janson, S. King, A. Koenig, J. Lodwick, S. Tecot, D. Watts, J. J. Wiens, and J. van Woerden for discussions, suggestions, and encouragement. For training in phylogenetic comparative methods, JFG thanks the AnthroTree Workshop supported by the National Science Foundation (BCS-0923791) and the National Evolutionary Synthesis Center (NSF grant EF-0905606), and specifically C. Nunn. While conducting this study, JFG was supported by a Graduate Research Fellowship from the National Science Foundation. We thank all who contributed to the long-term data used here, and without whom this project would not have been possible. The governments of Costa Rica, Indonesia, Kenya, Madagascar, Tanzania, and Uganda provided permission for our field studies. Given the long-term nature of this fieldwork, it is not possible to acknowledge all funding sources and collaborators; study-specific acknowledgments and Institutional Animal Care and Use Committee compliance are available as online supplemental information. Any opinions, findings, and conclusions, or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation or other granting agency.
Footnotes
Supporting Information
The following supporting information is available for this article:
Appendix S1. Supplemental Acknowledgements.
Supporting Information may be found in the online version of this article.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
LITERATURE CITED
- Alemseged Z. An integrated approach to taphonomy and faunal change in the Shungura Formation (Ethiopia) and its implication for hominid evolution. J. Hum. Evol. 2003;44:451–478. doi: 10.1016/s0047-2484(03)00012-5. [DOI] [PubMed] [Google Scholar]
- Alexander RD. The evolution of social behaviour. Annu. Rev. Ecol. Syst. 1974;5:325–382. [Google Scholar]
- Altmann J, Altmann SA, Hausfater G, McCuskey SA. Life history of yellow baboons: physical development, reproductive parameters, and infant mortality. Primates. 1977;18:315–330. [Google Scholar]
- Arnold C, Matthews L, Nunn C. The 10kTrees website: a new online resource for primate phylogeny. Evol. Anthropol. 2010;19:114–118. [Google Scholar]
- Barnosky AD. Taphonomy and herd structure of the extinct Irish elk, Megaloceros giganteus. Science. 1985;228:340–344. doi: 10.1126/science.228.4697.340. [DOI] [PubMed] [Google Scholar]
- Batschelet E. Circular statistics in biology. New York: Academic Press; 1981. [Google Scholar]
- Behrensmeyer AK. Fossils in the making. Chicago, IL: Univ. of Chicago Press; 1980. [Google Scholar]
- Behrensmeyer AK, Kidwell SM. Taphonomy’s contributions to paleobiology. Paleobiology. 1985;11:105–119. [Google Scholar]
- Behrensmeyer AK, Kidwell SM, Gastaldo RA. Taphonomy and paleobiology. Paleobiology. 2000;26:103–147. [Google Scholar]
- Berger J, Dulamtseren S, Cain S, Enkkhbileg D, Lichtman P, Namshir Z, Wingard G, Reading R. Back-casting sociality in extinct species: new perspectives using mass death assemblages and sex ratios. Proc. R. Soc. B. 2001;268:131–139. doi: 10.1098/rspb.2000.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borries C, Larney E, Lu A, Ossi K, Koenig A. Costs of group size: lower developmental and reproductive rates in larger groups of leaf monkeys. Behav. Ecol. 2008;19:1186–1194. [Google Scholar]
- Boyce MS. Seasonality and patterns of natural selection for life histories. Am. Nat. 1979;114:569–583. [Google Scholar]
- Brockman DK, van Schaik C, editors. Seasonality in primates: studies of living and extinct human and non-human primates. New York: Cambridge Univ. Press; 2005. [Google Scholar]
- Bronikowski AM, Altmann J, Brockman DK, Cords M, Fedigan LM, Pusey A, Stoinski T, Morris WF, Strier KB, Alberts SC. Aging in the natural world: comparative data reveal similar mortality patterns across primates. Science. 2011;331:1325–1328. doi: 10.1126/science.1201571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos FA, Fedigan LM. Behavioral adaptations to heat stress and water scarcity in white-faced capuchins (Cebus capucinus) in Santa Rosa National Park, Costa Rica. Am. J. Phys. Anthropol. 2009;138:101–111. doi: 10.1002/ajpa.20908. [DOI] [PubMed] [Google Scholar]
- Carnegie S, Fedigan L, Melin A. Reproductive seasonality in female capuchins (Cebus capucinus) in Santa Rosa (Area de Conservación Guanacaste), Costa Rica. Int. J. Primatol. 2011;32:1076–1090. [Google Scholar]
- Chapman CA, Lambert JE. Habitat alteration and the conservation of African primates: case study of Kibale National Park, Uganda. Am. J. Primatol. 2000;50:169–185. doi: 10.1002/(SICI)1098-2345(200003)50:3<169::AID-AJP1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Wasserman MD, Gillespie TR, Speirs ML, Lawes MJ, Saj TL, Ziegler TE. Do nutrition, parasitism, and stress have synergistic effects on red colobus populations living in forest fragments? Am. J. Phys. Anthropol. 2006;131:525–534. doi: 10.1002/ajpa.20477. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Saj TL, Snaith TV. Temporal dynamics of nutrition, parasitism, and stress in colobus monkeys: implications for population regulation and conservation. Am. J. Phys. Anthropol. 2007;134:240–250. doi: 10.1002/ajpa.20664. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Chapman LJ, Jacob AL, Rothman JM, Omeja P, Reyna-Hurtado R, Hartter J, Lawes MJ. Tropical tree community shifts: implications for wildlife conservation. Biol. Conserv. 2010a;143:366–374. [Google Scholar]
- Chapman CA, Speirs ML, Hodder SAM, Rothman JM. Colobus monkey parasite infections in wet and dry habitats: implications for climate change. Afr. J. Ecol. 2010b;48:555–558. [Google Scholar]
- Cheney D, Seyfarth R, Fischer J, Beehner J, Bergman T, Johnson S, Kitchen D, Palombit R, Rendall D, Silk J. Reproduction, mortality, and female reproductive success in chacma baboons of the Okavango Delta, Botswana. In: Swedell L, Leigh S, editors. Reproduction and fitness in baboons: behavioral, ecological, and life history perspectives. New York: Springer; 2006. pp. 147–176. [Google Scholar]
- Cheney DL, Wrangham RW. Predation. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate societies. Chicago, IL: Univ. of Chicago Press; 1987. pp. 227–239. [Google Scholar]
- Cheney DL, Lee PC, Seyfarth RM. Behavioral correlates of non-random mortality among free-ranging female vervet monkeys. Behav. Ecol. Sociobiol. 1981;9:153–161. [Google Scholar]
- Cheney DL, Seyfarth RM, Fischer J, Beehner J, Bergman T, Johnson SE, Kitchen DM, Palombit RA, Rendall D, Silk JB. Factors affecting reproduction and mortality among baboons in the Okavango Delta, Botswana. Int. J. Primatol. 2004;25:401–428. [Google Scholar]
- Condit R. Ecological implications of changes in drought patterns: shifts in forest composition in Panama. Clim. Change. 1998;39:413–427. [Google Scholar]
- Cords M. Mixed-species association of Cercopithecus monkeys in the Kakamega Forest, Kenya. Berkeley, CA: Univ. of California Press; 1987. [Google Scholar]
- Cords M, Chowdhury S. Life history of Cercopithecus mitis stuhlmanni in the Kakamega Forest, Kenya. Int. J. Primatol. 2010;31:433–455. [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sunderland, MA: Sinauer Associates; 2004. [Google Scholar]
- Dittus WPJ. The social regulation of population density and age-sex distribution in the toque monkey. Behaviour. 1977;63:281–322. [Google Scholar]
- Dittus WPJ. The social regulation of primate populations: a synthesis. In: Lindburg D, editor. The macaques: studies in ecology, behavior and evolution. New York: Van Nostrand Reinhold; 1980. pp. 263–286. [Google Scholar]
- Dunbar R. Primate social systems. Ithaca, NY: Cornell Univ. Press; 1988. [Google Scholar]
- Dunbar RIM. Demographic and life history variables of a population of gelada baboons (Theropithecus gelada) J. Anim. Ecol. 1980;49:485–506. [Google Scholar]
- Dunham AE, Erhart EM, Overdorff DJ, Wright PC. Evaluating effects of deforestation, hunting, and El Nino events on a threatened lemur. Biol. Conserv. 2008;141:287–297. [Google Scholar]
- Dunham AE, Erhart EM, Wright PC. Global climate cycles and cyclones: consequences for rainfall patterns and lemur reproduction in southeastern Madagascar. Glob. Change Biol. 2010;17:219–222. [Google Scholar]
- Ekernas LS, Cords M. Social and environmental factors influencing natal dispersal in blue monkeys, Cercopithecus mitis stuhlmanni. Anim. Behav. 2007;73:1009–1020. [Google Scholar]
- Fedigan LM, Jack K. Neotropical primates in a regenerating costa rican dry forest: a comparison of howler and capuchin population patterns. Int. J. Primatol. 2001;22:689–713. [Google Scholar]
- Fedigan LM, Carnegie SD, Jack KM. Predictors of reproductive success in female white-faced capuchins (Cebus capucinus) Am. J. Phys. Anthropol. 2008;137:82–90. doi: 10.1002/ajpa.20848. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies from molecular sequences—inference and reliability. Annu. Rev. Genet. 1988;22:521–565. doi: 10.1146/annurev.ge.22.120188.002513. [DOI] [PubMed] [Google Scholar]
- Fleagle JG. Primate locomotion and diet. In: Chivers D, Wood B, Bisborough A, editors. Food acquisition and processing in primates. New York: Plenum Press; 1984. pp. 105–117. [Google Scholar]
- Fleagle JG. Primate adaptation and evolution. San Diego, CA: Academic Press; 1999. [Google Scholar]
- Frankie GW, Rizzardi M, Vinson SB, Griswold TL, Ronchi P. Changing bee composition and frequency on a flowering legume, Andira inermis (Wright) Kunth ex DC. during el Nino and La Nina Years (1997, 1999) in Northwestern Costa Rica. J. Kans. Entomol. Soc. 2005;78:100–117. [Google Scholar]
- Freckleton R, Harvey P, Pagel M. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 2002;160:712–726. doi: 10.1086/343873. [DOI] [PubMed] [Google Scholar]
- Frich P, Alexander L, Della-Marta P, Gleason B, Haylock M, Klein Tank A, Peterson T. Observed coherent changes in climatic extremes during the second half of the twentieth century. Clim. Res. 2002;19:193–212. [Google Scholar]
- Genovart M, Negre N, Tavecchia G, Bistuer A, Parpal LS, Oro D. The young, the weak and the sick: evidence of natural selection by predation. PLoS ONE. 2010;5:e9774. doi: 10.1371/journal.pone.0009774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie TR, Lonsdorf EV, Canfield EP, Meyer DJ, Nadler Y, Raphael J, Pusey AE, Pond J, Pauley J, Mlengeya T. Demographic and ecological effects on patterns of parasitism in eastern chimpanzees (Pan troglodytes schweinfurthii) in Gombe National Park, Tanzania. Am. J. Phys. Anthropol. 2010;143:534–544. doi: 10.1002/ajpa.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall J. Population dynamics during a 15 year period in one community of free-living chimpanzees in the Gombe National Park, Tanzania. Z. Tierpsychol. 1983;61:1–60. [Google Scholar]
- Goodall J. The chimpanzees of Gombe: patterns of behavior. Cambridge, MA: Harvard Univ. Press; 1986. [Google Scholar]
- Gordon WF, Vinson SB, Newstrom LE, Barthell JF. Nest site and habitat preferences of centris bees in the Costa Rican dry forest. Biotropica. 1988;20:301–310. [Google Scholar]
- Grine FE. Dental evidence for dietary differences in Australopithecus and Paranthropus: a quantitative analysis of permanent molar microwear. J. Hum. Evol. 1986;15:783–822. [Google Scholar]
- Groves CP. Primate taxonomy. Washington, DC: Smithsonian Books; 2001. [Google Scholar]
- Guernier V, Hochberg ME, Guegan JF. Ecology drives the worldwide distribution of human diseases. PLoS Biol. 2004;2:740–746. doi: 10.1371/journal.pbio.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WJ. Demographic consequences of a food and water shortage to desert chacma baboons, Papio ursinus. Int. J. Primatol. 1985;6:451–462. [Google Scholar]
- Harris TR, Chapman CA, Monfort SL. Small folivorous primate groups exhibit behavioral and physiological effects of food scarcity. Behav. Ecol. 2010;21:46–56. [Google Scholar]
- Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, Samuel MD. Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296:2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- Huffman MA, Wrangham RW. Diversity of medicinal plant use by chimpanzees in the wild. In: Wrangham RW, McGrew WC, deWall FB, Heltne PGH, editors. Chimpanzee cultures. Boston: Harvard Univ. Press; 1994. pp. 129–148. [Google Scholar]
- Huffman MA, Gotoh S, Turner LA, Hamai M, Yoshida K. Seasonal trends in intestinal nematode infection and medicinal plant use among chimpanzees in the Mahale Mountains, Tanzania. Primates. 1997;38:111–125. [Google Scholar]
- IPCC. Climate change 2001: the scientific basis. In: Houghton JT, Ding Y, Griggs DJ, Noguer M, van der Linden P, Dai X, Maskell K, Johnson CA, editors. Contribution of working group I to the third assessment report of the intergovernmental panel on climate change. New York: Cambridge University Press; 2001. p. 881. [Google Scholar]
- Irwin M, Raharison JL, Wright P. Spatial and temporal variability in predation on rainforest primates: do forest fragmentation and predation act synergistically? Anim. Conserv. 2009;12:220–230. [Google Scholar]
- Isbell L, Young T, Jaffe K, Carlson A, Chancellor R. Demography and life histories of sympatric patas monkeys, Erythrocebus patas, and vervets, Cercopithecus aethiops, in Laikipia, Kenya. Int. J. Primatol. 2009;30:103–124. doi: 10.1007/s10764-009-9332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbell LA. Contest and scramble competition: patterns of female aggression and ranging behaviour among primates. Behav. Ecol. 1991;2:143–155. [Google Scholar]
- Isbell LA. Predation on primates: ecological patterns and evolutionary consequences. Evol. Anthropol. 1994;3:61–71. [Google Scholar]
- IUCN. IUCN Red List of threatened species. [Accessed March 11, 2010];2010 Available at http://www.iucnredlist.org. [Google Scholar]
- Jammalamadaka SR, Sengupta A. Topics in circular statistics. River Edge, NJ: World Scientific; 2001. [Google Scholar]
- Janson C, Verdolin J. Seasonality of primate births in relation to climate. In: Schaik CV, Brockman DK, editors. Seasonality in primates: studies of living and extinct human and nonhuman primates. New York: Cambridge Univ. Press; 2005. pp. 307–350. [Google Scholar]
- Jin T, Wang DZ, Zhao Q, Yin LJ, Qin DG, Ran WZ, Pan WS. Reproductive parameters of wild Trachypithecus leucocephalus: seasonality, infant mortality and interbirth interval. Am. J. Primatol. 2009;71:558–566. doi: 10.1002/ajp.20688. [DOI] [PubMed] [Google Scholar]
- Jones JH. The force of selection on the human life cycle. Evol. Hum. Behav. 2009;30:305–314. doi: 10.1016/j.evolhumbehav.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JH. Primates and the evolution of long, slow life histories. Curr. Biol. 2011;21:R708–R717. doi: 10.1016/j.cub.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpanty SM, Wright PC. Predation on lemurs in the rainforest of Madagascar by multiple predator species: observations and experiments. In: Gursky S, Nekaris KAI, editors. Primate anti-predator strategies. New York: Springer; 2007. p. 7799. [Google Scholar]
- Keele BF, Jones JH, Terio KA, Estes JD, Rudicell RS, Wilson ML, Li YY, Learn GH, Beasley TM, Schumacher-Stankey J, et al. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature. 2009;460:515–519. doi: 10.1038/nature08200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharin VV, Zwiers FW, Zhang X, Hegerl GC. Changes in temperature and precipitation extremes in the IPCC ensemble of global coupled model simulations. J. Clim. 2007;20:1419–1444. [Google Scholar]
- King SJ, Morelli TL, Arrigo-Nelson S, Ratelolahy FJ, Godfrey LR, Wyatt J, Tecot S, Jernvall J, Wright PC. Morphometrics and pattern of growth in wild sifakas (Propithecus edwardsi) at Ranomafana National Park, Madagascar. Am. J. Primatol. 2011;73:155–172. doi: 10.1002/ajp.20881. [DOI] [PubMed] [Google Scholar]
- Koenig A. Competitive regimes in forest-dwelling Hanuman langur females (Semnopithecus entellus) Behav. Ecol. Sociobiol. 2000;48:93–109. [Google Scholar]
- Kühl HS, Elzner C, Moebius Y, Boesch C, Walsh PD. The price of play: self-organized infant mortality cycles in chimpanzees. PLoS ONE. 2008;3:e2440. doi: 10.1371/journal.pone.0002440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurten B. Variation and dynamics of a fossil antelope population. Paleobiology. 1983;9:62–69. [Google Scholar]
- Lin JC, Nichol KL. Excess mortality due to pneumonia or influenza during influenza seasons among persons with acquired immunodeficiency syndrome. Arch. Intern. Med. 2001;161:441–446. doi: 10.1001/archinte.161.3.441. [DOI] [PubMed] [Google Scholar]
- Lonsdorf EV, Murray CM, Travis DA, Gilby IC, Chosy J, Goodall J, Pusey AE. A retrospective analysis of factors correlated to chimpanzee (Pan troglodytes schweinfurthii) respiratory health at Gombe National Park, Tanzania. EcoHealth. 2011;8:26–35. doi: 10.1007/s10393-011-0683-0. [DOI] [PubMed] [Google Scholar]
- Lubinski PM, O’Brien CJ. Observations on seasonality and mortality from a recent catastrophic death assemblage. J. Archaeol. Sci. 2001;28:833–842. [Google Scholar]
- Lyman RL. Vertebrate taphonomy. New York: Cambridge Univ. Press; 1994. [Google Scholar]
- Marshall AJ, Boyko CM, Feilen KL, Boyko RH, Leighton M. Defining fallback foods and assessing their importance in primate ecology and evolution. Am. J. Phys. Anthropol. 2009;140:603–614. doi: 10.1002/ajpa.21082. [DOI] [PubMed] [Google Scholar]
- Martin PR, Bonier F, Moore IT, Tewksbury JJ. Latitudinal variation in the asynchrony of seasons: implications for higher rates of population differentiation and speciation in the tropics. Ideas Ecol. Evol. 2009;2:9–17. [Google Scholar]
- Martins EP, Diniz-Filho JA, Housworth EA. Adaptive constraints and the phylogenetic comparative method: a computer simulation test. Evolution. 2002;56:1–13. [PubMed] [Google Scholar]
- McCarthy T, Bloem A, Larkin R. Observations on the hydrology and geohydrology of the Okavango Delta. S. Afr. J. Geol. 1988;1001:101–117. [Google Scholar]
- McDonald JH. Handbook of biological statistics. Baltimore, MD: Sparky House Publishing; 2009. [Google Scholar]
- Merceron G, Escarguel G, Angibault JM, Verheyden-Tixier H, Begun D. Can dental microwear textures record inter-individual dietary variations? PLoS ONE. 2010;5:e9542. doi: 10.1371/journal.pone.0009542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihlbachler MC. Demography of late Miocene rhinoceroses (Teleoceras proterum and Aphelops malacorhinus) from Florida: linking mortality and sociality in fossil assemblages. Paleobiology. 2003;29:412–428. [Google Scholar]
- Milton K. Factors influencing leaf choice by howler monkeys: a test of some hypotheses of food selection by generalist herbivores. Am. Nat. 1979;114:362–378. [Google Scholar]
- Milton K. Effects of bot fly (Alouattamyia baeri) parasitism on a free-ranging howler (Alouatta palliata) population in Panama. J. Zool. 1996;239:39–63. [Google Scholar]
- Nelson RJ. Seasonal immune function and sickness responses. Trends Immunol. 2004;25:187–192. doi: 10.1016/j.it.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Nunn C, van Schaik CP. A comparative approach to reconstructing the socioecology of extinct primates. In: Plavcan JM, Jungers WL, Kay RF, van Schaik CP, editors. Reconstructing behavior in the primate fossil record. New York: Kluwer Academic/Plenum; 2001. pp. 159–216. [Google Scholar]
- Nunn CL, Altizer S. Infectious diseases in primates: behavior, ecology and evolution. Oxford, U.K: Oxford University Press; 2006. [Google Scholar]
- Nunn CL, Altizer S, Jones KE, Sechrest W. Comparative tests of parasite species richness in primates. Am. Nat. 2003;162:597–614. doi: 10.1086/378721. [DOI] [PubMed] [Google Scholar]
- Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Nick I. Caper: comparative analyses of phylogenetics and evolution in R. R package version 0.4. 2011 Available at http://CRAN.R-project.org/package=caper. [Google Scholar]
- Otis J, Froehlich J, Thorington R. Seasonal and age-related differential mortality by sex in the mantled howler monkey, Alouatta palliata. Int. J. Primatol. 1981;2:197–205. [Google Scholar]
- Paaijmans KP, Read AF, Thomas MB. Understanding the link between malaria risk and climate. Proc. Natl. Acad. Sci. USA. 2009;106:13844–13849. doi: 10.1073/pnas.0903423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis E, Claude J, Strimmer K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- Parmesan C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006;37:637–669. [Google Scholar]
- Parmesan C, Yohe GA. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- Pazol K, Cords M. Seasonal variation in feeding behavior, competition and female social relationships in a forest dwelling guenon, the blue monkey (Cercopithecus mitis stuhlmanni), in the Kakamega Forest, Kenya. Behav. Ecol. Sociobiol. 2005;58:566–577. [Google Scholar]
- Pedersen AB, Jones KE, Nunn CL, Altizer S. Infectious diseases and extinction risk in wild mammals. Conserv. Biol. 2007;21:1269–1279. doi: 10.1111/j.1523-1739.2007.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penteriani V, Del Mar Delgado M, Bartolommei P, Maggio C, Alonso-Alvarez C, Holloway GJ. Owls and rabbits: predation against substandard individuals of an easy prey. J. Avian Biol. 2008;39:215–221. [Google Scholar]
- Perry S. Conformism in the food processing techniques of white-faced capuchin monkeys (Cebus capucinus) Anim. Cogn. 2009;12:705–716. doi: 10.1007/s10071-009-0230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochron ST, Tucker WT, Wright PC. Demography, life history, and social structure in Propithecus diadema edwardsi from 1986–2000 in Ranomafana National Park, Madagascar. Am. J. Phys. Anthropol. 2004;125:61–72. doi: 10.1002/ajpa.10266. [DOI] [PubMed] [Google Scholar]
- Pusey AE, Oehlert GW, Williams JM, Goodall J. Influence of ecological and social factors on body mass of wild chimpanzees. Int. J. Primatol. 2005;26:3–31. [Google Scholar]
- Pusey AE, Wilson ML, Anthony Collins D. Human impacts, disease risk, and population dynamics in the chimpanzees of Gombe National Park, Tanzania. Am. J. Primatol. 2008;70:738–744. doi: 10.1002/ajp.20567. [DOI] [PubMed] [Google Scholar]
- R Core Development Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- Reed KE, Fleagle JG. Geographic and climatic control of primate diversity. Proc. Natl. Acad. Sci. USA. 1995;92:7874–7876. doi: 10.1073/pnas.92.17.7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard A. Primates in nature. New York: W.H. Freeman and Company; 1985. [Google Scholar]
- Rogers RR. Taphonomy of three dinosaur bone beds in the Upper Cretaceous Two Medicine Formation of northwestern Montana: evidence for drought-related mortality. Palaios. 1990;5:394–413. [Google Scholar]
- Rohlf FJ. Comparative methods for the analysis of continuous variables: geometric interpretations. Evolution. 2001;55:2143–2160. doi: 10.1111/j.0014-3820.2001.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Semple S, Cowlishaw G, Bennett PM. Immune system evolution among anthropoid primates: parasites, injuries and predators. Proc. R. Soc. Lond. B Biol. Sci. 2002;269:1031–1037. doi: 10.1098/rspb.2001.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith TV, Chapman CA. Towards an ecological solution to the folivore paradox: patch depletion as an indicator of within-group scramble competition in red colobus. Behav. Ecol. Sociobiol. 2005;59:185–190. [Google Scholar]
- Snaith TV, Chapman CA. Primate group size and socioecological models: do folivores really play by different rules? Evol. Anthropol. 2007;16:94–106. [Google Scholar]
- Stanford CB, Wallis J, Matama H, Goodall J. Patterns of predation by chimpanzees on red colobus monkeys in Gombe National Park, 19821991. Am. J. Phys. Anthropol. 1994;94:213–228. doi: 10.1002/ajpa.1330940206. [DOI] [PubMed] [Google Scholar]
- Steenbeek R, van Schaik CP. Competition and group size in Thomas’s langurs (Presbytis thomasi): the folivore paradox revisited. Behav. Ecol. Sociobiol. 2001;49:100–110. [Google Scholar]
- Sterck EHM, Steenbeek R. Female dominance relationships and food competition in the sympatric Thomas langur and long-tailed macaque. Behaviour. 1997;134:749–774. [Google Scholar]
- Sterck EHM, Willems EP, van Hooff JA, Wich SA. Female dispersal, inbreeding avoidance and mate choice in Thomas langurs (Presbytis thomasi) Behaviour. 2005;142:845–868. [Google Scholar]
- Strier KB. Primate behavioral ecology. Boston: Pearson Allyn and Bacon; 2007. [Google Scholar]
- Strier KB, Altmann J, Brockman DK, Bronikowski AM, Cords M, Fedigan LM, Lapp H, Liu X, Morris WF, Pusey AE. The primate life history database: a unique shared ecological data resource. Meth. Ecol. Evol. 2010;1:199–211. doi: 10.1111/j.2041-210X.2010.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhsaker TT. The red colobus monkey. Chicago, IL: University of Chicago Press; 1975. [Google Scholar]
- Struhsaker TT. Ecology of an African rain forest: logging in Kibale and the conflict between conservation and exploitation. Gainesville, FL: Univ. of Florida Press; 1997. [Google Scholar]
- Teaford MF, Glander KE. Dental microwear in live, wild trapped Alouatta palliata from Costa Rica. Am. J. Phys. Anthropol. 1991;85:313–319. doi: 10.1002/ajpa.1330850310. [DOI] [PubMed] [Google Scholar]
- Teaford MF, Glander KE. Dental microwear and diet in a wild population of mantled howling monkeys (Alouatta palliata) In: Norconk MA, Rosenberger AL, Garber PA, editors. Adaptive radiations of neotropical primates. New York: Plenum Press; 1996. pp. 433–449. [Google Scholar]
- Teaford MF, Oyen OJ. In vivo and in vitro turnover in dental microwear. Am. J. Phys. Anthropol. 1989;80:447–460. doi: 10.1002/ajpa.1330800405. [DOI] [PubMed] [Google Scholar]
- Tebaldi C, Hayhoe K, Arblaster JM, Meehl GA. Going to the extremes. Clim. Change. 2006;79:185–211. [Google Scholar]
- Teelen S. Influence of chimpanzee predation on the red colobus population at Ngogo, Kibale National Park, Uganda. Primates. 2008;49:41–49. doi: 10.1007/s10329-007-0062-1. [DOI] [PubMed] [Google Scholar]
- van Dijk J, Sargison ND, Kenyon F, Skuce PJ. Climate change and infectious disease: helminthological challenges to farmed ruminants in temperate regions. Animal. 2010;4:377–392. doi: 10.1017/S1751731109990991. [DOI] [PubMed] [Google Scholar]
- van Schaik C. Phenological changes in a Sumatran rain forest. J. Trop. Ecol. 1986;2:327–347. [Google Scholar]
- van Schaik CP, Janson CH, editors. Infanticide by males and its implications. London: Cambridge Univ. Press; 2000. [Google Scholar]
- Vitone ND, Altizer S, Nunn CL. Body size, diet and sociality influence the species richness of parasitic worms in anthropoid primates. Evol. Ecol. Res. 2004;6:183–199. [Google Scholar]
- Walsh PD, Biek R, Real LA. Wave-like spread of Ebola Zaire. PLoS Biol. 2005;3:1946–1953. doi: 10.1371/journal.pbio.0030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DP. Mountain gorilla reproduction and sexual behavior. Am. J. Primatol. 1991;24:211–225. doi: 10.1002/ajp.1350240307. [DOI] [PubMed] [Google Scholar]
- Watts DP. Seasonality in the ecology and life histories of mountain gorillas (Gorilla gorilla beringei) Int. J. Primatol. 1998;19:929–948. [Google Scholar]
- Wich SA, Steenbeek R, Sterck EHM, Korstjens AH, Willems EP, Schaik CPV. Demography and life history of Thomas langurs (Presbytis thomasi) Am. J. Primatol. 2007;69:641–651. doi: 10.1002/ajp.20386. [DOI] [PubMed] [Google Scholar]
- Williams JM, Lonsdorf EV, Wilson ML, Schumacher-Stankey J, Goodall J, Pusey AE. Causes of death in the Kasekela chimpanzees of Gombe National Park, Tanzania. Am. J. Primatol. 2008;70:766–777. doi: 10.1002/ajp.20573. [DOI] [PubMed] [Google Scholar]
- Wrangham R. Feeding behaviour of chimpanzees in Gombe National Park, Tanzania. In: Clutton-Brock TH, editor. Primate ecology: studies of feeding and ranging behavior in lemurs, monkeys and apes. New York: Academic Press; 1977. pp. 503–538. [Google Scholar]
- Wrangham RW. An ecological model of female-bonded primate groups. Behaviour. 1980;75:262–300. [Google Scholar]
- Wright P. Demography and life history of free-ranging Propithecus diadema edwardsi in Ranomafana national park, Madagascar. Int. J. Primatol. 1995;16:835–854. [Google Scholar]
- Wright P, Arrigo-Nelson S, Hogg KL, Bannon B, LynMorelli T, Wyatt J, Harivelo AL, Ratelolahy F. Habitat disturbance and seasonal fluctuations of lemur parasites in the rain forest of Ranomafana National Park, Madagascar. In: Huffman MA, Chapman CA, editors. Primate parasite ecology: the dynamics and study of host-parasite relationships. New York: Cambridge Univ. Press; 2009. pp. 311–330. [Google Scholar]
- Wright PC. Impact of predation risk on the behaviour of Propithecus diadema edwardsi in the rain forest of Madagascar. Behaviour. 1998;135:483–512. [Google Scholar]
- Wright PC. Lemur traits and Madagascar ecology: coping with an island environment. Yearb. Phys. Anthropol. 1999;42:31–72. doi: 10.1002/(sici)1096-8644(1999)110:29+<31::aid-ajpa3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Zimmerman JK, Wright SJ, Calderón O, Pagan MA, Paton S. Flowering and fruiting phenologies of seasonal and aseasonal neotropical forests: the role of annual changes in irradiance. J. Trop. Ecol. 2007;23:231–251. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.