Abstract
Background. Adiponectin (APN) possesses anti-inflammatory and antiatherogenic effects. Atrial fibrillation (AF) is burdened by enhanced systemic inflammation and platelet activation, as documented by increased blood levels of soluble CD40L (sCD40L). The interplay between APN and platelet activation in AF is still undefined. Materials and Methods. Circulating levels of APN and sCD40L were measured in 257 anticoagulated nonvalvular AF patients. Exclusion criteria were as follows: prosthetic heart valves, cardiac revascularization in the previous year, severe cognitive impairment, chronic infectious or autoimmune diseases, and active cancer. Results. Mean age was 72.9 (±8.7) years and 41.6% were female. Serum APN and plasmatic sCD40L were inversely correlated (R −0.626, P < 0.001). A progressive increase of sCD40L across tertiles of CHA2DS2-VASc score was observed (rS 0.473, P < 0.001), whilst APN was inversely correlated (rS −0.463, P < 0.001). A multivariable linear regression analysis showed that CHA2DS2-VASc score (B −0.227, P < 0.001) and sCD40L (B −0.524, P < 0.001) correlated to APN. Conclusions. AF patients at high risk of stroke disclose low and high levels of APN and sCD40L, respectively, suggesting a role for APN if it favors platelet activation in vivo in this clinical setting. Enhancing APN levels may be a future goal to reduce the risk of vascular outcomes in AF patients.
1. Introduction
Atrial fibrillation (AF) is the most frequent supraventricular cardiac arrhythmia in the general population. Patients affected by AF, despite the recent introduction of novel oral anticoagulants, show an increased risk for ischemic vascular complications, such as ischemic stroke and cardiovascular mortality [1].
Several evidences suggest that AF is burdened by an enhanced systemic inflammatory status [2] and platelet activation [3, 4], as shown by the enhanced release of soluble CD40 ligand (sCD40L), which may affect AF-related thromboembolic events [5].
AF is characterized by the simultaneous presence of different atherosclerotic risk factors, frequently represented by arterial hypertension, diabetes, obesity, and dyslipidemia [6]. In particular, obesity is a well-known recognized risk factor for developing AF, and more recently weight loss has been associated with improved cardiac symptoms and reduced cardiac remodeling in AF patients [7, 8].
Adiponectin (APN) is the most abundant adipokine produced by adipose tissue, acting as an insulin-sensitizer and anti-inflammatory molecule [9, 10]. In addition to its metabolic properties, APN exerts antiatherogenic effects [11], and reduced serum APN levels have been found in patients with obesity, insulin resistance, and type 2 diabetes [12].
Data regarding APN levels in AF are controversial. Low serum APN levels have been found in paroxysmal AF patients compared to controls [13], whilst higher APN levels have been described in patients with permanent AF, compared to paroxysmal AF and controls [14]. Recently, Hernandez-Romero et al. have shown an association between low APN levels and cardiovascular outcomes in patients affected by AF [15]. Among the mechanisms accounting for such association, the interplay between APN and platelet activation could be considered as platelets play a key role in precipitating acute coronary and cerebrovascular disease [16]. Thus, APN is an antioxidant molecule, which inhibits platelet activation via lowering platelet oxidative stress [17, 18]. So far, data on APN interplay with platelet activation in AF patients are lacking. The aim of the study has been to examine the relationship between APN and platelet activation, as assessed by sCD40L, and to evaluate the association with CHA2DS2-VASc score in a cohort of anticoagulated nonvalvular AF patients.
2. Material and Methods
2.1. Study Design and Patient Selection
The study included 257 consecutive patients with AF who were referred to the Atherothrombosis Center of the Department of Internal Medicine and Medical Specialties of “Sapienza” University of Rome. All patients were treated with oral vitamin K antagonists according to CHA2DS2-VASc score [19] and the international normalized ratio was maintained in a therapeutic range of 2.0-3.0.
Exclusion criteria included presence of prosthetic heart valves, cardiac revascularization in the previous year, severe cognitive impairment (Alzheimer's disease and Parkinson's disease), chronic infectious diseases, autoimmune systemic diseases, and active cancer.
At enrollment, medical history, anthropometric data, and electrocardiogram were recorded and a sample of blood was collected from all patients. Arterial hypertension was defined as elevated blood pressure (≥140/≥90 mmHg) or taking antihypertensive therapy [20] regimen; diabetes was defined as a casual plasma glucose ≥200 mg/dL (11.1 mmol/L), fasting plasma glucose ≥126 mg/dL (7.0 mmol/L), or antidiabetic treatment [21]. Heart failure (HF) was defined as the presence of signs and symptoms typical of heart failure or reduced ejection fraction (EF ≤ 40%) [22]. Metabolic syndrome (MetS) was defined according to modified ATP-III criteria: abdominal obesity, given as waist circumference (in men >102 cm/>40, in women >88 cm/>35); triglycerides ≥150 mg/dL, HDL cholesterol (men <40 mg/dL, women <50 mg/dL); blood pressure ≥130/≥85 mmHg; fasting glucose ≥100 mg/dL [23].
All patients provided a written informed consent. The study protocol was approved by the local ethical board of “Sapienza” University of Rome and was carried out according to the principles of the Declaration of Helsinki [24].
2.2. Laboratory Analysis
Total serum APN levels were measured with a commercial immunoassay (Tema Ricerca, Italy) and expressed as ng/mL. Intra-assay and interassay coefficients of variation were 6% and 8%, respectively.
Platelet activation was assessed by the release of sCD40L. Blood samples were collected without stasis to minimize platelet activation from subjects who had fasted for at least 12 hours, directly mixed in a vacutainer (Vacutainer Systems, Belliver Industrial Estate) with 1 part of 3,8% Na citrate (ratio 9 : 1) and immediately centrifuged for 20 minutes at 2000 rpm at −4°C. Plasma samples were stored at −80°C until use.
Plasma levels of sCD40L were evaluated by immunoassay (Quantikine CD40 ligand, R&D Systems) and expressed as ng/mL. Intra-assay and interassay coefficients of variation were 6% and 7%, respectively.
2.3. Statistical Analysis
Categorical variables were reported as counts (percentages) and continuous variables as mean ± standard deviation (SD) or median and interquartile range (IQR) unless otherwise indicated. Independence of categorical variables was tested by χ 2 test. Normal distribution of parameters was assessed by Kolmogorov-Smirnov test. Student's unpaired t-test and Pearson product-moment correlation analysis were used for normally distributed continuous variables. After dividing population according to tertiles of CHA2DS2-VASc score, APN and sCD40L were analyzed. Group comparisons were performed using analysis of variance (ANOVA). Appropriate nonparametric tests (Mann-Whitney U-test, Kruskal-Wallis test, and Spearman rank correlation test [rS]) were employed for all the other variables. Only P values lower than 0.05 were considered as statistically significant. All tests were two-tailed and analyses were performed using computer software packages (SPSS-18.0, SPSS Inc.).
2.4. Sample Size
We calculated that 48 patients per group were required to have a 90% chance of detecting, as significant at the 5% level, a difference for APN levels between groups of 2 ng/mL with SD = 3 ng/mL.
3. Results
Baseline characteristics of all patients are reported in Table 1. Mean age was 72.9 (±8.7) years and 41.6% were female. One-hundred sixteen patients (45.1%) had paroxysmal AF, whilst 141 (54.9%) had persistent/permanent AF.
Table 1.
Baseline characteristics.
| Anthropometric and metabolic data | |
|---|---|
| Age (years) | 72.9 ± 8.7 |
| Female gender (%) | 41.6 |
| Body mass index (kg/m2) | 27.0 ± 4.3 |
| CHA2DS2-VASc score# | 3 (2–4) |
| Adiponectin# (ng/mL) | 5.6 (3.2–8.6) |
| sCD40L# (ng/mL) | 49 (40–70) |
|
| |
| Cardiovascular risk factors | |
|
| |
| Hypertension (%) | 88.3 |
| Diabetes mellitus (%) | 17.9 |
| Heart failure (%) | 16.7 |
| History of stroke/TIA (%) | 14.0 |
| History of MI (%) | 23.0 |
| Metabolic syndrome (%) | 51.0 |
|
| |
| Concomitant therapies | |
|
| |
| (i) Antiplatelets (%) | 11.3 |
| (ii) ACE inhibitor/ARBs (%) | 68.1 |
| (iii) β blockers (%) | 45.1 |
| (iv) Calcium channel blockers (%) | 33.9 |
| (v) Statins (%) | 45.5 |
| (vi) Antiarrhythmic drugs (%) | 34.2 |
#Data are expressed as median and interquartile range.
sCD40L: soluble CD40 ligand, TIA: transient ischemic attack, MI: myocardial infarction, ACE: angiotensin converting enzyme, and ARBs: angiotensin receptor blockers.
Median adiponectin value was 5.6 [3.2–8.6] ng/mL and median sCD40L was 49 [40–70] ng/mL. A significant inverse correlation between adiponectin and sCD40L was found (R −0.626, P < 0.001) (Figure 1).
Figure 1.
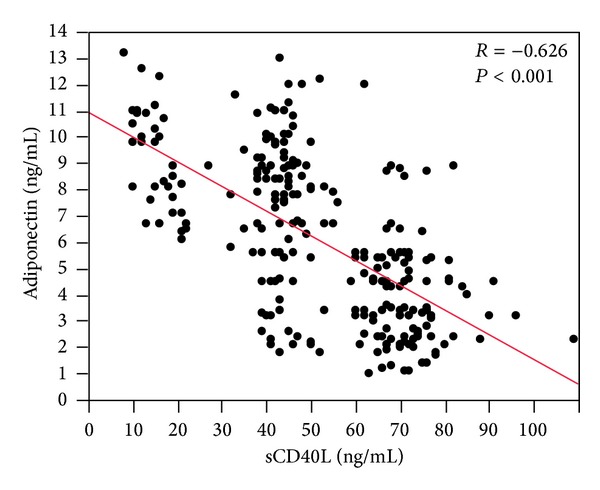
Linear regression analysis between sCD40L and adiponectin levels.
After dividing population according to tertiles of CHA2DS2-VASc score, a significant difference across tertiles was found both for sCD40L (P < 0.001) and APN (P < 0.001) (Table 2) (Figures 2(a) and 2(b)).
Table 2.
Median values of adiponectin and sCD40L according to tertiles of CHA2DS2-VASc score.
| CHA2DS2-VASc score | P value | |||
|---|---|---|---|---|
| 1st tertile | 2nd tertile | 3rd tertile | ||
| Adiponectin | 8.4 (5.5–9.2) | 6.5 (4.5–8.7) | 3.1 (2.3–4.3) | <0.001 |
| sCD40L | 44 (40.0–47.5) | 45 (22–68) | 70 (67–74) | <0.001 |
sCD40L: soluble CD40 ligand.
Figure 2.
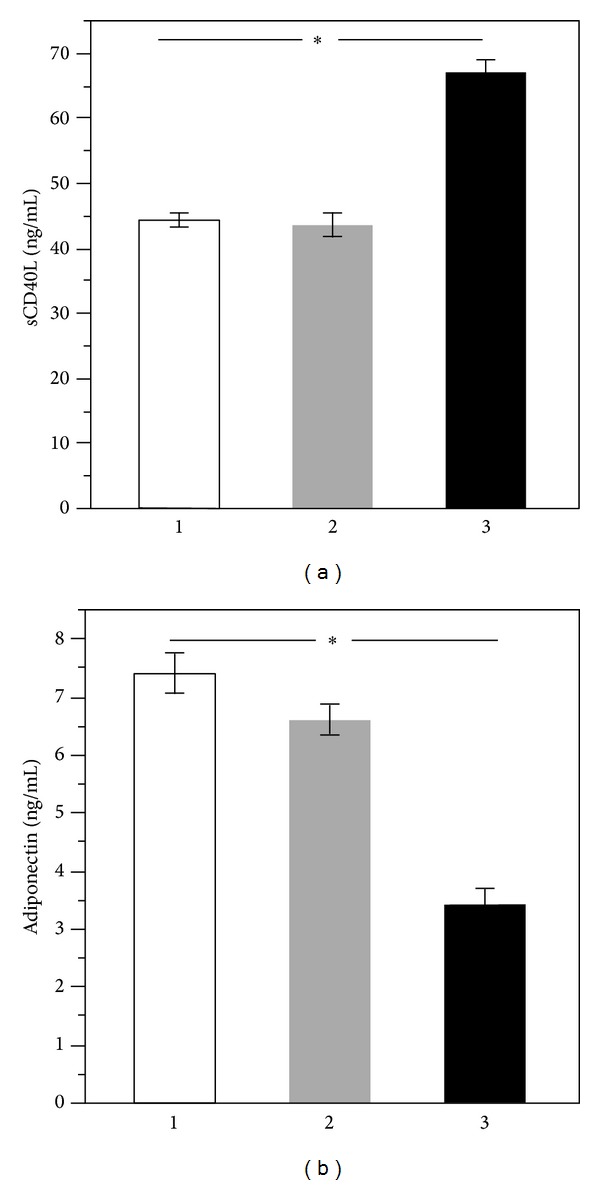
Median values of sCD40L (a) and adiponectin (b) according to tertiles of CHA2DS2-VASc score.
In particular, a progressive increase of sCD40L across tertiles of CHA2DS2-VASc score was observed (rS 0.473, P < 0.001) (Table 2). On the contrary, APN inversely correlated with tertiles of CHA2DS2-VASc score (rS −0.463, P < 0.001) (Table 2).
After adjustment for potential confounding factors, such as age, BMI, smoking habits, and diabetes, multivariable linear regression analysis showed that only CHA2DS2-VASc score (R 2 0.431, B −0.227, P < 0.001) and sCD40L (R 2 0.392, B −0.524, P < 0.001) were independently correlated to adiponectin.
4. Discussion
The present study shows an inverse relationship between serum APN and CHA2DS2-VASc score, suggesting that lower antioxidant and higher inflammatory conditions are detectable in patients at a higher risk of stroke; the inverse relation between APN and sCD40L also suggests a role for oxidative stress in enhancing platelet activation in vivo.
Experimental and observational studies demonstrated that APN is directly involved in generating cardiovascular ischemic complications in the general population [25] and in different clinical settings at risk of vascular complications [15, 26–28]. Evidence derived from the in vitro study suggested that APN was able to inhibit the macrophage tissue factor, a key molecule promoting thrombus formation in disrupted plaques [29]. In APN-knockout mice, APN deficiency was associated with enhanced thrombus formation and platelet aggregation [11]. The interplay between APN and platelet activation has been explored in patients at risk of vascular complications [30, 31]. Thus, patients with MetS express receptors for APN on the platelet surface; platelet incubation with APN resulted in platelet aggregation inhibition and impaired CD40L release [31]. Similar results were observed in patients with type 2 diabetes [32], in which spontaneous platelet aggregation was inhibited by APN.
Clinical studies demonstrated that APN levels correlated with coronary artery disease severity [27], atherothrombotic and lacunar stroke types in men [28], and with incident heart failure in the physicians' health study [26]. Concerning AF, a recent study showed that APN levels were associated with cardiovascular events in anticoagulated AF female patients [15]. To investigate the mechanism potentially accounting for such inverse association, we analyzed the interplay between APN and sCD40L, which is a marker of platelet activation as it derives prevalently from CD40L released by the activated platelet [33, 34]. The inverse correlation between APN and sCD40L may provide novel insights on in vivo platelet activation in this setting as it suggests that the antioxidant status predisposes platelet activation. Such hypothesis is biologically plausible as previous studies consistently showed that oxidative stress is implicated in platelet activation via several mechanisms including formation of isoprostanes, which are chemically stable eicosanoids with proaggregating property [35], and an inactivation of nitric oxide, a powerful antioxidant molecule [36]. Also, platelet incubation with antioxidants different than APN similarly results in inhibition of platelet aggregation [37]. A previous study from our group [5] demonstrated that platelet activation, as assessed by circulating levels of sCD40L, is predictive of vascular outcomes in AF patients but the mechanism accounting for platelet activation was not explored. The present study provides insight into these findings suggesting that the antioxidant status may account for platelet activation in AF.
Another finding of the present study is the inverse correlation between APN and CHA2DS2-VASc score indicating that the risk of stroke is higher and the antioxidant status is lower. This finding is consistent with a previous study showing that serum levels of vitamin E, another molecule with antioxidant property, are inversely related to CHA2DS2-VASc score and predict cardiovascular outcomes in AF patients [38].
From our data it is, therefore, arguable that patients at a higher risk of stroke disclose a lower antioxidant status in association with platelet activation, both changes being potentially implicated in precipitating vascular outcomes by favoring atherosclerotic progression and thrombosis.
In conclusion, the present study shows that AF patients at high risk of stroke disclose low and high levels of APN and sCD40L, respectively, suggesting a role for APN in favoring platelet activation in vivo in this clinical setting. Enhancing APN levels may be a future goal to reduce the risk of vascular outcomes in AF patients.
Acknowledgments
This work was supported by a Grant, University of Rome “Sapienza,” year 2011, Project no. C26A11J528 to Giacomo Frati. The authors thank Fondazione Roma.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Camilla Calvieri and Giacomo Frati have equal contribution.
References
- 1.Biondi-Zoccai G, Malavasi V, D'Ascenzo F, et al. Comparative effectiveness of novel oral anticoagulants for atrial fibrillation: evidence from pair-wise and warfarin-controlled network meta-analyses. HSR Proceedings in Intensive Care and Cardiovascular Anesthesia. 2013;5(1):40–54. [PMC free article] [PubMed] [Google Scholar]
- 2.Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. Journal of the American College of Cardiology. 2012;60(22):2263–2270. doi: 10.1016/j.jacc.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 3.Choi JH, Cha JK, Huh JT. Adenosine diphosphate-induced platelet aggregation might contribute to poor outcomes in atrial fibrillation-related ischemic stroke. Journal of Stroke and Cerebrovascular Diseases. 2013 doi: 10.1016/j.jstrokecerebrovasdis.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Lip GYH, Patel JV, Hughes E, Hart RG. High-sensitivity C-reactive protein and soluble CD40 ligand as indices of inflammation and platelet activation in 880 patients with nonvalvular atrial fibrillation: relationship to stroke risk factors, stroke risk stratification schema, and prognosis. Stroke. 2007;38(4):1229–1237. doi: 10.1161/01.STR.0000260090.90508.3e. [DOI] [PubMed] [Google Scholar]
- 5.Ferro D, Loffredo L, Polimeni L, et al. Soluble CD40 ligand predicts ischemic stroke and myocardial infarction in patients with nonvalvular atrial fibrillation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(12):2763–2768. doi: 10.1161/ATVBAHA.107.152777. [DOI] [PubMed] [Google Scholar]
- 6.Violi F, Loffredo L. Thromboembolism or atherothromboembolism in atrial fibrillation? Circulation: Arrhythmia and Electrophysiology. 2012;5(6):1053–1055. doi: 10.1161/CIRCEP.112.979229. [DOI] [PubMed] [Google Scholar]
- 7.Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. The Journal of the American Medical Association. 2013;310(19):2050–2060. doi: 10.1001/jama.2013.280521. [DOI] [PubMed] [Google Scholar]
- 8.Cavarretta E, Casella G, Cali B, et al. Cardiac remodeling in obese patients after laparoscopic sleeve gastrectomy. World Journal of Surgery. 2013;37(3):565–572. doi: 10.1007/s00268-012-1874-8. [DOI] [PubMed] [Google Scholar]
- 9.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circulation Research. 2005;96(9):939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 10.Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Annals of the New York Academy of Sciences. 2010;1212:E1–E19. doi: 10.1111/j.1749-6632.2010.05875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato H, Kashiwagi H, Shiraga M, et al. Adiponectin acts as an endogenous antithrombotic factor. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26(1):224–230. doi: 10.1161/01.ATV.0000194076.84568.81. [DOI] [PubMed] [Google Scholar]
- 12.Han SH, Quon MJ, Kim J, Koh KK. Adiponectin and cardiovascular disease. Response to therapeutic interventions. Journal of the American College of Cardiology. 2007;49(5):531–538. doi: 10.1016/j.jacc.2006.08.061. [DOI] [PubMed] [Google Scholar]
- 13.Choi BJ, Heo JH, Choi IS, et al. Hypoadiponectinemia in patients with paroxysmal atrial fibrillation. Korean Circulation Journal. 2012;42(10):668–673. doi: 10.4070/kcj.2012.42.10.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimano M, Shibata R, Tsuji Y, et al. Circulating adiponectin levels in patients with atrial fibrillation. Circulation Journal. 2008;72(7):1120–1124. doi: 10.1253/circj.72.1120. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez-Romero D, Jover E, Marin F, et al. The prognostic role of the adiponectin levels in atrial fibrillation. European Journal of Clinical Investigation. 2013;43(2):168–173. doi: 10.1111/eci.12028. [DOI] [PubMed] [Google Scholar]
- 16.Freynhofer MK, Bruno V, Wojta J, Huber K. The role of platelets in athero-thrombotic events. Current Pharmaceutical Design. 2012;18(33):5197–5214. doi: 10.2174/138161212803251899. [DOI] [PubMed] [Google Scholar]
- 17.Carnevale R, Pignatelli P, Di Santo S, et al. Atorvastatin inhibits oxidative stress via adiponectin-mediated NADPH oxidase down-regulation in hypercholesterolemic patients. Atherosclerosis. 2010;213(1):225–234. doi: 10.1016/j.atherosclerosis.2010.08.056. [DOI] [PubMed] [Google Scholar]
- 18.Wang WQ, Zhang HF, Gao GX, Bai Q, Li R, Wang X. Adiponectin inhibits hyperlipidemia-induced platelet aggregation via attenuating oxidative/nitrative stress. Physiological Research. 2011;60(2):347–354. doi: 10.33549/physiolres.932044. [DOI] [PubMed] [Google Scholar]
- 19.Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European heart rhythm association. European Heart Journal. 2012;33(21):2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 20.Mancia G, Fagard R, Narkiewicz K, et al. 2013 Practice guidelines for the management of arterial hypertension of the European society of hypertension (ESH) and the European society of cardiology (ESC): ESH/ESC task force for the management of arterial hypertension. Journal of Hypertension. 2013;31(10):1925–1938. doi: 10.1097/HJH.0b013e328364ca4c. [DOI] [PubMed] [Google Scholar]
- 21.Rydén L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the task force on diabetes, pre-diabetes, and cardiovascular diseases of the European society of cardiology (ESC) and developed in collaboration with the European association for the study of diabetes (EASD) European Heart Journal. 2013;34(39):3035–3087. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 22.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European society of cardiology. Developed in collaboration with the heart failure association (HFA) of the ESC. European Journal of Heart Failure. 2012;14(8):803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 23.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American heart association/national heart, lung, and blood institute scientific statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 24.World Medical Association. World medical association declaration of Helsinki. Ethical principles for medical research involving human subjects. Bulletin of the World Health Organization. 2001;79(4):373–374. [PMC free article] [PubMed] [Google Scholar]
- 25.Hao G, Li W, Guo R, et al. Serum total adiponectin level and the risk of cardiovascular disease in general population: a meta-analysis of 17 prospective studies. Atherosclerosis. 2013;228(1):29–35. doi: 10.1016/j.atherosclerosis.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Djousse L, Wilk JB, Hanson NQ, Glynn RJ, Tsai MY, Gaziano JM. Association between adiponectin and heart failure risk in the physicians' health study. Obesity. 2013;21(4):831–834. doi: 10.1002/oby.20260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel JV, Abraheem A, Dotsenko O, et al. Circulating serum adiponectin levels in patients with coronary artery disease: relationship to atherosclerotic burden and cardiac function. Journal of Internal Medicine. 2008;264(6):593–598. doi: 10.1111/j.1365-2796.2008.02007.x. [DOI] [PubMed] [Google Scholar]
- 28.Kantorova E, Chomova M, Kurca E, et al. Leptin, adiponectin and ghrelin, new potential mediators of ischemic stroke. Neuro Endocrinology Letters. 2011;32(5):716–721. [PubMed] [Google Scholar]
- 29.Okamoto Y, Ishii S, Croce K, et al. Adiponectin inhibits macrophage tissue factor, a key trigger of thrombosis in disrupted atherosclerotic plaques. Atherosclerosis. 2013;226(2):373–377. doi: 10.1016/j.atherosclerosis.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Missiou A, Wolf D, Platzer I, et al. CD40L induces inflammation and adipogenesis in adipose cells—a potential link between metabolic and cardiovascular disease. Thrombosis and Haemostasis. 2010;103(4):788–796. doi: 10.1160/TH09-07-0463. [DOI] [PubMed] [Google Scholar]
- 31.Restituto P, Colina I, Varo JJ, Varo N. Adiponectin diminishes platelet aggregation and sCD40L release. Potential role in the metabolic syndrome. The American Journal of Physiology—Endocrinology and Metabolism. 2010;298(5):E1072–E1077. doi: 10.1152/ajpendo.00728.2009. [DOI] [PubMed] [Google Scholar]
- 32.Hara K, Omori K, Sumioka Y, Aso Y. Spontaneous platelet aggregation evaluated by laser light scatter in patients with type 2 diabetes: effects of short-term improved glycemic control and adiponectin. Translational Research. 2012;159(1):15–24. doi: 10.1016/j.trsl.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 33.André P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation. 2002;106(8):896–899. doi: 10.1161/01.cir.0000028962.04520.01. [DOI] [PubMed] [Google Scholar]
- 34.Antoniades C, Bakogiannis C, Tousoulis D, Antonopoulos AS, Stefanadis C. The CD40/CD40 ligand system. Linking inflammation with atherothrombosis. Journal of the American College of Cardiology. 2009;54(8):669–677. doi: 10.1016/j.jacc.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 35.Pignatelli P, Carnevale R, Di Santo S, et al. Inherited Human gp91phox deficiency is associated with impaired isoprostane formation and platelet dysfunction. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(2):423–434. doi: 10.1161/ATVBAHA.110.217885. [DOI] [PubMed] [Google Scholar]
- 36.Walford G, Loscalzo J. Nitric oxide in vascular biology. Journal of Thrombosis and Haemostasis. 2003;1(10):2112–2118. doi: 10.1046/j.1538-7836.2003.00345.x. [DOI] [PubMed] [Google Scholar]
- 37.Gao LG, Cao J, Mao QX, Lu XC, Zhou XL, Fan L. Influence of omega-3 polyunsaturated fatty acid-supplementation on platelet aggregation in humans: a meta-analysis of randomized controlled trials. Atherosclerosis. 2013;226(2):328–334. doi: 10.1016/j.atherosclerosis.2012.10.056. [DOI] [PubMed] [Google Scholar]
- 38.Cangemi R, Pignatelli P, Carnevale R, et al. Cholesterol-adjusted vitamin E serum levels are associated with cardiovascular events in patients with non-valvular atrial fibrillation. International Journal of Cardiology. 2013;168(4):3241–3247. doi: 10.1016/j.ijcard.2013.04.142. [DOI] [PubMed] [Google Scholar]


