Abstract
Background
Disparities in US breast cancer mortality between older Black and White women have increased in the last twenty years. Regular mammography use is important for early detection and treatment: its utilization among older Blacks especially in counties with high Black mortality is of interest, but its extent and determinants are unknown.
Methods
We used Medicare claims for Black and White women 65–74 years old in 203 counties with the highest Black breast cancer mortality. Outcomes over six years were: screening, i.e., ≥1 screening mammogram, and regular screening, i.e., ≥3 mammograms. With logistic regressions, we examined the independent effect of race on screening controlling for individual and county-level factors.
Results
Of 406,602 beneficiaries, 17% were Black. Screening and regular screening was significantly lower among Blacks (51.6% vs. 56.9%; 32.9% vs 43.1%, respectively). Controlling for covariates, including use of cervical cancer screening, flu shots, or lipids tests, Black women were more likely to have screening (OR 1.23, CI: 1.20–1.25), but not regular screening (OR 0.95, CI: 0.93 – 0.97) than White women. County-level managed care penetration was negatively associated with screening and regular screening.
Conclusions
In Medicare enrollees from these counties, breast cancer screening was low. Black women had same or better odds of screening than White women. Some health care factors, e.g., managed care, were negatively associated with screening. Further studies on the determinants of mammography utilization in older women from these counties are warranted.
Keywords: Cancer, disparities, mammography, screening, Black women
Introduction
Over 200,000 women are newly diagnosed with breast cancer, and nearly 40,000 succumb to this disease in the US annually.1 After Medicare started reimbursing for screening mammograms in the early 90’s, increasing trends in breast cancer mortality among older White and Black women were reversed, and have since decreased.2 However, rates decreased less rapidly for Black women leading to persisting and increasing disparities.2 Further, in most of the 203 US counties where 75% of all breast cancer deaths for older Black women occurred in 1999–2005, the county-level age adjusted mortality rates for older Black women were above those for White women.3 The temporal link between Medicare’s reimbursement for screening mammography and the unequal rates of decline in breast cancer mortality which followed, make it important to further understand screening mammography utilization in counties with high breast cancer mortality.
The Centers for Disease Control and Prevention (CDC) recommends that women 50 to 74 years of age receive at least one mammogram every two years,4 and the American Cancer Society recommends yearly screening regardless of age for women in good health.5 Despite these guidelines, several studies reported differences in screening rates among older Black and White women.6–8 These were limited by follow-up periods of two years or less, thereby not evaluating the uptake of regular screening over longer periods.6–11 Given the importance of repeated screening, there is a need to understand whether older women are screened regularly as recommended, and what factors may predispose or enable them to do so. For example, having primary care visits or other preventive services are individual-level factors associated with higher likelihoods of screening.6,11–13 Community-level factors, for example a county’s socioeconomic status or availability and type of medical resources such as managed care organizations or medical schools, also affect health care utilization as well as health outcomes.8,12,14–19 In a previous study, we found that counties with a higher proportion of hospitals associated with medical schools were less likely to have disparities in breast cancer mortality for Black and White older women,3 leading us to hypothesize that the presence of medical schools may be associated with better breast cancer screening and early detection.
The objective of this paper was to assess the uptake of breast cancer screening in women 65 to 74 year old from counties with most of the breast cancer deaths in Black older women. We examined screening over a period of 6 years, and the factors at the individual and county level that were associated with this screening behavior.
Methods
Population
We obtained Medicare claims data for outpatient procedures, physician visits and inpatient stays for 1,000,000 White and Black women age 65 years old and older randomly selected from 203 US counties. These counties had 14 or more breast cancer deaths in older Black women in 1999–2005.3 Counties were mainly urban counties in the Eastern US. On average 25.7% of their population was Black females, 7.0% had less than 9 years of school, 14.6% were uninsured, and 12.4% lived below poverty, and the average median annual household income was $42,457. The average breast cancer mortality was 117 per 100,000 in White and 137.5 in Black older women.
Our sample included women continuously enrolled in fee-for-service Medicare for 6 years from 2001–2006 (N=414,453). Because recommendations differ across organizations for women 75 and older, we limited our analyses to women 65 to 74 years of age. We included women in fee-for service plans because claims data are not available for women in managed care plans. We excluded women who had claims indicating breast cancer in the first year of follow-up, i.e., in 2001 (N=7,851). Breast cancer was identified using International Classification of Disease-version 9 (ICD-9) codes 174-174.9 and 233, ICD-9 procedure codes 40.11,40.23, 40.3, 85.2, 85.21, 85.22, 85.23, 85.4x, and Current Procedure Terminology (CPT) /Health Care Financing Administration Common Procedure Coding System (HCPCS) codes 19120, 19125, 19126, 19160, 19162, 19180, 19182, 19184-7, 19200. 19211-6, 19220, 19224-9, 19240, 19250-5, and 38740-38745 for breast cancer related procedures.
Outcomes
Mammograms were identified using claims from outpatient and physician visits, and CPT /HCPCS codes (76090-76092,G0202,G0204, G0206) or ICD-9 codes (V76.12, 87.37) for unilateral and bilateral mammography or for screening mammography.20 We included screening and diagnostic billing codes and adapted the algorithm proposed by Smith-Bindman to differentiate screening and diagnostic mammograms. We defined “screening” as at least one screening mammogram, and “regular screening” as at least three mammograms, screening and diagnostic, in the six years of follow-up. In the latter definition, we included diagnostic mammograms to account for the possibility of women skipping or delaying one or more screening mammograms because of a diagnostic procedure, and thus not meeting the definition of regular screening when they should.
In sensitivity analyses, we re-defined screening as having one or more screening or diagnostic mammograms in the six-year follow-up period. Further, we re-defined regular screening as the receipt of three screening mammograms in the follow-up period, thus not including diagnostic mammograms.
Analysis
We used logistic regressions to test the association of screening with race and selected covariates based on the Behavioral Model of Access to Care.14 The model posits that access to medical care depends on three main factors: need, predisposing, and enabling. The need factors are related to co-morbid conditions that may make a woman more likely to access care and receive advice about breast screening. We included in our analytic model comorbid conditions identified by the Elixhauser comorbidity algorithm.21 The predisposing factors were age and the use of other preventive tests in 2001, i.e., cervical cancer screening, lipid tests, and flu shots. These variables were used to represent the woman’s attitude toward preventive care, as it relates to other cancers, other chronic diseases, and infectious diseases. Moreover, given the limits of the claims data in providing other individual level information, we included community level predisposing factors: these served as proxy for individual predisposing characteristics and also to adjust for the direct effect of community level factors on access to care.
Community level factors were defined at the county level using data from the Area Resource File,22 which contains information on demographics and socioeconomics, physicians, hospitals, and other resources from sources like the US Census Bureau, the American Medical Association, the American Hospital Association, and the Center for Medicare and Medicaid Services.22 We considered the county because the availability of resources, particular medical resources, may be determined at this geographic level, and not at lower levels such as ZIP codes or census tracts.23 Based on the 75th percentile value of each variable, we dichotomized these variables and defined counties where women resided to be:
Older: if greater than 16.2% of the county residents were women ages 65 years and older. This variable ranged in value from 6.4% to 26.5%, with a mean of 14.7%. The mean is similar to the 14% US mean for men and women 65 and older.
Low education: if the proportion of county population with 9 years of school or less was above 8.1%. This variable ranged in value from 2.4% to 20.1%, with a mean of 6.6%. This is similar to the US mean of 6% in 2012.
The individual-level enabling factor included in our model was a variable indicating high emergency room (ER) utilization. This variable was chosen to represent the availability and access, or lack of, to good quality care. In the sample, the mean percent of claims from ER departments was 3.5%. We defined high ER utilizers women whose percent of claims from ER visits was greater or equal to the value of the 75th percentile for the sample, that is 4.1%.
Further, we used community level data to adjust for economic status. Based on the 75th percentile value of each variable, we defined counties as “Poor” if the proportion of county population that lived below the poverty level was more than 13.6%. This variable ranged in value from 4.1% to 27.5%, with a mean of 11.2%. In comparison, the mean proportion of US population living below poverty was 14.3% in 2007–2011.
Among the enabling factors, we also considered the type of medical care available to women in our sample. Because the presence of managed care organizations and the availability of primary care doctors have been found to be associated with the uptake of breast cancer screening, we defined “High managed care penetration” those counties where the managed care penetration was above 24.4% (range 0–47.3%, mean 15.6%), and counties “With abundant primary care resources” as those with a number of primary care physicians per 100,000 persons 65 and older of 220.7 or more (range 25.3– 435.9, mean 180.5). Moreover, since the presence of medical schools may also be associated with higher levels of screening, we defined counties “With high medical school presence” as those counties where the proportion of hospitals associated with medical schools was above 57.1% (range 0 –100%, mean 42.3%).
Results
Of the 406,602 women identified, approximately 17% of women were Black (Table 1). Blacks were younger than Whites, less likely to have used preventive services, and more likely to have comorbid conditions, greater ER utilization, and to come from poorer and less educated counties or from counties with fewer primary care resources, lower managed care penetration, and higher proportions of hospitals associated with medical schools (Table 1).
Table 1.
Descriptive statistics for women 65 to 74 years old enrolled in Medicare in 2001–2006, from 203 selected US counties
| All women | Black | White | Pa | |
|---|---|---|---|---|
| N | 406,602 | 70,941 | 335,661 | |
| Percentage | 100.0 | 17.4 | 82.6 | |
| Women’s characteristics (%) | ||||
| Age 70–74 | 48.2 | 44.4 | 49.0 | <0.0001 |
| Any comorbidity | 66.3 | 73.3 | 64.9 | <0.0001 |
| Breast cancer during follow-up | 6.1 | 5.4 | 6.3 | <0.0001 |
| Preventive services received in 2001 (%) | ||||
| Cervical cancer screening | 39.3 | 28.7 | 41.5 | <0.0001 |
| Flu shot | 59.9 | 42.4 | 63.6 | <0.0001 |
| Lipids Test | 60.8 | 56.7 | 61.6 | <0.0001 |
| High ER utilizationb (%) | 25.2 | 36.9 | 22.7 | <0.0001 |
| County characteristics (%) | ||||
| Olderc | 25.4 | 17.5 | 27.1 | <0.0001 |
| Low educationd | 24.9 | 33.8 | 23.1 | <0.0001 |
| Poore | 24.8 | 40.8 | 21.5 | <0.0001 |
| High managed care penetrationf | 24.9 | 19.0 | 26.2 | <0.0001 |
| With high medical school presenceg | 23.0 | 26.7 | 22.3 | <0.0001 |
| With abundant primary care resourcesh | 24.1 | 17.7 | 25.5 | <0.0001 |
P value for bivariate association of race with listed variables
High ER utilization = Person with >4.1% of claims from ER
Older = county with > 16.2% of the county residents being women age 65 years old and older
Low education = county with > 8.1% of the population having ≤9 years of school
Poor = county with > 13.6% of county population living below the poverty level
High managed care penetration = county with > 24.4% managed care penetration
County with high medical school presence = county where >57.1% of hospitals being associated with medical schools
County with abundant primary care resources = county with >220.7 primary care physicians per 100,000 persons 65 and older.
Overall, 56% of women had screening (≥1 screening mammogram) over the six years of follow-up, 51.6% of Black and 56.9% of White women (unadjusted OR 0.81, CI: 0.80–0.82) (Table 2). Controlling for age, number of comorbidities, other preventive measures (cervical cancer screening, lipids tests or flu shot), high ER utilization, and county level variables, odds of screening were statistically greater for Black than for White women (OR 1.23 CI: 1.20–1.25) (Table 2). Moreover, screening was positively associated with having comorbid conditions or other preventive services at the beginning of the follow-up period, and having high ER utilization. Of county level variables, only managed care penetration had a significant association with screening: 40.7% of women in counties with high penetration had screening compared to 61.0% in counties with low penetration (adjusted OR 0.69, CI 0.67–0.70). Moreover, although differences were modest, women from counties with greater presence of medical schools were less likely to have screening than women in counterpart counties (53.2% vs 56.8%); however, in adjusted analyses, odds of screening were higher for women in counties with greater presence of medical schools (adjusted OR 1.11 CI 1.09–1.13).
Table 2.
Women with screening, i.e., at least one screening mammogram in the six year follow-up period: unadjusted and adjusted odds ratios (OR) and 95% Confidence Intervals (CI)
| Women with Screening |
OR of Screening | ||||
|---|---|---|---|---|---|
| % | Unadjusted | 95% CI | Adjusted | 95% CI | |
| All women | 56.0 | -- | -- | -- | -- |
| Women’s characteristics | |||||
| White | 56.9 | ||||
| Black | 51.6 | 0.81 | 0.80–0.82 | 1.23 | 1.20–1.25 |
| Age 65–69 | 56.3 | ||||
| Age 70–74 | 55.5 | 0.97 | 0.96–0.98 | 0.88 | 0.86–0.89 |
| No comorbidities | 30.7 | ||||
| Any comorbidity | 68.8 | 4.97 | 4.90–5.04 | 2.34 | 2.29–2.38 |
| Preventive services | |||||
| Cervical cancer screening | 87.6 | 12.88 | 12.67–13.11 | 7.59 | 7.45–7.74 |
| No cervical cancer screening | 35.4 | ||||
| Flu shot | 74.4 | 7.28 | 7.18–7.39 | 3.36 | 3.30–3.42 |
| No flu shots | 28.5 | ||||
| Lipids tests | 73.1 | 6.54 | 6.45–6.63 | 2.12 | 2.08–2.16 |
| No lipid tests | 29.4 | ||||
| ER utilization | |||||
| Higha | 61.3 | 1.34 | 1.32–1.36 | 1.10 | 1.08–1.12 |
| Low | 54.1 | ||||
| County | |||||
| Older County b | 50.9 | 0.77 | 0.76–0.78 | 0.90 | 0.89–0.92 |
| Younger county | 57.6 | ||||
| Low education c | 51.9 | 0.81 | 0.80–0.82 | 0.84 | 0.82–0.86 |
| Higher education | 57.3 | ||||
| Poord | 52.3 | 0.83 | 0.82–0.84 | 0.93 | 0.91–0.95 |
| Non poor | 57.1 | ||||
| High managed care penetration e | 40.7 | 0.44 | 0.43–0.44 | 0.69 | 0.67–0.70 |
| Low managed care penetration | 61.0 | ||||
| With high medical school presence f | 53.2 | 0.87 | 0.86–0.88 | 1.11 | 1.09–1.13 |
| Lower medical school presence | 56.8 | ||||
| With abundant primary care resources g | 54.2 | 0.91 | 0.90–0.93 | 1.04 | 1.02–1.06 |
| Without abundant primary care resources | 56.5 | ||||
High ER utilization = Person with >4.1% of claims from ER
Older = county with > 16.2% of the county residents being women age 65 years old and older
Low education = county with > 8.1% of the population having ≤9 years of school
Poor = county with > 13.6% of county population living below the poverty level
High managed care penetration = county with > 24.4% managed care penetration
County with high medical school presence = county where >57.1% of hospitals being associated with medical schools
County with abundant primary care resources = county with >220.7 primary care physicians per 100,000 persons 65 and older.
Approximately 41% of women had regular screening (≥3 mammograms, screening or diagnostic) in the follow-up period, 32.9% of Black and 43.1% of White women (unadjusted OR 0.59 CI: 0.58–0.61). This association was only marginally significant in adjusted analyses (OR 0.95, CI: 0.93 – 0.97) (Table 3). Regular screening was significantly and positively associated with having comorbid conditions and receiving preventive services, but not associated with high ER utilization. Of community factors, regular screening was negatively and significantly associated with living in a county with high managed care penetration.
Table 3.
Women with regular screening, i.e., at least 3 mammograms in the six year follow-up period: unadjusted and adjusted odds ratios (OR) and 95% Confidence Intervals (CI)
| Women with Regular Screening |
OR of Regular Screening | ||||
|---|---|---|---|---|---|
| % | Unadjusted | 95%CI | Adj | 95%CI | |
| All Women | 41.3 | -- | -- | ||
| Women’s characteristics | |||||
| White | 43.1 | ||||
| Black | 32.9 | 0.65 | 0.64–0.66 | 0.95 | 0.93–0.97 |
| Age 65–69 | 41.7 | ||||
| Age 70–74 | 40.9 | 0.96 | 0.95–0.98 | 0.90 | 0.89–0.92 |
| No comorbidities | 21.2 | ||||
| Any comorbidity | 51.5 | 3.94 | 3.88–4.00 | 1.98 | 1.94–2.02 |
| Breast cancer during follow-up | 72.7 | 4.12 | 4.01–4.24 | 3.07 | 2.97–3.17 |
| No breast cancer | 39.3 | ||||
| Preventive services | |||||
| Cervical cancer screening | 72.2 | 9.57 | 9.43–9.71 | 5.94 | 5.85–6.04 |
| No cervical cancer screening | 21.3 | ||||
| Flu shot | 57.8 | 6.87 | 6.77–6.98 | 3.35 | 3.30–3.41 |
| No flu shots | 16.6 | ||||
| Lipids tests | 55.2 | 5.02 | 4.96–5.09 | 1.69 | 1.66–1.72 |
| No lipid tests | 19.7 | ||||
| ER utilization | |||||
| Higha | 41.4 | 1.00 | 0.99–1.02 | 0.87 | 0.86–0.89 |
| Low | 41.3 | ||||
| County | |||||
| Older County b | 38.6 | 0.86 | 0.85–0.88 | 0.99 | 0.97–1.01 |
| Younger county | 42.2 | ||||
| Low education c | 36.7 | 0.78 | 0.76–0.79 | 0.85 | 0.84–0.87 |
| Higher education | 42.8 | ||||
| Poord | 37.3 | 0.80 | 0.79–0.82 | 0.95 | 0.93–0.97 |
| Non poor | 42.6 | ||||
| High managed care penetration e | 29.4 | 0.51 | 0.50–0.51 | 0.73 | 0.72–0.75 |
| Low managed care penetration | 45.2 | ||||
| With high medical school presence f | 38.7 | 0.87 | 0.86–0.88 | 1.08 | 1.06–1.10 |
| Lower medical school presence | 42.1 | ||||
| With abundant primary care resources g | 40.8 | 0.97 | 0.96–0.99 | 1.06 | 1.04–1.08 |
| Without abundant primary care resources | 41.5 | ||||
High ER utilization = Person with >4.1% of claims from ER
Older = county with > 16.2% of the county residents being women age 65 years old and older
Low education = county with > 8.1% of the population having ≤9 years of school
Poor = county with > 13.6% of county population living below the poverty level
High managed care penetration = county with > 24.4% managed care penetration
County with high medical school presence = county where >57.1% of hospitals being associated with medical schools
County with abundant primary care resources = county with >220.7 primary care physicians per 100,000 persons 65 and older.
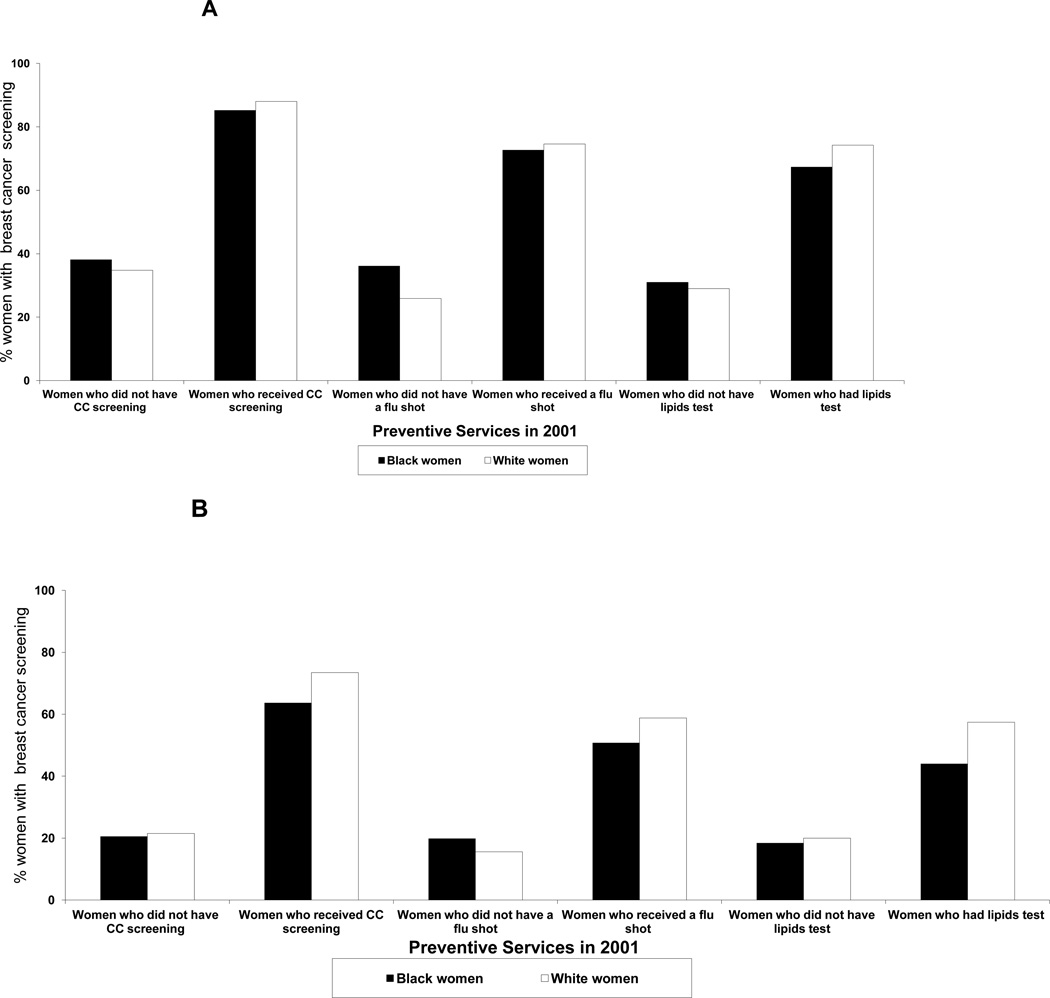
We further examined screening for women with and without other preventive services (Figures 1A and 1B). Among women who received other preventive services in 2001, screening (≥1 screening mammogram) was similar for White and Black women and, in some cases, slightly higher for Whites. Among women who did not receive other preventive services, screening was slightly higher for Black women, especially among women who did not receive a flu shot. Similarly, regular screening (≥3 mammograms, screening or diagnostic) was higher for White women among women with preventive services, but not among women without preventive services in 2001. All differences in proportions were significant at P<0.0001.
Figure 1.
A: Percentage of White and Black Older Women with Breast Cancer Screening in 2001–2006 by receipt of other preventive services in 2001 (Cervical Cancer Screening, Flu Shot, Lipid Tests), (Medicare Claims Data 2001–2006). Screening is defined as at least one screening mammogram in the six year follow-up period. CC = Cervical Cancer.
B: Percentage of White and Black Older Women with Regular Breast Cancer Screening in 2001–2006 by receipt of other preventive services in 2001 (Cervical Cancer Screening, Flu Shot, Lipid Tests), (Medicare Claims Data 2001–2006). Regular Screening is defined as at least three mammograms in the six year follow-up period. CC = Cervical Cancer.
In sensitivity analyses, we found that screening rates were slightly higher when we re-defined screening as having at least one screening or diagnostic mammogram: in this case, 59.6% of women had screening, 55.3% of Black and 60.5% of White women. When we re-defined regular screening considering only screening mammograms, we found that 26.6% of women had regular screening, 18.9% of Blacks and 28.2% of Whites.
Discussion
In US counties where most of the breast cancer deaths for older Black women occur, only about 56% of women 65–74 years old received at least one screening mammogram in a 6 year follow-up period starting in 2001, and about 41% had three or more mammograms. This latter proportion would be even lower, 26.6%, if only screening mammograms were considered. Thus, recommendations of obtaining one mammogram every two years are not followed by the majority of older women in these counties.
From a policy perspective, understanding why screening rates among older women are low is fundamental. Low breast cancer screening rates among Medicare beneficiaries have been reported previously. In a five year period following Medicare coverage for screening mammograms (1993 to 1997), only 57% of Medicare beneficiaries in one state had one or more screening mammograms.24 Further, in two year periods from 2000 to 2005, screening was found in fewer than 50% of women.6–8 Similarly, among older women with breast cancer between 1993 and 2005, only 49% had screening mammograms before diagnosis.13 While these rates are worrisome, our finding that 41% or fewer women received regular screening is of particular concern as it is the repeat mammography that is extremely beneficial for detecting small tumor masses that could undergo early medical treatment. Recently, the results from a 30 year follow-up study revealed a highly significant reduction in breast cancer mortality among women with regular mammography as compared to women without regular mammography (RR 0.69; 95% CI 0.56 – 0.84).25 Furthermore, it is especially important to understand screening uptake among older American women in the counties selected for this study. As mentioned above, most of the breast cancer deaths for older Black women occur in these counties, and in most, mortality rates are higher for Black than for White women.3 Therefore, improving breast cancer screening rates in these targeted areas is bound to improve the outcomes of older Black American women.
Having access to medical care remains an important predictor of screening as illustrated by greater screening rates for women who receive care for other comorbid conditions.12,13 However, access to breast cancer screening also depends on the organization of medical care and resources available.8,12,16–19 We found that women in counties with high managed care penetration were less likely to receive one or more mammograms in six years than women in counties with lower managed care penetration. Although women in our sample were not in managed care plans, their care may have been affected by managed care companies.16 Managed care companies may encourage physician practices for their enrollees that spill over to fee-for-service patients receiving care from the same physicians.16 Alternatively, managed care–induced physician practices may lead to changes in the availability of breast cancer screening facilities or personnel that affect patients in managed and non-managed care plans.16 An in-depth qualitative investigation may be needed to determine whether and why managed care companies in these counties examined here have practices that discourage breast cancer screening in older women. However, it is to be noted that in these same, managed care penetration was not associated with county-level breast cancer mortality rates or with having disparities in mortality.3 In addition, our findings are contrary to studies that have found no effect or positive effects on breast cancer screening of being managed care enrollees or living in areas with high managed care penetration.16,26,27 For example, Baker et al. found a positive effect among women ages 40–75 years old.16
Furthermore, while having a usual source of care and a recommendation from a physician are found to be crucial factors for breast cancer screening.12,19,28 we found that availability of primary care physicians had only minimal effects on screening in these counties. Similarly negligible was the effect of the presence of medical schools. Previously, we found that the presence of medical schools was associated with counties having lower breast cancer mortality and no disparities for older women.3 We, thus, had hypothesized that medical schools may be better positioned to promote and provide screening; however, we did not find that to be the case. Further studies to understand how the health care system in these areas of the US may hinder breast cancer screening are necessary.
It is concerning that in the selected counties, screening rates were lower for older Black women. This is not a surprising finding since previous studies have also reported similar differences.6–8 However, our multivariable adjusted analyses indicated no differences in screening between Black and White women, and even higher odds for Black women of having at least one screening mammogram in six years. Others had similar findings when adjusting for socio-economic status.29,30 In our study, higher odds of screening for Black women became apparent when we included utilization of other preventive services in the model. In particular, among women who had not received other preventive services, such as flu shots, Black women were more likely than Whites to have breast cancer screening. This may signify that for older Black women, mammograms are accessible even if women do not access care for other preventive services, or that the breast cancer screening behavior differs from other preventive health care seeking behaviors. It may be that programs to increase uptake of, and reduce barriers to, breast cancer screening in this population successfully improved screening.
It is clear that much remains to be understood about breast cancer screening among older women residing in these counties. There are various factors we cannot investigate in this study. For example, the attitude, access, and support for preventive services, and in particular breast cancer screening, in the White and Black communities of these counties.28,31 While the number of physicians per capita did not seem to affect screening in this population, understanding physicians’ attitude toward breast cancer and screening, and the relation with older patients, and if indeed these are impacted by the managed care or medical school presence, may also shed light on why screening rates are low.28,31,32 Additionally, we do not know what breast cancer screening programs are available. Evidence-based programs have been implemented in these counties, including those that aim at reducing barriers to screening for Black and low income women.33 However, the reach to the county population and in particular to older women is not known.
This study has some limitations. First, we detected mammograms only if they were paid by Medicare, and thus screening rates may be lower than those found in studies that used self-report of mammography use.9,10 It may be that women in our sample received mammograms for which Medicare was not billed, or self-reported rates are an overestimation of the true screening rates.34,35 Second, we used an algorithm to identify screening mammograms. However, the use of claims has been found to be reliable to examine mammography screening.36 Third, we did not have data at the individual level that may be important to explain utilization of mammograms, e.g., economic status, education, social support, perceived breast cancer risk, or fatalistic attitudes.10,16,37–48 Fourth, we did not use information on adjacent counties, which may be those with richer resources where women travel to receive medical care. And lastly, we used limited information on health care utilization and utilization of other preventive services.
In summary, in 203 counties where most of breast cancer deaths in Black older women occur, more than half of women Medicare beneficiaries did not receive regular screening in a six-year period. For Black women, mammography utilization was less tied to a predisposition for, or availability of, other prevention services, than for White women. This may indicate that access to mammography screening may be separate from access to other preventive services through the health care system, and/or that women may have been more receptive to interventions that focused on improving the uptake of this particular cancer screening. Moreover, characteristics of the local health care system may affect breast cancer screening, in particular the penetration of managed care companies or the presence of medical schools. Therefore, the breast cancer screening utilization of older women in these US counties and the factors contributing to it warrant further investigation.
Acknowledgments
Funding source: This work was funded by the National Cancer Institute (U54CA118948). M. Virk-Baker and T. Nagy were supported in part by the UAB Cancer Prevention and Control Training Program (R25CA047888). R. Levine was partially supported by the National Center for Minority Health and Health Disparities (2P20MD000516-05A1). The opinions expressed herein are those of the authors and not necessarily those of the NIH or any other organization with which the authors are affiliated.
Footnotes
None of the authors have any financial disclosures to make.
References
- 1.American Cancer Society. Cancer Facts and Figures. Atlanta, GA: 2013. [Google Scholar]
- 2.Levine RS, Kilbourne BE, Baltrus PA, et al. Black-white disparities in elderly breast cancer mortality before and after implementation of medicare benefits for screening mammography. J Health Care Poor Underserved. 2008;19(1):103–134. doi: 10.1353/hpu.2008.0019. [DOI] [PubMed] [Google Scholar]
- 3.Pisu M, Wang D, Martin MY, Baltrus P, Levine RS. Presence of medical schools may contribute to reducing breast cancer mortality and disparities. J Health Care Poor Underserved. 2010 Aug;21(3):961–976. doi: 10.1353/hpu.0.0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Breast cancer screening. [Accessed March 19, 2011]; http://www.cdcgov/cancer/breast/basic_info/screening.htm.
- 5.American Cancer Society. American Cancer Society recommendations for early breast cancer detection in women without breast symptoms. [Accessed March 20, 2011]; http://www.cancer.org/Cancer/BreastCancer/MoreInformation/BreastCancerEarlyDetection/breast-cancer-early-detection-acs-recs. [Google Scholar]
- 6.Kagay CR, Quale C, Smith-Bindman R. Screening mammography in the American elderly. Am J Prev Med. 2006 Aug;31(2):142–149. doi: 10.1016/j.amepre.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 7.Bynum JP, Braunstein JB, Sharkey P, Haddad K, Wu AW. The influence of health status, age, race on screening mammography in elderly women. Arch Intern Med. 2005 Oct 10;165(18):2083–2088. doi: 10.1001/archinte.165.18.2083. [DOI] [PubMed] [Google Scholar]
- 8.Elkin EB, Ishill NM, Snow JG, et al. Geographic access and the use of screening mammography. Med Care. 2010 Apr;48(4):349–356. doi: 10.1097/MLR.0b013e3181ca3ecb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelaher M, Stellman JM. The impact of medicare funding on the use of mammography among older women: implications for improving access to screening. Prev Med. 2000 Dec;31(6):658–664. doi: 10.1006/pmed.2000.0759. [DOI] [PubMed] [Google Scholar]
- 10.Williams BA, Lindquist K, Sudore RL, Covinsky KE, Walter LC. Screening mammography in older women. Effect of wealth and prognosis. Arch Intern Med. 2008 Mar 10;168(5):514–520. doi: 10.1001/archinternmed.2007.103. [DOI] [PubMed] [Google Scholar]
- 11.Koya DL, Chen JG, Smith TG, Moran WP. Screening mammography use in Medicare beneficiaries reflects 4-year mortality risk. Am J Med. 2011 Apr;124(4):369, e361–e368. doi: 10.1016/j.amjmed.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Schueler KM, Chu PW, Smith-Bindman R. Factors associated with mammography utilization: a systematic quantitative review of the literature. J Womens Health (Larchmt) 2008 Nov;17(9):1477–1498. doi: 10.1089/jwh.2007.0603. [DOI] [PubMed] [Google Scholar]
- 13.Yasmeen S, Xing G, Morris C, Chlebowski RT, Romano PS. Co-morbidities and mammography use interact to explain racial/ethnic disparities in breast cancer stage at diagnosis. Cancer. 2011 Jul 15;117(14):3252–3261. doi: 10.1002/cncr.25857. [DOI] [PubMed] [Google Scholar]
- 14.Andersen RM, Davidson PL. Improving Access to Care in America: Individual and Contextual Indicators. In: Andersen R, Rice TH, Kominski GF, editors. Changing the U.S Health Care System: Key Issues in Health Services, Policy, and Management. San Francisco: Jossey-Bass Health Series; 2001. [Google Scholar]
- 15.Coughlin SS, Uhler RJ, Bobo JK, Caplan L. Breast cancer screening practices among women in the United States, 2000. Cancer Causes Control. 2004 Mar;15(2):159–170. doi: 10.1023/B:CACO.0000019496.30145.62. [DOI] [PubMed] [Google Scholar]
- 16.Baker LC, Phillips KA, Haas JS, Liang SY, Sonneborn D. The effect of area HMO market share on cancer screening. Health Serv Res. 2004 Dec;39(6 Pt 1):1751–1772. doi: 10.1111/j.1475-6773.2004.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elting LS, Cooksley CD, Bekele BN, et al. Mammography capacity impact on screening rates and breast cancer stage at diagnosis. Am J Prev Med. 2009 Aug;37(2):102–108. doi: 10.1016/j.amepre.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Mobley LR, Kuo TM, Clayton LJ, Evans WD. Mammography facilities are accessible, so why is utilization so low? Cancer Causes Control. 2009 Aug;20(6):1017–1028. doi: 10.1007/s10552-009-9295-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coughlin SS, Leadbetter S, Richards T, Sabatino SA. Contextual analysis of breast and cervical cancer screening and factors associated with health care access among United States women, 2002. Soc Sci Med. 2008 Jan;66(2):260–275. doi: 10.1016/j.socscimed.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Smith-Bindman R, Miglioretti DL, Lurie N, et al. Does utilization of screening mammography explain racial and ethnic differences in breast cancer? Ann Intern Med. 2006 Apr 18;144(8):541–553. doi: 10.7326/0003-4819-144-8-200604180-00004. [DOI] [PubMed] [Google Scholar]
- 21.Elixhauser A, Steiner C, Harris DR, Coffey RM. Co-morbidity measures for use with administrative data. Med Care. 1998 Jan;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Area Resource File. ( http://arf.hrsa.gov/).
- 23.Litaker D, Tomolo A. Association of contextual factors and breast cancer screening: finding new targets to promote early detection. J Womens Health (Larchmt) 2007 Jan-Feb;16(1):36–45. doi: 10.1089/jwh.2006.0090. [DOI] [PubMed] [Google Scholar]
- 24.Harrison RV, Janz NK, Wolfe RA, Tedeschi PJ, Huang X, McMahon LF., Jr 5-Year mammography rates and associated factors for older women. Cancer. 2003 Mar 1;97(5):1147–1155. doi: 10.1002/cncr.11172. [DOI] [PubMed] [Google Scholar]
- 25.Tabar L, Vitak B, Chen TH, et al. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology. 2011 Sep;260(3):658–663. doi: 10.1148/radiol.11110469. [DOI] [PubMed] [Google Scholar]
- 26.Haas JS, Phillips KA, Sonneborn D, McCulloch CE, Liang SY. Effect of managed care insurance on the use of preventive care for specific ethnic groups in the United States. Med Care. 2002 Sep;40(9):743–751. doi: 10.1097/00005650-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Tye S, Phillips KA, Liang SY, Haas JS. Moving beyond the typologies of managed care: the example of health plan predictors of screening mammography. Health Serv Res. 2004 Feb;39(1):179–206. doi: 10.1111/j.1475-6773.2004.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez ED, Khoury AJ, Dailey AB, Hall AG, Chisholm LR. Screening mammography: a cross-sectional study to compare characteristics of women aged 40 and older from the deep South who are current, overdue, and never screeners. Womens Health Issues. 2009 Nov-Dec;19(6):434–445. doi: 10.1016/j.whi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Purc-Stephenson RJ, Gorey KM. Lower adherence to screening mammography guidelines among ethnic minority women in America: a meta-analytic review. Prev Med. 2008 Jun;46(6):479–488. doi: 10.1016/j.ypmed.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rakowski W, Rogers ML, Dominick GM, Clark MA. Understanding reversals of association between cancer screening and race/ethnicity. Cancer Epidemiol Biomarkers Prev. 2012 Sep;21(9):1450–1457. doi: 10.1158/1055-9965.EPI-11-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schootman M, Kinman E, Farria D. Rural-urban differences in ductal carcinoma in situ as a proxy for mammography use over time. J Rural Health. 2003 Fall;19(4):470–476. doi: 10.1111/j.1748-0361.2003.tb00584.x. [DOI] [PubMed] [Google Scholar]
- 32.Young RF, Schwartz K, Booza J. Medical barriers to mammography screening of African American women in a high cancer mortality area: implications for cancer educators and health providers. J Cancer Educ. 2011 Jun;26(2):262–269. doi: 10.1007/s13187-010-0184-9. [DOI] [PubMed] [Google Scholar]
- 33.Austin S, Martin MY, Levine RS, Pisu M. Breast cancer screening interventions in selected counties across US regions. Cancer Causes Control. 2010 Dec;21(12):2165–2172. doi: 10.1007/s10552-010-9636-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holt K, Franks P, Meldrum S, Fiscella K. Mammography self-report and mammography claims: racial, ethnic, and socioeconomic discrepancies among elderly women. Med Care. 2006 Jun;44(6):513–518. doi: 10.1097/01.mlr.0000215884.81143.da. [DOI] [PubMed] [Google Scholar]
- 35.Cronin KA, Miglioretti DL, Krapcho M, et al. Bias associated with self-report of prior screening mammography. Cancer Epidemiol Biomarkers Prev. 2009 Jun;18(6):1699–1705. doi: 10.1158/1055-9965.EPI-09-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith-Bindman R, Quale C, Chu PW, Rosenberg R, Kerlikowske K. Can Medicare billing claims data be used to assess mammography utilization among women ages 65 and older? Med Care. 2006 May;44(5):463–470. doi: 10.1097/01.mlr.0000207436.07513.79. [DOI] [PubMed] [Google Scholar]
- 37.Benjamins MR, Kirby JB, Bond Huie SA. County characteristics and racial and ethnic disparities in the use of preventive services. Prev Med. 2004 Oct;39(4):704–712. doi: 10.1016/j.ypmed.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 38.Schootman M, Jeffe DB, Reschke AH, Aft RL. Disparities related to socioeconomic status and access to medical care remain in the United States among women who never had a mammogram. Cancer Causes Control. 2003 Jun;14(5):419–425. doi: 10.1023/a:1024941626748. [DOI] [PubMed] [Google Scholar]
- 39.Meissner HI, Breen N, Taubman ML, Vernon SW, Graubard BI. Which women aren't getting mammograms and why? (United States) Cancer Causes Control. 2007 Feb;18(1):61–70. doi: 10.1007/s10552-006-0078-7. [DOI] [PubMed] [Google Scholar]
- 40.Barrett K, Legg J. Demographic and health factors associated with mammography utilization. Am J Health Promot. 2005 Jul-Aug;19(6):401–405. doi: 10.4278/0890-1171-19.6.401. [DOI] [PubMed] [Google Scholar]
- 41.Breast cancer screening and socioeconomic status--35 metropolitan areas, 2000 and 2002. MMWR Morb Mortal Wkly Rep. 2005 Oct 7;54(39):981–985. [PubMed] [Google Scholar]
- 42.Fowler BA. The influence of social support relationships on mammography screening in African-American women. J Natl Black Nurses Assoc. 2007 Jul;18(1):21–29. [PubMed] [Google Scholar]
- 43.Taylor VM, Thompson B, Montano DE, Mahloch J, Johnson K, Li S. Mammography use among women attending an inner-city clinic. J Cancer Educ. 1998 Summer;13(2):96–101. doi: 10.1080/08858199809528524. [DOI] [PubMed] [Google Scholar]
- 44.Messina CR, Lane DS, Glanz K, et al. Relationship of social support and social burden to repeated breast cancer screening in the women's health initiative. Health Psychol. 2004 Nov;23(6):582–594. doi: 10.1037/0278-6133.23.6.582. [DOI] [PubMed] [Google Scholar]
- 45.Katapodi MC, Facione NC, Miaskowski C, Dodd MJ, Waters C. The influence of social support on breast cancer screening in a multicultural community sample. Oncol Nurs Forum. 2002 Jun;29(5):845–852. doi: 10.1188/02.ONF.845-852. [DOI] [PubMed] [Google Scholar]
- 46.Eisner EJ, Zook EG, Goodman N, Macario E. Knowledge, attitudes, and screening behavior of women ages 65 and older on mammography screening and Medicare: results of a national survey. Women Health. 2002;36(4):1–18. doi: 10.1300/J013v36n04_01. [DOI] [PubMed] [Google Scholar]
- 47.Jones AR, Thompson CJ, Oster RA, et al. Breast cancer knowledge, beliefs, and screening behaviors among low-income, elderly black women. J Natl Med Assoc. 2003 Sep;95(9):791–797. 802-795. [PMC free article] [PubMed] [Google Scholar]
- 48.Paskett ED, Tatum C, Rushing J, et al. Racial differences in knowledge, attitudes, and cancer screening practices among a triracial rural population. Cancer. 2004 Dec 1;101(11):2650–2659. doi: 10.1002/cncr.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]