Abstract
The chemical composition, antioxidant and antimicrobial activities, and the preservative effect of Thymus capitata essential oil against Listeria monocytogenes inoculated in minced beef meat were evaluated. The essential oil extracted was chemically analyzed by gas chromatography-mass spectrometry. Nineteen components were identified, of which carvacrol represented (88.89%) of the oil. The antioxidant activity was assessed in vitro by using both the DPPH and the ABTS assays. The findings showed that the essential oil exhibited high antioxidant activity, which was comparable to the reference standards (BHT and ascorbic acid) with IC50 values of 44.16 and 0.463 μg/mL determined by the free-radical scavenging DPPH and ABTS assays, respectively. Furthermore, the essential oil was evaluated for its antimicrobial activity using disc agar diffusion and microdilution methods. The results demonstrated that the zone of inhibition varied from moderate to strong (15–80 mm) and the minimum inhibition concentration values ranged from 0.32 to 20 mg/mL. In addition, essential oil evaluated in vivo against Listeria monocytogenes showed clear and strong inhibitory effect. The application of 0.25 or 1% (v/w) essential oil of T. capitata to minced beef significantly reduced the L. monocytogenes population when compared to those of control samples (P-value <0.01).
1. Introduction
The problems of spoilage and food poisoning, mainly by oxidation processes or by microorganism activity, during production and storage are still concerns for both the food industry and consumers, despite the use of synthetic chemical additives and various preservation methods [1–3]. However, the side effects of some synthetic antioxidants used in food processing, such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA), have already been documented. They showed carcinogenic effects in living organisms [4, 5]. Consequently, there has been increasing interest in developing new types of effective and nontoxic natural antioxidant and antimicrobial compounds both to prevent the growth of food- borne and spoiling microbes and to extend the shelf-life of foods [6, 7]. In this context, medicinal and aromatic plants have emerged as an alternative to synthetic products, used not only in traditional medicine but also in a number of food and pharmaceutical products, due to their high content of phenolic compounds, their nutritional properties, and bioactivity [8].
Thymus capitata is a Mediterranean herb of the Lamiaceae family that grows mainly in northern Tunisia [9]. This species is an aromatic plant, mostly used (fresh or dried) as a spice, in some Tunisian traditional meat dishes, both for its preservative qualities and its savory taste. In Tunisian folk medicine, Thymus species are well known as medicinal plants because of their biological and pharmacological properties, which include antiasthmatic, antiseptic, antimycotic, spasmolytic, anti-inflammatory, antimicrobial and, antioxidant activities [9–12]. Recently, Thymus species essential oils (EOs) and their components gained increasing importance because of their wide acceptance by consumers and other exploitations and potential multipurpose functional use [9].
Generally, the essential oils (EOs) are aromatic and volatile liquids extracted from plant materials, such as flowers, roots, bark, leaves, seeds, peel, fruits, wood, and whole plant. They are considered to be plant secondary metabolites, which play an important role in plant defense as they often possess antimicrobial and antioxidant properties [13–15]. For these reasons, EOs have been primarily used, in the food industry, as flavoring agents in food system and can be used as natural antimicrobials in food preservation (extending shelf-life) [15, 16] against a wide range of food spoiling microbes.
Previous phytochemical studies of the genus Thymus EOs have reported the presence of a number of bioactive compounds, including carvacrol, thymol, p-cymene, and γ-terpinene, which have been reported to have many biological activities [3, 9, 11]. Figueiredo et al. in 2008 [11] have demonstrated that EOs of Portuguese T. capitata presented great chemical homogeneity characterized by a relatively high amount of carvacrol.
In addition, to our knowledge, there are no published studies that have evaluated the preservative effect of T. capitata EO against L. monocytogenes in minced meat, the causative agent of listeriosis, one of the most virulent foodborne diseases. Human infection predominantly occurs as a result of occasional contamination of ready-to-eat and raw food products, particularly meat products [17, 18]. Listeriosis has been associated with a mortality rate as high as 30–40% [19]. The ubiquitous prevalence of this pathogen in nature, its ability to proliferate at temperature near 0°C, and its resistance to certain preservatives has resulted in an extensive effort to develop processes to control its growth in foods [20].
Today, different strategies are applied in order to control pathogens in meats, and interest has been focused on the application of EOs as a safe and effective alternative to chemical preservative. Their application in controlling pathogens could reduce the risk of foodborne outbreak and assure consumers safe meat products. The chemical composition and antimicrobial properties of EOs extracted from diverse plant species have been demonstrated using a variety of experimental methods [21, 22].
The purposes of the present work are (i) to evaluate the chemical composition of Tunisian T. capitata EOs by GC-MS and compare it to previous published works, (ii) to confirm in vitro the antioxidant activity of this EO, and (iii) to assess in vitro its antimicrobial activities against a selected group of bacteria strains. Besides, this study was also designed to determine the efficacy of T. capitata EO in inhibiting L. monocytogenes growth in model minced beef meat during refrigerated storage.
2. Materials and Methods
2.1. Materials and Chemicals
Chemicals: 2,2-diphenyl-2-picrylhydrazyl hydrate (DPPH), 2,6-di-tert-butyl-4-methylphenol (BHT), 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), ascorbic acid, dimethyl sulfoxide (DMSO), potassium persulfate, and all reagents were purchased from Sigma (St. Louis, MO, USA), Fluka Chemie (Buchs, Switzerland), and Merck (Nottingham, UK).
2.2. Plant Materials
The aerial parts of T. capitata were collected from Zaghouan region (north Tunisia) in June 2010. The samples species were identified and confirmed by a specialist in botany. The freshly cut plants were sorted out and dried in the shade at ambient temperature for two weeks. Dried samples were grounded into powder, packed in paper bags, and stored in the dark in a dry place.
2.3. Preparation of the Essential Oils
The dried powder aerial parts of plant were submitted to hydrodistillation process in a clevenger-type apparatus for 3 hours according to the method recommended in the current European Pharmacopoeia 6.0 in 2008 [23]. The EO collected was then dried over anhydrous sodium sulphate (Na2SO4), filtered, and stored at 4°C in the dark for further use.
2.4. Chemical Composition of Essential Oil
2.4.1. Apparatus
GC-MS analysis of the essential oil was carried out with Hewlett Packard 7890 A GC equipped with a 5975 mass selective detector and an HP-5 MS capillary column (30 m × 0.25 mm id, film thickness 0.25 μm). For GC/MS detection, the ion source was set to 230°C with electron ionization energy of 70 eV. Scanning range was varied from 40 to 550 atomic mass units (amu). Helium was used as the carrier gas at a flow rate of 0.8 mL/min. One μL of diluted oil in hexane (1/100, v/v) was injected manually in splitless mode. The oven program temperature was programmed from 60°C to 250°C with a rate of 4°C/min and then held constant for 5 min.
2.4.2. Qualitative and Quantitative Analyses of EO
The identification of the chemical compounds of EO was based on mass spectral library (Wiley 275.L, 8th edition) and/or with standards when available and confirmed by comparison of their GC retention indices either with those of authentic standards injected under the same chromatographic conditions or with data published in the literature, as described by Adams in 2007 [24].
2.5. Quantification of Total Antioxidant Activity
The literature outlines different approaches for the determination of the antioxidant activities of the plant extracts. Therefore, generally different methodological approaches lead to scattered results, which are hardly comparable and sometimes conflicting [25, 26]. For that reason, we combined two complementary techniques, based on DPPH and ABTS free radical-scavenging activity.
2.5.1. DPPH Radical-Scavenging Assay
Radical-scavenging activity (RSA) of plant extracts against stable DPPH was determined by spectrophotometry. EOs extracts at different concentrations (0.1; 0.25; 0.5; 1; 5; 10; 50; 100; 200 μg/mL) were mixed with the same volume of 0.2 mM methanolic DPPH solution. Samples were kept in the dark for 30 min at room temperature, and absorption-decrease was measured. Absorption of negative control containing the same amount of methanol and DPPH solution was prepared and measured in the same time. The experiment was carried out in triplicate. RSA of extracts was measured by the method described by Brand-Williams et al. in 1995 [27] but slightly modified as shown below:
| (1) |
where AB is AB absorption of blank sample at t = 0 min and AA is the tested sample absorption at t = 30 min.
The antioxidant activity was also expressed as IC50, which was defined as effective concentration of the sample (in μg/mL) at which 50% of DPPH radicals are scavenged. BHT and ascorbic acid were used as positive control. Each assay was repeated 3 times. The average result and standard deviation were reported.
2.5.2. ABTS Activity
ABTS radical-scavenging activity of EOs was determined according to Re et al. in 1999 [28]. The ABTS solution was diluted with methanol, to absorbance of 0.7 at 734 nm. After the addition of 950 μL of diluted ABTS solution to 50 μL of plant EOs, the mixture was incubated at 37°C for 10 min, and then the absorbance was measured at 734 nm. Tests were carried out in triplicate. Butylated hydroxytoluene (BHT) and ascorbic acid (AA) were used as positive controls.
The ABTS radical-scavenging activity of the sample was calculated by the following equation:
| (2) |
where Abs control is the absorbance of ABTS radical + methanol and Abs sample is the absorbance of ABTS radical + sample (EO/standard).
Sample concentration providing 50% inhibition (IC50) was obtained plotting the inhibition percentage against sample concentrations.
2.6. Antimicrobial Screening
2.6.1. Microorganisms and Growth Conditions
The EO was tested against a large panel of microorganisms. Bacteria were obtained from international culture collections ATCC and the local culture collection of Pasteur Institute of Tunis. They included 8 Gram-positive bacteria and 16 Gram-negative bacteria (Table 1). The bacterial strains were cultivated in Luria Bertani Medium (LB) (Oxoid Ltd., UK) at 37°C except for Bacillus species, which were incubated at 30°C. Working cultures were prepared by inoculating a loopful of each test bacteria in 5 mL of Luria Bertani Medium (LB) (Oxoid Ltd., UK) and incubated at 37°C for 18 hours.
Table 1.
Bacteria strains used.
| Gram-negative bacteria | Gram-positive bacteria |
|---|---|
| Escherichia coli ATCC 25922 | Enterococcus faecalis ATCC 11700 |
| Enterobacter cloacae ATCC 13097 | Listeria monocytogenes ATCC 19118 |
| Proteus mirabilis ATCC 29906 | Staphylococcus aureus ATCC 6538 |
| Pseudomonas aeruginosa ATCC 27853 | Staphylococcus aureus ATCC 25923 |
| Pseudomonas aeruginosa ATCC 9027 | Staphylococcus aureus ATCC 6538 |
| Salmonella enteritidis ATCC 502 | Streptococcus pyogenes ATCC 12344 |
| Salmonella salamae ATCC 6633 | Bacillus cereus ATCC 11778 |
| Salmonella typhimurium ATCC 14028 |
Bacillus cereus
(food isolate) |
| Shigella flexneri ATCC 29903 |
Bacillus subtilis
(food isolate) |
| Yersinia enterocolitica ATCC 23715 | |
| Klebsiella oxytoca (clinical isolate) | |
|
Morganella morganii
(clinical isolate) |
|
|
Pseudomonas aeruginosa
(clinical isolate) |
|
| Salmonella anatum (food isolate) | |
| Shigella sonnei (clinical isolate) | |
| Vibrio cholerae (clinical isolate) |
2.6.2. Disc-Diffusion Method
The paper disc-diffusion method was employed for the determination of EO antimicrobial activity [29]. Briefly, suspension in LB of the tested microorganism (0.1 mL of 107-108 cells per mL) was spread on the solid LB media plates. Paper discs (9 mm in diameter) were individually impregnated with 12 μL of the oil and then placed on the inoculated plates. We did not use the DMSO to facilitate the solubilization of EO in LB-Agar. However, in order to accelerate diffusion of the essential oil, plates were placed at 4°C for 2 hours and were then incubated at 37°C for 24 hours. The diameters of the inhibition zones were measured in millimeters. All tests were performed in duplicate and repeated three times. Streptomycin B (15 μg/mL) and chloramphenicol (30 μg/mL) were used as positive controls.
2.6.3. Determination of the Minimum Inhibitory Concentration
The Minimal Inhibitory Concentrations (MICs) of the EO against the tested microorganisms were determined by the broth microdilution method [30]. All tests were performed in LB, supplemented with DMSO (the highest final concentration 0.1%). Microbial strains were cultured overnight at 37°C and were suspended in LB medium to give a final density of 5 × 105 CFU/mL, which was confirmed by viable counts. Geometric dilutions ranging from 0.039 mg/mL to 20 mg/mL of the EOs were prepared in 96-well microtiter plate (Iwaki brand, Asahi Techno Glass, Japan), including one growth control (LB+DMSO), and one sterility control (LB+DMSO+ test oil). Plates were subsequently incubated under normal atmospheric conditions at 37°C for 24 hours and under vigorous agitation. The wells were then examined for evidence of growth indicated by the presence of white “pellets” on their bottoms. MICs values were determined as the lowest EO concentration that inhibited visible growth of the tested microorganism. The negative controls were set up with DMSO in amounts corresponding to the highest quantity present in the test solution (0.1%). The tests were performed three times.
2.7. Inhibitory Effect of the EO against Listeria Inoculated in Minced Beef Meat
The in situ efficacy of the EO was evaluated against L. monocytogenes in a minced beef meat model according to the procedure described by Careaga et al. in 2003 [31] but with a slight modification.
2.7.1. Preparation of Meat Beef
Freshly postrigor lean beef muscles were obtained from a slaughter house in Tunis,Tunisia. Each piece was immersed in boiling water for 5 min, in order to reduce the number of the microorganisms attached to the beef muscle surface. The cooked surface of the muscle was eliminated with sterile knives under aseptic conditions.
2.7.2. Treatment of Minced Beef
Prior to minced beef contamination with Listeria monocytogenes and the addition of EO, beef muscles were also examined for any contamination by bacteria (aerobic psychrotrophic flora) and the tested pathogens (results not shown). In order to evaluate the antimicrobial activity of T. capitata EO in a meat beef sample, the pieces of meat prepared as above were minced in a sterile grinder, and portions of 25 ± 0.1 g were put in high-density polyethylene bags. The meat samples were inoculated with L. monocytogenes in concentration of 105 CFU/g of meat and mixed homogeneously for 3 min at room temperature to ensure proper distribution of the pathogen. Following homogenization, the T. capitata EO was dissolved in 10% DMSO and was subsequently added at different concentrations (0.02; 0.06; 0.1; 1; 1.5; 2 and 3 % (v/w)) to the inoculated samples. To obtain uniform distribution of the added compounds, treated meat samples were then homogenized by means of a Stomacher 400 Seward (London,UK) used at a normal speed for 5 min. All bags containing these samples of meat were stored at 7°C and examined at 0, 3, 6, 9, 12, and 15 days of storage for L. monocytogenes enumeration. The untreated samples (controls) were added to sterile water (instead of EO), inoculated with the test bacteria, and stored under the same conditions as the tested samples. Three replicates of each experiment were performed in all cases.
2.7.3. Bacterial Enumeration
A microbiological analysis was performed on the meat, with the aim to assess quantitatively and qualitatively the background microflora. L. monocytogenes count was done adding 250 mL of Muller-Hinton broth to the 25 g in the polyethylene bag. The samples were homogenized for one min and incubated at 37°C for 6 hours. From this pre-enrichment, the L. monocytogenes was determined by the plate colony count technique. After serial 10-fold dilution with physiological saline solution, 100 μL of each sample was spread onto surfaces of the Muller- Hinton agar medium followed by incubation at 37°C for 24 hours. Sterile saline water was added to the untreated control, inoculated with the test bacteria instead of T. capitata EO stored under the same conditions as the other samples.
2.8. Statistical Analysis
The inhibitory concentration 50% (IC50 values) for antioxidant activities was calculated by nonlinear regression analysis using the Graphpad Prism version 5.0. The dose-response curve was obtained by plotting the percentage of inhibition versus the concentrations. Correlations between inhibition activity and EO concentration were evaluated using Spearman's correlation test [32]. Statistical significance of the differences between the treated and the control sample means was evaluated by Welch 2-sample t-test. Repeated ANOVA test [33] was used to check overall difference in activity tendency and EO concentration effect. A P value <0.05 was considered to imply significance; however, corrections for multiple testing were carried out when necessary. All computations were performed using The R software 2.11 version (http://www.r-project.org/).
3. Results and Discussion
3.1. Chemical Composition of the Extracted Essential Oils
Table 2 shows the chemical constituents, their relative percentage of the total chromatogram area and Kovats index of T. capitata EO.
Table 2.
Chemical composition of the essential oil isolated from the aerial parts of Thymus capitata from Zaghouan region (Tunisia).
| Compounds | Retention time | %a | RIb | Method of identificationc |
|---|---|---|---|---|
| 1-Octen-3-ol | 6.434 | 0.25 | 987.179 | RI, MS |
| Beta-myrcene | 6.749 | 0.11 | 1020.159 | RI, MS |
| α-Terpinen | 7.481 | 0.15 | 1018.808 | RI, MS |
| p-Cymene | 7.699 | 1.14 | 1026.317 | RI, MS |
| γ-Terpinene | 8.677 | 0.40 | 1060.006 | RI, MS |
| Sabinene hydrate | 8.946 | 0.09 | 1069.27 | RI, MS |
| Linalol | 9.902 | 1.57 | 1101.99 | RI, MS |
| Borneol | 12.070 | 1.06 | 1169.64 | RI, MS |
| Terpinen-4-ol | 12.431 | 1.41 | 1180.90 | RI, MS |
| α-Terpineol | 12.866 | 0.29 | 1194.47 | RI, MS |
| Trans-dihydrocarvone | 13.066 | 0.11 | 1200.07 | RI, MS |
| Beta-citral | 14.474 | 0.24 | 1243.84 | RI, MS |
| Carvone | 14.600 | 0.18 | 1247.70 | RI, MS |
| Citral | 15.481 | 0.33 | 1274.69 | RI, MS |
| Thymol | 16.202 | 0.51 | 1296.78 | RI, MS |
| Carvacrol | 16.688 | 88.98 | 1311.93 | RI, MS |
| Caryophyllene | 20.264 | 0.63 | 1425 | RI, MS |
| Caryophyllene epoxide | 25.191 | 1.08 | 1589.88 | RI, MS |
| Dodecyl acrylate | 28.144 | 0.44 | 1695.47 | RI, MS |
| Total | 98.97 |
(2) Compounds are listed according to their elution on HP-5MS capillary column.
aPeak area of essential oil components.
bKovats retention indices relative to C9–C20 n-alkanes on the HP-5MS capillary column.
cComponents were identified based on their KI on HP-5MS capillary column and GC-MS data.
GC-MS analysis of the volatile constituents of the EO allowed the identification of 19 compounds representing 98.97% of the total oil. Carvacrol was the major one with 88.98%. The other identified components were minor. These results are in line with those reported by Napoli et al. [34]. The chemical composition of this EO showed that it is rich in oxygen containing monoterpenes (94.98%). Monoterpene hydrocarbons or both sesquiterpene and oxygen containing sesquiterpene were represented at about 2% each. This wealth of oxygen-containing monoterpenes (OM), especially carvacrol, can enhance the value of this EO as an active natural product. The major product carvacrol was described as a strong antibacterial molecule [9, 11] and it is now considered one of the products singled out for their pharmacological effects. These results are in accordance with previous studies [35, 36], which demonstrated that carvacrol was the main compound of T. capitata oils with 75% and 65.8%, respectively.
On the other hand, there are many reports on the chemical composition of other oils isolated from the plants belonging to the genus of thymus. Tomaino et al. in 2005 [37] reported that the major constituents of thyme EO were carvacrol, thymol, and p-cymene and they can reach the following percentages: 48.9%, 45.3%, and 26.19%, respectively, while Jaafari et al. in 2007 [38] found that these same constituents are the main components in thyme EO from Morocco and can hit the following percentages: 85%, 42%, and 23%, respectively. These variations in the composition of the EO could be due to factors such as plant age, plant part, development stage, the geographical localization, harvesting period, temperature, and environmental factors prevailing in the Mediterranean regions and principally by chemotype since they influence the plant biosynthetic pathways and consequently, the relative proportion of the main characteristic compounds [39].
3.2. Antioxidant Activity
Two complementary colorimetric methods, namely the DPPH and ABTS assays are compared to the reference standards butylated hydroxyl toluene (BHT) and ascorbic acid (AA), and the results are presented in Figure 1. The DPPH and the ABTS radicals are the two most widely used and stable chromogen compounds to measure the antioxidant activity of biological material [40]. In addition, the model of the DPPH radical-scavenging and ABTS radical cation decolorization assay can be used to evaluate the antioxidant activities in a relatively short time compared with other methods [41, 42]. In the present study, the capacity of the EO to scavenge the free radicals DPPH• and ABTS+• and their reducing power was determined on the basis of their concentration providing 50% inhibition (IC50) and the lower IC50 value reflects high radical-scavenging activity [43].
Figure 1.
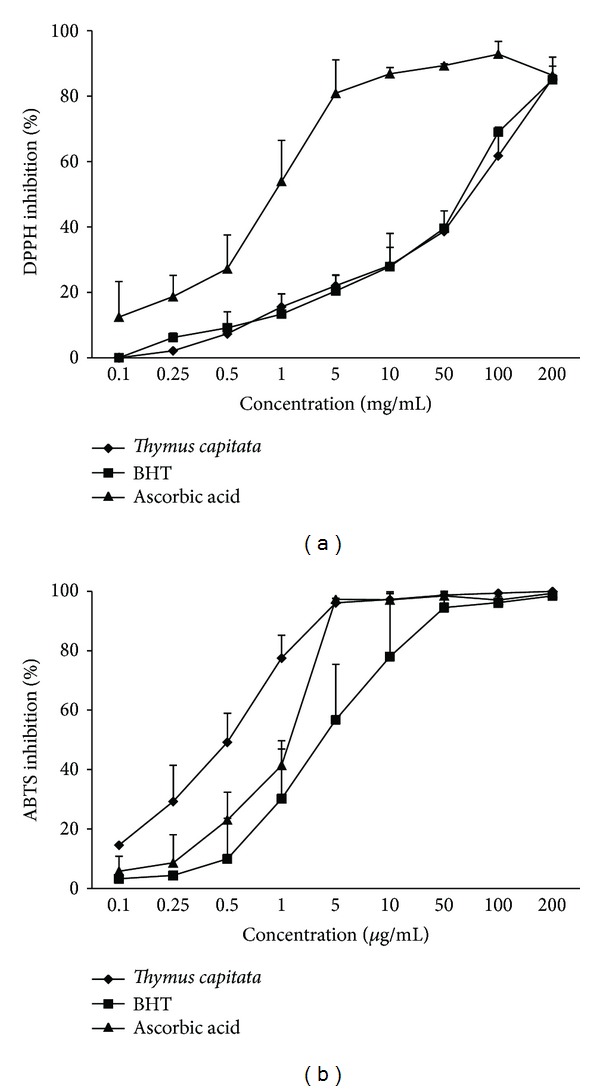
The antioxidant activities of Thymus capitata essential oil as determined by DPPH (a) and ABTS (b) free radical-scavenging activity. The absorbance values were converted to scavenging effects (%) and data plotted as the means of replicate scavenging effect (%) values. (Results are expressed as means ± standard deviation of three measurements.)
3.2.1. DPPH Free Radical-Scavenging Activity
The effect of antioxidant on DPPH radical-scavenging was conceived to their hydrogen-donating ability [44]. DPPH is a stable free radical that accepts on electron or hydrogen radical to become a stable diamagnetic molecule [43].
From the analysis of Figure 1(a), we can conclude that the radical-scavenging activity of the EO and positive controls increased with increasing concentration (Spearman correlations r = 0.856 with P values <0.0001). Furthermore, the results obtained in this study indicated that the T. capitata EO exhibited a high DPPH radical-scavenging activity and its percentage inhibition reached 85.44 ± 1.06% at a concentration of 200 μg/mL. The graph (Figure 1(a)) showed that the radical-scavenging activity of T. capitata EO was 44.16 ± 0.809 μg/mL, which appeared lower than of synthetic antioxidants BHT and ascorbic acid, with values of IC50 = 39.97 ± 1.64 μg/mL and 1.136 ± 0.305 μg/mL, respectively.
3.2.2. ABTS Free Radical-Scavenging Activity
Similar to DPPH, the decolorization of ABTS radical reflects the capacity of an antioxidant species to donate electron or hydrogen atoms to inactivate this radical cation [45]. The ABTS results were in good agreement with DPPH method that the scavenging activity of the EO was increased with the increasing concentration (Spearman correlations r = 0.89 with P values <0.0001). From the analysis of Figure 1(b), we can conclude that the T. capitata EO exhibited higher ABTS radical-scavenging activity (99.98 ± 0.01%), which was comparable to that of BHT (98.46 ± 0.95%) and ascorbic acid (99.33 ± 0.59%) for the same concentration 200 μg/mL. These findings were confirmed by calculating the IC50 values for the T. capitata EO (IC50 = 0.463 ± 0.122 μg/mL), which was found to be significantly (P < 0,05) better than that of BHT (IC50 = 3.204 ± 3.541 μg/mL) and ascorbic acid (IC50 = 1.126 ± 0.19 μg/mL). These results are in agreement with previous studies [46, 47], which showed that greater antioxidant potential of several Thymus species EOs could be related to the nature of phenolic compounds and their hydrogen ability. Besides, it could be ascribed to the oxygenated types of compounds, such as carvacrol and thymol [26, 48]. Moreover, the activities of EOs of Thymus species depend on several structural features of the molecules and are primarily attributed to the high reactivity of hydroxyl group substituent [49].
Scavenging the ABTS radical by the T. capitata EO was found to be much higher than that of DPPH radical. These differences can be explained by the mechanism of the involved reaction. The ABTS radical reactions involve electron transfer and take place at a much faster rate compared to DPPH radicals [50]. Furthermore, various factors like stereoselectivity of the radicals or the solubility of the tested sample in different testing systems and functional groups present in the bioactive compounds have been reported to affect the capacity of the sample to react and quench different radicals [51]. Wang et al. in 1998 [52] showed that some compounds which have ABTS+ scavenging activity may not show DPPH scavenging activity.
3.3. Antimicrobial Activity
In the present study, the in vitro antimicrobial activities of T. capitata EO against the studied microorganisms were qualitatively and quantitatively assessed by the presence or absence of inhibition zones and MIC values, respectively (Table 3). The results obtained from the disc- diffusion method indicated that EO exerted a strong antibacterial activity against all tested strains. Results were comparable to those of the antibiotics (chloramphenicol and streptomycin), used as positive controls. The size of the inhibition zone of T. capitata EO varied from 15 to 80 mm, while the inhibition zones of the chloramphenicol and streptomycin ranged from 18–27 mm to 12–22 mm, respectively.
Table 3.
Antibacterial activity of essential oil from Thymus capitata, using paper disc-diffusion method and microdilution test.
| Strains | Disc-diffusion method (DD) | MIC | ||
|---|---|---|---|---|
| Thymbra capitata (L.) | Antibiotics | Thymbra capitata (L.) | ||
| a | b | |||
| Pseudomonas aeruginosa ATCC 27853 | 23 | 21 | 12 | 10 |
| Pseudomonas aeruginosa ATCC 9027 | 15 | 19 | 13 | 20 |
| Pseudomonas aeruginosa (clinical isolate) | 17 | 22 | 16 | 20 |
| Escherichia coli ATCC 25922 | 70 | NA | 12 | 2.5 |
| Enterococcus faecalisATCC 11700 | 60 | 20 | 14 | 2.5 |
| Enterobacter cloacae ATCC 13097 | 80 | 18 | 13 | 5 |
| Salmonella typhimurium ATCC 14028 | 50 | 22 | 15 | 2.5 |
| Salmonella enteritidis ATCC 502 | 80 | 21 | 13 | 5 |
| Salmonella salamae ATCC 6633 | 75 | 22 | 15 | 5 |
| Salmonella anatum (food isolate) | 80 | 20 | 18 | 2.5 |
| Shigella flexneri ATCC 29903 | 80 | 18 | 15 | 2.5 |
| Shigella sonnei (clinical isolate) | 80 | 20 | 14 | 1.25 |
| Staphylococcus aureus ATCC 2592 | 20 | 20 | 22 | 5 |
| Staphylococcus aureus ATCC 6538 | 75 | 23 | NT | 0.32 |
| Streptococcus pyogenes ATCC 12344 | 75 | 21 | NT | 2.5 |
| Listeria monocytogenes ATCC 19118 | 70 | 23 | 16 | 5 |
| Morganella morganii (clinical isolate) | 75 | NT | NT | 1.25 |
| Klebsiella oxytoca (clinical isolate) | 70 | 21 | 15 | 2.5 |
| Vibrio cholerae (clinical isolate) | 80 | NT | NT | 0.63 |
| Yersinia enterocolitica ATCC 23715 | 80 | NT | NT | 10 |
| Proteus mirabilis ATCC 29906 | 45 | NT | NT | 5 |
| Bacillus cereus ATCC 11768 | 50 | 20 | 16 | 0.63 |
| Bacillus cereus (food isolate) | 80 | NA | NA | 1.25 |
| Bacillus subtilis (food isolate) | 70 | 27 | 15 | 5 |
(3) Disc-diffusion method. Inhibition zone in diameter around the discs impregnated with 12 μL of essential oil. The diameter (9 mm) of the disc is included.
MIC: minimal inhibitory concentration; values given as mg/mL for the essential oils.
a: Chloramphenicol (30 μg/μL); b: streptomycin B (10 μg/μL); NT: not tested; NA: not active.
Referring to the large inhibition zones observed with disk-diffusion method for T. capitata EO, the MIC values were determined by the microdilution broth assay (Table 3). The results of the MIC values against tested Gram-positive and Gram-negative bacteria varied from 0.32 to 5 mg/mL and from 0.63 to 20 mg/mL, respectively. We found that the antibacterial activity of the EO depends on its concentration and the tested bacteria strain. Interestingly, we have found that Staphylococcus aureus ATCC 6538 is the most sensitive tested microorganism, with the lowest MIC value (0.32 mg/mL), and it was closely followed by Bacillus cereus ATCC 11768. This antimicrobial spectrum obtained with the EO of T. capitata is comparable in most cases to the one reported by Bounatirou et al. in 2007 [9]. In addition, Vibrio cholerae (clinical isolate) is the most sensitive Gram-negative bacteria with the lowest MIC value (0.63 mg/mL). Our results confirmed that Gram-positive bacteria were more susceptible to the antimicrobial properties of EO than Gram-negative ones. These differences could be attributed in part to the great complexity of the double membrane-containing cell envelope in Gram-negative bacteria compared to the single membrane structure of the positive ones [53, 54]. These differences may be attributed also to the presence of the lipopolysaccharides in the outer membrane of the Gram-negative bacteria, which make them inherently resistant to external agents, such as hydrophilic dyes, antibiotics, detergents, and lipophilic compounds [14, 55]. However, the ability of EOs to disrupt the permeability barrier of cell membrane structures and the accompanying loss of chemiosmotic control is the most likely reason for its lethal action [56]. The EOs can coagulate the cytoplasm and damage lipids and proteins [3]. Their mechanism of action would be similar to other phenolics, that is, the disturbance of the proton motive force, electron flow, active transport, and coagulation of cell contents. Instead, enzymes such as ATPases are known to be located in the cytoplasmic membrane and to be bordered by lipid molecules [3, 13].
Generally, antimicrobial activities of the EOs are difficult to correlate with a specific compound due to their complexity and variability; nevertheless, some investigators reported that there is a relationship between the chemical composition of the most abundant components in the EO and the antimicrobial activity [57, 58]. In the present study, carvacrol was the main component of T. capitata EO. It has been reported to be biocidal, resulting in bacterial membrane perturbations that lead to leakage of intracellular ATP and potassium ions and ultimately cell death [59, 60]. Previous studies [61] mentioned that carvacrol at concentrations of 0.5% and 1% shows antibacterial activity against Shigella sonnei and Shigella flexneri. Besides, it has been reported that carvacrol causes perturbation in the bacterial membrane and thus potentially can exert antibacterial activity also at intracellular sites [60, 62]. These results are in accordance with the earlier findings [12, 54] that showed that Thymus species' essential oils rich in carvacrol were demonstrated to be potent antimicrobial in vitro.
However, other constituents, such as terpinene and p-cymene have been shown to display relatively good activity due to their potential synergistic or antagonistic effects [10, 63].
3.4. The Effects of the EOs on L. monocytogenes Inoculated in Minced Beef Meat
In this part of our work, we studied in vivo the anti-Listeria activity of different concentration of T. capitata EO when inoculated in minced beef meat, as well as the effect of EO on the extension of shelf life and the preservation of the freshness of meats. It is well known that not all microbiologists demonstrated that decontamination of meat is required or even desirable. It has been argued by Jay in 1996 [64] that high levels of indigenous nonpathogenic microorganisms may have a protective effect on meat and its products, by out-competing the pathogens. Despite this fact, our samples were decontaminated in order to reduce the number of factors involved in the microorganisms' growth in such food model and to avoid interferences of colonies on plating agar. The bacteria count, which is related to survival time of L. monocytogenes in our processed food model following treatment with various concentrations (0.01; 0.05; 0.25, and 1.25% (v/w)) of T. capitata EO, was presented in Figure 2. Results showed that the initially recorded population of Listeria monocytogenes in untreated samples (control) increased approximately from 5log CFU/g to 7.13 log CFU/g during 15 days of storage. However, data from each of the four preparations showed a gradual decrease in the bacteria count with the increasing EO concentration. It appears that the used concentrations are higher than those applied for the in vitro tests. This cannot be misleading, because it is well established that intrinsic factors such as composition (e.g., proteins, fat) as well as extrinsic factors (temperature, oxygen limitation) of the food affect the behavior of bacteria in food ecosystems and may act synergistically with preservatives such as antimicrobial agents [32]. Indeed, food components, such as proteins and fat, are known to bind and/or solubilized phenolic compounds, reducing their availability for antimicrobial activity. Furthermore, it has been reported by many authors that antimicrobial activity of spice is lower in food systems than in microbiological media [65].
Figure 2.
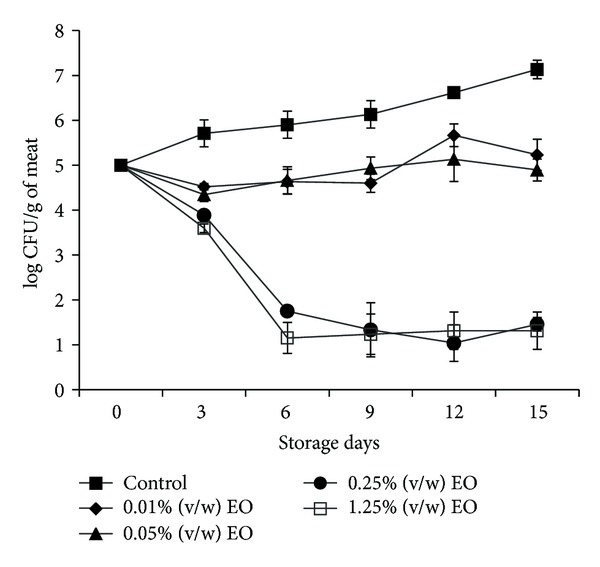
Time-related survival of Listeria monocytogenes at 7°C following treatment with increasing concentrations of Thymus capitata essential oil. Bacteria were supplemented in minced beef meat samples at 105 CFU/g of meat. Values are the average of three individual replicates.
Indeed, a reduction of 4×log/g in the level of L. monocytogenes was recorded in 3 days of storage with a concentration of 0.25 or 1.25% (v/w) of T. capitata EO, compared to the control (not treated), and those treated either with a concentration of 0.01 or 0.05% (v/w) of T. capitata EO. The differences in the values were statistically significant (P values <0.001). Thus, at the end of experimentation (15 days of storage), bacteria count in minced beef treated with a concentration of 0.25 and 1.25% (v/w) of T. capitata EO decreased and reached 1.45 and 1.13×log CFU/g, respectively. However, we did not notice immediate lethal (bactericidal) effects on L. monocytogenes when T. capitata EO was applied as described by other studies [66, 67], but we observed a strong inhibitory activity against L. monocytogenes. These discrepancies between our results and others that found full lethal effects can be explained by the fact that the activity depends on the type, composition and the concentration of the EO, the strain, and the dose of target microorganism inoculated in the meat. In this study, we used high inoculum (105 CFU/g) before treating mince beef compared to low inoculum (103 CFU/g) used by Hsouna et al. in 2011 [67]. Taken together, these results demonstrated that EOs derived from T. capitata have a great potential in terms of activity against the tested strains of L. monocytogenes. Thus, the dose-related inhibitory activity suggests the possibility of using this product as meat preservative. In agreement with our findings, Djenane et al. in 2011 [14] showed a high decrease of bacteria load when minced beef is treated with Pistacia lentiscus and Satureja montana EOs against Listeria monocytogenes CECT 935.
These results are in accordance with previous studies reveling that thyme EO significantly reduced viable counts of Listeria monocytogenes in Russian-type salad during one-week storage at 10°C when combined with Enterocin AS-48 (30–60 μg/g) [68] and exhibited a reduction about 0.25% of initial populations of L. monocytogenes in minced pork by 2 and 2.3×log CFU/g after 8 days of storage at 4°C and 8°C [69]. In fact, the potent antimicrobial activities of T. capitata EO observed in this study can be attributed to the presence of high concentration of carvacrol, which has a well-documented antibacterial potential [14].
4. Conclusion
In conclusion, this study focused on the correlation between the chemical concentration and the effectiveness of T. capitata EO as an antioxidant and antimicrobial. The results of this work show that T. capitata EO can exhibit strong antioxidant and antimicrobial activity, probably due to its particular chemical composition, mainly the high amounts of carvacrol. In the second part, L. monocytogenes populations in minced beef treated with essential oil were significantly lower than those in control samples throughout the storage period. The application of 0.25 or 1% EOs (v/w) of T. capitata EO to minced beef coupled with low temperature storage can reduce the potential of L. monocytogenes contamination. So, this EO can be used for the preservation of meats against L. monocytogenes and for increasing their shelf life. All results obtained herein suggest that the T. capitata EO exhibited a bioprotector effect and therefore it could be used in many biotechnological fields as a natural preservative ingredient of food and/or pharmaceutical industries.
Acknowledgments
This work was supported by a grant from the Ministère de l'Enseignement Supérieur, de la Recherche Scientifique et de la Technologie (Tunisia). The authors are very grateful to Dr. Ghrabi-Gammar Zeineb (INAT) for identification of the harvested plants used in this study and Dr. Chokri Messaoud (INSAT) for his help on the GC and GC-MS analyses. The authors equally thank Dr. Nazek Gallas (Institut Pasteur de Tunis, Tunisia) for providing them with bacteria.
Abbreviations
- EOs:
Essential oils
- T. capitata:
Thymus capitata
- GC-MS:
gas chromatography, mass-spectrometry.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Alzoreky NS, Nakahara K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. International Journal of Food Microbiology. 2003;80(3):223–230. doi: 10.1016/s0168-1605(02)00169-1. [DOI] [PubMed] [Google Scholar]
- 2.Shan B, Cai Y-Z, Brooks JD, Corke H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. International Journal of Food Microbiology. 2007;117(1):112–119. doi: 10.1016/j.ijfoodmicro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Viuda-Martos M, Mohamadyb MA, Fernández-Lópeza J, et al. In vitro antioxidant and antibacterial activities of essentials oils obtained from Egyptian aromatic plants. Food Control. 2011;22:1715–1722. [Google Scholar]
- 4.Tenore GC, Ciampaglia R, Arnold NA, et al. Antimicrobial and antioxidant properties of the essential oil of Salvia lanigera from Cyprus. Food and Chemical Toxicology. 2011;49(1):238–243. doi: 10.1016/j.fct.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Iverson F. Phenolic antioxidants: health protection branch studies on butylated hydroxyanisole. Cancer Letters. 1995;93(1):49–54. doi: 10.1016/0304-3835(95)03787-W. [DOI] [PubMed] [Google Scholar]
- 6.Mohd Nazri NAA, Ahmat N, Adnan A, Syed Mohamad SA, Syaripah Ruzaina SA. In vitro antibacterial and radical scavenging activities of Malaysian table salad. African Journal of Biotechnology. 2011;10(30):5728–5735. [Google Scholar]
- 7.Rawdkuen S, Suthiluk P, Kamhangwong D, Benjakul S. Antimicrobial activity of some potential active compounds against food spoilage microorganisms. African Journal of Biotechnology. 2012;11:13914–13921. [Google Scholar]
- 8.Phillipson JD. Phytochemistry and pharmacognosy. Phytochemistry. 2007;68(22–24):2960–2972. doi: 10.1016/j.phytochem.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 9.Bounatirou S, Smiti S, Miguel MG, et al. Chemical composition, antioxidant and antibacterial activities of the essential oils isolated from Tunisian Thymus capitatus . Food Chemistry. 2007;105(1):146–155. [Google Scholar]
- 10.Nejad Ebrahimi S, Hadian J, Mirjalili MH, Sonboli A, Yousefzadi M. Essential oil composition and antibacterial activity of Thymus caramanicus at different phenological stages. Food Chemistry. 2008;110(4):927–931. doi: 10.1016/j.foodchem.2008.02.083. [DOI] [PubMed] [Google Scholar]
- 11.Figueiredo AC, Barroso JG, Pedro LG, Salgueiro L, Miguel MG, Faleiro ML. Portuguese thymbra and thymus species volatiles: chemical composition and biological activities. Current Pharmaceutical Design. 2008;14(29):3120–3140. doi: 10.2174/138161208786404218. [DOI] [PubMed] [Google Scholar]
- 12.Jamali CA, El Bouzidi L, Bekkouche K, et al. Chemical composition, antioxidant and anticandidal activities of essential oils from different wild Moroccan Thymus species. Chemistry & Biodiversity. 2012;9:1188–1197. doi: 10.1002/cbdv.201200041. [DOI] [PubMed] [Google Scholar]
- 13.Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. International Journal of Food Microbiology. 2004;94(3):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Djenane D, Yangüela J, Montañés L, Djerbal M, Roncalés P. Antimicrobial activity of Pistacia lentiscus and Satureja montana essential oils against Listeria monocytogenes CECT 935 using laboratory media: efficacy and synergistic potential in minced beef. Food Control. 2011;22(7):1046–1053. [Google Scholar]
- 15.Hyldgaard M, Mygind T, Meyer RL. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Frontiers in Microbiology. 2012;25:1–24. doi: 10.3389/fmicb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bajpai VK, Rahman A, Dung NT, Huh MK, Kang SC. In vitro inhibition of food spoilage and foodborne pathogenic bacteria by essential oil and leaf extracts of Magnolia liliflora Desr. Journal of Food Science. 2008;73(6):M314–M320. doi: 10.1111/j.1750-3841.2008.00841.x. [DOI] [PubMed] [Google Scholar]
- 17.Bubonja-Sonje M, Giacometti J, Abram M. Antioxidant and antilisterial activity of olive oil, cocoa and rosemary extract polyphenols. Food Chemistry. 2011;127(4):1821–1827. [Google Scholar]
- 18.McLauchlin J, Mitchell RT, Smerdon WJ, Jewell K. Listeria monocytogenes and listeriosis: a review of hazard characterisation for use in microbiological risk assessment of foods. International Journal of Food Microbiology. 2004;92(1):15–33. doi: 10.1016/S0168-1605(03)00326-X. [DOI] [PubMed] [Google Scholar]
- 19.Datta AR. Listeria monocytogenes. In: Miliotis MD, Bier JW, editors. International Handbook of Foodborne Pathogens. New York, NY, USA: Marcel Dekker; 2003. pp. 105–121. [Google Scholar]
- 20.Farber JM, Peterkin PI. Listeria monocytogenes, a food-borne pathogen. Microbiological Reviews. 1991;55(3):476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Djenane D, Yangüela J, Amrouche T, Boubrit S, Boussad N, Roncalés P. Chemical composition and antimicrobial effects of essential oils of Eucalyptus globulus, Myrtus communis and Satureja hortensis against Escherichia coli O157:H7 and Staphylococcus aureus in minced beef. Food Science and Technology International. 2011;17(6):505–515. doi: 10.1177/1082013211398803. [DOI] [PubMed] [Google Scholar]
- 22.Ozkan G, Sagdic O, Gokturk RS, Unal O, Albayrak S. Study on chemical composition and biological activities of essential oil and extract from Salvia pisidica . LWT—Food Science and Technology. 2010;43(1):186–190. [Google Scholar]
- 23.European Pharmacopoeia 6.0. Determination of essential oils in herbal drugs. 2.8.12, 2008.
- 24.Adams RP. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th edition. Carol Stream, Ill, USA: Allured; 2007. [Google Scholar]
- 25.Mantle D, Anderton JG, Falkous G, Barnes M, Jones P, Perry EK. Comparison of methods for determination of total antioxidant status: application to analysis of medicinal plant essential oils. Comparative Biochemistry and Physiology B. 1998;121(4):385–391. [Google Scholar]
- 26.Ruberto G, Baratta MT. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chemistry. 2000;69(2):167–174. [Google Scholar]
- 27.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Science and Technology. 1995;28(1):25–30. [Google Scholar]
- 28.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 1999;26(9-10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 29.NCCLS (National Committee for Clinical Laboratory Standards) Performance Standards for Antimicrobial Susceptibility Testing. 9th edition. Wayne, Pa, USA: International Supplement. M100-S9; 1999. [Google Scholar]
- 30.NCCLS (National Committee for Clinical Laboratory Standards) Performance Standards for Antimicrobial Disc Susceptibility Test. 6th edition. Wayne, Pa, USA: Approved Standard. M2-A6; 1997. [Google Scholar]
- 31.Careaga MÓ, Fernández E, Dorantes L, Mota L, Jaramillo ME, Hernandez-Sanchez H. Antibacterial activity of Capsicum extract against Salmonella typhimurium and Pseudomonas aeruginosa inoculated in raw beef meat. International Journal of Food Microbiology. 2003;83(3):331–335. doi: 10.1016/s0168-1605(02)00382-3. [DOI] [PubMed] [Google Scholar]
- 32.Best DJ, Roberts DE. Algorithm AS 89: the upper tail probabilities of spearman's rho. Journal of the Royal Statistical Society. 1975;24:377–379. [Google Scholar]
- 33.Chambers JM, Freeny AE, Heiberger RM. Analysis of variance, designed experiments. In: Chambers JM, Hastie TJ, editors. Statistical Models in S. Pacific Grove, Calif, USA: Wadsworth & Brooks/Cole; 1992. [Google Scholar]
- 34.Napoli EM, Curcuruto G, Ruberto G. Screening of the essential oil composition of wild Sicilian thyme. Biochemical Systematics and Ecology. 2010;38(4):816–822. [Google Scholar]
- 35.Palmeira-De-Oliveira A, Gaspar C, Palmeira-De-Oliveira R, et al. The anti-Candida activity of Thymbra capitata essential oil: effect upon pre-formed biofilm. Journal of Ethnopharmacology. 2012;140(2):379–383. doi: 10.1016/j.jep.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 36.Salgueiro LR, Pinto E, Gonçalves MJ, et al. Chemical composition and antifungal activity of the essential oil of Thymbra capitata . Planta Medica. 2004;70(6):572–575. doi: 10.1055/s-2004-827162. [DOI] [PubMed] [Google Scholar]
- 37.Tomaino A, Cimino F, Zimbalatti V, et al. Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chemistry. 2005;89(4):549–554. [Google Scholar]
- 38.Jaafari A, Mouse HA, Rakib EM, et al. Chemical composition and antitumor activity of different wild varieties of Moroccan thyme. Brazilian Journal of Pharmacognosy. 2007;17(4):477–491. [Google Scholar]
- 39.Verma RS, Verma RK, Chauhan A, Yadav AK. Seasonal variation in essential oil content and composition of Thyme, Thymus serpyllum l. Cultivated in Uttarakhand hills. Indian Journal of Pharmaceutical Sciences. 2011;73(2):233–235. doi: 10.4103/0250-474x.91570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, Wang X, Li Y, Li P, Wang H. Polyphenolic compounds and antioxidant properties of selected China wines. Food Chemistry. 2009;112(2):454–460. [Google Scholar]
- 41.Koleva II, Van Beek TA, Linssen JPH, De Groot A, Evstatieva LN. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochemical Analysis. 2002;13(1):8–17. doi: 10.1002/pca.611. [DOI] [PubMed] [Google Scholar]
- 42.Ozgen M, Reese RN, Tulio AZ, Jr., Scheerens JC, Miller AR. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. Journal of Agricultural and Food Chemistry. 2006;54(4):1151–1157. doi: 10.1021/jf051960d. [DOI] [PubMed] [Google Scholar]
- 43.Patel-Rajesh M, Patel-Natvar J. In vitro antioxidant activity of coumarin compounds by DPPH, Super oxide and nitric oxide free radical scavenging methods. Journal of Advanced Pharmaceutical Technology & Research. 2011;1:52–68. [Google Scholar]
- 44.Chen Y, Xie M-Y, Nie S-P, Li C, Wang Y-X. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum . Food Chemistry. 2008;107(1):231–241. [Google Scholar]
- 45.Olajuyigbe OO, Afolayan AJ. Phytochemical assessment and antioxidant activities of alcoholic and aqueous extracts of acacia mearnsii de wild. International Journal of Pharmacology. 2011;7(8):856–861. [Google Scholar]
- 46.Tepe B, Sokmen M, Akpulat HA, Daferera D, Polissiou M, Sokmen A. Antioxidative activity of the essential oils of Thymus sipyleus subsp. sipyleus var. sipyleus and Thymus sipyleus subsp. sipyleus var. rosulans. Journal of Food Engineering. 2005;66(4):447–454. [Google Scholar]
- 47.Guimarães R, Sousa MJ, Ferreira ICFR. Contribution of essential oils and phenolics to the antioxidant properties of aromatic plants. Industrial Crops and Products. 2010;32(2):152–156. [Google Scholar]
- 48.Piccaglia R, Marotti M, Giovanelli E, Deans SG, Eaglesham E. Antibacterial and antioxidant properties of Mediterranean aromatic plants. Industrial Crops and Products. 1993;2(1):47–50. [Google Scholar]
- 49.Číž M, Čížová H, Denev P, Kratchanova M, Slavov A, Lojek A. Different methods for control and comparison of the antioxidant properties of vegetables. Food Control. 2010;21(4):518–523. [Google Scholar]
- 50.Ennajar M, Bouajila J, Lebrihi A, et al. Chemical composition and antimicrobial and antioxidant activities of essential oils and various extracts of Juniperus phoenicea l. (Cupressacees) Journal of Food Science. 2009;74(7):M364–M371. doi: 10.1111/j.1750-3841.2009.01277.x. [DOI] [PubMed] [Google Scholar]
- 51.Adedapo AA, Jimoh FO, Afolayan AJ, Masika PJ. Antioxidant activities and phenolic contents of the methanol extracts of the stems of Acokanthera oppositifolia and Adenia gummifera . BMC Complementary and Alternative Medicine. 2008;8, article 54 doi: 10.1186/1472-6882-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang M, Li J, Rangarajan M, et al. Antioxidative phenolic compounds from Sage (Salvia officinalis) Journal of Agricultural and Food Chemistry. 1998;46:4869–4873. [Google Scholar]
- 53.Busatta C, Mossi AJ, Rodrigues MRA, Cansian RL, De Oliveira JV. Evaluation of Origanum vulgare essential oil as antimicrobial agent in sausage. Brazilian Journal of Microbiology. 2007;38(4):610–616. [Google Scholar]
- 54.Bagamboula CF, Uyttendaele M, Debevere J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri . Food Microbiology. 2004;21(1):33–42. [Google Scholar]
- 55.Gupta SM, Arif M, Ahmed Z. Antimicrobial activity in leaf, seed extract and seed oil of Jatropha curcas L. plant. Journal of Applied and Natural Science. 2011;3:102–105. [Google Scholar]
- 56.Cox SD, Mann CM, Markham JL, Gustafson JE, Warmington JR, Wyllie SG. Determining the antimicrobial actions of tea tree oil. Molecules. 2001;6(2):87–91. [Google Scholar]
- 57.Farag RS, Daw ZY, Hewedi FM, El-Baroty GSA. Antimicrobial activity of some Egyptian spice essential oils. Journal of Food Protection. 1989;52:665–667. doi: 10.4315/0362-028X-52.9.665. [DOI] [PubMed] [Google Scholar]
- 58.Deans SG, Svoboda KP. The antimicrobial properties of marjoram (Origanum majorana L.) volatile oil. Flavour and Fragrance Journal. 1990;5(3):187–190. [Google Scholar]
- 59.Knowles JR, Roller S, Murray DB, Naidu AS. Antimicrobial action of carvacrol at different stages of dual-species biofilm development by Staphylococcus aureus and Salmonella enterica serovar typhimurium. Applied and Environmental Microbiology. 2005;71(2):797–803. doi: 10.1128/AEM.71.2.797-803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ultee A, Kets EPW, Smid EJ. Mechanisms of action of carvacrol on the food-borne pathogen. Applied and Environmental Microbiology. 1999;65(10):4606–4610. doi: 10.1128/aem.65.10.4606-4610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bagamboula CF, Uyttendaele M, Debevere J. Antimicrobial effect of spices and herbs on Shigella sonnei and S. flexneri . Journal of Food Protection. 2003;66(4):668–673. doi: 10.4315/0362-028x-66.4.668. [DOI] [PubMed] [Google Scholar]
- 62.Cristani M, D’Arrigo M, Mandalari G, et al. Interaction of four monoterpenes contained in essential oils with model membranes: implications for their antibacterial activity. Journal of Agricultural and Food Chemistry. 2007;55(15):6300–6308. doi: 10.1021/jf070094x. [DOI] [PubMed] [Google Scholar]
- 63.Vardar-Ünlü G, Candan F, Sókmen A, et al. Antimicrobial and antioxidant activity of the essential oil and methanol extracts of Thymus pectinatus Fisch. et Mey. Var. pectinatus (Lamiaceae) Journal of Agricultural and Food Chemistry. 2003;51(1):63–67. doi: 10.1021/jf025753e. [DOI] [PubMed] [Google Scholar]
- 64.Jay JM. Microorganisms in fresh ground meats: the relative safety of products with low versus high numbers. Meat Science. 1996;43(1):S59–S66. doi: 10.1016/0309-1740(96)00055-1. [DOI] [PubMed] [Google Scholar]
- 65.Shelef LA, Jyothi EK, Bulgarelli MA. Growth of enteropathogenic and spoilage bacteria in sage-containing broth and foods. Journal of Food Science. 1984;49(3):737–740. [Google Scholar]
- 66.Hayouni EA, Chraief I, Abedrabba M, et al. Tunisian Salvia officinalis L. and Schinus molle L. essential oils: their chemical compositions and their preservative effects against Salmonella inoculated in minced beef meat. International Journal of Food Microbiology. 2008;125(3):242–251. doi: 10.1016/j.ijfoodmicro.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 67.Hsouna AB, Trigui M, Mansour RB, Jarraya RM, Damak M, Jaoua S. Chemical composition, cytotoxicity effect and antimicrobial activity of Ceratonia siliqua essential oil with preservative effects against Listeria inoculated in minced beef meat. International Journal of Food Microbiology. 2011;148(1):66–72. doi: 10.1016/j.ijfoodmicro.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 68.Antonio CM, Abriouel H, López RL, Omar NB, Valdivia E, Gálvez A. Enhanced bactericidal activity of enterocin AS-48 in combination with essential oils, natural bioactive compounds and chemical preservatives against Listeria monocytogenes in ready-to-eat salad. Food and Chemical Toxicology. 2009;47(9):2216–2223. doi: 10.1016/j.fct.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 69.Aureli P, Costantini A, Zolea S. Antimicrobial activity of some plant essential oils against Listeria monocytogenes . Journal of Food Protection. 1992;55:344–348. doi: 10.4315/0362-028X-55.5.344. [DOI] [PubMed] [Google Scholar]


