Abstract
Rodent studies employing cortical removal techniques, ranging from transient deactivation to surgical ablation of cortex, reveal the importance of auditory cortical integrity in detecting short silent gaps in white noise (2–15 ms; [1,2]). Processing limits for longer gaps under decorticate conditions in rats remain unknown. Determining the temporal threshold for sub-cortical resolution of gaps in noise could, however, shed light on both normal hierarchical processing of acoustic temporal stimuli, as well as the etiology of processing anomalies following developmental cortical disruption [3,4,5,6,7,8]. To address these important issues, we assessed whether intact rats, as well as those with induced developmental cortical disruptions (microgyria), could resolve silent gaps of 20 to 100 ms in duration when embedded in white noise, during functional deactivation of auditory cortex. Results showed that both intact rats, as well as those with cortical malformations resulting from early focal disruptions of neuronal migration, could resolve silent gaps of 100 ms duration under cortical deactivation (KCl). However, only intact rats could reliably detect 75 ms gaps, suggesting possible sub-cortical anomalies in subjects with early cortical disturbances.
Keywords: Auditory temporal processing, Sub-cortical, Startle response, Developmental injury, Learning impairment
Introduction
Silent gap detection tasks are frequently used to assess temporal processing in the auditory system [9, 5, 10, 11]. In humans, rapid temporal processing of acoustic information is developmentally important to the acquisition of normal speech and language [12, 13, 14]. Given that auditory cortical circuits have been shown to play an important role in the processing of rapidly changing acoustic information in rodents [1,2] and humans [14, 15, 16], it follows that early disruption of cortical development (e.g., neuronal migration) could alter subsequent auditory temporal discrimination. In fact, cumulative findings show that developmental cortical disruption results in widespread alterations of cortical anatomy and physiology, in rodent models and also developmentally disabled populations with evidence of neural anomalies [17, 18, 19, 20]. For example, postmortem brains of human dyslexics have been shown to exhibit focal cortical malformations indicating anomalies of early neuronal migration (microgyria, ectopia; [20]), and recent human studies show that abnormal cortical structure and physiology are associated with deficits in language development and reading [14, 15, 16, 21, 22]. Concomitant evidence shows that these language disruptions are correlated with basic deficits in rapid auditory processing that may manifest early in infancy [14]. Importantly, rodents with induced and spontaneous focal cortical migrational anomalies (microgyria, ectopia) also show deficits in processing short duration acoustic stimuli when compared to shams [3, 4, 5]. Taken together, this convergent information highlights an important role for early cortical integrity in subsequent auditory processing, and suggests that developmental disruptions of cortex (including key processes such as neuronal migration) may lead to subsequent sensory processing anomalies in both humans and animal models, which may in turn relate to developmental disruption of language-related processes in humans.
Studies evaluating the consequences of cortical malformations (e.g., microgyria and ectopia) continue to reveal evidence of subsequent global changes in both anatomy and circuitry, including in structures distal to the cortex (such as thalamic nuclei; [4, 7, 8, 17]). For example, rats with microgyric malformations of cortex induced on postnatal day one (P1) exhibit significant shifts in medial geniculate nucleus (MGN) cell size, with more small and fewer large neurons compared to shams [4,7]. Similar abnormalities in cell size are seen in the lateral and medial geniculate nuclei of the thalamus in dyslexic brains examined post mortem [8]. In rodents, these shifts in cell size are accompanied by changes in cortico-cortical and cortico-thalamic connectivity [17]. Reports have also shown decreases in interneuron population, up-regulation of excitatory synapses, and increases in excitatory inputs beyond the malformations - all of which may interfere with regional communication within and beyond cortex [19, 23]. These developmentally dependent shifts in brain organization are likely to alter a wide range of behavioral processes at various levels. Although some or all of these findings may relate to (or interact with) the emergence of associated rapid auditory processing deficits, it is still unclear whether and how cortical developmental disruption affects sensory processing thresholds, as well as whether these behavioral changes reflect secondary functional alterations to sub-cortical structures (such as the MGN) or the direct effects of cortical alterations per se.
Teasing apart the contributions of sub-cortical and cortical function to auditory temporal processing thresholds as measured at the behavioral level may ultimately provide insight into both normal and pathological neurodevelopmental conditions such as dyslexia [24]. With this in mind, the present research sought to address two fundamental issues related to normal and pathological auditory temporal processing.
The first issue concerns whether discrimination of auditory temporal cues is specifically dependent on auditory cortex in rats, or if processing of these behaviorally relevant cues can occur sub-cortically. For example, studies have shown that both reversible deactivation and aspiration lesions of auditory cortex (A1) in rats lead to impairments in detection of short duration silent gaps in white noise (2–15 ms for deactivation and 2–10 ms after ablation; [1, 2]). However, discrimination of longer duration silent gaps in noise (> 20 ms) under cortical deactivation has not been examined. Yet the assessment of auditory temporal processing thresholds using gap durations of 20–100 ms could help identify whether auditory cortex is the primary contributor to temporal acuity in developmentally normal rats [25].
The second issue concerns evidence of impaired rapid gap detection in rats with cortical developmental disruption (microgyria, [5]), indicating that this type of pathology can degrade subsequent temporal acuity [9, 4, 5, 13, 14]. Given data indicating wide spread changes to connectivity and morphology of cortical and sub-cortical structures following developmental perturbation of cortex, it seems plausible that non-cortically dependent temporal processing could be secondarily degraded as a result of an initial cortical developmental pathology [26]. By evaluating temporal processing capabilities of intact rats as well as rats with developmentally disrupted cortex (microgyria) we can determine whether temporal processing deficits observed in microgyric rats are primarily a product of cortical dysfunction alone, versus more widespread changes to brain organization (possibly including thalamic function).
In summary, the current study sought to address two fundamental questions; 1) Are developmentally normal, functionally decorticate adult rodents able to process temporally relevant acoustic signals (silent gaps in white noise, duration 20–100 ms)?; 2) Does developmental cortical injury (microgyria) impair the processing of these signals (as compared to shams) when measured under functional deactivation of auditory cortex? To address these questions, male rats with focal lesions of cortex induced on P1 (a procedure known to disrupt neuronal migration and lead to microgyric malformations), along with sham littermates, were assessed for rapid auditory processing as juveniles (P23), using a gap detection task in an adapted startle reduction paradigm. Subsequently, gap detection thresholds for the same subjects were assessed in adulthood (P70+), under both normal conditions (saline) and also functional deactivation of auditory cortex (using topical KCl).
Experimental Procedures
Subjects
Subjects included 31 male Wistar rats (sham n=16, microgyric n=15) born to purchased dams (Charles River Laboratory, Wilmington, MA) at the University of Connecticut. Subjects were right or left ear marked and housed into pairs at P21 using a 12:12 light/dark cycle with food and water available ad lib. Juvenile behavioral testing began at P23 for all subjects. All procedures were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, including adequate measures to minimize pain and discomfort. The Institutional Animal Care and Use committee (IACUC) at the University of Connecticut approved all procedures.
Induction of P1 freezing lesion (microgyria)
On the day of surgery (P1), litters were culled to 10 pups (8 male, 2 female), with male pups randomly assigned to receive double-pair freezing lesion or sham surgery. Females were retained to equalize litter size and avoid all-male litters. Prior to surgery, subjects were cryogenically anesthetized using crushed ice. Surgeries involved a 1 mm incision, followed by the placement of a 2 mm stainless steel probe cooled to −70°C on the skullcap. Focal lesions were induced as a double-pair (two to each hemisphere) as previously described [5]. This procedure has been shown to lead to formation of cortical microgyria (see figure 2) [4, 5]. Sham subjects received similar treatment but with a room temperature probe. After surgery, pups were individually marked with inkpad injections, warmed under a lamp and placed back with the mother.
Figure 2.
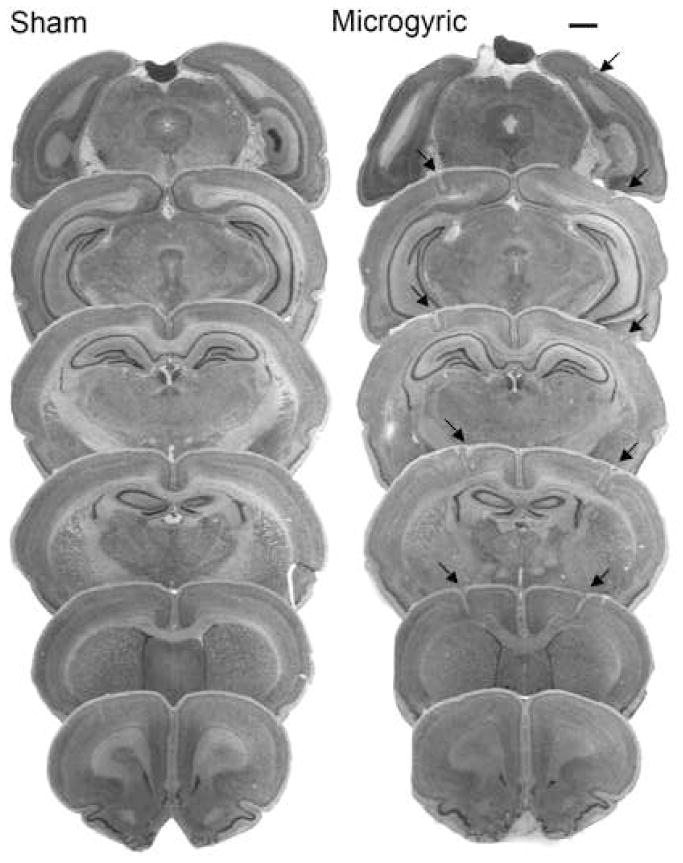
Overlaid photomicrographs showing sham sections and the extent of bilateral microgyria induced on postnatal day one (P1) in a typical case (scale bar 500μm).
Adult Craniotomy (preparation for auditory cortical deactivation)
Craniotomy surgeries started on P70 for the first 4 subjects. Subsequently, a maximum of 4 surgeries were performed each day for 7 days, totaling 31 surgeries over 8 days. On the day of craniotomy, subjects were anesthetized with ketamine (70mg/kg ip) and xylazine (8mg/kg ip) and placed in a stereotaxic instrument with blunt ear bars (to avoid damaging the tympanic membrane). A two-cm-long midline incision was made starting at bregma. The underlying tissue was removed and a pair of 4-mm-diameter bores were made, centered 6 mm posterior to bregma and 5 mm lateral to the sagittal suture, approximately overlaying Te1-3 (auditory cortex; [27]). The dura was left intact. Prior to lightly stapling the skin, antibiotic ointment soaked cotton pellets were placed into the skull openings. Adult behavioral assessments began the following day.
Adult Saline/KCL Application Procedure
Twenty-four hours after craniotomy surgery, subjects were briefly anesthetized with isofluorane (4% vapor), staples were removed, and the antibiotic pellets were replaced with cotton pellets saturated in a .9% sterile saline solution. Behavioral testing began 30 min after the application of saline. On the following day, the same procedure was followed, however instead of saline, cotton pellets were saturated in a 25% sterile KCl solution and placed over each of the skull openings overlying auditory cortex. The effects of KCl application were visible almost immediately after animals awoke from anesthesia. These effects included motor slowing and diminished posture, and visibly contrasted the saline condition (which evidenced no such symptoms). The methods described above approximate those used by Ison et al. [1].
Histology
At the end of behavioral testing, subjects were weighed, anesthetized with ketamine/xylazine (100/15 mg/kg), and transcardially perfused with saline followed by 10% phosphate buffered formalin. The brains were removed, neonatal lesions were visually confirmed (appearing as surface indentations on cortex), and the location verified, and brains were bottled in formalin and shipped to GDR at Beth Israel Deaconess Medical Center for histological preparation. All subjects identified as microgyric showed evidence of focal malformations, and no such malformations were seen in sham brains.
Behavioral testing: startle reduction
Juvenile auditory testing began on P23 and involved a startle response paradigm that has been discussed extensively elsewhere [4, 5]. Briefly, the startle modification paradigm involves the presentation of an auditory cue prior to a startle-eliciting stimulus (SES). The SES produces an acoustic startle reflex (ASR), and if the preceding auditory cue is detected, the intensity of the ASR is reduced accordingly. For testing, subjects were placed on a load cell platform (Med Associates, Georgia, VT, USA), which measured the subject’s ballistic motor response to the SES in mV. Signals were acquired and passed through a linear load cell amplifier (PHM-250-60) into a Biopac MP100WS acquisition system (Biopac Systems, Santa Barbra, CA) connected to two Macintosh computers, which recorded the subject’s movement and ASR as a mV signal. The maximum peak value defining the ASR for each trial was extracted from the 200 ms following the onset of the SES, and this ASR represents a dependent variable. Auditory stimuli were generated using a Pentium 4 Dell PC with custom programmed software and a Tucker Davis Technologies (RP2) real time processor. Stimulus files were played through a Marantz integrated amplifier connected to nine Cambridge Sound Works speakers, with sound levels calibrated by sound-level meter [4]. Each pair of platforms had one speaker centered and mounted 30 cm above. Attenuated response scores (ATT) were calculated from the peak ASR, using the formula ([mean cued response/mean uncued response] × 100). In this formula, absolute response scores (as measured by load-cell displacement for each subject’s startle response) for cued and uncued trials are expressed as a ratio, multiplied by 100 (thus ATT scores represent a percentage). ATT scores approaching 100% indicate no detection of the cue, and ATT scores were analyzed as a second dependent variable for all tasks.
Single tone
In the current study, all subjects received one day of a normal single tone procedure (NST; see [4] for details), prior to silent gap testing, to verify normal hearing and startle function. The single tone test session was comprised of 104 trials (cued or uncued), presented in a pseudo-random order on one day. Uncued trials consisted of a silent background followed by the 105 dB, 50 ms SES. On cued trials a 75 dB, 7 ms, 2,300 Hz tone was presented 50 ms prior to the SES (the “cue”). Trials were variable in duration (16 – 24 sec) to prevent anticipation of the SES.
Long silent gap
The long silent gap session (0–100 ms gaps) included 300 trials, each consisting of the presentation of a variable duration long silent gap (0, 2, 5, 10, 20, 30, 40, 50, 75, or 100 ms) embedded in continuous 75 dB broadband white noise. The gap was presented 50 ms prior to a 105 dB burst of white noise. The uncued trials used a “gap” of 0 ms. The cue-burst interval for each task was maintained at 50 ms [4, 5]. The long silent gap task was presented in the juvenile period to provide experience for the subsequent short silent gap task, and also in adulthood to assess gap detection following application of KCl/saline to auditory cortex.
Short silent gap
A short silent gap procedure (using gap durations of 0, 2, 3, 4, 5, 6, 7, 8, 9 and 10 ms) was also presented in the juvenile period, in order to assess the shortest detectable gap as a function of Treatment (microgyria or sham) [5]. All parameters except for the gap durations were identical to those in the long silent gap condition.
Results
Brain analysis
Analysis of postmortem brains revealed similar location and size of microgyric malformations in all of the subjects that received P1 freezing lesion treatment (sensorimotor cortex (SM-1), with some extension into frontal, temporal and occipital cortices). None of the sham subjects showed any cortical malformations.
Behavioral analysis
Initial analysis of subject scores under KCl deactivation revealed an unusually high degree of variability for both sham and microgyric rats. Accordingly, a criterion of two or more standard deviations from the treatment group mean was used as an outlier formula for removal of subjects from further analyses. It is unclear what factors may have contributed to such aberrant ATT scores in a small number of subjects. However, a roughly equal number of subjects from each treatment condition were removed from final analyses, suggesting that the anomalous scores were not related to the presence or absence of microgyria (sham n=3, microgyric n=4). The total number of subjects for each Treatment group utilized in final analyses was 24 (sham n=13, microgyric n=11).
Single tone
An ANOVA comparing attenuated response scores indicated no significant Treatment effect for normal single tone detection. Thus, sham and microgyric subjects performed equally well on this task in the juvenile period. Paired samples t-tests using mean absolute response scores (cue vs. uncue) showed significant differences (p < .05) between cued and uncued responses for both groups, indicating significant and equivalent baseline auditory discrimination and startle in both groups.
Juvenile Long silent gap
A repeated measures ANOVA for Treatment (2 levels) × Gap (9 levels) on ATT scores from the long silent gap (0–100 ms) task showed no significant Treatment effect for the juvenile period (F1,22; p = ns). However, sham subjects could detect gaps down to 10 ms (10, 20, 30, 40, 50, 75, 100 ms gaps) (F1, 12; p < .05), whereas microgyric subjects were only able to discriminate down to 20 ms gaps (20, 30, 40, 50, 75, 100 ms gaps) (F1, 10; p < .05).
Juvenile Short silent gap
A repeated measures ANOVA for Treatment (2 levels) × Gap (9 levels) on ATT scores from the short silent gap (0–10) task showed a significant Treatment effect, [F1, 22; p < .035 one-tail], with shams showing overall better gap detection than microgyrics (see figure 3). A multivariate ANOVA was computed to assess differences in performance at individual gaps. Results showed that shams were significantly better than microgyrics at detecting gaps of 3, 4 and 5 ms in duration (p < .05). Paired samples t-tests using mean absolute response scores (cue vs. uncue) also revealed that sham subjects could detect all of the short silent gaps (2–10 ms, F1, 12; p < .05), whereas microgyrics could only detect down to the 4 ms gap (4–10 ms, F1, 10; p < .05). These results replicate previous findings showing that focal developmental disruption of cortex (e.g., microgyria) leads to robust and predictable rapid auditory processing (RAP) deficits later in life [4, 5].
Figure 3.
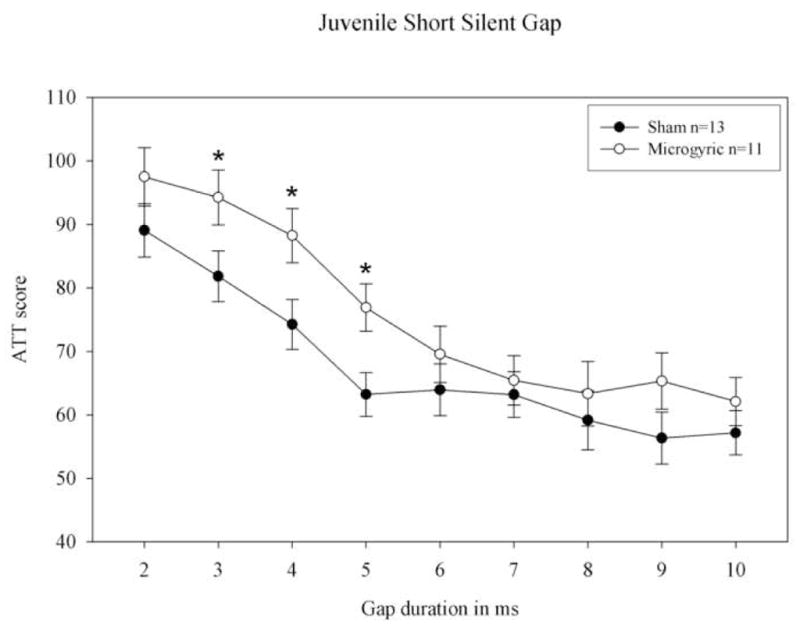
Juvenile short (0–10 ms) silent gap attenuated response (ATT) scores for sham and microgyric rats. Shams showed significantly better gap detection compared to the lesion group (*= p < .05).
Adult Sham and Microgyria: Saline/KCl
A repeated measures ANOVA for Saline/KCl (2 levels) × Gap (9 levels) was conducted to assess the performance of sham subjects under saline versus KCl application to auditory cortex. Results showed a highly significant main effect of Saline/KCl [F1,12; p < .01] indicating that shams had significantly lower (i.e., better) attenuation scores under saline as compared to KCl application (see figure 4). Under saline, shams could detect down to a 5 ms silent gap (F1, 12; p < .05). However, under KCl, the threshold for gap detection was shifted to 75 ms (with shams discriminating both 75 and 100 ms gaps; F1, 12; p < .05). A repeated measures ANOVA for Saline/KCl (2 levels) × Gap (9 levels) was also conducted to assess the performance of microgyric subjects under saline versus KCl application to auditory cortex. Results again revealed a significant main effect of Saline/KCl (F1, 10; p < .01), indicating that subjects had significantly lower (i.e., better) attenuation scores under saline as compared to KCl application. Under saline, microgyric subjects were able to detect down to a 5 ms silent gap, as evidenced by paired samples t-tests comparing mean absolute response scores (cued vs. uncued) at the 2–100 ms gap durations (F1, 10; p < .05). However, under KCl, the threshold for microgyrics appeared to shift to 100 ms, with subjects significantly detecting only the 100 ms gap (F1, 10; p < .05). Finally, a repeated measures ANCOVA for Treatment (2 levels; sham, microgyria) × Gap (2 levels, 75–100 ms) under KCl application with NST as a covariate, revealed a non-significant trend toward worse performance in microgyrics during cortical deactivation [F1, 1; p = .09, one-tail].
Figure 4.
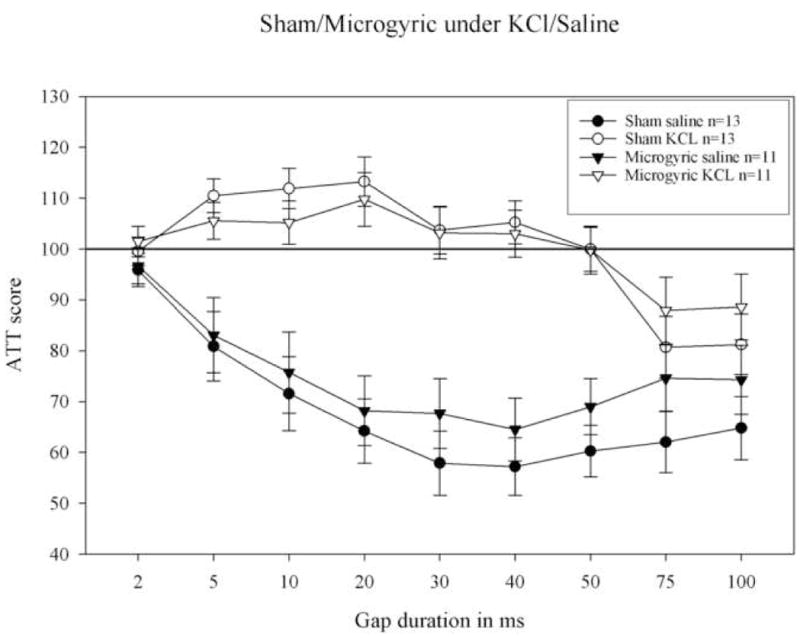
Long silent gap (0–100) attenuation response scores (ATT scores) for sham and microgyric rats in adulthood following saline and KCl application to auditory cortex. Note a significant effect of KCl, with both groups performing markedly better under saline. Sham subjects significantly detected the 75 and 100 ms gaps under KCl deactivation of auditory cortex. Microgyric subjects could only significantly detect the 100 ms gap under KCl (paired samples t-test for mean cued and uncued startle amplitude at each gap duration). ATT scores approaching 100 indicate chance performance (failure to discriminate the cued and uncued trials).
Discussion
At a general level, our current results support the view that cortex is not required for the processing of long duration (75–100 ms) temporally relevant acoustic information in rats, at least as measured by the adapted startle reduction paradigm described here. Thus, although we confirmed evidence of impaired detection of brief silent gaps (2–20 ms) under functional decortication, (similar to results reported by Ison et al., [1] and Bowen et al., [2]), we also found that at longer gap durations, developmentally normal (sham) subjects were able to show significant detection (as evidenced by attenuation of the startle response). Interestingly, subjects with developmental disruptions of cortex (microgyria), who have previously been shown to exhibit changes in thalamic morphology and hemispheric connectivity, were also able to resolve silent gaps of 100 ms in duration under KCl. These findings indicate that sub-cortical structures functioning during reversible deactivation of auditory cortex can process temporally important signals in the 75 – 100 ms range. Evidence that functionally decorticate rats are able to temporally resolve long duration silent gaps in white noise provides valuable information for future investigations of the behavioral relevance of sub-cortical structures in temporal acoustic processing, particularly following developmental disruptions that could have cascading effects on cortical and sub-cortical morphology and connectivity.
In the current study, we were unable to obtain clear evidence that deficits in the processing of rapid silent gaps (0–10 ms) observed in juvenile microgyric subjects persist when the threshold for silent gap detection is shifted under cortical deactivation. This in turn indicates that neurodevelopmental alterations in cortex could be the direct cause of rapid auditory processing deficits seen in microgyric rodents (i.e., with sub-cortical acoustic temporal processing intact). However, it is important to note that microgyric rats were unable to significantly detect the 75 ms silent gaps under KCl, even though shams showed significant detection. While this is not the major finding of the current study, it does suggest that further testing will be required, specifically using a more fine-grained array of gaps between 75 and 100 ms, to fully determine whether temporal processing deficits seen in microgyric rats under normal conditions are also seen in microgyric rats under cortical deactivation. Such a result would support the hypothesis that thalamic anomalies in subjects with early cortical disruption (e.g., microgyria) may contribute to acoustic processing abnormalities (e.g., RAP deficits), and may in turn account in part for processing anomalies seen in developmentally disrupted populations such as those with language disorders (e.g., dyslexia, [8, 13, 21, 22, 24].
Conclusions
We have shown that sham rats are capable of detecting long-duration silent gaps (75 & 100 ms) following cortical deactivation using KCl, indicating that sub-cortical structures can effectively process temporally relevant stimuli at these longer durations in rats. In addition, we found that rats with focal developmental disruptions (e.g., microgyria) are able to detect only silent gaps of 100 ms under KCl. Although no significant between group difference (sham vs. microgyric) was seen during cortical deactivation, the fact that microgyric rats were unable to detect 75 ms silent gaps suggests that future research may reveal subtle processing deficits within the 75–100 ms range in microgyric rats as compared to shams under cortical deactivation. Such findings would indicate functional alterations in sub-cortical processing that might contribute to RAP deficits evidenced under normal conditions in microgyric rats, as well as within dyslexic and language disabled populations. The current findings highlight the importance of assessing subcortical processing contributions to the behavioral assessment of RAP deficits, and may have important implications for our understanding of neural mechanisms contributing to processing deficits in human language impaired populations.
Figure 1.
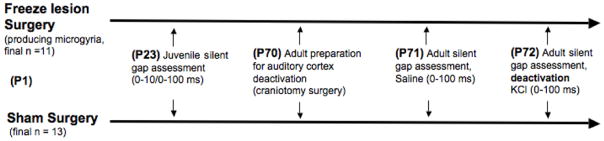
Surgery and behavior testing timeline for microgyria and sham groups.
Acknowledgments
Research supported by NIH Grant HD20806
References
- 1.Ison JR, O’Connor K, Bowen G, Bocirnea A. Temporal resolution of gaps in noise by the rat is lost with functional decortication. Behavioral Neuroscience. 1991;105(1):33–40. doi: 10.1037//0735-7044.105.1.33. [DOI] [PubMed] [Google Scholar]
- 2.Bowen PG, Lin D, Merritt K, Ison T, Ison JR. Auditory cortex lesions in the rat impair both temporal acuity and noise increment thresholds, revealing a common neural substrate. Cereb Cortex. 2003;13:815–822. doi: 10.1093/cercor/13.8.815. [DOI] [PubMed] [Google Scholar]
- 3.Clark M, Rosen G, Tallal P, Fitch R. Impaired processing of complex auditory stimuli in rats with induced cerebrocortical microgyria: An animal model of developmental language disabilities. J Cogn Neurosci. 2000;12(5):828–39. doi: 10.1162/089892900562435. [DOI] [PubMed] [Google Scholar]
- 4.Peiffer A, Rosen G, Fitch RH. Rapid auditory processing and MGN morphology in microgyric rats reared in varied acoustic environments. Brain Research Dev Brain Res. 2002;138(2):187–93. doi: 10.1016/s0165-3806(02)00472-8. [DOI] [PubMed] [Google Scholar]
- 5.Threlkeld SW, McClure MM, Rosen GD, Fitch RH. Developmental timeframes for induction of microgyria and rapid auditory processing deficits in the rat. Brain Res. 2006;1109:22–31. doi: 10.1016/j.brainres.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Rosen G, Burstein D, Galaburda A. Changes in Efferent and Afferent Connectivity in Rats With Induced Cerebrocortical Microgyria. J Comp Neurol. 2000;418:423–440. [PubMed] [Google Scholar]
- 7.Herman AE, Galaburda AM, Fitch RH, Carter AR, Rosen GD. Cerebral microgyria, thalamic cell size and auditory temporal processing in male and female rats. Cerebral Cortex. 1997;7(5):453–64. doi: 10.1093/cercor/7.5.453. [DOI] [PubMed] [Google Scholar]
- 8.Galaburda A, Menard M, Rosen G. Evidence for aberrant auditory anatomy in developmental dyslexia. Proc Natl Acad Sci USA. 1994;91:8010–8013. doi: 10.1073/pnas.91.17.8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman J, Peiffer A, Matthew C, Benasich A, Fitch RH. Age and experience-related improvements in gap detection in the rat. Dev Brain Res. 2004;152:83–91. doi: 10.1016/j.devbrainres.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Phillips DP. Auditory gap detection, perceptual channels, and temporal resolution in speech perception. J Am Acad Audiol. 1999;10(6):343–54. [PubMed] [Google Scholar]
- 11.Trehub SE, Schneider BA, Henderson JL. Gap detection in infants, children and adults. J Acoust Soc Am. 1995;98:2532–2541. doi: 10.1121/1.414396. [DOI] [PubMed] [Google Scholar]
- 12.Tallal P, Piercy M. Defects of non-verbal auditory perception in children with developmental aphasia. Nature. 1973;241(5390):468–9. doi: 10.1038/241468a0. [DOI] [PubMed] [Google Scholar]
- 13.Tallal P. Improving language and literacy is a matter of time. Nature Reviews. 2004;4:721–728. doi: 10.1038/nrn1499. [DOI] [PubMed] [Google Scholar]
- 14.Benasich A, Choudhury N, Friedman JT, Realpe-Bonilla T, Chojnowska C, Cou Z. The infant as a prelinguistic model for language learning impairments: predicting from event-related potentials to behavior. Neuropsychologia. 2006;44(3):396–411. doi: 10.1016/j.neuropsychologia.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bishop DV, McArthur GM. Immature cortical responses to auditory stimuli in specific language impairment: evidence from ERPs to rapid tone sequences. Dev Sci. 2004;7(4):F11–8. doi: 10.1111/j.1467-7687.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- 16.Bishop DV, McArthur GM. Individual differences in auditory processing in specific language impairment: a follow-up study using event-related potentials and behavioral thresholds. Cortex. 2005;1(3):327–41. doi: 10.1016/s0010-9452(08)70270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosen G, Windzio H, Galaburda A. Unilateral Induced Neocortical Malformation and the Formation of Ipsilateral and Contralateral Barrel Fields. Neuroscience. 2001;103(4):931–939. doi: 10.1016/s0306-4522(01)00044-6. [DOI] [PubMed] [Google Scholar]
- 18.Gabel LA, LoTurco JJ. Layer I ectopias and increased excitability in murine neocortex. J Neurophysiol. 2002;87:2471–9. doi: 10.1152/jn.2002.87.5.2471. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs KM, Prince DA. Excitatory and inhibitory postsynaptic currents in a rat model of epileptogenic microgyria. J Neurophysiol. 2005;93(2):687–96. doi: 10.1152/jn.00288.2004. [DOI] [PubMed] [Google Scholar]
- 20.Galaburda A, Sherman G, Rosen G, Aboitiz F, Geschwind N. Developmental Dyslexia: Four Consecutive Patients with Cortical Abnormalities. Ann Neurol. 1985;18(2):222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- 21.Chang BS, Ly J, Bodell A, Apse KA, Ravenscroft RS, Sheen VL, Doherty MJ, et al. Reading impairment in the neuronal migration disorder of perientricular nodular herterotopia. Neurology. 2005;64:799–803. doi: 10.1212/01.WNL.0000152874.57180.AF. [DOI] [PubMed] [Google Scholar]
- 22.de Vasconcelos Hage SR, Cendes F, Montenegro MA, Abramides DV, Guimaraes CA, Guerreiro MM. Specific language impairment: linguistic and neurobiological aspects. Arq Neuropsiquiatr. 2006;64(2A):173–180. doi: 10.1590/s0004-282x2006000200001. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs KM, Hwang BJ, Prince DA. Focal epileptogenesis in a rat model of polymicrogyria. J Neurophysiol. 1999;81(1):159–73. doi: 10.1152/jn.1999.81.1.159. [DOI] [PubMed] [Google Scholar]
- 24.Habib M. The neurological basis of developmental dyslexia: An overview and working hypothesis. Brain. 2000;123:2373–2399. doi: 10.1093/brain/123.12.2373. [DOI] [PubMed] [Google Scholar]
- 25.Winer J, Miller L, Lee C, Schreiner C. Auditory thalamocortical transformation: structure and function. TRENDS in Neurosciences. 2005;28(5):255–263. doi: 10.1016/j.tins.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Herman A, Galaburda A, Fitch RH, Carter A, Rosen G. Cerebral microgyria, thalamic cell size and auditory temporal processing in male and female rats. Cerebral cortex. 1997;7:453–464. doi: 10.1093/cercor/7.5.453. [DOI] [PubMed] [Google Scholar]
- 27.Paxinos G, Watson C. The rat brain. New York: Academic Press; 1986. [Google Scholar]


