Abstract
Given that human adolescents place a high value on social interactions—particularly while consuming alcohol—the current study utilized a novel social drinking paradigm to examine rewarding and aversive properties of ethanol in non-water deprived rats that were housed and tested in groups of five same-sex littermates. On postnatal day (P) 34 (adolescents) or P69 (adults), rats were habituated to the testing apparatus for 30 min. On the next day, animals were placed into the test apparatus and given 30-min access to a supersaccharin solution (3% sucrose; 0.125% saccharin), followed immediately by an intraperitoneal injection of ethanol (0, 0.25, 0.5, 1.0, 1.5 g/kg). Subsequent intake of the supersacharrin solution was assessed on three consecutive test days. Adolescent males were less sensitive to ethanol’s aversive effects than adult males, with adolescent males maintaining an aversion on all three test days only at the 1.5 g/kg dose, whereas adults demonstrated aversions across test days to 1 and 1.5 g/kg. Adolescent females maintained aversions to 1 and 1.5 g/kg across days, whereas adult females continued to show an aversion to the 1.5 g/kg dose only. These opposite patterns of sensitivity that emerged among males and females at each age in the propensity to maintain an ethanol-induced taste aversion under social conditions may contribute to age- and sex-related differences in ethanol intake. Testing in social groups may be useful for future work when studying rodent models of adolescent alcohol use given the importance that human adolescents place on drinking in social settings.
Adolescence is the transition from childhood to adulthood characterized by a number of behavioral, hormonal, and neural changes that are highly conserved across mammalian species [1]. Although there is no specific incident that determines the beginning and end of adolescence, in humans, the transition from immaturity to maturity is thought to roughly include the second decade of life [2], with a late adolescent/emerging adulthood transition continuing into the twenties [3]. In rodents, neural, behavioral, and hormonal alterations associated with adolescence occur from postnatal (P) day 28–42 (early-to-mid adolescence), with late adolescence/emerging adulthood continuing from P42–55 [4]. A notable feature of adolescence is an increase in importance of and time spent interacting with peers, an age-specific characteristic evident in both humans [5] and rodents [6, 7] and thought to reflect associations beneficial for the transition into adulthood [8].
In addition to a greater focus on peer-directed social interactions, adolescence is a period in life during which alcohol use is commonly initiated [9], with levels exceeding those observed in adulthood [10]. The higher rates of alcohol intake observed in human adolescents have likewise been supported in rodent models [11, 12]. Social interactions appear to play a role in these adolescent-typical increases in alcohol use. For instance, adolescents report consuming alcohol mostly for its ability to encourage and facilitate ease of interactions with peers [see 13, for review]. Similarly, animal models have demonstrated that low doses of ethanol enhance social interactions in adolescents [7, 14], whereas this effect is not observed in adults under normal, non-stressful, conditions [14]. On the other hand, when compared with adults, adolescents are relatively insensitive to the social inhibition that emerges at moderate-to-high ethanol doses [14]. This adolescent-typical combination of enhanced sensitivity to ethanol-induced social facilitation coupled with an insensitivity to adverse effects (e.g., social inhibition) of ethanol may promote ethanol consumption in adolescents.
One of numerous adverse effects of ethanol to which adolescents are less responsive than adults is its ability to serve as an unconditioned stimulus in a conditioned taste aversion (CTA) paradigm [15, 16]. There is evidence supporting a negative relationship between the magnitude of CTA in adolescence and later ethanol consumption in adulthood [16]. Indeed, ethanol intake and ethanol CTA have been generally found to be negatively correlated [17], supporting the suggestion that aversive properties of ethanol may discourage drinking. Studies to date exploring ethanol’s aversive effects in adolescence have typically been conducted in singly or pair-housed animals, although recent data from our laboratory has demonstrated that exposure to a social peer during ethanol intoxication in the CTA paradigm attenuated sensitivity to ethanol’s aversive effects in adolescents but not adults [18]. Given the importance of social interactions for adolescents, further development of social models to examine age differences in ethanol aversions are likely to yield data of potential relevance to human adolescents.
Sex differences in ethanol intake are particularly prevalent in adult rodents, with adult females typically consuming greater levels of ethanol than adult males [11]. These sex differences in ethanol intake patterns, however, are often less apparent in adolescent rodents [11, 19]. These findings correspond with the human data demonstrating that sex differences in ethanol intake begin to appear in late-adolescence, albeit in the opposite direction (i.e., greater number of drinks consumed in men than women). In prior work, our laboratory observed a lack of sex differences in ethanol-induced CTA in adult animals, whereas adolescent females exposed to a social partner displayed both a conditioned taste preference at a low dose of ethanol and ethanol-induced CTA at higher doses–effects not observed in adolescent males [18].
The present study explored age and sex differences in ethanol CTA among non-deprived animals housed and tested in groups of five same-sex littermates. A wide ethanol dose range was used in order to assess potential taste preferences as well as taste aversions to ethanol among animals conditioned and tested in a social setting.
Subjects
The experimental subjects consisted of 170 Sprague-Dawley rats bred in our colony at Binghamton University. All animals were kept in a temperature-controlled vivarium maintained on a 12:12-hr light/dark cycle (lights on at 0700). Litters were culled to five males and five females within 24 hours after birth, with pups weaned on P21 and housed thereafter in plastic cages with five same-sex littermates. All animals were treated in accordance with the animal care guidelines established by the National Institutes of Health under protocols approved by the Binghamton University Institutional Animal Care and Use Committee. Animals had ad libitum access to food and water throughout the duration of the experiment.
Procedure
To examine the effects of ethanol sensitivity in the CTA paradigm, a 2 age (adolescent, adult) × 2 sex × 5 ethanol dose (0, 0.25, 0.5, 1.0, 1.5 g/kg) experimental design was used. On P34 (adolescents) and P69 (adults), each set of littermates housed together was habituated to the test context by placing the littermates together in the context (i.e., a white opaque plastic cage [50.8 × 40.64 × 20.32 cm]) for 30 min. and then returning them to their home cage until the next day. On conditioning day, cage mates were again placed together in the testing context and videotaped (SONY handycam) while being given a 30-min access to a novel sweetened supersaccharin solution containing 3% sucrose and .125% saccharin in water—a modification of the “supersac” intake procedure [20] used previously in our laboratory [21]. Immediately thereafter, bottles were removed and each animal in the group was injected intraperitoneally with a different challenge dose of ethanol. Ethanol dose was varied altering the volume of the 12.6% (v/v) ethanol solution in physiological saline, with control animals given saline at a volume equivalent to the highest ethanol dose. After injection, animals were returned to their home cage and left undisturbed for 24 hr. For the following three days post-conditioning (i.e., test days), cage mates were placed into the testing context while being given access to the supersaccharin solution for 30 min. On these three days of testing, animals received no injections and were immediately placed back into their home cages following each drinking session. Prior to all sessions, each experimental subject was weighed and given a unique mark across its back to enable individual animal identification on the video recordings of the conditioning and test sessions.
Statistics
Intake values from the conditioning and post-injection test days were examined for outliers, with 16 animals removed prior to data analyses (final n=7–10/group). Data from 11 animals were removed due to low baseline intake—i.e., less than 1 ml: 3 Adolescent females (one each from the 0.5, 1.0, and 1.5 g/kg groups); 4 Adolescent males (one from the 0, 0.25, 1.0, 1.5 g/kg groups); and 4 Adult females (one from the 0 and 1.5 g/kg groups; 2 from the 1.0 g/kg group). Data from five additional animals were eliminated due to exceeding outlier criteria of ±2 standard deviations from the mean: one Adolescent female and 2 Adolescent males from the 0 g/kg groups; and 2 Adult females (one from the 0.25 and 1.5 g/kg groups). Individual consumption was estimated by determining time spent drinking (sec) during the 30-min videotaped session by each animal in the group; this number for each animal was then transformed to percentage of the total intake time of the group, and multiplied by total ml of intake of the group to obtain an intake estimate for each animal. Preliminary work from our laboratory confirmed a significant correlation between time spent drinking and volume consumed of a supersaccharin solution in individually-housed adolescent male (r=0.92) and female (r=0.90) rats as well as their adult counterparts (r=0.92, r=0.97, respectively).
Baseline intake data assessed prior to ethanol injection on conditioning day were analyzed using a 2 age (adolescent, adult) × 2 sex × 5 ethanol dose (0, 0.25, 0.5, 1.0, 1.5 g/kg) analysis of variance (ANOVA), whereas intake data across the three test days were analyzed via a 2 age (adolescent, adult) × 2 sex × 5 ethanol dose (0, 0.25, 0.5, 1.0, 1.5 g/kg) × 3 (test day) mixed-factor ANOVA, with test day treated as a repeated measure. The supersaccharin intake across test days data violated the assumption of homogeneity of variance and were transformed using a square root transformation to improve homogeneity prior to analyses. Fisher’s LSD test was used to determine significant effects and interactions. Ethanol-induced CTA was indexed via significant decreases in supersaccharin intake relative to corresponding saline-injected controls. All data are graphed using raw data for ease of interpretation and reflect mean ± SEM.
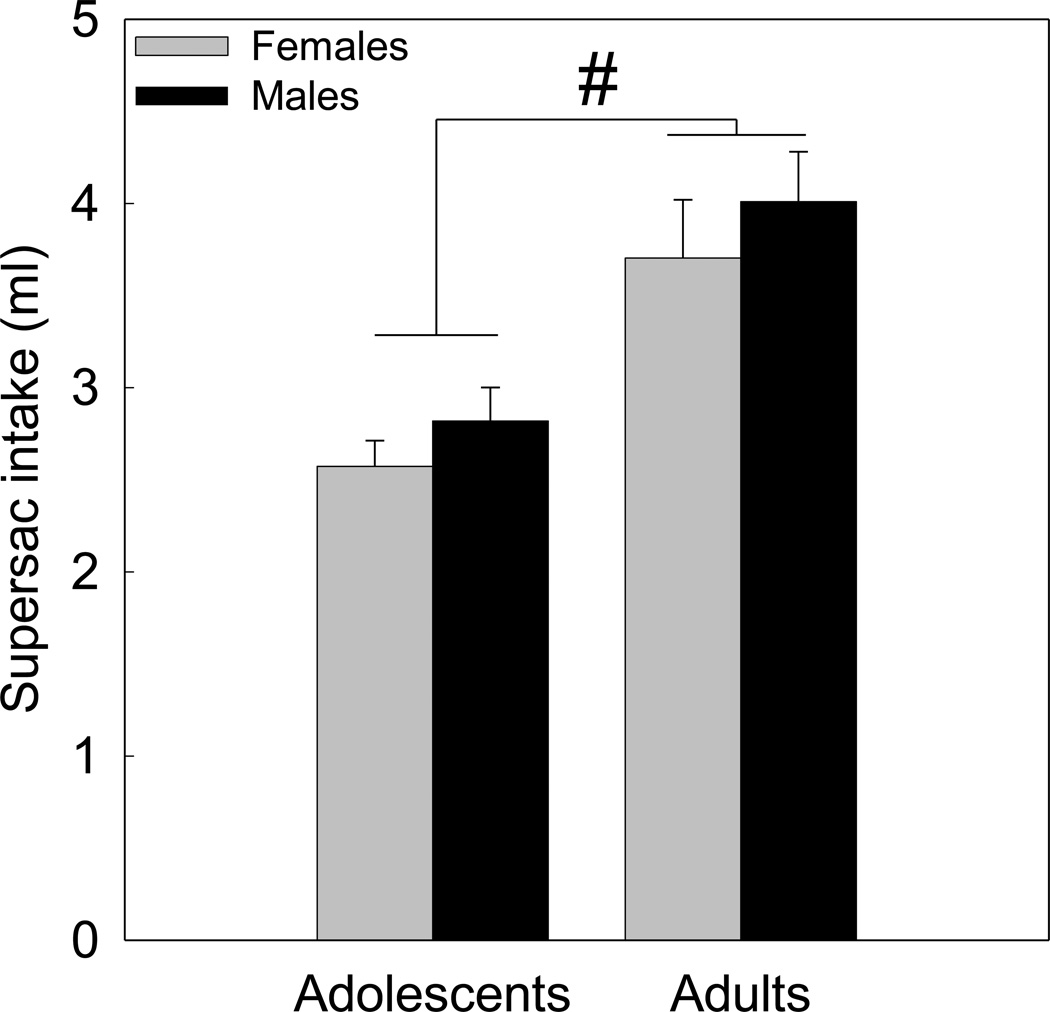
The factorial ANOVA of baseline supersaccharin intake revealed a significant main effect of age [F(1,155) = 23.83, p<0.000001], with adult animals of both sexes consuming more of the supersaccharin solution than adolescent males and females (Figure 1).
Figure 1.
Baseline supersaccharin (supersac) intake (ml) in adolescent and adult, male and female rats. # reflects a significant main effect of age.
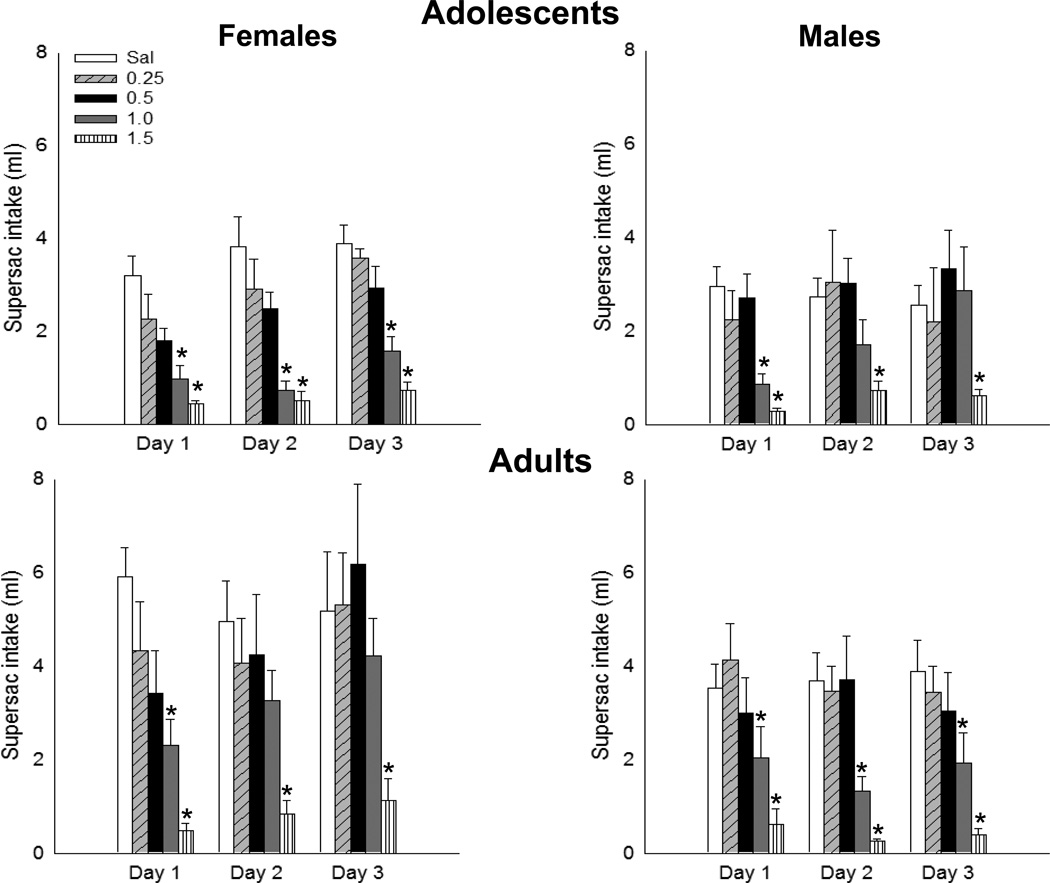
Analysis of the test day intake revealed main effects of age [F(1,134) = 9.41, p<0.01] and dose [F(4,134) = 22.55, p<0.00001] as well as day × sex [F(2,268) = 7.02, p<0.001], day × dose [F8,268) = 2.15, p<0.05], and day × age × sex × dose [F(8,268) = 2.86, p<0.05] interactions. Fisher’s post-hoc test showed that, in adolescent females, significant decreases in supersaccharin intake relative to saline-injected controls emerged after conditioning with 1 and 1.5 g/kg ethanol and were maintained across the three test days (Figure 2, top left). Adolescent males consumed significantly less supersaccharin solution on test day 1 than saline-conditioned animals after conditioning with 1 and 1.5 g/kg ethanol, maintaining an aversion on subsequent testing days only at the 1.5 g/kg dose (Figure 2, top right). In adults, females displayed an aversion to 1 g/kg ethanol only on day 1, whereas an aversion to 1.5 g/kg ethanol was maintained across all three test days (Figure 2, bottom left). Adult males expressed and maintained aversions to 1 and 1.5 g/kg ethanol across all three test days (Figure 2, bottom right).
Figure 2.
Supersaccharin (supersac) intake (ml) in adolescent (top panels) and adult (bottom panels) rats on the three days post-ethanol injection. * reflects a significant difference from same age- and sex- saline-injected control animal.
Both age- and sex-related differences in the expression and extinction of a CTA to ethanol were seen in this study, where animals were conditioned and tested in a social context. Adolescent males and adult females showed an attenuated sensitivity to the aversive properties of ethanol relative to their same age, opposite sex counterparts. Age differences in dose of ethanol required to maintain CTA across days were also evident in both sexes, with adolescent males requiring a higher dose (1.5 g/kg) than adult males (1.0 g/kg) to express ethanol CTA on all test days. An opposite age-related pattern of ethanol sensitivity was evident in females. Although 1.5 g/kg ethanol was sufficient to produce a CTA in adult males in the current study, we have previously shown that this dose of ethanol elicits a conditioned place preference (CPP) in adult male rats [22]. It is not uncommon for the same dose of a drug of abuse to result in a place preference and taste aversion [23, 24]. Aside from obvious differences associated with place and taste conditioning (e.g., spatial location vs flavor), differences in procedural manipulations likely contributed to the opposite results found. It is possible that number of injections and timing of ethanol administration played a role, given that rats in the CPP study were conditioned for four days and injected immediately before placement into the place conditioning chamber on each day, whereas rats in the current study were injected once immediately after being removed from the test chamber. It has been shown that rats conditioned with multiple pairings of ethanol at moderate doses (i.e., 1 g/kg) do show a preference for the ethanol-paired side [24, 25] and a CTA [24]. Thus, it is possible that multiple pairings of ethanol may reduce the aversive properties of ethanol associated with a particular context; however, it should be noted that multiple injections of moderate doses of ethanol do continue to produce CTA in rats [15], demonstrating that the differences in acquisition of preference and avoidance to the same dose of a drug is dependent on the type of conditioning paradigm used.
A sex difference in sensitivity to the aversive effects of ethanol in this CTA test was seen in adolescents (with adolescent males expressing an aversion only on test day 1 after 1 g/kg, whereas adolescent females maintained an ethanol-induced CTA at this intermediate dose on all three test days. These findings support those of Vetter-O’Hagen and colleagues (2009) demonstrating that when tested in the presence of a conspecific, adolescent males were less sensitive to the aversive effects of ethanol than adolescent females. In contrast, although the study by Vetter-O’Hagen et al. (2009) revealed no evidence for sex differences in adults, sex differences in ethanol CTA were evident in the present study where adults were tested at 1 g/kg, with females being less sensitive than males. It should be noted, however, that no sex differences were apparent on day 1, with these differences emerging on subsequent test days. The reduced sensitivity to EtOH-induced CTA in females corresponds with previous research in adults tested over multiple days [26]. An insensitivity of adult females to the aversive effects of ethanol may permit them to consume more ethanol than their adult male counterparts before experiencing aversive effects that presumably serve to moderate intake.
While age differences in the dose required to maintain ethanol-induced aversions over days were evident in both males and females, the effects were opposite at each sex. The need for a greater dose of ethanol in males to maintain CTA over days in adolescence than in adulthood is consistent with numerous reports demonstrating that adult males are more sensitive to the aversive effects of ethanol in a CTA paradigm, regardless of testing procedures [15, 16, 18]. Yet, an opposite pattern of age-related sensitivity to EtOH-induced CTA emerged in females, with it being the adult females who exhibited only transient aversions to the 1 g/kg ethanol dose, whereas their adolescent counterparts demonstrated and maintained aversions to 1 and 1.5 g/kg ethanol. It is unclear why adult females would be less sensitive to the aversive effects of ethanol than adult males and adolescent females. One possibility is that social housing and testing of females may have reduced anxiety that may be associated with typical CTA test circumstances where animals are not housed and tested in large social groups, decreasing their sensitivity to the aversive properties of ethanol. Indeed, adult females have been shown to be more affected by isolate-housing in terms of suppressing social interactions than are males [27]. Estrous cycle was not determined or controlled, and hence it is also possible that variations across days and among females in stage of the estrous cycle could have increased variability in the data sufficiently to disrupt expression of CTA among adult females tested at the intermediate dose. Previous research has shown that reproductive status (i.e., stage of estrous) influences taste reactivity in female rats [28]. However, the possibility that gonadal hormones influenced the aversive properties of ethanol in females appears unlikely, in that we have previously shown no differences in ethanol-induced CTA among females that were pre- or post-pubertally ovariectomized when they were compared with age-matched sham-operated or non-manipulated controls [21].
Both adolescent and adult animals of each sex demonstrated an aversion to the highest ethanol doses on the first day, with differences emerging among the groups by the second and third test days. Differences in rate of extinction that emerged between males and females at each age are likely due to the strength of CTA acquisition. Prior evidence suggests that an animal’s resistance to extinction is a particularly sensitive indicator of how strongly it acquires a conditioned response, with extinction delayed in animals that have acquired stronger conditioned responses [e.g., 29]. Resistance to extinction has also been observed to change as a function of age, with aged and middle-aged mice taking longer to extinguish a lithium-chloride-induced CTA than younger mice [30]. Thus, the persistent aversion seen over test days in adolescent females and adult males after conditioning with the 1 g/kg dose likely reflects stronger CTA at this dose than that seen in adolescent males and adult females who no longer displayed a significant aversion by the second test day following conditioning at this dose.
A limitation of the current study is that we did not directly compare animals trained and tested in a social context versus in isolation to determine the possible role that group housing/testing per se plays in the development of an ethanol CTA. However, comparisons with other studies performed in our laboratory found ethanol-induced CTAs in isolate- and pair-housed adults at similar doses (e.g., 1 and 1.5 g/kg) as used here [15, 18]. In those studies, however, adolescents did not demonstrate an aversion to the 1 g/kg dose (as was observed on test day 1 here), with aversions to the 1.5 g/kg dose even emerging only in one of the studies [15]. The seeming enhanced sensitivity to ethanol CTA among adolescents seen under the social training/test conditions used in the present study relative to animals trained/tested alone as in prior work [15, 18] could be related to the social circumstances of conditioning/testing, or to other procedural variables, such as the use of different CS’s (e.g., saccharin, sucrose, and supersaccharin). The current study used a highly palatable tastant, supersaccharin, to encourage intake of the CS without the need for water deprivation. Yet, there is evidence suggesting that the hedonic value of the CS influences the strength of the CTA [31], with for instance evidence that rats exposed to a lower hedonic-valued CS demonstrate a stronger lithium chloride CTA than those exposed to a CS with a higher hedonic value [31]. Although not tested in that study [31], it is possible that differences in the hedonic value of the CS may also alter sensitivity to the US; thus, rats may require different doses of ethanol to induce a CTA depending on the value placed on the CS by the rats. Although care should be taken when interpreting CTA results that use highly palatable solutions, the results of the current study are in line with those using more neutral CSs in finding similar age-related differences in sensitivity to ethanol-induced CTA [16, 18].
The observed sex and age differences in the propensity to form ethanol CTA may contribute to age- and sex-related differences in ethanol intake. For instance, the insensitivity of adolescent males to the aversive effects of ethanol relative to their adult counterparts may permit the elevated ethanol consumption levels typically seen during adolescence, particularly among males [11, 12]. Likewise, adult females may consume more ethanol than their male counterparts [11, 18] perhaps in part because they are not as sensitive to the aversive consequences of ethanol as adult males. Given that human adolescents typically drink in social settings and place high value on social interactions while consuming alcohol, testing animals in familiar social groups as in the present study may prove useful in future work using basic animal models to explore potential contributors to elevated ethanol use in human adolescents.
Highlights.
Both adolescents and adults developed EtOH-induced CTA in a social context
Adults consumed more supersac than adolescents at baseline, regardless of sex
In males, adolescents were less sensitive to EtOH-induced CTA than adults
In females, adolescents were more affected by EtOH CTA than adults
Adult females recovered from a CTA to EtOH sooner than adult males
Acknowledgments
The work presented in this manuscript was funded by R01 AA017355, P50 AA017823, U01 AA19972 to Linda P. Spear.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose.
References
- 1.Spear LP. The Behavioral Neuroscience of Adolescence. New York: W. W. Norton & Company; 2010. [Google Scholar]
- 2.Lerner RM, Galambos NL. Adolescent development: challenges and opportunities for research, programs, and policies. Annual review of psychology. 1998;49:413–446. doi: 10.1146/annurev.psych.49.1.413. [DOI] [PubMed] [Google Scholar]
- 3.Arnett JJ. Emerging adulthood. A theory of development from the late teens through the twenties. The American psychologist. 2000;55:469–480. [PubMed] [Google Scholar]
- 4.Vetter-O'Hagen CS, Spear LP. Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Developmental psychobiology. 2012;54:523–535. doi: 10.1002/dev.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Csikszentmihalyi M, Larson R, Prescott S. The ecology of adolescent activity and experience. Journal of Youth and Adolescence. 1977;6:281–294. doi: 10.1007/BF02138940. [DOI] [PubMed] [Google Scholar]
- 6.Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behavioural brain research. 2008;188:398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trezza V, Baarendse PJ, Vanderschuren LJ. Prosocial effects of nicotine and ethanol in adolescent rats through partially dissociable neurobehavioral mechanisms. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:2560–2573. doi: 10.1038/npp.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neuroscience and biobehavioral reviews. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- 9.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on drug use: 2012 Overview, Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research, The University of Michigan; 2013. [Google Scholar]
- 10.Administration SAaMHS. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. NSDUH Series H-44, HHS Publication No (SMA) 12-4713. [Google Scholar]
- 11.Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcoholism, clinical and experimental research. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- 12.Tambour S, Brown LL, Crabbe JC. Gender and age at drinking onset affect voluntary alcohol consumption but neither the alcohol deprivation effect nor the response to stress in mice. Alcoholism, clinical and experimental research. 2008;32:2100–2106. doi: 10.1111/j.1530-0277.2008.00798.x. [DOI] [PubMed] [Google Scholar]
- 13.Kuntsche E, Knibbe R, Gmel G, Engels R. Why do young people drink? A review of drinking motives. Clinical psychology review. 2005;25:841–861. doi: 10.1016/j.cpr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcoholism, clinical and experimental research. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- 15.Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male sprague-dawley rats: impact of age and stress. Alcoholism, clinical and experimental research. 2010;34:2106–2115. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schramm-Sapyta NL, DiFeliceantonio AG, Foscue E, Glowacz S, Haseeb N, Wang N, et al. Aversive effects of ethanol in adolescent versus adult rats: potential causes and implication for future drinking. Alcoholism, clinical and experimental research. 2010;34:2061–2069. doi: 10.1111/j.1530-0277.2010.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vetter-O'Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol and alcoholism. 2009;44:547–554. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcoholism, clinical and experimental research. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- 20.Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behavioural pharmacology. 2008;19:1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morales M, Spear LP. Differences in sensitivity to ethanol-induced conditioned taste aversions emerge after pre- or post-pubertal gonadectomy in male and female rats. Behavioural brain research. 2013;240:69–75. doi: 10.1016/j.bbr.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morales M, Varlinskaya EI, Spear LP. Evidence for conditioned place preference to a moderate dose of ethanol in adult male Sprague-Dawley rats. Alcohol. 2012;46:643–648. doi: 10.1016/j.alcohol.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker LA. LSD produces place preference and flavor avoidance but does not produce flavor aversion in rats. Behavioral neuroscience. 1996;110:503–508. doi: 10.1037//0735-7044.110.3.503. [DOI] [PubMed] [Google Scholar]
- 24.Lopez M, Cantora R. Opposite effects of ethanol on taste and place conditioning in rats. Revista Iberoamericana de Psicología y Salud. 2010;1:207–221. [Google Scholar]
- 25.Bozarth MA. Evidence for the rewarding effects of ethanol using the conditioned place preference method. Pharmacology Biochemistry and Behavior. 1990;35:485–487. doi: 10.1016/0091-3057(90)90191-j. [DOI] [PubMed] [Google Scholar]
- 26.Sherrill LK, Berthold C, Koss WA, Juraska JM, Gulley JM. Sex differences in the effects of ethanol pre-exposure during adolescence on ethanol-induced conditioned taste aversion in adult rats. Behavioural brain research. 2011;225:104–109. doi: 10.1016/j.bbr.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starkey NJ, Normington G, Bridges NJ. The effects of individual housing on 'anxious' behaviour in male and female gerbils. Physiology & behavior. 2007;90:545–552. doi: 10.1016/j.physbeh.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Clarke SN, Ossenkopp KP. Taste reactivity responses in rats: influence of sex and the estrous cycle. The American journal of physiology. 1998;274:R718–R724. doi: 10.1152/ajpregu.1998.274.3.R718. [DOI] [PubMed] [Google Scholar]
- 29.Annau Z, Kamin LJ. The conditioned emotional response as a function of intensity of the US. Journal of comparative and physiological psychology. 1961;54:428–432. doi: 10.1037/h0042199. [DOI] [PubMed] [Google Scholar]
- 30.Springer AD, Fraley SM. Extinction of a conditioned taste aversion in young, mid-aged, and aged C57/BL6 mice. Behavioral and neural biology. 1981;32:282–294. doi: 10.1016/s0163-1047(81)92333-5. [DOI] [PubMed] [Google Scholar]
- 31.Roll JM. The effect of manipulating CS hedonic value on the magnitude of a conditioned taste aversion. Current Psychology. 1994;13:226–232. [Google Scholar]