Abstract
Adolescence is a time of dramatic changes including rapid physical growth, the onset of sexual maturation, the activation of new drives and motivations, and a wide array of social and affective changes and challenges. This review focuses on behavioral changes in this interval and is organized by the claim that a key set of these adolescent changes are part of a more general re-orientation of social behavior. More specifically we hypothesize that pubertal maturation is associated with the activation of social and motivational tendencies, which in turn influence behavior and emotion in adolescence depending upon interactions with social context. We focus on evidence for two examples of these motivational changes: 1) increases in sensation seeking (motivational tendency to want to experience high-intensity, exciting experiences) and 2) stronger natural interest in—and pursuit of—contact with peers and potential romantic partners. We consider how these motivational changes contribute to the broader social re-orientation of adolescence, including exploration of social experiences, the development of skills and knowledge relevant to taking on adult social roles, individuation from family, and the establishment of an individual identity, all of which represent core developmental tasks during this period in the life span (Blakemore, 2008; Dahl & Spear, 2004; Steinberg & Morris, 2000). The paper also emphasizes the importance of investigating and understanding the direct influences of puberty on behavior and disentangling these from the broader set of changes during adolescent development.
The onset of adolescence is a time of dramatic biological, behavioral, and social changes. These include rapid physical growth, sexual maturation, and emotional changes that range from igniting romantic interests to increased self-consciousness and social anxieties. Adolescence is also a time of new social challenges such as increasing academic pressures, competition with peers, and difficulties learning to balance desires for immediate gratification with an understanding of the importance of long-term goals and consequences (Dahl & Spear, 2004). Amidst this myriad of adolescent changes there are also sharply increasing rates of problems with the control of behavior and emotion despite the fact that the regulatory capacities are improving across this interval of development. As discussed elsewhere in this issue, adolescence involves ongoing development of brain structure (Lenroot & Giedd, in press; Paus, in press), sleep (Feinberg & Campbell, in press), and brain function (Somerville, Jones, & Casey, in press), including gradual increases in capacities for cognitive control and executive function (Luna, Padmanabhan, & O'Hearn, in press).
In the current paper, we examine pubertal maturation in relation to several aspects of adolescent behavior, with an emphasis on the increase in reproductive hormones that are instrumental to pubertal development. We review emerging evidence that pubertal maturation is closely associated with a set of affective (emotional and motivational) changes, which in turn influence some behavioral tendencies. We put forth the hypothesis that many (if not most) of the behavioral changes associated with pubertal maturation are linked to activational effects on specific motivational tendencies—including increased sensation seeking, and stronger natural attraction to peer and romantic contexts—and that the subsequent influences on individual behavior are highly variable depending upon the social context as well as underlying individual differences in temperament/personality.
Understanding these puberty-specific changes in behavior represents an important dimension of normal development in adolescence; it also has broad clinical and social policy relevance. Interactions between these motivational tendencies and the social contexts that amplify these tendencies are relevant to understanding the health paradox of adolescence. That is, adolescence represents one of the healthiest periods of the life span with respect to physical health, yet overall morbidity and mortality rates increase 200% (Centers for Disease Control and Prevention, 2009; Ozer, Macdonald, & Irwin, 2002; Resnick, et al., 1997). Rates of accidents, suicide, homicide, depression, alcohol and substance abuse, HIV, Hepatitis C, unwanted pregnancies, anorexia and bulimia rise sharply in this developmental period (Force, 1996; Ozer, et al., 2002). On the one hand, these various types of adolescent health problems appear heterogeneous; on the other hand, the majority of these health consequences reflect difficulties with control of emotion and behavior.
An important framework for understanding some of these affective changes at puberty can be understood within the broader social re-orientation of adolescence. As noted by recent reviews on the social neuroscience of adolescence (e.g. Blakemore, 2008; Nelson, Leibenluft, McClure, & Pine, 2005) adolescence is marked by changes in social cognition and by functional and structural development of brain networks implicated in social processing. We hypothesize that the pubertal rise in reproductive hormones activates motivational tendencies—including appetitive motivations in the realm of social goals and rewards—that help to facilitate this social re-orientation. These are evident in adolescents' increasing motivations to attract friends and romantic partners, to attain social status, and more generally, in their natural tendencies to pay more attention to, care about, and react to peer, romantic, and sexual contexts.
The activation of these motivational tendencies can have positive effects on behavior – specifically, natural inclinations leading to learning and exploration of social environments in ways that contribute to acquiring relevant knowledge and skills. Yet, stronger attraction to peer and romantic pursuits--particularly in combination with increased sensation seeking--can contribute to a wide range of risky adolescent behaviors associated with negative health consequences, especially in some social contexts.
Before returning to these clinical and social policy implications in more detail, we will first review the evidence for puberty-specific changes in behavior. To begin, we will briefly describe the hormonal and physical changes of puberty and outline the limitations and challenges to research on puberty and behavior, and then describe emerging areas of progress in disentangling puberty-specific neurobehavioral changes. Next, we focus on two areas of investigation where there is strong evidence for puberty-specific behavioral changes--sensation-seeking and motivation for social status--and then consider these in relation to our model emphasizing motivational tendencies.
Pubertal Development
Adolescence can be defined as that awkward period between the onset of sexual maturation and the attainment of adult roles and responsibilities (Dahl & Spear, 2004). The transition into adolescence is marked by pubertal development, which includes activation of the hypothalamic-pituitary-gonadal (HPG) axis and the hypothalamic-pituitary-adrenal (HPA) axis (see Buck Louis, et al., 2008 for a detailed overview of pubertal development). Puberty is triggered by increases in the frequency and amplitude of nocturnal pulses of gonadotropin-releasing hormone (GnRH) in the hypothalamus. There is little research on the age at which GnRH pulses begin in humans, but studies of nonhuman primates suggest that there are individual differences in the function of the GnRH system and in the response to factors that influence GnRH function (Centeno et al., 2007). Given this, and given sex differences in puberty onset in humans, it is difficult to estimate the age at which increased GnRH pulsing begins in humans. A critical frequency of GnRH pulses activates pituitary release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) pulses, which in turn, activate the gonads. In girls, this leads to ovarian secretion of estradiol, progesterone, and ovarian androgens, and eventually to the development of ovulatory menstrual cycles. In boys, LH pulses lead to testicular secretion of androgens. In addition to gonadal development, puberty also involves important changes such as increases in adrenal androgens, rapid growth in body size, changes in distribution of body fat, and development of secondary sex characteristics.
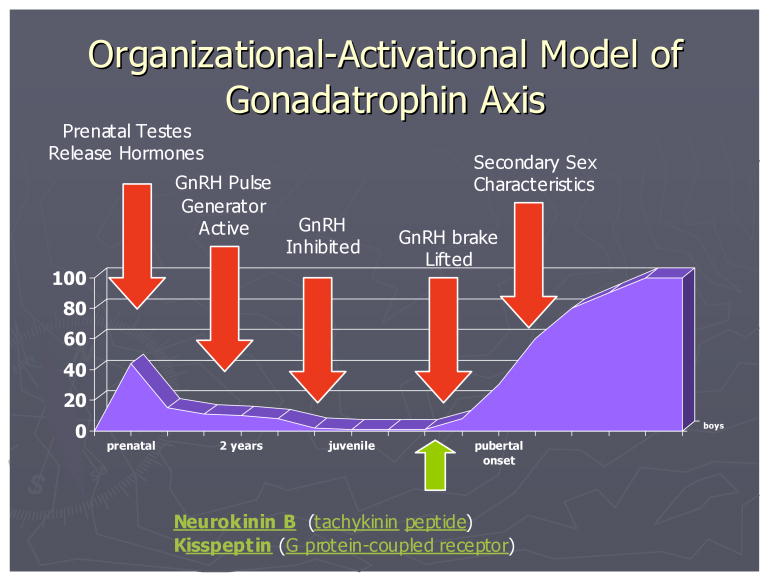
Importantly, puberty represents a re-activation of specific neuroendocrine systems. That is, the reproductive hormones that increase sharply at puberty represent a second re-activation of neuroendocrine axes that were also active in infancy [see figure 1 for an illustration of this pattern for males]. This two-phase pattern of the effects of reproductive hormones in development has been described in relation to the organizational-activational hypothesis (Phoenix, Goy, & Young, 1967; see Romeo, 2003 for a more recent review). This model focuses on the observation that the same hormones that initially organize sex differences in the body and the brain during fetal and early postnatal life are also the hormones that exert activational effects on behavior during puberty.
FIGURE 1.
Illustration of an organizational-activational model for boys, showing early activation of GnRH pulsing, a period of quiescence, then re-activation of the GnRH pulsing at the onset of puberty.
More recently, evidence from animal research on hormonal influences on behavior indicates that sex-steroid hormones can also have activational effects on brain development at puberty (Schulz, Molenda-Figueira, & Sisk, 2009). That is, puberty can be viewed as a period of re-activation of hormonal influences on behavior and the brain. Brain development is highly sensitive to hormone influence perinatally, but the activational effects of hormones do not simply occur during perinatal development, as once thought. Examples of puberty-related organization of behavior are evident in both males and females, as illustrated by the Syrian hamster model of pubertal hormone influence. In male hamsters, the organizing influence of pubertal hormones has been studied by castration after perinatal hormone influence but before puberty. As a result, testosterone at puberty appears to influence increases in male hamsters' sexual behavior and aggressive behavior (Schulz & Sisk, 2006), and their reduction in anxiety-related locomotor activity in a novel environment (Primus & Kellogg, 1989). In female hamsters, which have been less extensively studied, ovarian hormones at puberty influence food-guarding and feeding behaviors (Field, Whishaw, Forgie, & Pellis, 2004; Swithers, McCurley, Hamilton, & Doerflinger, 2008). At a neural level, organizational effects of pubertal hormones include cellular-level changes in brain circuits, such as testosterone influences on increases in white matter volume (Perrin, et al., 2008). Importantly, pubertal hormones influence behavior directly but also influence experience, which in turn influences behavior (Schulz, et al., 2009). Thus, there are several paths from hormones to behavior in adolescence.
In humans, variability in the onset and progression of puberty exists, and there is evidence that environmental influences contribute to this variability. Specifically, endocrine-disrupting chemicals, nutrition, and body size seem to play a role in this variability, but more research is needed to understand the nature of these factors (Buck Louis, et al., 2008). There also appear to be some cultural differences in secular trends toward earlier onset of puberty in the United States, with more evidence for decrease in the age of pubertal onset in Mexican-American and African-American youth than in European-American youth (Himes, 2006). However, there is stronger evidence for a secular trend in girls than in boys, and in girls, this trend appears to be present for breast development and menarche rather than for other signs of puberty (Euling, et al., 2008). It is not known exactly what triggers the onset of puberty, although genetic factors and experience appear to contribute. Emerging genetic evidence now suggests that the kisspeptin-GPR54 regulatory system plays a role in puberty onset through influence on GnRH release (Buck Louis, et al., 2008; Plant, 2006).
Measurement of puberty in humans is typically conducted through classification of physical development into stages originally defined by Tanner (Marshall & Tanner, 1968). This system includes 5 stages on two scales: one for breast development in girls and gonadal development in boys; and one for pubic hair development in boys and girls. Classification with this system can be conducted either through physical exam and rating by a trained medical or research professional or through self-report based on descriptions of visual depictions of the stages. There are important concerns about the reliability and validity of pubertal staging given the atheoretical basis for the approach and the somewhat arbitrary nature of the 5-point scale itself. Other means of assessing puberty include menarche in girls, level of circulating reproductive hormones (e.g., luteinizing hormone), bone age determination, and measurement of uterine and ovarian volume (Buck Louis, et al., 2008). Each method has advantages and disadvantages (for a broad discussion of both conceptual and methodological issues regarding measures of puberty see Dorn, Dahl, Woodward, & Biro, 2006; Shirtcliff, Dahl, & Pollak, 2009).
In humans, the strongest evidence for puberty-specific influences on behavior to date is in the domain of romantic interest and sexual motivation (Richards, Crowe, Larson, & Swarr, 1998; Udry, 1987). There is also evidence that some changes in emotional intensity are more closely linked to pubertal maturation rather than age, including measures of parent-adolescent conflict (Steinberg, 1988, 1989; Steinberg & Morris, 2000). In addition, there is strong evidence that at least some aspects of sensation-seeking increase at puberty (Steinberg, 2004). These changes, along with the general increase in salience and pursuit of social goals will be considered within the larger framework of social re-orientation that is central to adolescence.
Challenges in Research on Puberty and Behavior
It is essential to point out that we are currently limited in our understanding of puberty-specific changes in behavior at least in part because few studies have been designed in ways to specifically address the impact of pubertal maturation on behavioral development and the relevant developing neural systems. Some important aspects of cognitive development change with age, not puberty (Velanova, Wheeler, & Luna, 2008), and describing the difference has important implications for understanding which developmental changes are linked to puberty and which are linked to other aspects of adolescent development (e.g., social experiences).
First of all, it is important to note that a large number of the most influential studies of adolescent cognitive, emotional, and brain development have contained no measures of puberty at all (e.g. Giedd, et al., 1999; Gogtay, et al., 2004; see Keating, 2004 for review). For example, studies have compared a group of participants with an age range in the teenage years with a group of adults or a group of younger children, but have not examined pubertal development in terms of either sexual maturation or circulating hormone levels to determine pubertal status of their adolescent participants. Because adolescence is defined by both pubertal maturation and social changes it is therefore difficult to disentangle the effects of social context on adolescence from the effects of puberty. Secondly, even among the studies that have obtained objective measures of puberty, these are often based on questionnaire or self-rating of maturation, and it is often difficult to disentangle age effects from pubertal maturation in samples with a broad age range, because in most such samples, age and puberty are closely correlated with each other (and age is measured with greater precision than categories of pubertal stage) (Shirtcliff, et al., 2009).
However, there have been a few studies that have utilized cross-sequential or longitudinal designs with measures of reproductive hormones, such as the Great Smoky Mountain Study (Costello, et al., 1996) from which several puberty-specific findings have emerged, including strong evidence for a direct link between puberty and risk for affective disorders (Angold, 2003; Angold, Costello, Erkanli, & Worthman, 1999; Angold, Erkanli, Silberg, Eaves, & Costello, 2002; Angold, Worthman, & Costello, 2003). Another example of a study design that allows disentanglement of puberty-specific effects is illustrated by Martin et al. (2002). They selected 208 children in a narrow age range (11-14 years of age) and examined the influence of pubertal maturation on sensation-seeking as well as nicotine, alcohol, and marijuana use and found that self-reported puberty, but not age, was correlated with increased sensation-seeking.
Recent progress in this area of research is promising, with more efforts to examine the role of puberty in adolescent behavior and experience more explicitly. Developmentalists are now considering the role of pubertal maturation in the psychophysiology of affective reactivity (Quevedo, Benning, Gunnar, & Dahl, 2009; Silk, et al., 2009) and stress responding (e.g. Stroud, et al., 2009).
Most recently, a study we are conducting at the University of Pittsburgh is examining pubertal development, genetic factors, reward-related and threat-related brain function, inhibitory control, action monitoring, and affect regulation in 125 adolescents in the narrow age range of 11-13 years. By selecting a limited age range and recruiting girls to be somewhat younger than boys, and then re-studying the sample in a longitudinal design, we will be able to examine pubertal influences on brain function and behavior without the usual confounding by age effects. To date, we have found that pubertal maturation—independent of the effects of age—influences both brain structure and brain function relevant to social reorientation. Specifically, sexual maturation, measured by Tanner stage of physical development is associated with increased medial temporal lobe volume in boys, and circulating testosterone level measured by bloodspot sampling and hormone assay is associated with decreased total gray matter volume in girls (Bramen, Dahl, Forbes, & Sowell, under review). Sexual maturation and testosterone are associated with reduced striatal reactivity and increased medial prefrontal reactivity in response to winning in a monetary reward functional magnetic resonance imaging paradigm (Forbes, Phillips, et al., under review). Sexual maturation is associated with decreased amygdala and ventrolateral prefrontal reactivity to ambiguous social threats—that is, fearful and neutral facial expressions—but no change in reactivity to unambiguous social threats—that is, angry facial expressions (Forbes, Hariri, Phillips, Ryan, & Dahl, under review). Together, these findings indicate that puberty influences the development of brain systems that play a role in processing affective and social stimuli. As noted in animal studies of re-organization of the brain by pubertal hormones, hormones can exert influences on neural circuits at cellular levels (Schulz, et al., 2009).
Adolescent Behavior Influenced by Puberty
Several perspectives indicate that puberty has an important influence on adolescent—and eventually adult—behavior. Models of hormones and behavior suggest that puberty has an organizational effect on brain development and adolescent behavior, creating a foundation for long-term patterns of behavior, including problem behaviors such as psychopathology (Sisk & Zehr, 2005). Research on brain structure in adolescents with atypical patterns of reproductive hormones (e.g., congenital adrenal hyperplasia) or genetic disorders involving sex chromosomes provides additional evidence for the role of hormones in brain development (Giedd, et al., 2006).
Although changes in hormone levels represent an obvious and strongly influential component of puberty, associations between puberty and behavior cannot be assumed to reflect direct effects of hormones on behavior. Based on animal and basic studies, it is clear that the timing and patterning of hormone release (e.g., pulses of hormones at a key frequency, or rates of rise of hormone level) can represent the biological signal (see Knobil, 2005 for a classic example of research on hormonal pulse signals). There also is evidence that hormones can have different influences on behavior during puberty than in adulthood and depending on the behavioral context. An example of this is support for the challenge model of testosterone-aggression associations (Archer, 2006), which predicts that while circulating testosterone levels increase to adult levels during puberty, testosterone does not produce a direct increase in aggression during adolescence. In adulthood, the testosterone-aggression association is different. In adults, testosterone increases with circumstances such as competition among males for mates, and aggression also increases. Evidence from human and non-human primate studies indicates that testosterone does not necessarily have a direct relation to aggressive behavior. Rather, testosterone increases motivation to attain higher status, and the effect of testosterone level on behavior is dependent on social and developmental context (Wallen, 2001).
Sensation-Seeking
One key area of research relevant to these issues in human adolescent development is the well documented change in sensation-seeking that occurs during adolescence. Prior to reviewing the data in this area, it is important to emphasize that the term sensation-seeking has been used broadly to include a range of impulsive and reckless behaviors, however the central component of sensation-seeking can be understood as a motivational tendency: wanting/liking high-sensation high-arousal experiences. Thus, the increase in sensation-seeking that appears to occur at the onset of adolescence (and may be linked directly to the rise in reproductive hormones) is an example of the type of motivational tendency described in our model.
Sensation-seeking, or the pursuit of high-intensity, exciting experiences, occurs more frequently in adolescents than in either children or adults. Notably, sensation-seeking tendencies are correlated more strongly with puberty than age (Spear, 2000; Steinberg, 1999). According to Arnett (1992, 1994; Arnett & Balle-Jensen, 1993), sensation-seeking is one of the developmental contributors to risk behaviors and is more likely to emerge during adolescence than any other time period. In a study of 1,053 Danish youth (12-20 years of age), sensation-seeking was found to be related to most types of risk behaviors (e.g. sex without contraception, marijuana use, cigarette smoking) (Arnett & Balle-Jensen, 1993). Thus, the positive thrill associated with risk-taking sometimes has a greater influence on behavioral choices than the cognitive understanding of possible negative consequences associated with a particular behavior. In similar ways, peer pressure, concerns about social rejection, and the desire to be popular can sharply impact adolescents' behavior.
One of the first studies to demonstrate the specific link between sensation-seeking and puberty was conducted by Martin et al. (2002) in a design that focused on adolescents within a narrow age range of 11-14 years. In this study, sensation-seeking was correlated not with age but with pubertal maturation. Boys and girls with more advanced pubertal development had higher ratings of sensation seeking and greater drug use (Martin, et al., 2002).
A recent study by Steinberg and colleagues (2008) has further elucidated changes in sensation seeking during adolescent development. In addition, the study aimed at disentangling sensation-seeking (as an appetitive motivation for exciting experiences), and impulsivity, which is sometimes associated with sensation seeking, but reflects quick actions that occur without consideration of consequences. Using behavioral (e.g., Iowa Gambling Task, delay discounting) and self-report measures (Zuckerman, 1971) in a large sample of adolescents, the authors reported that sensation-seeking and impulsivity follow different developmental patterns. Sensation-seeking increases from age 10, peaks between 13-16 years, and then declines. Impulsivity, on the other hand, steadily declines from age 10 to age 30. The authors interpret their findings as pointing to explanations of the increased risk-taking that occurs during early adolescence, when both sensation-seeking and impulsivity are high.
Motivational Tendencies: Studies of Social Dominance and Sexual Behavior
While social behavior changes importantly with pubertal maturation, research on social dominance suggests that changes cannot simply be attributed to hormones whose levels change at puberty. Social context—including composition of the social group, rank within the group, and social experience—also changes meaningfully during adolescence and can interact with hormones to exert its own influence on social behavior. To illustrate this, we describe some compelling studies of sexual behavior in non-human primates by Wallen and colleagues. These studies illustrate the relation of reproductive hormones to social dominance and sexual behavior.
Extended to humans, these non-human primate studies underscore the critical importance of motivational tendencies for social dominance rather than any simple effect on behavior. Because there are similarities in pubertal development in humans and non-human primates—including the pattern of GnRH release across development and the hormones involved in the HPG axis—non-human primates, particularly rhesus monkeys, are considered a valuable animal model for puberty in humans (Plant, 2001, 2008). For example, humans and non-human primates both exhibit a clear period of quiescence between perinatal HPG activity and pubertal HPG activity. Clearly, more research is needed that is designed to disentangle this complex set of changing behaviors of adolescence and to more rigorously test the hypothesis that motivation to achieve social dominance in humans increases with increasing levels of reproductive hormones during puberty. In addition, studies to examine the separate and overlapping contributions of hormones, social environment, and social customs to these behaviors in humans are also needed.
A valuable contribution of the non-human primate literature on testosterone and social behavior to understanding the role of hormones in social behavior is its emphasis on the influence of both reproductive hormones and social context. These two factors can interact to influence behavior. For example, sexual behavior in female rhesus monkeys is influenced both by social context (e.g., pair vs. multi-animal group) and by ovarian cycle phase, with some behaviors more frequent in particular social contexts at certain cycle phases (Wallen & Winston, 1984). Similarly, social rank in females influences age at first ovulation (Zehr, Van Meter, & Wallen, 2005), with higher-rank females more likely to ovulate at an early age. This finding underscores the influence of social context on pubertal development. Thus, when considering the role of puberty in social dominance behavior in humans, it is critical to consider more than simply hormone influences. A more comprehensive approach to examining puberty and behavior, then, will also take into account social experiences, settings, and developmental history.
For example, there is an interaction between testosterone level and sexual experience in predicting female-mounting behavior in male rhesus monkeys (Wallen, 2001). During puberty, there is an increase in the tendency for male monkeys to demonstrate mounting behavior toward females. This change in social behavior occurs during a period in which testosterone levels are increasing. Wallen and colleagues observed the mounting behavior of male rhesus monkeys living in large, multi-male and multi-female social groups. These males were observed for approximately 100 hours at age 1.5 years, which is during the juvenile stage and before puberty, and then again for approximately 55 hours during the mating season at 3.5 years, at which puberty is beginning. Blood samples were collected weekly and assayed for testosterone. Notably, they found that testosterone levels were not directly related to development of mounting behaviors during puberty. Instead, sexual experience, defined as the ejaculatory reflex, was associated with behavior independent of testosterone level. Even low-testosterone male monkeys exhibited high proportions of female-mounting behaviors if they had had a successful sexual experience. Wallen and colleagues interpret their data as indicating that testosterone levels are important in motivating the appetite for social behavior at a level sufficient for naïve males to overcome hesitation to approach mature females. However once an animal has had the rewarding experience of a successful sexual encounter, motivation from other sources (e.g., reward-learning) is sufficient, and hormone levels are no longer associated with this behavior. Similarly, when androgen levels in adult male monkeys are reduced through a single-dose pharmacologic manipulation, changes in sexual behavior depend upon social context. In these “testicular suppression” studies, male rhesus monkeys were given a single dose of antide, a gonadotropin-releasing hormone antagonist that reduces androgen levels to castration levels. After testicular suppression, male sexual behavior decreased more rapidly when males were placed in multiple-male social groups, in which competition for mates is present, than when they were placed in single-male social groups, where there is no competition (Davis-daSilva & Wallen, 1989). Male sexual behavior also decreased more slowly in group contexts than in male-female pair contexts (Wallen, 1999; Wallen, Eisler, Tannenbaum, Nagell, & Mann, 1991). In addition, in multiple-male social groups, social rank and sexual experience influence changes in sexual behavior after testicular suppression. Notably, sexual behavior decreased more rapidly after testicular suppression in low-ranking male monkeys and inexperienced male monkeys than in high-ranking or sexually experienced monkeys (Wallen, 1999).Together, these findings indicate that both during puberty and during adulthood, social context and social experience play an important role in regulating the influence of hormones on behavior. While reproductive hormones are critical to social behaviors, the behaviors they influence are sensitive to social context.
Conclusions
In sum, the current review of pubertal influences on behavior indicates that many dramatic changes occur in behaviors related to increasing social appetite and social re-orientation at adolescence. Sensation-seeking and motivation for social dominance are examples of behaviors subject to pubertal influence.
Although these examples provide a compelling view of the role of puberty in adolescent social development and later adult behavior, research on the influence of puberty on adolescent social development requires further elaboration. Findings must be replicated, and the multiple sources of influence on puberty itself and on the behaviors it influences remain to be sorted out. The combination of early organizing effects of hormones and early social experiences can set the stage for pubertal development and associated behavior changes, and the experience of pubertal development itself—both in terms of hormone changes and concurrent social experiences—shapes adolescent development and presumably later behavior.
Given the intriguing findings to date on the contributions of biological factors to adolescent social behavior, it will be exciting for future work to extend this line of investigation to individual differences in the association of puberty and social behavior. Just as it is crucial to describe normal, mean-level developmental patterns of adolescent behavior, it is important to examine the variety of trajectories through this developmental period. Adolescence is a formative time for identity, status, and functioning, and it is also a time in which many long-term behavior patterns, including several forms of psychopathology (Paus, Keshavan, & Giedd, 2008), first emerge. With more careful attention to the biological and social bases for behavior, more developmental appreciation of social behavior, and more detailed examination of pubertal influences on behavior, we will have an opportunity to deepen understanding of key aspects of human development in ways that have great clinical and social policy relevance. Advancing our understanding of the neurobehavioral underpinnings of adolescent vulnerabilities related to affective changes at puberty has implications relevant to a wide range of behavioral and emotional health problems with onset in adolescence. A deeper, more mechanistic understanding of these maturational problems can provide leverage for developing more effective early interventions, including both clinical and policy level efforts. These are most evident in relation to sensation-seeking, risk-taking, reckless behaviors, alcohol and other substance use problems (see Windle, et al., 2008), accidents and relevant driving policies (see Dahl, 2008), aggression, health problems related to risky sexual behaviors, and the development of affective disorders (see Steinberg, et al., 2006).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angold A. Adolescent depression, cortisol and DHEA. Psychological Medicine. 2003;33:573–581. doi: 10.1017/s003329170300775x. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychological Medicine. 1999;29(5):1043–1053. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- Angold A, Erkanli A, Silberg J, Eaves L, Costello EJ. Depression scale scores in 8-17-year-olds: effects of age and gender. Journal of Child Psychology and Psychiatry. 2002;43(8):1052–1063. doi: 10.1111/1469-7610.00232. [DOI] [PubMed] [Google Scholar]
- Angold A, Worthman CM, Costello EJ. Puberty and Depression. New York: Cambridge University Press; 2003. [Google Scholar]
- Archer J. Testosterone and human aggression: An evaluation of the challenge hypothesis. Neuroscience and Biobehavioral Reviews. 2006;30(3):319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Arnett J. Reckless behavior in adolescence: A developmental perspective. Developmental Review. 1992;12:339–373. [Google Scholar]
- Arnett J. Sensation seeking: A new conceptualization and a new scale. Personality and Individual Differences. 1994;16:289–296. [Google Scholar]
- Arnett J, Balle-Jensen L. Cultural bases of risk behavior: Danish adolescents. Child Development. 1993;64:1842–1855. doi: 10.1111/j.1467-8624.1993.tb04217.x. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nature Reviews, Neuroscience. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Bramen JE, Dahl RE, Forbes EE, Sowell ER. Pubertal status predicts medial temporal lobe and cortical gray matter volume in normally developing adolescents under review. [Google Scholar]
- Buck Louis GM, Gray LE, Jr, Marcus M, Ojeda SR, Pescovitz OH, Witchel SF, et al. Environmental factors and puberty timing: Expert panel research needs. Pediatrics. 2008;121(Suppl 3):S192–207. doi: 10.1542/peds.1813E. [DOI] [PubMed] [Google Scholar]
- Centeno ML, Sanchez RL, Cameron JL, Bethea CL. Hypothalamic gonadotrophin-releasing hormone expression in female monkeys with different sensitivity to stress. Journal of Neuroendocrinology. 2007;19(8):594–604. doi: 10.1111/j.1365-2826.2007.01566.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Surveillance for Violent Deaths - National Violent Death Reporting System, 16 States, 2006. Morbidity and Mortality Weekly Report: Surveillance Summaries. 2009;58(SS-1) Retrieved from http://www.cdc.gov/mmwr/PDF/ss/ss5801.pdf. [PubMed] [Google Scholar]
- Costello EJ, Angold A, Burns BJ, Stangl DK, Tweed DL, Erkanli A, et al. The great smokey mountains study of youth: Goals, design, methods, and the prevalence of DSM-III-R disorders. Archives of General Psychiatry. 1996;53(12):1129–1136. doi: 10.1001/archpsyc.1996.01830120067012. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Biological, developmental, and neurobehavioral factors relevant to adolescent driving risks. American Journal of Preventive Medicine. 2008;35(3 Suppl):S278–284. doi: 10.1016/j.amepre.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Spear LP. Adolescent brain development. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Davis-daSilva M, Wallen K. Suppression of male rhesus testicular function and sexual behavior by a gonadotropinreleasing-hormone agonist. Physiology and Behavior. 1989;45:963–968. doi: 10.1016/0031-9384(89)90222-9. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Dahl RE, Woodward HR, Biro F. Defining the boundaries of early adolescence: A user's guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science. 2006;10(1):30–56. [Google Scholar]
- Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, Sorensen TI, et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: Panel findings. Pediatrics. 2008;121(Suppl 3):S172–191. doi: 10.1542/peds.2007-1813D. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Campbell I. Sleep & circadian rhythms during adolescence in press. [Google Scholar]
- Field EF, Whishaw IQ, Forgie ML, Pellis SM. Neonatal and pubertal, but not adult, ovarian steroids are necessary for the development of female-typical patterns of dodging to protect a food item. Behavioral Neuroscience. 2004;118(6):1293–1304. doi: 10.1037/0735-7044.118.6.1293. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Phillips ML, Ryan ND, Dahl RE. Neural systems of social threat processing in adolescents: Influence of pubertal maturation and relation to real-world negative affect and depressive symptoms under review. [Google Scholar]
- Forbes EE, Phillips ML, Manuck SB, Moyles DL, Tarr JA, Ryan ND, et al. Pubertal maturation, neural reactivity to reward, and adolescents' mood in natural environments under review. [Google Scholar]
- Force, USPST. Guide to clinical preventive services. Alexandria, VA: International Medical Publishing; 1996. [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, et al. Puberty-related influences on brain development. Molecular and Cellular Endocrinology. 2006;254-255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes JH. Examining the evidence for recent secular changes in the timing of puberty in US children in light of increases in the prevalence of obesity. Molecular and Cellular Endocrinology. 2006;254-255:13–21. doi: 10.1016/j.mce.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Keating DP. Cognitive and brain development. In: Lerner RJ, Steinberg LD, editors. Handbook of Adolescent Psychology. 2nd. Hoboken, NJ: Wiley; 2004. pp. 45–84. [Google Scholar]
- Knobil E. Discovery of the hypothalamic gonadotropin-releasing hormone pulse generator and of its physiologic significance. 1992. American Journal of Obstetrics and Gynecology. 2005;193(5):1765–1766. doi: 10.1016/j.ajog.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain and Cognition. doi: 10.1016/j.bandc.2009.10.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O'Hearn K. What has fMRI told us about the development of cognitive control through adolescence. Brain and Cognition. doi: 10.1016/j.bandc.2009.08.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annual Review of Medicine. 1968;19(283-300) doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ, et al. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(12):1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Ozer EM, Macdonald T, Irwin CEJ. Adolescent health care in the United States: Implications and projections for the new millennium. In: Mortimer JT, Larson RW, editors. Changing Adolescent Experience: Societal Trends and the Transition to Adulthood. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: Myelin or axon? Brain and Cognition. doi: 10.1016/j.bandc.2009.06.002. in press. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews. Neuroscience. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin JS, Herve PY, Leonard G, Perron M, Pike GB, Pitiot A, et al. Growth of white matter in the adolescent brain: Role of testosterone and androgen receptor. Journal of Neuroscience. 2008;28(38):9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix C, Goy R, Young W. Sexual behavior: General aspects. In: Martini L, Ganong W, editors. Neuroendocrinology. Vol. 2. New York: Academic Press; 1967. [Google Scholar]
- Plant TM. Neurobiological bases underlying the control of the onset of puberty in the rhesus monkey: A representative higher primate. Frontiers in Neuroendocrinology. 2001;22(2):107–139. doi: 10.1006/frne.2001.0211. [DOI] [PubMed] [Google Scholar]
- Plant TM. The male monkey as a model for the study of the neurobiology of puberty onset in man. Molecular and Cellular Endocrinology. 2006;254-255:97–102. doi: 10.1016/j.mce.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Plant TM. Hypothalamic control of the pituitary-gonadal axis in higher primates: Key advances over the last two decades. Journal of Neuroendocrinology. 2008;20(6):719–726. doi: 10.1111/j.1365-2826.2008.01708.x. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Developmental Psychobiology. 1989;22(6):633–643. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Quevedo KM, Benning SD, Gunnar MR, Dahl RE. The onset of puberty: Effects on the psychophyiology of defensive and appetitive motivation. Development and Psychopathology. 2009;21(1):27–45. doi: 10.1017/S0954579409000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick MD, Bearman PS, Blum RW, Bauman KE, Harris KM, Jones J, et al. Protecting adolescents from harm. Findings from the National Longitudinal Study on Adolescent Health. JAMA. 1997;278(10):823–832. doi: 10.1001/jama.278.10.823. [DOI] [PubMed] [Google Scholar]
- Richards MH, Crowe PA, Larson R, Swarr A. Developmental patterns and gender differences in the experience of peer companionship during adolescence. Child Development. 1998;69(1):154–163. [PubMed] [Google Scholar]
- Romeo RD. Puberty: A period of both organizational and activational effects of steriod hormones on neurobehavioural development. Journal of Neuroendocrinology. 2003;15:1185–1192. doi: 10.1111/j.1365-2826.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Hormones and Behavior. 2009;55(5):597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Molecular and Cellular Endocrinology. 2006;254-255:120–126. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: Correspondence between hormonal and physical development. Child Development. 2009;80(2):327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Whalen DJ, Ostapenko LJ, Ladouceur CD, Dahl RE. Pubertal changes in emotional information processing: Pupillary, behavioral, and subjective evidence during emotional word identification. Development and Psychopathology. 2009;21(1):7–26. doi: 10.1017/S0954579409000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology. 2005;26(3-4):163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey B. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. doi: 10.1016/j.bandc.2009.07.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Reciprocal relation between parent-child distance and pubertal maturation. Developmental Psychology. 1988;24:122–128. [Google Scholar]
- Steinberg L. Pubertal maturation and parent-adolescent distance: An evolutionary perspective. In: Adams G, Montemayor R, Gullotta T, editors. Advances in Adolescent Development. Vol. 1. Beverly Hills, CA: Sage; 1989. [Google Scholar]
- Steinberg L. Adolescence. Fifth. New York: McGraw-Hill College; 1999. [Google Scholar]
- Steinberg L. Risk-taking in adolescence: What changes, and why? Annals of the New York Academy of Sciences. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Developmental Psychology. 2008;44(6):1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Dahl R, Keating D, Kupfer DJ, Masten AS, Pine DS. The study of developmental psychopathology in adolescence: Integrating affective neuroscience with the study of context. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology. 2. Vol. 2. Hoboken, NJ: John Wiley & Sons; 2006. pp. 710–741. [Google Scholar]
- Steinberg L, Morris A. Adolescent development. Annual Review of Psychology. 2000;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, et al. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21(1):47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swithers SE, McCurley M, Hamilton E, Doerflinger A. Influence of ovarian hormones on development of ingestive responding to alterations in fatty acid oxidation in female rats. Hormones and Behavior. 2008;54(3):471–477. doi: 10.1016/j.yhbeh.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udry J. Hormonal and social determinants of adolescent sexual initiation. In: Bancroft J, editor. Adolescence and Puberty. Oxford University Press; 1987. pp. 70–87. [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral Cortex. 2008;18(11):2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen K. Risky business: Social context and hormonal modulation of primate sexual desire. In: Wallen K, Schneider J, editors. Reproduction in Context. Cambridge, MA: MIT Press; 1999. pp. 289–323. [Google Scholar]
- Wallen K. Sex and context: Hormones and primate sexual motivation. Hormones and Behavior. 2001;40(2):339–357. doi: 10.1006/hbeh.2001.1696. [DOI] [PubMed] [Google Scholar]
- Wallen K, Eisler JA, Tannenbaum PL, Nagell KM, Mann DR. Antide (Nal-Lys GnRH antagonist) suppression of pituitary-testicular function and sexual behavior in groupliving rhesus monkeys. Physiology and Behavior. 1991;50:429–435. doi: 10.1016/0031-9384(91)90090-b. [DOI] [PubMed] [Google Scholar]
- Wallen K, Winston LA. Social complexity and hormonal influences on sexual behavior in rhesus monkeys (Macaca mulatta) Physiology and Behavior. 1984;32(4):629–637. doi: 10.1016/0031-9384(84)90318-4. [DOI] [PubMed] [Google Scholar]
- Windle M, Spear LP, Fuligni AJ, Angold A, Brown JD, Pine D, et al. Transitions into underage and problem drinking: Developmental processes and mechanisms between 10 and 15 years of age. Pediatrics. 2008;121(Suppl 4):S273–289. doi: 10.1542/peds.2007-2243C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr JL, Van Meter PE, Wallen K. Factors regulating the timing of puberty onset in female rhesus monkeys (Macaca mulatta): Role of prenatal androgens, social rank, and adolescent body weight. Biology of Reproduction. 2005;72(5):1087–1094. doi: 10.1095/biolreprod.104.027755. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Dimensions of sensation seeking. Journal of Consulting and Clinical Psychology. 1971;36:45–52. doi: 10.1037/h0026047. [DOI] [PubMed] [Google Scholar]