Abstract
The aims of this study were to investigate regional white matter microstructural differences between very preterm (<30 weeks’ gestational age and/or <1250g) and full term (≥37 weeks’ gestational age) infants at term corrected age with diffusion tensor imaging, and to explore perinatal predictors of diffusion measures, and the relationship between regional diffusion measures and neurodevelopmental outcomes at age 7 years in very preterm children. Mean (p=0.003), axial (p=0.008), and radial diffusivity (p=0.003) in total white matter were increased in very preterm compared with full term infants, with similar fractional anisotropy in the two groups. There was little evidence that group-wise differences were specific to any of the 8 regions studied for each hemisphere. Perinatal white matter abnormality and intraventricular hemorrhage (grade III or IV) were associated with increased diffusivity in the white matter of very preterm infants. Higher white matter diffusivity measures of the inferior occipital and cerebellar region at term equivalent age were associated with increased risk of impairments in motor and executive function at 7 years in very preterm children, but there was little evidence for associations with IQ or memory impairment. In conclusion, myelination is likely disrupted or delayed in very preterm infants, especially those with perinatal brain abnormality. Altered diffusivity at term-equivalent age helps explain impaired functioning at 7 years. This study defines the nature of microstructural alterations in very preterm infant white matter, assists in understanding the associated risk factors, and is the first study to reveal an important link between inferior occipital and cerebellar white matter disorganization in infancy, and executive and motor functioning 7 years later.
Keywords: Diffusion tensor, MRI, neonate, premature, neurodevelopment
1. INTRODUCTION
Despite reduced mortality rates for very preterm infants over the past few decades, very preterm birth still remains one of the largest causes of morbidity for newborns. 30–60% of very preterm children will experience cognitive impairments and learning disabilities (Anderson and Doyle, 2003; Holsti et al., 2002; Taylor et al., 2004). In particular, IQ (Kerr-Wilson et al., 2012), executive functioning and memory impairments (Anderson and Doyle, 2004; Luu et al., 2011) have been reported in very preterm children. Approximately 8% of very preterm children will develop cerebral palsy (Ancel et al., 2006), while up to 40% will display mild motor deficits (Holsti et al., 2002; Williams et al., 2010).
Very preterm infants often face a range of medical complications following birth. Very preterm infants are born with an immature respiratory system (MacArthur and Dezoete, 1992) and lack of oxygen to the immature brain may result in hypoxic ischemic insults, which can lead to white matter abnormality, a serious and debilitating consequence of very preterm birth (Volpe, 2005). Specifically, prematurity is often associated with damage to pre-myelinating oligodendrocytes, resulting in hypomyelination, white matter atrophy and ventriculomegaly (Billiards et al., 2008; Blumenthal, 2004; Volpe, 2009). White matter around the lateral ventricles and the centrum semiovale is particularly vulnerable in very preterm infants, and can lead to periventricular leukomalacia (Banker and Larroche, 1962). These relationships suggest that brain abnormality in very preterm infants may be targeted to particular regions and microstructural properties of the white matter. Therefore, a greater understanding of microstructural properties of the very preterm brain is required. Diffusion tensor imaging can provide sensitive measures of microstructural alterations to the cerebral white matter in order to further study the impact of very preterm birth.
Diffusion tensor imaging has been used to report white matter microstructural alterations in children (Constable et al., 2008; Counsell et al., 2008) and adolescents (Nagy et al., 2009) born preterm. A few groups have also used diffusion tensor imaging to define the microstructural development of cerebral white matter in the whole brain of very preterm infants compared with full term infants (Yung et al., 2007), as well as the central white matter and posterior limb of the internal capsule (Huppi et al., 1998), centrum semiovale (Counsell et al., 2006; Krishnan et al., 2007; Neil et al., 1998), frontal white matter, corpus callosum and corona radiata (Anjari et al., 2007; Rose et al., 2008). Although some differences in microstructure between very preterm and full term infants have been identified, a comprehensive evaluation of regional white matter microstructural organization in a large and representative cohort of very preterm infants is required. Furthermore, previous literature has largely failed to report on axial and radial diffusivity, which can provide further details as to the nature of microstructural alterations, such as disruption or delay to myelination (Budde et al., 2007; Song et al., 2003).
Apart from white matter abnormality (Arzoumanian et al., 2003; Cheong et al., 2009; Counsell et al., 2003; Counsell et al., 2006; Huppi et al., 2001; Miller et al., 2002), there has been little exploration of other potential perinatal causes of white matter microstructural alterations in very preterm infants. Although a few studies suggest there are functional consequences for reduced anisotropy in various white matter regions in preterm two year olds (Counsell et al., 2008), preterm 12 year olds (Constable et al., 2008), and very low birth weight 15 year olds (Skranes et al., 2007), no study has yet determined if preterm infant white matter microstructure can predict childhood neurodevelopmental functioning. The current study will provide an important addition to the literature by comprehensively exploring regional white matter microstructural alterations at term-equivalent age, including axial and radial diffusivity, as predictors of neurodevelopmental outcomes during childhood in a large and representative cohort, enabling understanding of early causes for later abnormal functioning. Earlier identification of those destined for later impairments is important for counseling, intervention and monitoring of high-risk infants.
The aims of this study were: 1) to determine overall and regional brain differences in white matter microstructure between very preterm and full term infants at term-equivalent age, 2) to determine perinatal factors that are associated with white matter microstructural measures at term-equivalent age in very preterm infants, and 3) to explore the relationship between regional white matter microstructural measures at term-equivalent age and neuropsychological outcomes at 7 years of age in very preterm infants. It was hypothesized that very preterm infants would have lower fractional anisotropy values, but increased mean, axial and radial diffusivity values within the whole brain white matter compared with full term infants, with some regions being more sensitive to prematurity than others (e.g. frontal regions where white matter is last to mature, and periventricular regions that are more vulnerable to injury). It was also hypothesized that qualitative white matter abnormality would be associated with reduced diffusivity in very preterm infants. Finally, it was hypothesized that lower fractional anisotropy and higher diffusivity in frontal regions would be associated with reduced IQ and executive functioning at age 7 years, lower fractional anisotropy and higher diffusivity within motor regions would be associated with poorer movements, and lower fractional anisotropy and higher diffusivity in the temporal area would be associated with poorer memory.
2. MATERIALS AND METHODS
2.1. Subjects
Between July 2001 and December 2003, a prospective observational cohort study of very preterm infants was conducted at the Royal Women’s and Royal Children’s Hospitals in Melbourne, Australia. Of 348 eligible very preterm infants born <30 weeks’ gestational age and/or <1250 g without congenital anomalies, 224 surviving infants were recruited. At the same time, 46 clinically healthy full term infants (≥37 weeks’ gestational age) free of neonatal complications, congenital and chromosomal abnormalities were recruited from the Royal Women’s Hospital postnatal ward or via response to advertising in recruiting hospitals. Informed parental consent was obtained for all participants and the study was approved by the Research and Ethics Committee at the Royal Women’s Hospital. Of those infants recruited, 43% (n=116) were included in the current study (96 very preterm and 20 full term). The remaining infants had no diffusion scan (n=135), the diffusion image was unsuccessful or of insufficient quality for further analysis due to movement or imaging artifact (n=6), or structural scans were of insufficient quality for tissue segmentation and regional parcellation (n=13).
Perinatal data on gestational age at birth and at scan, weight at birth, sex, multiple births, cardio-respiratory treatments, corticosteroid exposure and neonatal infection were obtained by chart review. White matter abnormality was qualitatively assessed from magnetic resonance images at term-equivalent age by a qualified neurologist using a grading system assessing cystic lesions, focal signal abnormality, myelination in the posterior limb of the internal capsule and corona radiata, callosal thinning, lateral ventricular diameter, and white matter volume measured via biparietal diameter. White matter abnormality was graded as: Grade I, normal white matter; Grade II, mild white matter abnormality; Grade III, moderate white matter abnormality; Grade IV, severe white matter abnormality (Kidokoro et al., 2013). Intraventricular hemorrhage was recorded as the highest grade from serial ultrasound scans throughout the neonatal intensive care course. Birth weight standard deviation z-score was computed relative to the British Growth Reference data (Cole et al., 1998). Intrauterine growth restriction was defined as birth weight standard deviation z-score more than 2 standard deviations below the mean weight for gestational age from the reference data.
2.2. Scanning
All infants were scanned at term-equivalent age (38–42 weeks’ gestational age) in a 1.5 Tesla General Electric Signa MRI scanner (Milwaukee, Wisconsin) during natural sleep. Each infant underwent whole brain structural 3D T1 spoiled gradient recalled imaging with 0.8–1.6 mm coronal slices; flip angle 45°; repetition time 35ms; echo time 9ms; field of view 21 × 15cm2; matrix 256 × 192, as well as T2 dual echo fast spin echo sequences with interleaved acquisition (1.7 – 3mm coronal slices; repetition time 4000msec; echo time 60 / 160msec; field of view 22 × 16cm2; matrix 256 × 192, interpolated 512 × 512). Linescan diffusion imaging sequences (4 – 6 mm axial slices; 2 images at b = 5 s/mm2; 6 non-collinear gradient directions at b = 700 s/mm2) were acquired within the same session.
2.3. Image segmentation and parcellation
Whole brain tissue segmentation and regional parcellation were carried out as previously reported (Thompson et al., 2007). In brief, brain tissues were segmented (Warfield, 1996; Warfield et al., 2000) and each segmented infant brain image was parcellated into eight regions within each hemisphere: dorsal prefrontal, orbitofrontal, premotor, subgenual, sensorimotor, midtemporal, parieto-occipital, and inferior occipital and cerebellum using the Talairach parcellation scheme (Peterson et al., 2000; Thompson et al., 2007).
2.4. Diffusion image processing
Diffusion images were eddy current corrected and corrected for simple head motion using FSL (Jenkinson and Smith, 2001). Brain extraction of the baseline image was performed using FSL’s brain extraction tool (Smith, 2002). Local linear fitting of the diffusion tensor model at each voxel was achieved using FSL’s DTIfit tool (Behrens et al., 2003b). Sums of squares error plots were inspected for assessment of the tensor fit and artefact detection. Probabilistic diffusion orientation and local diffusion parameters were characterized and estimated at each voxel using Markov Chain Monte Carlo sampling using FSL’s Bayesian estimation of diffusion parameters obtained using sampling techniques (Behrens et al., 2003a; Behrens et al., 2003b).
An averaged brain diffusion map was extracted (FSL’s brain extraction tool) and registered to the T1 image using FSL’s linear registration tool (Jenkinson and Smith, 2001). The resulting transformation matrix was applied to the fractional anisotropy, mean, axial and radial diffusivity images. All registrations were checked for quality of alignment to the target.
2.5. Measuring white matter diffusivity
The segmented image (Fig. 1A) was separated into myelinated white matter (Fig. 1B) and unmyelinated white matter (Fig. 1C). The unmyelinated white matter volume was eroded using a spherical spatial filtering kernel with a radius of one voxel to eliminate voxels misclassified as unmyelinated white matter at the interface of gray matter and cerebrospinal fluid due to partial volume effects (Thompson et al., 2007; Xue et al., 2007) (Fig. 1D). A total white matter mask was created by combining the myelinated and eroded unmyelinated white matter volumes (Fig. 1E). The 16-region parcellation (8 regions in each hemisphere) was also applied to this white matter mask.
Figure 1.
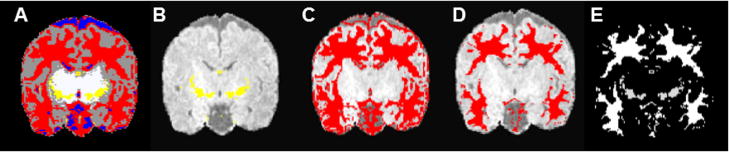
(A) Tissue segmentation map, (B) extracted myelinated white matter volume, (C) extracted unmyelinated white matter volume, (D) eroded unmyelinated white matter volume, (E) binary total white matter volume (myelinated and eroded unmyelinated white matter volumes combined).
The registered average diffusion maps (Fig. 2A) were multiplied by the binary white matter volumes for the whole brain and each region (Fig. 2B) to obtain white matter diffusion estimates for fractional anisotropy, mean, axial and radial diffusivity within each of the 16 regions (8 regions measured in the 2 hemispheres, Fig. 2C).
Figure 2.
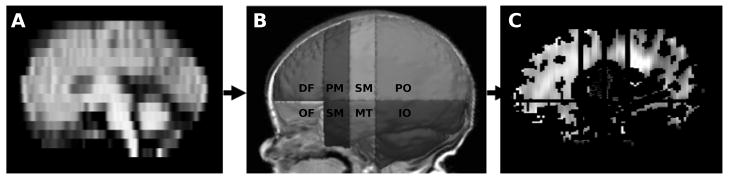
Steps involved in obtaining regional white matter diffusion measures: After transforming the mean diffusion image (A) into original T1 image space (in which regional parcellation was performed) (B), binary white matter volumes for each of the 16 regions are multiplied by each of the diffusion maps (fractional anisotropy, mean, axial and radial diffusivity). Mean diffusivity is shown (C). DF= dorsal prefrontal, OF= orbitofrontal, PM = premotor, SG = subgenual, SM = sensorimotor, MT = midtemporal, PO = parieto-occipital, IO = interior occipital.
2.6. Neuropsychological outcomes
At 7 years corrected age, a detailed neuropsychological assessment was conducted for 99 of the 116 infants with MRI data (80 very preterm and 19 full term). For the purposes of this study the neuropsychological domains of interest were general intelligence (IQ), motor skills, executive function, and memory. IQ was estimated using the 4-subtest Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). The Movement Assessment Battery for Children – second edition was administered to evaluate motor skills, where the total raw test score was used (Henderson et al., 2007). Performance on the total raw score for the Tower of London task, which is a measure of planning ability, was included as a measure of executive function (Anderson et al., 1996). Finally, the total number of words recalled across trials 1–5 of list A on the California Verbal Learning Test – Children’s Version was used as a measure of memory and learning (Delis et al., 1994). For all measures, impairment was defined as <1 standard deviation from the mean raw score of the full term children.
2.7. Statistical analyses
Data were analyzed using Stata Release 12.0 and SAS version 9.2 (SAS Institute Inc., Cary, NC). Diffusion parameters and 7 year outcomes were approximately normal, hence parametric analyses were used. Differences between groups for infant and 7 year cohort characteristics were analyzed using χ2 tests for categorical variables, and independent samples T-tests for continuous variables.
Linear regression was used to explore differences between very preterm and full term infants in global white matter for each diffusion measure, adjusting for gestational age at scan (aim 1). Comparison of regional white matter microstructure between very preterm and full term infants was performed using a mixed model for each diffusion value which included all regions in a single model similar to that previously used (Peterson et al., 2003; Thompson et al., 2007). Correlations among parcellated brain regions within the same infant were modeled by including a random effect for each infant. Heteroscedasticity between regions of the brain was modeled by allowing both the within-subject and between-subject variability to vary by region. Models included two within-subject factors: region (eight levels) and hemisphere (two levels). The primary between-subjects factor of interest was group (very preterm and full term). Two- and three-way interactions between group, region and hemisphere were tested to explore the possibility that the effect of prematurity on white matter microstructure varied by region and hemisphere. Gestational age at scan was included in all models as a covariate.
The relationships between perinatal variables and white matter diffusion values (aim 2) were ascertained using a single linear regression model fitted for each diffusion measure including all perinatal variables and all regions in the brain. Again random effects were used to allow for the correlation between multiple observations within each individual. Clinically relevant perinatal variables included: sex, gestational age at birth, birth weight standard deviation z-score (a measure of intrauterine growth restriction), and an indicator for brain abnormality defined as either white matter abnormality (grade III and IV) or intraventricular hemorrhage (grade III and IV); many of which have been previously associated with cerebral changes in prematurity. Gestational age at scan was included in all models as a covariate.
Finally, the associations between white matter diffusion measures and impairment at 7 years (aim 3) were assessed using logistic regression. Separate models were fitted for each regional diffusion measure and outcome combination firstly with only gestational age at scan as a covariate, then additionally age at 7 year assessment and brain abnormality, to determine if associations were independent of these potential confounders.
Although no formal adjustment is made for multiple comparisons, aims 1 and 2 were assessed using a single model for each diffusion measure to reduce multiple comparisons, and for aim 3 our results are interpreted as exploratory, and we consider the overall pattern of relationships rather than focusing on individual p-values.
3. RESULTS
3.1. Subjects
Characteristics of the 116 subjects included in this study are reported in Table 1. As expected, multiple births were more common in the very preterm group and there were neonatal complications in the very preterm group that did not occur in the full term group. Birth weight standard deviation score was higher in the very preterm group (p=0.005) but sex was similar between groups (p=0.5). Of note, full term infants were slightly older than very preterm infants at the time of scan, but this was not statistically significant (p=0.3). At 7 years, very preterm children had higher rates of intellectual (Wechsler Abbreviated Scale of Intelligence IQ, p= 0.009), executive (Tower Of London, p=0.01), and motor (Movement Assessment Battery for Children, p=0.002) impairments compared with full term children, but not memory impairments (California Verbal Learning Test, p=0.9) (Table 1).
Table 1.
Characteristics of the cohort.
| Perinatal Characteristics | Very preterm (n=96) | Full term (n=20) |
|---|---|---|
| Antenatal corticosteroid administration, n (%) | 84 (88) | 0 (0) |
| Gestational age at birth (wk), mean (SD) | 27.7 (1.8) | 38.9 (1.1) |
| Male sex, n (%) | 49 (51) | 12 (60) |
| Multiple births, n (%) | 41 (43) | 0 (0) |
| Birth weight (g), mean (SD) | 990 (219) | 3300 (510) |
| Birth weight SD score, mean (SD) | −0.5 (0.9) | 0.09 (0.9) |
| Intrauterine growth restrictiona, n (%) | 8 (8) | 1 (5) |
| Bronchopulmonary dysplasiab, n (%) | 32 (33) | 0 (0) |
| Postnatal corticosteroid therapyc,d, n (%) | 9 (9) | 0 (0) |
| Proven necrotizing enterocolitis, n (%) | 3 (3)f | 0 (0) |
| Proven sepsis, n (%) | 37 (39) | 0 (0) |
| IVH (any grade), n (%) | 11 (11) | 0 (0) |
| IVH (grade III/IV), n (%) | 5 (5) | 0 (0) |
| WM abnormality (any grade), n (%) | 50 (52) | 1 (5) |
| WM abnormality (grade III/IV), n (%) | 17 (18) | 0 (0) |
| Brain abnormalitye, n (%) | 20 (21) | 0 (0) |
| Gestational age at scan (wk), mean (SD) | 40.3 (1.8) | 41.0 (1.3) |
| 7 year outcomes | Very preterm (n=80) | Full term (n=19) |
| Age at assessment (years) | 7.4 (0.2) | 7.6 (0.2) |
| Impairedf IQ, n (%) | 34 (35.4) | 2 (10.0) |
| Impairedf,g executive function, n (%) | 36 (37.5) | 3 (15.0) |
| Impairedf,h memory and learning, n (%) | 11 (14.1) | 3 (15.8) |
| Impairedf,i motor, n (%) | 35 (36.5) | 1 (5.0) |
Defined as birth weight SD score >2 SD below mean weight for gestational age,
Required oxygen at 36 weeks gestational age,
Postnatal dexamethasone, usual dose 0.15 mg/kg per day for 3 days, reducing over 10 days: total dose 0.89 mg/kg,
missing in one participant,
White matter (WM) abnormality grade III/IV or intraventricular hemorrhage (IVH) grade III/IV. SD = standard deviation,
Impairment defined as < 1 SD from mean of full-term children,
missing in 4 participants,
missing in 2 participants,
missing in 8 participants.
3.2. White matter diffusion
On average very preterm infants had higher mean, axial and radial diffusivity in the total white matter compared with full term infants, although there was little difference in fractional anisotropy between the two groups (Table 2).
Table 2.
Comparison of fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) within the total white matter of the very preterm (VPT) and full term (FT) brain.
| VPT, mean (SD) | FT, mean (SD) | Mean Difference (95% CI)* | p | |
|---|---|---|---|---|
| FA | 0.16 (0.02) | 0.16 (0.02) | −0.002 (−0.011, 0.007) | 0.69 |
| MD (×10−3 mm2/s) | 1.47 (0.08) | 1.41 (0.07) | 0.06 (0.02, 0.10) | 0.003 |
| AD (×10−3 mm2/s) | 1.71 (0.10) | 1.64 (0.08) | 0.07 (0.02, 0.11) | 0.008 |
| RD (×10−3 mm2/s) | 1.36 (0.07) | 1.30 (0.07) | 0.06 (0.02, 0.09) | 0.003 |
Adjusted for gestational age at scan. SD = standard deviation, CI = confidence interval.
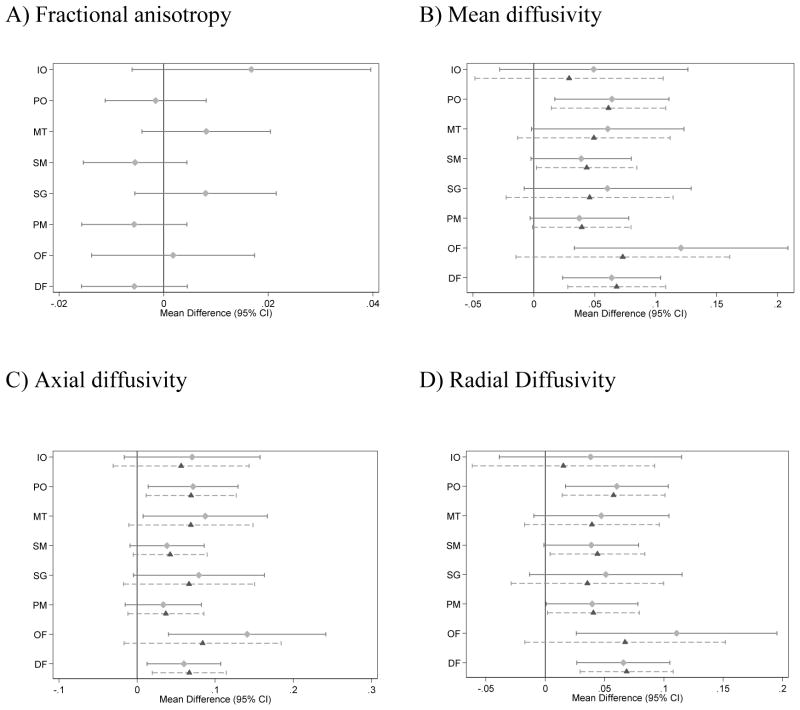
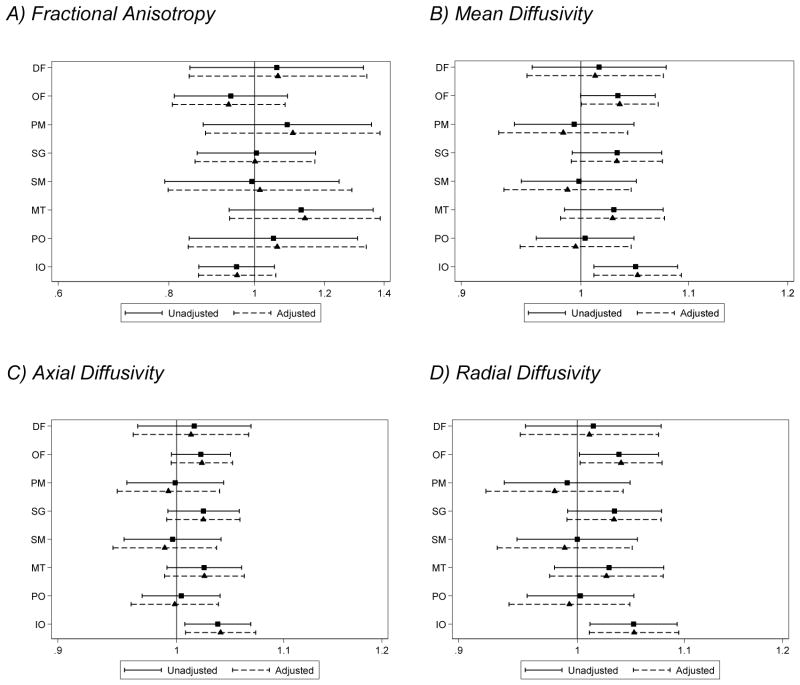
There was little evidence that the group difference varied by region for any of the diffusion measurements (group x region interaction p > 0.2 for all variables, Table 3). For mean, axial and radial diffusivity there was some evidence that the effect of group (very preterm vs. full term) differed by hemisphere (p-value for the group x hemisphere interaction <0.05, Table 3), where elevations in mean, axial and radial diffusivity in very preterm compared with full term infants were generally more pronounced for the right hemisphere (Fig. 3). There was no evidence for a three-way interaction between region, group, and hemisphere (Table 3).
Table 3.
Summary of interactions from fitting mixed models to total white matter diffusion measures, for group (very preterm vs. full term), region and hemisphere differences
| Fractional anisotropy | Mean diffusivity | Axial diffusivity | Radial diffusivity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | p | F | p | F | p | F | p | |
| Region x Group | 7 | 1.24 | 0.27 | 0.37 | 0.92 | 0.47 | 0.86 | 0.39 | 0.91 |
| Hemisphere x Group | 1 | 0.01 | 0.92 | 5.09 | 0.02 | 4.16 | 0.04 | 5.03 | 0.03 |
| Region x Hemisphere x Group | 7 | 1.08 | 0.37 | 1.27 | 0.26 | 1.26 | 0.27 | 1.18 | 0.31 |
Note: Results are F statistics and corresponding p-values from a single mixed model for each outcome. Df = degrees of freedom used in the F-test.
Figure 3.
Estimated differences in very preterm compared with full term infants for diffusion measures in the white matter, by region, adjusted for gestational age at the time of scan (n=96 very preterm and 20 full term infants). (A) fractional anisotropy, (B) mean diffusivity (×10−3mm2/s), (C) axial diffusivity (×10−3mm2/s), (D) radial diffusivity (×10−3mm2/s). Note, the Fractional anisotropy model does not allow the effect of group to vary by hemisphere as indicated in table 3. For mean, axial and radial diffusivity, the solid line represents the right hemisphere and the dashed line the left hemisphere.
DF = dorsal prefrontal, OF = orbitofrontal, PM = premotor, SG = subgenual, SM = sensorimotor, MT = mid-temporal, PO = parieto-occipital, IO = inferior occipital and cerebellum, CI = confidence interval.
3.3. Perinatal factors
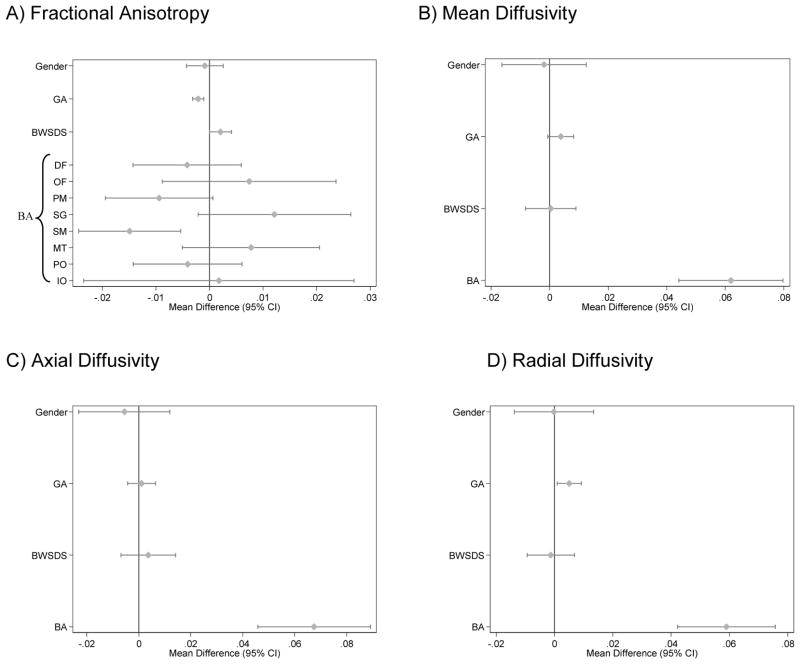
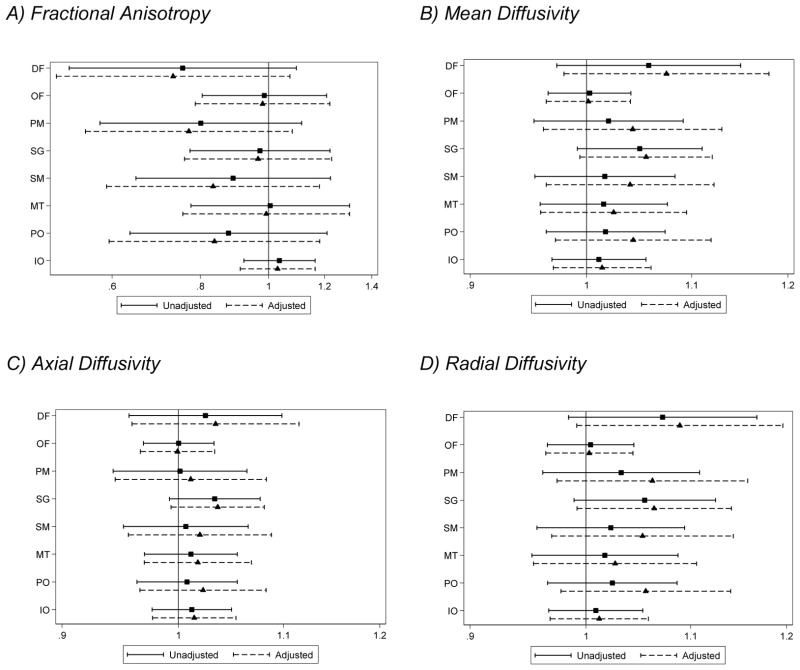
There was strong evidence that brain abnormality was associated with lower fractional anisotropy and higher mean, axial and radial diffusivity in the white matter of very preterm infants (Fig. 4). In addition, there was weak evidence that increasing gestational age was associated with lower fractional anisotropy and higher radial diffusivity (Fig. 4). The only evidence for a region-specific effect on fractional anisotropy values was for brain abnormality, where the strongest evidence was for a reduction in fractional anisotropy within the sensorimotor region of infants with brain abnormality (Fig. 4). There was little evidence for a region-specific effect of perinatal predictors on mean, axial or radial diffusivity.
Figure 4.
Relationships between perinatal variables and diffusion parameters in very preterm infants (n=95). (A) Fractional anisotropy, (B) mean, (C) axial, and (D) radial diffusivity (×10−3mm2/s), adjusting for gestational age at scan. Note, there was no evidence that the effect of perinatal variables varied by region with the exception of brain abnormality and fractional anisotropy. Estimates are regression parameters and 95% confidence intervals (CI), representing the change in the diffusion variable for each unit change in the perinatal predictor, adjusted for gestational age at the time of scan and all other variables shown.
GA = gestational age at birth, BWSDS = birth weight standard deviation z-score, and BA = brain abnormality: defined as having grade III/IV white matter abnormality or grade III/IV intraventricular hemorrhage, DF= dorsal prefrontal, OF= orbitofrontal, PM = premotor, SG = subgenual, SM = sensorimotor, MT = midtemporal, PO = parieto-occipital, IO = inferior occipital.
3.4. Neurodevelopmental outcomes at age 7 years
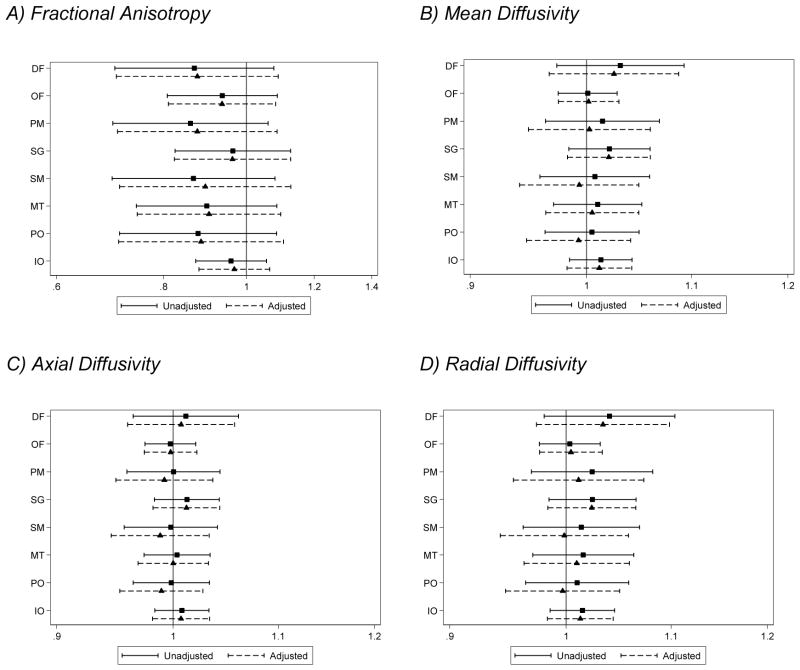
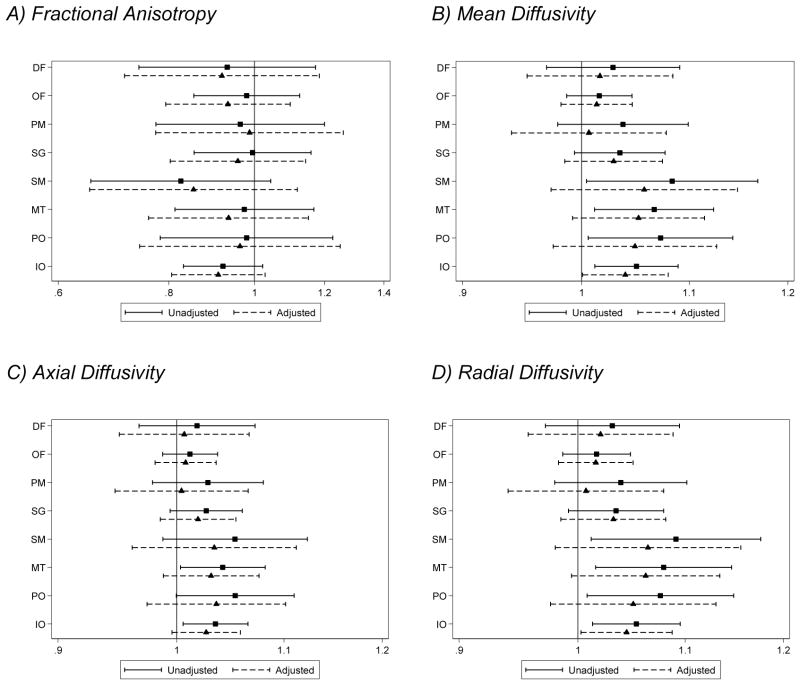
There was little evidence that diffusion measures were associated with IQ (Fig. 5.1). There was evidence for associations between increased diffusivities (particularly mean and radial diffusivity) within the posterior sub-regions of the brain (sensorimotor, midtemporal, inferior occipital and cerebellum and parieto-occipital regions) and impaired scores on the Movement Assessment Battery for Children. However, this relationship diminished after adjusting for brain abnormality and age at 7 year assessment (Fig. 5.2). Higher white matter diffusivity values in the inferior occipital and cerebellum region were associated with impaired executive functioning. The evidence for these associations was unaltered after adjusting for brain abnormality. There was also some evidence for an association between higher mean and radial diffusivity in the orbitofrontal region and impaired executive functioning (Fig. 5.3). There was little evidence that diffusion measures were associated with impaired memory scores (Fig. 5.4).
Figure 5.1.
Associations between white matter (A) fractional anisotropy, (B) mean, (C) axial, and (D) radial diffusivity values for each region at term-equivalent age and impairment in intelligence at age 7 years (assessed using the Wechsler Abbreviated Scale of Intelligence). Solid lines represent odds ratios of being impaired per percentage increase in fractional anisotropy, and per 0.01 ×10−3mm2/s increase in diffusivity and 95% confidence intervals adjusted for gestational age at the time of scan, and dashed lines represent odds ratios and 95% confidence intervals adjusted for gestational age at the time of scan, corrected age at the 7 year assessment and brain injury at term-equivalent age.
DF= dorsal prefrontal, OF= orbitofrontal, PM = premotor, SG = subgenual, SM = sensorimotor, MT = midtemporal, PO = parieto-occipital, IO = interior occipital and cerebellum.
Figure 5.2.
Associations between white matter (A) fractional anisotropy, (B) mean, (C) axial, and (D) radial diffusivity values for each region at term-equivalent age and impairment in movement at age 7 years (assessed using the Movement Assessment Battery for Children – second edition). Solid lines represent odds ratios of being impaired per percentage increase in fractional anisotropy, and per 0.01 × 10−3mm2/s increase in diffusivity 95% confidence intervals adjusted for gestational age at the time of scan, and dashed lines represent odds ratios and 95% confidence intervals adjusted for gestational age at the time of scan, corrected age at the 7 year assessment and brain injury at term-equivalent age.
DF= dorsal prefrontal, OF= orbitofrontal, PM = premotor, SG = subgenual, SM = sensorimotor, MT = midtemporal, PO = parieto-occipital, IO = interior occipital and cerebellum.
Figure 5.3.
Associations between white matter (A) fractional anisotropy, (B) mean, (C) axial, and (D) radial diffusivity values for each region at term-equivalent age and impairment in executive functioning at age 7 years (assessed using the Tower Of London task). Solid lines represent odds ratios of being impaired per percentage increase in fractional anisotropy, and per 0.01 × 10−3mm2/s increase in diffusivity and 95% confidence intervals adjusted for gestational age at the time of scan, and dashed lines represent odds ratios and 95% confidence intervals adjusted for gestational age at the time of scan, corrected age at the 7 year assessment and brain injury at term-equivalent age.
DF= dorsal prefrontal, OF= orbitofrontal, PM = premotor, SG = subgenual, SM = sensorimotor, MT = midtemporal, PO = parieto-occipital, IO = interior occipital and cerebellum.
Figure 5.4.
Associations between white matter (A) fractional anisotropy, (B) mean, (C) axial, and (D) radial diffusivity values for each region at term-equivalent age and impairment in memory and learning at age 7 years (assessed using the California Verbal Learning Test). Solid lines represent odds ratios of being impaired per percentage increase in fractional anisotropy, and per 0.01 × 10−3mm2/s increase in diffusivity and 95% confidence intervals adjusted for gestational age at the time of scan, and dashed lines represent odds ratios and 95% confidence intervals adjusted for gestational age at the time of scan, corrected age at the 7 year assessment and brain injury at term-equivalent age.
DF= dorsal prefrontal, OF= orbitofrontal, PM = premotor, SG = subgenual, SM = sensorimotor, MT = midtemporal, PO = parieto-occipital, IO = interior occipital and cerebellum.
4. DISCUSSION
4.1. Whole brain white matter microstructure
White matter diffusivity values were increased in very preterm infants compared with full term infants, including mean, axial and radial diffusivity values. Mean diffusivity is a reflection of overall brain water content (Neil et al., 1998), therefore higher mean diffusivity may be a result of fewer or less densely packed fibers, fewer macromolecules and oligodendrocytes, or increased extra-cellular space which may be caused by fewer neurons or glia (Barkovich, 2005; Dubois et al., 2006; Le Bihan, 2003; Pierpaoli et al., 2001). Considering mean diffusivity decreases with age (Huppi et al., 1998; Neil et al., 1998; Partridge et al., 2004), it is assumed that higher mean diffusivity corresponds to less mature white matter. Radial diffusivity has been associated with the health and maturation of myelin (Budde et al., 2007; Concha et al., 2006; Song et al., 2003; Song et al., 2005). A higher radial diffusivity in very preterm infants would suggest delay or disruption to the myelination of axons, or less compact or less dense fiber bundles than in full term infants (Mukherjee et al., 2002). Others have also found that radial diffusivity is altered in very preterm infants compared with full term controls (Gao et al., 2009; Haynes et al., 2005; Partridge et al., 2004). Axial diffusivity decreases during maturation (Berman et al., 2005; Partridge et al., 2005), and hence high neonatal axial diffusivity values may reflect immaturity of the fiber cytoskeleton (Gao et al., 2009; Mukherjee et al., 2002) and increased water content within the brain (Mukherjee et al., 2002).
Contrary to our original hypothesis, we found little evidence of a difference in fractional anisotropy within the white matter of very preterm compared with full term infants. Reductions in fractional anisotropy have been attributed to a reduction in axial diffusivity or an increase in radial diffusivity (Anjari et al., 2007). Considering the current study revealed increases in diffusivity in all directions in very preterm infants, i.e. in both axial and radial diffusivity, the possibility of finding anisotropic differences may have been reduced. A previous study did find evidence that lower whole brain white matter anisotropy was associated with prematurity, however that study involved children aged 8–11 years (Yung et al., 2007), indicating differences may not be apparent until later in development.
4.2. Regional white matter microstructure
While we found little evidence that the difference in diffusion values between very preterm and full term infants varied by region, there was potentially a larger difference between the groups in the orbitofrontal region, although there was large overlaps in the 95% confidence intervals for the group differences across the regions. Regional vulnerabilities may be related to the developmental trajectory for white matter maturation: first to mature are the posterior and central sensorimotor areas, and lastly in the more anterior frontal and peripheral regions (Berman et al., 2005; Brody et al., 1987; Dubois et al., 2006; Flechsig, 1901; Kinney et al., 1988; Volpe, 2000).
Using different image analysis methods to the current study, other groups have reported regional vulnerabilities with diffusion imaging in very preterm infants. Tract based spatial statistics studies have revealed lower fractional anisotropy values in the frontal white matter (Anjari et al., 2007) and coronal radiata (Rose et al., 2008) in very preterm compared with full term infants. Many studies have also revealed vulnerability of the centrum semiovale in very preterm infants (Anjari et al., 2007; Counsell et al., 2006; Krishnan et al., 2007; Neil et al., 1998), which would be located within the premotor and sensorimotor regions. Huppi et al. (1998) found that mean diffusivity was higher and anisotropy lower for very preterm infants compared with full term infants at term-equivalent age in the central white matter and posterior limb of the internal capsule (Huppi et al., 1998), which would correspond to sensorimotor, premotor and midtemporal regions. Other studies have found alterations to white matter microstructure in very preterm children (Constable et al., 2008; Counsell et al., 2008), adolescents (Nagy et al., 2009) and adults (Allin et al., 2011; Eikenes et al., 2011) compared with their term-born counterparts which would suggest that the alterations seen at term-equivalent in very preterm infants may persist.
4.3. Hemispheric differences
There was some evidence that the microstructural differences between full term and very preterm infants differed according to hemisphere, with larger differences between the groups in mean, axial and radial diffusivity in the right hemisphere. There is evidence to suggest that left hemisphere myelination is more rapid than the right in many brain regions during early development (Deoni et al., 2011). This developmental asymmetry may explain why very preterm infants, who are more prone to oligodendrocyte and myelination disruptions, are more vulnerable to right hemisphere microstructural alteration.
4.4. Perinatal predictors
Brain abnormality had a negative influence on diffusion measures within the white matter. Hypoxic-ischemia, infection and inflammation are known to be the major causes of brain abnormality, leading to excitotoxicity, and potentially necrosis, apoptosis, astrogliosis and microgliosis as well as damage to and reduction of pre-myelinating oligodendrocytes. Increased diffusion in the injured very preterm brain may also be a result of edema (Calvert and Zhang, 2005). For some regions there were positive associations between brain abnormality and fractional anisotropy, where others had negative associations. In particular, there was a strong association between brain abnormality and reduced fractional anisotropy within the sensorimotor region. The sensorimotor region predominantly contains the corticospinal white matter tracts. Our results are consistent with others who reported lower fractional anisotropy and higher diffusivity in association with white matter injury in preterm infants (Bonifacio et al., 2010) and adolescents (Feldman et al., 2012). Studies specifically examining regional changes in diffusion values have also found associations between reduced fractional anisotropy (in addition to increased radial and axial diffusivity) and white matter abnormalities (Arzoumanian et al., 2003; Huppi et al., 2001; Miller et al., 2002). Furthermore, diffuse excessive high signal intensity in the white matter of very preterm infants has been associated with altered diffusion values within regional white matter (Cheong et al., 2009; Counsell et al., 2006).
There was some evidence that increasing gestational age was associated with lower fractional anisotropy and higher radial diffusivity in very preterm infants. This is a somewhat unexpected finding, as others report increasing anisotropy and reduced diffusivity with increasing gestational age (Huppi et al., 1998; Partridge et al., 2004). However these studies examined specific white matter regions rather than whole brain white matter. Examining all white matter across the brain in the current study may lead to interference by crossing fibres. Alternatively, the unexpected findings may be attributed to smaller white matter volumes in younger infants, leading to greater partial volume effects and cerebrospinal fluid contamination (Feldman et al., 2012).
4.5. Neurodevelopmental outcomes at age 7 years
Infant diffusivity values were related to 7 year outcomes in a region-specific way. In particular, there was evidence that higher diffusivity in the inferior occipital and cerebellum region was associated with poorer outcomes at age 7 years, namely impaired motor outcome, impaired memory and learning, and impaired executive functioning. Anatomically, the white matter of the inferior occipital and cerebellum region possibly contains cerebellar tracts such as the cerebellothalamic, corticopontocerebellar and spinocerebellar tracts, as well as portions of the posterior corpus callosum, inferior corticospinal and corticopontine tracts, optic radiations, posterior and temporal projections of the superior longitudinal fascicles, and the inferior longitudinal fascicles. Although cerebellar tracts have not been specifically investigated in prematurity, damage to the cerebellum has been associated with impaired functioning in those born preterm (Allin et al., 2005; Tam et al., 2011). Specifically, literature has revealed associations between cerebellar tract integrity and cognitive and motor functioning (Salmi et al., 2010) and has suggested that there may be a role for the cerebellum in executive functioning (Koziol et al., 2012). Thus the contribution of cerebellar white matter to adverse outcomes in these neuropsychological domains in very preterm populations may be important, and requires further investigation. The posterior corpus callosum (Thompson et al., 2012), motor pathways (Hoon et al., 2009), superior (Frye et al., 2010), and inferior longitudinal fasciculus (Skranes et al., 2007) have previously been associated with impaired cognitive and motor functioning in the preterm literature. Therefore, damage to such white matter fibers may be a factor contributing to the association between altered inferior occipital and cerebellum white matter and impaired neurodevelopmental functioning in the current study.
There was also some evidence of associations between increased diffusivities within the whole brain, sensorimotor, midtemporal and parieto-occipital regions and impaired motor outcome, however these associations appeared to be mediated by brain abnormality. These regions contain the corticospinal and corticopontine white matter fibers, which are known to be associated with movement. Our findings are consistent with others who have found associations between white matter microstructure in the motor pathways and motor development (Drobyshevsky et al., 2007; Rose et al., 2007; Skranes et al., 2007).
There was weak evidence for an association between higher orbitofrontal white matter mean and radial diffusivity and impaired performance on the Tower Of London task which taps planning and working memory. These findings indicate a possible link between altered myelination in frontal regions and impaired executive functioning, which is consistent with the notion that higher-order cognitive functions are largely subserved by prefrontal regions. Others have shown specific posterior-to-frontal white matter tract fractional anisotropy reductions associated with executive dysfunction in very low birth weight adolescents (Skranes et al., 2009), as well as associations between white matter abnormality and impaired executive functioning in very preterm 4 year-olds (Woodward et al., 2011).
The current study found little evidence of associations between diffusion measures and impaired IQ or memory. In contrast, Skranes et al. reported lower IQ in relation to lower fractional anisotropy in the external capsule, inferior and middle superior fasciculus in very low birth weight adolescents (Skranes et al., 2007), and others have also found associations between diffusion values and IQ in preterm populations (Feldman et al., 2012; Krishnan et al., 2007). Furthermore, one group has previously reported associations between altered white matter microstructure and impaired memory scores in young adults born preterm (Allin et al., 2011).
4.6. Limitations
The diffusion values reported in the current study are averaged over a diffuse area of white matter, representing many tract populations with crossing fibers. Certainly no deductions can be made about integrity of specific fiber populations, as the method of regional parcellation was not tract-specific. The diffusion tensor imaging results presented in this study require confirmation by other studies using more technologically advanced diffusion acquisition protocols, higher field strength, increased image resolution, more gradient directions, and improved signal to noise ratio, as well as more advanced analysis algorithms and techniques. The images acquired for this study did not meet the minimum requirements for analysis with tract-based spatial statistics.
4.7. Conclusions and implications
In this study, diffusion tensor imaging has provided neurobiological evidence for the global and localized alterations to the white matter of very preterm infants compared with term-born controls. The very preterm infant white matter was less mature than that of full term infants at term-equivalent age. The differences in microstructure appeared to be a result of increased water content and immaturity of the cytoskeleton, and disruption or delay to myelination, as implied by higher mean, axial and radial diffusivity values. There was little evidence of differences in fractional anisotropy between full term and very preterm infants, and little evidence that the group differences varied by region. Brain abnormality was found to be negatively associated with white matter organization in the very preterm infant, emphasizing the importance of prevention and treatment of brain abnormality in order to protect white matter from the adverse effects of prematurity. Importantly, this study is the first to show neuroanatomically specific regional changes in white matter diffusivity values at term-equivalent and the consequences for neurodevelopmental functioning at 7 years. Higher diffusivity in the inferior occipital and cerebellum region at term-equivalent was most associated with impaired neurodevelopmental functioning at 7 years, including impaired motor and executive function, which may be a reflection of abnormal cerebellar or other inferior occipital and cerebellum white matter fibers. There was some suggestion of associations between higher diffusivity in the orbitofrontal region and reduced executive functioning, as well as increased diffusivity in the sensorimotor, midtemporal and parieto-occipital regions and impaired motor outcome. These findings warrant further investigation. Insights gained into the early microstructural alterations of the white matter from this study may contribute to the development of tailored clinical interventions which are likely to improve long-term functional outcomes for very preterm infants. Some promising clinical interventions include magnesium sulfate given to women threatening to deliver preterm to reduce the risk of cerebral palsy in their child (Doyle et al., 2007), and caffeine treatment in the first weeks after birth, which has been shown not only to improve neurodevelopmental outcomes in childhood (Schmidt et al., 2012; Schmidt et al., 2007), but also alter diffusion values at term-equivalent age in a direction consistent with improved maturation of the brain in infants <1250 g birth weight (Doyle et al., 2010).
Highlights.
Very preterm infants have altered white matter diffusion compared with controls
Diffusion alterations may reflect watery white matter and altered myelination
Disrupted white matter organization is related to qualitative brain abnormalities
Altered neonatal white matter diffusivity predicts impairment at 7 years of age
Inferior and cerebellar regions related to motor and executive functioning
Acknowledgments
We gratefully thank Merilyn Bear for recruitment, Hong X Wang for image processing, Michael Kean and the radiographers at the Royal Children’s Hospital, the VIBeS and Developmental imaging teams at the Murdoch Childrens Research Institute for their ideas and support, as well as the families and children who participated in this study.
This work was supported by the National Health and Medical Research Council of Australia [237117 and 546519 to L.W.D., 400317 to G.F.E., 628371 and 491209 to P.J.A., the Australian Postgraduate Award to D.K.T.); the National Institute of Health [R01 RR021885, R01 GM074068, R01 EB008015, P30 HD018655 to S.K.W.]; the United Cerebral Palsy Foundation (USA) to T.E.I.; the Leila Y. Mathers Charitable Foundation (USA) to G.F.E.; the Brown Foundation (USA) to G.F.E.; and the Victorian Government’s Operational Infrastructure Support Program to D.K.T., and P.J.A.
Abbreviations
- FSL
functional magnetic resonance imaging of the brain’s software library
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allin MP, Salaria S, Nosarti C, Wyatt J, Rifkin L, Murray RM. Vermis and lateral lobes of the cerebellum in adolescents born very preterm. Neuroreport. 2005;16 (16):1821–1824. doi: 10.1097/01.wnr.0000185014.36939.84. [DOI] [PubMed] [Google Scholar]
- Allin MPG, Kontis D, Walshe M, Wyatt J, Barker GJ, Kanaan RAA, McGuire P, Rifkin L, Murray RM, Nosarti C. White Matter and Cognition in Adults Who Were Born Preterm. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0024525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancel PY, Livinec F, Larroque B, Marret S, Arnaud C, Pierrat V, Dehan M, N’Guyen S, Escande B, Burguet A, Thiriez G, Picaud JC, Andre M, Breart G, Kaminski M, Group ES. Cerebral palsy among very preterm children in relation to gestational age and neonatal ultrasound abnormalities: the EPIPAGE cohort study. Pediatrics. 2006;117 (3):828–835. doi: 10.1542/peds.2005-0091. [DOI] [PubMed] [Google Scholar]
- Anderson P, Anderson V, Lajoie G. The Tower of London Test: Validation and standardisation for pediatric populations. The Clinical Neuropsychologist. 1996;10(1):54–65. [Google Scholar]
- Anderson P, Doyle LW. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA. 2003;289 (24):3264–3272. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Doyle LW. Executive functioning in school-aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics. 2004;114 (1):50–57. doi: 10.1542/peds.114.1.50. [DOI] [PubMed] [Google Scholar]
- Anjari M, Srinivasan L, Allsop JM, Hajnal JV, Rutherford MA, Edwards AD, Counsell SJ. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. Neuroimage. 2007;35 (3):1021–1027. doi: 10.1016/j.neuroimage.2007.01.035. [DOI] [PubMed] [Google Scholar]
- Arzoumanian Y, Mirmiran M, Barnes PD, Woolley K, Ariagno RL, Moseley ME, Fleisher BE, Atlas SW. Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. Am J Neuroradiol. 2003;24 (8):1646–1653. [PMC free article] [PubMed] [Google Scholar]
- Banker BQ, Larroche JC. Periventricular leukomalacia of infancy. A form of neonatal anoxic encephalopathy. Arch Neurol. 1962;7:386–410. doi: 10.1001/archneur.1962.04210050022004. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ. Magnetic resonance techniques in the assessment of myelin and myelination. J Inherit Metab Dis. 2005;28 (3):311–343. doi: 10.1007/s10545-005-5952-z. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003a;6 (7):750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003b;50 (5):1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Berman JI, Mukherjee P, Partridge SC, Miller SP, Ferriero DM, Barkovich AJ, Vigneron DB, Henry RG. Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. Neuroimage. 2005;27 (4):862–871. doi: 10.1016/j.neuroimage.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Billiards SS, Haynes RL, Folkerth RD, Borenstein NS, Trachtenberg FL, Rowitch DH, Ligon KL, Volpe JJ, Kinney HC. Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol. 2008;18 (2):153–163. doi: 10.1111/j.1750-3639.2007.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal I. Periventricular leucomalacia: a review. Eur J Pediatr. 2004;163 (8):435–442. doi: 10.1007/s00431-004-1477-y. [DOI] [PubMed] [Google Scholar]
- Bonifacio SL, Glass HC, Chau V, Berman JI, Xu D, Brant R, Barkovich AJ, Poskitt KJ, Miller SP, Ferriero DM. Extreme premature birth is not associated with impaired development of brain microstructure. J Pediatr. 2010;157(5):726–732. e721. doi: 10.1016/j.jpeds.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody BA, Kinney HC, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. J Neuropathol Exp Neurol. 1987;46 (3):283–301. doi: 10.1097/00005072-198705000-00005. [DOI] [PubMed] [Google Scholar]
- Budde MD, Kim JH, Liang HF, Schmidt RE, Russell JH, Cross AH, Song SK. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med. 2007;57 (4):688–695. doi: 10.1002/mrm.21200. [DOI] [PubMed] [Google Scholar]
- Calvert JW, Zhang JH. Pathophysiology of an hypoxic-ischemic insult during the perinatal period. Neurol Res. 2005;27 (3):246–260. doi: 10.1179/016164105X25216. [DOI] [PubMed] [Google Scholar]
- Cheong JL, Thompson DK, Wang HX, Hunt RW, Anderson PJ, Inder TE, Doyle LW. Abnormal white matter signal on MR imaging is related to abnormal tissue microstructure. Am J Neuroradiol. 2009;30 (3):623–628. doi: 10.3174/ajnr.A1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;17 (4):407–429. [PubMed] [Google Scholar]
- Concha L, Gross DW, Wheatley BM, Beaulieu C. Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. Neuroimage. 2006;32 (3):1090–1099. doi: 10.1016/j.neuroimage.2006.04.187. [DOI] [PubMed] [Google Scholar]
- Constable RT, Ment LR, Vohr BR, Kesler SR, Fulbright RK, Lacadie C, Delancy S, Katz KH, Schneider KC, Schafer RJ, Makuch RW, Reiss AR. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121 (2):306–316. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- Counsell SJ, Allsop JM, Harrison MC, Larkman DJ, Kennea NL, Kapellou O, Cowan FM, Hajnal JV, Edwards AD, Rutherford MA. Diffusion-weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics. 2003;112 (1 Pt 1):1–7. doi: 10.1542/peds.112.1.1. [DOI] [PubMed] [Google Scholar]
- Counsell SJ, Edwards AD, Chew AT, Anjari M, Dyet LE, Srinivasan L, Boardman JP, Allsop JM, Hajnal JV, Rutherford MA, Cowan FM. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008;131 (Pt 12):3201–3208. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- Counsell SJ, Shen Y, Boardman JP, Larkman DJ, Kapellou O, Ward P, Allsop JM, Cowan FM, Hajnal JV, Edwards AD, Rutherford MA. Axial and radial diffusivity in preterm infants who have diffuse white matter changes on magnetic resonance imaging at term-equivalent age. Pediatrics. 2006;117 (2):376–386. doi: 10.1542/peds.2005-0820. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test- Children’s Version. Psychological Corporation; San Antonio, TX: 1994. [Google Scholar]
- Deoni SC, Mercure E, Blasi A, Gasston D, Thomson A, Johnson M, Williams SC, Murphy DG. Mapping infant brain myelination with magnetic resonance imaging. J Neurosci. 2011;31 (2):784–791. doi: 10.1523/JNEUROSCI.2106-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle LW, Cheong J, Hunt RW, Lee KJ, Thompson DK, Davis PG, Rees S, Anderson PJ, Inder TE. Caffeine and brain development in very preterm infants. Ann Neurol. 2010;68 (5):734–742. doi: 10.1002/ana.22098. [DOI] [PubMed] [Google Scholar]
- Doyle LW, Crowther CA, Middleton P, Marret S. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. 2007;(3):CD004661. doi: 10.1002/14651858.CD004661.pub2. [DOI] [PubMed] [Google Scholar]
- Drobyshevsky A, Bregman J, Storey P, Meyer J, Prasad PV, Derrick M, MacKendrick W, Tan S. Serial diffusion tensor imaging detects white matter changes that correlate with motor outcome in premature infants. Dev Neurosci. 2007;29 (4–5):289–301. doi: 10.1159/000105470. [DOI] [PubMed] [Google Scholar]
- Dubois J, Hertz-Pannier L, Dehaene-Lambertz G, Cointepas Y, Le Bihan D. Assessment of the early organization and maturation of infants’ cerebral white matter fiber bundles: a feasibility study using quantitative diffusion tensor imaging and tractography. Neuroimage. 2006;30 (4):1121–1132. doi: 10.1016/j.neuroimage.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Eikenes L, Lohaugen GC, Brubakk AM, Skranes J, Haberg AK. Young adults born preterm with very low birth weight demonstrate widespread white matter alterations on brain DTI. NeuroImage. 2011;54 (3):1774–1785. doi: 10.1016/j.neuroimage.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Feldman HM, Lee ES, Loe IM, Yeom KW, Grill-Spector K, Luna B. White matter microstructure on diffusion tensor imaging is associated with conventional magnetic resonance imaging findings and cognitive function in adolescents born preterm. Dev Med Child Neurol. 2012;54 (9):809–814. doi: 10.1111/j.1469-8749.2012.04378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flechsig P. Developmental (myelogenetic) localisation of the cerebral cortex in the human. Lancet. 1901;158:1027–1030. [Google Scholar]
- Frye RE, Hasan K, Malmberg B, Desouza L, Swank P, Smith K, Landry S. Superior longitudinal fasciculus and cognitive dysfunction in adolescents born preterm and at term. Dev Med Child Neurol. 2010;52 (8):760–766. doi: 10.1111/j.1469-8749.2010.03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Lin W, Chen Y, Gerig G, Smith JK, Jewells V, Gilmore JH. Temporal and spatial development of axonal maturation and myelination of white matter in the developing brain. Am J Neuroradiol. 2009;30 (2):290–296. doi: 10.3174/ajnr.A1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes RL, Borenstein NS, Desilva TM, Folkerth RD, Liu LG, Volpe JJ, Kinney HC. Axonal development in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2005;484 (2):156–167. doi: 10.1002/cne.20453. [DOI] [PubMed] [Google Scholar]
- Henderson SE, Sugden DA, Barnett AL. Movement Assessment Battery for Children -2 second edition (Movement ABC-2) The Psychological Corporation; London, UK: 2007. [Google Scholar]
- Holsti L, Grunau RV, Whitfield MF. Developmental coordination disorder in extremely low birth weight children at nine years. J Dev Behav Pediatr. 2002;23 (1):9–15. doi: 10.1097/00004703-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Hoon AH, Jr, Stashinko EE, Nagae LM, Lin DD, Keller J, Bastian A, Campbell ML, Levey E, Mori S, Johnston MV. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol. 2009;51 (9):697–704. doi: 10.1111/j.1469-8749.2009.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA, Volpe JJ. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res. 1998;44 (4):584–590. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Murphy B, Maier SE, Zientara GP, Inder TE, Barnes PD, Kikinis R, Jolesz FA, Volpe JJ. Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics. 2001;107 (3):455–460. doi: 10.1542/peds.107.3.455. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5 (2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kerr-Wilson CO, Mackay DF, Smith GC, Pell JP. Meta-analysis of the association between preterm delivery and intelligence. J Public Health (Oxf) 2012;34 (2):209–216. doi: 10.1093/pubmed/fdr024. [DOI] [PubMed] [Google Scholar]
- Kidokoro H, Neil J, Inder TE. A new MRI assessment tool to define brain abnormalities in very preterm infants at term. Am J Neuroradiol. 2013 doi: 10.3174/ajnr.A3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol. 1988;47 (3):217–234. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- Koziol LF, Budding DE, Chidekel D. From movement to thought: executive function, embodied cognition, and the cerebellum. Cerebellum. 2012;11 (2):505–525. doi: 10.1007/s12311-011-0321-y. [DOI] [PubMed] [Google Scholar]
- Krishnan ML, Dyet LE, Boardman JP, Kapellou O, Allsop JM, Cowan F, Edwards AD, Rutherford MA, Counsell SJ. Relationship between white matter apparent diffusion coefficients in preterm infants at term-equivalent age and developmental outcome at 2 years. Pediatrics. 2007;120 (3):e604–609. doi: 10.1542/peds.2006-3054. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4 (6):469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Luu TM, Ment L, Allan W, Schneider K, Vohr BR. Executive and memory function in adolescents born very preterm. Pediatrics. 2011;127 (3):e639–646. doi: 10.1542/peds.2010-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur B, Dezoete A. Early beginnings: Development in children born preterm. Oxford University Press; Auckland: 1992. [Google Scholar]
- Miller SP, Vigneron DB, Henry RG, Bohland MA, Ceppi-Cozzio C, Hoffman C, Newton N, Partridge JC, Ferriero DM, Barkovich AJ. Serial quantitative diffusion tensor MRI of the premature brain: development in newborns with and without injury. J Magn Reson Imaging. 2002;16 (6):621–632. doi: 10.1002/jmri.10205. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Miller JH, Shimony JS, Philip JV, Nehra D, Snyder AZ, Conturo TE, Neil JJ, McKinstry RC. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. Am J Neuroradiol. 2002;23 (9):1445–1456. [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Ashburner J, Andersson J, Jbabdi S, Draganski B, Skare S, Bohm B, Smedler AC, Forssberg H, Lagercrantz H. Structural correlates of preterm birth in the adolescent brain. Pediatrics. 2009;124 (5):e964–972. doi: 10.1542/peds.2008-3801. [DOI] [PubMed] [Google Scholar]
- Neil JJ, Shiran SI, McKinstry RC, Schefft GL, Snyder AZ, Almli CR, Akbudak E, Aronovitz JA, Miller JP, Lee BC, Conturo TE. Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology. 1998;209 (1):57–66. doi: 10.1148/radiology.209.1.9769812. [DOI] [PubMed] [Google Scholar]
- Partridge SC, Mukherjee P, Berman JI, Henry RG, Miller SP, Lu Y, Glenn OA, Ferriero DM, Barkovich AJ, Vigneron DB. Tractography-based quantitation of diffusion tensor imaging parameters in white matter tracts of preterm newborns. J Magn Reson Imaging. 2005;22 (4):467–474. doi: 10.1002/jmri.20410. [DOI] [PubMed] [Google Scholar]
- Partridge SC, Mukherjee P, Henry RG, Miller SP, Berman JI, Jin H, Lu Y, Glenn OA, Ferriero DM, Barkovich AJ, Vigneron DB. Diffusion tensor imaging: serial quantitation of white matter tract maturity in premature newborns. Neuroimage. 2004;22 (3):1302–1314. doi: 10.1016/j.neuroimage.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, Duncan CC, Makuch RW, Gore JC, Ment LR. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284 (15):1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13 (6 Pt 1):1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- Rose J, Mirmiran M, Butler EE, Lin CY, Barnes PD, Kermoian R, Stevenson DK. Neonatal microstructural development of the internal capsule on diffusion tensor imaging correlates with severity of gait and motor deficits. Dev Med Child Neurol. 2007;49 (10):745–750. doi: 10.1111/j.1469-8749.2007.00745.x. [DOI] [PubMed] [Google Scholar]
- Rose SE, Hatzigeorgiou X, Strudwick MW, Durbridge G, Davies PS, Colditz PB. Altered white matter diffusion anisotropy in normal and preterm infants at term-equivalent age. Magn Reson Med. 2008;60 (4):761–767. doi: 10.1002/mrm.21689. [DOI] [PubMed] [Google Scholar]
- Salmi J, Pallesen KJ, Neuvonen T, Brattico E, Korvenoja A, Salonen O, Carlson S. Cognitive and motor loops of the human cerebro-cerebellar system. J Cogn Neurosci. 2010;22 (11):2663–2676. doi: 10.1162/jocn.2009.21382. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Anderson PJ, Doyle LW, Dewey D, Grunau RE, Asztalos EV, Davis PG, Tin W, Moddemann D, Solimano A, Ohlsson A, Barrington KJ, Roberts RS Caffeine for Apnea of Prematurity Trial I. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA. 2012;307 (3):275–282. doi: 10.1001/jama.2011.2024. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W Caffeine for Apnea of Prematurity Trial G. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357 (19):1893–1902. doi: 10.1056/NEJMoa073679. [DOI] [PubMed] [Google Scholar]
- Skranes J, Lohaugen GC, Martinussen M, Indredavik MS, Dale AM, Haraldseth O, Vangberg TR, Brubakk AM. White matter abnormalities and executive function in children with very low birth weight. Neuroreport. 2009;20 (3):263–266. doi: 10.1097/wnr.0b013e32832027fe. [DOI] [PubMed] [Google Scholar]
- Skranes J, Vangberg TR, Kulseng S, Indredavik MS, Evensen KA, Martinussen M, Dale AM, Haraldseth O, Brubakk AM. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130 (Pt 3):654–666. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17 (3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20 (3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26 (1):132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Tam EWY, Rosenbluth G, Rogers EE, Ferriero DM, Glidden D, Goldstein RB, Glass HC, Piecuch RE, Barkovich AJ. Cerebellar Hemorrhage on Magnetic Resonance Imaging in Preterm Newborns Associated with Abnormal Neurologic Outcome. J Pediatr. 2011;158 (2):245–250. doi: 10.1016/j.jpeds.2010.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HG, Minich NM, Klein N, Hack M. Longitudinal outcomes of very low birth weight: Neuropsychological findings. J Int Neuropsychol Soc. 2004;10 (2):149–163. doi: 10.1017/S1355617704102038. [DOI] [PubMed] [Google Scholar]
- Thompson DK, Inder TE, Faggian N, Warfield SK, Anderson PJ, Doyle LW, Egan GF. Corpus callosum alterations in very preterm infants: perinatal correlates and 2 year neurodevelopmental outcomes. Neuroimage. 2012;59 (4):3571–3581. doi: 10.1016/j.neuroimage.2011.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DK, Warfield SK, Carlin JB, Pavlovic M, Wang HX, Bear M, Kean MJ, Doyle LW, Egan GF, Inder TE. Perinatal risk factors altering regional brain structure in the preterm infant. Brain. 2007;130 (Pt 3):667–677. doi: 10.1093/brain/awl277. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neurology of the newborn. 4. W.B. Sanders; London: 2000. [Google Scholar]
- Volpe JJ. Encephalopathy of prematurity includes neuronal abnormalities. Pediatrics. 2005;116 (1):221–225. doi: 10.1542/peds.2005-0191. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8 (1):110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield S. Fast k-NN classification for multichannel image data. Pattern Recognition Letters. 1996;17 (7):713–721. [Google Scholar]
- Warfield SK, Kaus M, Jolesz FA, Kilinis R. Adaptive, template moderated, spatially varying statistical classification. Medical Image Analysis. 2000;4 (1):43–55. doi: 10.1016/s1361-8415(00)00003-7. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) The Psychological Corporation; 1999. [Google Scholar]
- Williams J, Lee KJ, Anderson PJ. Prevalence of motor-skill impairment in preterm children who do not develop cerebral palsy: a systematic review. Dev Med Child Neurol. 2010;52 (3):232–237. doi: 10.1111/j.1469-8749.2009.03544.x. [DOI] [PubMed] [Google Scholar]
- Woodward LJ, Clark CAC, Pritchard VE, Anderson PJ, Inder TE. Neonatal White Matter Abnormalities Predict Global Executive Function Impairment in Children Born Very Preterm. Dev Neuropsychol. 2011;36 (1):22–41. doi: 10.1080/87565641.2011.540530. [DOI] [PubMed] [Google Scholar]
- Xue H, Srinivasan L, Jiang S, Rutherford M, Edwards AD, Rueckert D, Hajnal JV. Automatic segmentation and reconstruction of the cortex from neonatal MRI. Neuroimage. 2007;38 (3):461–477. doi: 10.1016/j.neuroimage.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Yung A, Poon G, Qiu DQ, Chu J, Lam B, Leung C, Goh W, Khong PL. White matter volume and anisotropy in preterm children: a pilot study of neurocognitive correlates. Pediatr Res. 2007;61 (6):732–736. doi: 10.1203/pdr.0b013e31805365db. [DOI] [PubMed] [Google Scholar]