Abstract
Multi-site electron paramagnetic resonance (EPR) oximetry, using multi-probe implantable resonators, was used to measure the partial pressure of oxygen (pO2) in the brains of rats following normobaric hyperoxia and mild hypoxia. The cerebral tissue pO2 was measured simultaneously in the cerebral cortex and striatum in the same rats before, during, and after normobaric hyperoxia and mild hypoxia challenges. The baseline mean tissue pO2 values (±SE) were not significantly different between the cortex and striatum. During 30 min of 100% O2 inhalation, a statistically significant increase in tissue pO2 of all four sites was observed, however, the tissue pO2 of the striatum area was significantly higher than in the forelimb area of the cortex. Brain pO2 significantly decreased from the baseline value during 15 min of 15% O2 challenge. No differences in the recovery of the cerebral cortex and striatum pO2 were observed when the rats were allowed to breathe 30% O2. It appears that EPR oximetry using implantable resonators can provide information on pO2 under the experimental conditions needed for such a study. The levels of pO2 that occurred in these experiments are readily resolvable by multi-site EPR oximetry with multi-probe resonators. In addition, the ability to simultaneously measure the pO2 in several areas of the brain provides important information that could potentially help differentiate the pO2 changes that can occur due to global or local mechanisms.
1 Introduction
The cerebral tissue is the critical site for pO2-dependent physiological and pathophysiological processes in the brain. Therefore, tissue pO2 is the pertinent parameter for following these processes. In the past, surrogates had to be used for these values, such as the pO2 available in the vascular system. Recently, more direct measurements have been made using oxygen electrodes. However, these methods have limitations due to the trauma associated with the introduction of the probe and are difficult to use for repeated pO2 measurements. Electron paramagnetic resonance (EPR) oximetry has the potential to provide repeated non-invasive measurements of tissue pO2 at specific sites. This methodology has been successfully used to study the effects of various anesthetics, a hemoglobin modifier, brain tumors and ischemia/reperfusion on brain oxygenation [1, 2]. With the recent development of multi-site capability using multi-probe implantable resonators, it is now possible to gain insight into tissue pO2 in specific areas of the brain with excellent sensitivity and precise localization [3].
This paper summarizes the results obtained using four multi-probe implantable resonators in the brains of rats subjected to normobaric hyperoxia and mild hypoxia. We have also investigated the response of different regions of the brain to these challenges.
2 Materials and Methods
2.1. Multi-probe implantable resonators
Oxygen sensitive lithium phthalocyanine (LiPc) crystals and implantable resonators were synthesized and fabricated in our laboratory. The properties of LiPc have been reported earlier [4]. The resonator has two sets of loops, a larger loop on one end and four small loops on the other end. The large loop is used to couple inductively to the L-band EPR spectrometer. The small loops contain LiPc aggregates which are coated with a highly gas permeable biocompatible Teflon AF 2400® (Aldrich, Steinheim, Germany) [3, 5]. The small loops are implanted in the sites of interest and the larger loop is placed on the skull below the skin for coupling with the external loop resonator of the EPR spectrometer. The high density of the unpaired spins, combined with a narrow intrinsic line width of embedded LiPc crystals, allows measurements of pO2 in the brain with an area of ~ 0.1–0.12 mm2. Implantable resonators were calibrated before implantation into the brain and the EPR spectra reflect the average pO2 on the surface of each probe. The response to oxygen of the probes (EPR line width as a function of pO2) remained unchanged after implantation in the rats’ brains, which allowed the monitoring of oxygen during several weeks [3].
2.2. Animal preparation
All animal procedures were conducted in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of Dartmouth Medical School.
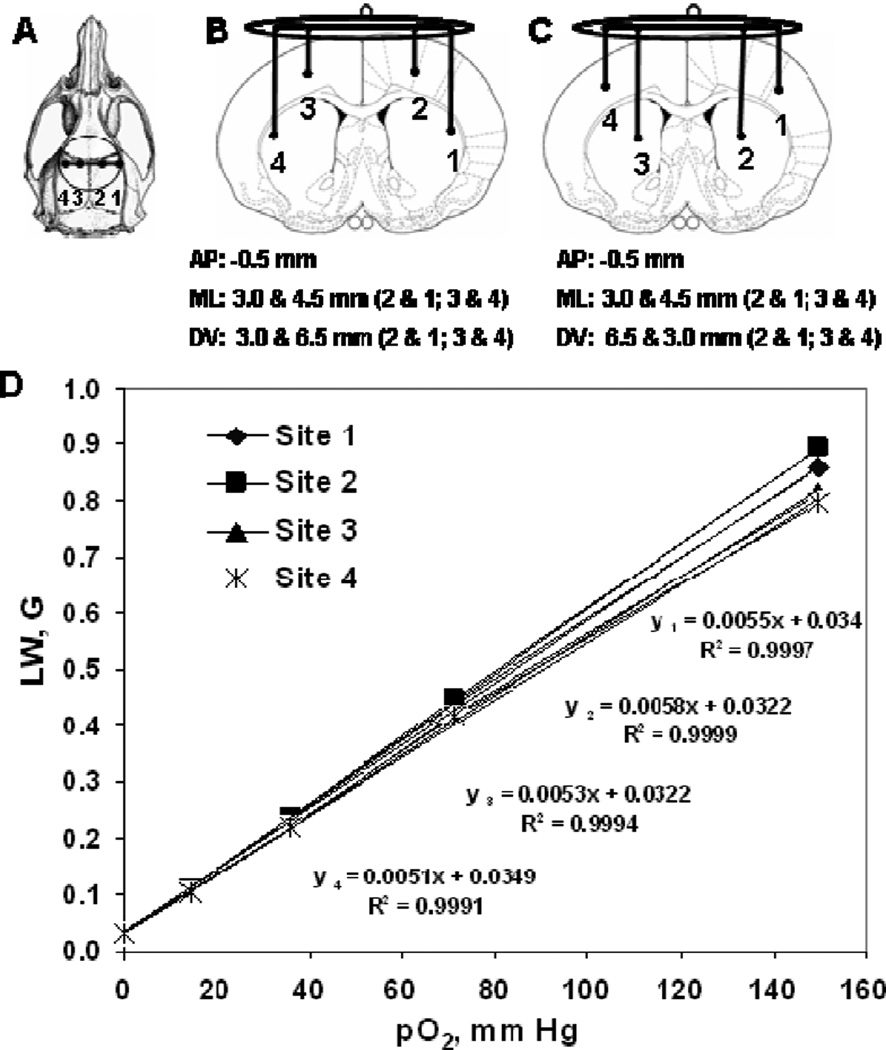
Eleven male Sprague-Dawley rats, 250–330 g (Charles River Laboratories, Wilmington, MA) were used. One week prior to the pO2 measurement, the rats were anesthetized with 2–2.5% isoflurane in 30% O2 through a nose cone and 4-probe implantable resonators were placed directly into the brain at a depth of 3.0 and 5.5 mm from the surface of the skull, through 1.0 mm drill holes located 3.0 and 4.5 mm from the midline (left and right) and 0.5 mm behind the bregma (Figure 1).
Fig. 1.
(A) Axial view of the rat skull with trephination positions of the implantable resonator; (B) Schematic of cross-section of a rat brain showing the implantable resonator in the forelimb area of the cortex (site 2 & 3) and striatum area (site 1 & 4); (C) Parietal cortex area (site 1 & 4) and striatum area (site 2 & 3); and (D) Typical calibration curves of a 4-probe implantable resonator. The four probes are indicated in the plot, showing the calibration curves for each probe.
The study has two groups of rats: (1) forelimb area of the cortex (site 2 & 3) and striatum (site 1 & 4) on each hemisphere, n=6; (2) the parietal cortex area (site 2 & 3) and striatum (site 1 & 4) on each hemisphere, n=5. The rats were anesthetized with 1.5% isoflurane and 30% oxygen and a baseline pO2 was measured for 30 min using multi-site EPR oximetry as described below. After the baseline pO2 measurements, animals were subjected sequentially to normobaric hyperoxia (FiO2 100% O2 for 30 min), normoxia (FiO2 30% O2 for 25 min), mild hypoxia (FiO2 15% O2 for 15 min) and normoxia (FiO2 30% O2 for 20 min). During EPR measurements, the temperature of the animals was monitored using a rectal probe and maintained at 37±0.5°C using a thermostatically-controlled heated pad and a flow of warm air.
2.3. Multi-site EPR Oximetry
EPR oximetry was performed with an L-band (1.2 GHz) EPR spectrometer with an external loop resonator specifically designed for in vivo experiments [6]. The rat was placed between the poles of the EPR magnet and the head positioned under the extended loop resonator and adjusted to obtain the maximum signal from the implanted probe. A set of coils capable of generating a magnetic field gradient in the direction of the magnetic field with a magnitude up to 3.0 G/cm was used to separate the EPR spectra from the probes of the implanted resonator [7]. Cerebral pO2 was determined by measuring the peak-to-peak line widths of the EPR spectra, which was then converted to pO2 by using appropriate calibration of the implantable resonator used in the study. The spectrometer parameters were: incident microwave power of 1–2 mW: modulation frequency 24 kHz, magnetic field 430 G, scan time 10 sec and modulation amplitude not exceeding one third of the peak-to-peak line width (typical 0.3–0.5 G).
2.4. Statistical Analysis
Data were analyzed by Student’s ”T” test. A paired “T” test was used to compare 30% O2 with the other interventions (100% O2 and 15% O2 inhalation) within the same group. Comparisons between the cerebral cortex and striatum in the same hemisphere at the same time point were made using Student’s “T” test for unpaired samples. The tests were two-sided, and a change with a p-value < 0.05 was considered statistically significant. All data are expressed as mean ± SE, n is the number of animals used in each group.
3 Results
Several features of these results are pertinent for the purpose of this report, which is to demonstrate the feasibility of these techniques. One important advantage of this new technique is the feasibility of carrying out oximetry at any depth. This considerably extends the application of EPR oximetry to almost any site where the resonator can be implanted. Secondly, the signal-to-noise ratios of the EPR spectra obtained with implantable resonators in the present study are significantly better than the spectra recorded from the LiPc crystals implanted directly at a similar, or even shallower depth, in the brain in previous studies [2, 8].
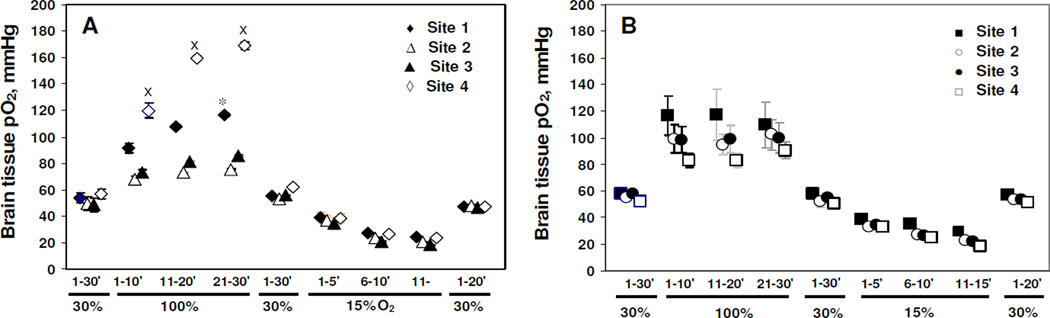
The average tissue pO2 values of the cerebral cortex and striatum are summarized in Figure 2. The baseline tissue pO2 values of the cerebral cortex and striatum are in the expected range, and were similar and stable during the baseline measurements. During the 30 min of 100% O2 inhalation, a statistically significant increase in the pO2 of the four sites was observed (p<0.05), but the responses of the cerebral cortex and striatum to this challenge were different. The tissue pO2 was significantly higher in the striatum area than in the forelimb area of the cortex (p<0.05). The tissue pO2 of the four sites decreased significantly from baseline during 15 min of mild hypoxia (p<0.05 and p<0.01). The tissue pO2 recovered in a similar manner in the cerebral cortex and striatum when the rats were allowed to breathe 30% O2.
Fig. 2.
The time course of mean brain tissue pO2 (mean ± SE) measured by multi-site EPR oximetry with a 4-probe implantable resonator in rats after normobaric hyperoxia (100% O2) and mild hypoxia (15% O2) challenges. A: Group1; n = 6; B: Group 2; n=5. *p<0.05 compared with pO2 in site 2; xp< 0.05 compared with pO2 in site 3 (unpaired Student’s t test).
4 Discussion
Multi-site EPR oximetry with multi-probe implantable resonators make it feasible to measure acute changes of the pO2 in several sites of the brain simultaneously and repeatedly over the entire experimental period. Furthermore, due to the immediate response of the LiPc embedded in the sensor probes to changes in pO2, this approach could potentially be used to measure the dynamics of pO2 changes over time [3]. Spatial resolution between sites in the range of 1.5 mm (site 1 and site 2; site 3 and site 4, Figure 1 B and C) can easily be achieved by applying minimal gradient power without significant distortion of the EPR signals. This is a particularly useful feature of multi-site EPR oximetry because the measurement from one region can be used as an internal control while the signals from other regions in the same or contralateral hemisphere could be monitored as indicators of the experimental procedures.
As summarized in Figure 2, the pO2 values at the four different sites in brain were stable over 30 min of baseline measurements and are similar to our recently reported values [3], but are higher than those reported earlier [2, 8]. This is likely due to the differences in breathing (ventilated vs. spontaneous procedure), level of FiO2, PaO2 and the concentration of anesthetics used in these various studies. A significant difference in pO2 between the forelimb area of the cortex and striatum during normobaric hyperoxia challenge in group 1, may reflect anatomical differences, possibly accentuated by the differences in response of blood flow in these two areas under 30% O2 and 100% O2 inhalation. Further studies are needed to assess the effect of normobaric hyperoxia on blood flow between the forelimb area of the cortex and striatum. There are some technical aspects and precautions necessary in using this approach. Due to some variation in the calibration observed at higher oxygen concentrations (Figure 1D), it is advised to calibrate each resonator before it is implanted, so that the EPR line width recorded from each probe (site) could be converted to pO2 by using its own calibration. In addition, it is essential that the multi-probe implantable resonators are implanted into tissue a few days in advance of the experiments to allow any tissue damage to heal that might occur due to the implantation of the probes.
In conclusion, the present results suggest that 1) normobaric hyperoxia and mild hypoxia breathing significantly enhances and decreases cerebral oxygenation, respectively, 2) the responses of the forelimb area of the cortex and striatum to normobaric hyperoxia are different, and 3) the ability to simultaneously measure the pO2 in several areas of the brain provides a unique possibility to investigate pO2 changes at different sites that might occur due to global or local mechanisms.
Acknowledgments
This work was supported by NIH grants PO1EB2180, and used the facilities of the EPR Center for Viable Systems (P41EB002032).
References
- 1.Hou H, Grinberg O, Williams B, Grinberg S, Yu H, Alvarenga DL, Wallach H, Buckey J, Swartz HM. Effect of oxygen therapy on brain damage and cerebral pO2 in transient focal cerebral ischemia in the rat. Physiol Meas. 2007;28:963–976. doi: 10.1088/0967-3334/28/8/017. [DOI] [PubMed] [Google Scholar]
- 2.Hou H, Khan N, O'Hara JA, et al. Increased oxygenation of intracranial tumors by efaproxyn (efaproxiral), an allosteric hemoglobin modifier: In vivo EPR oximetry study. Int J Radiat Oncol Biol Phys. 2005;61:1503–1509. doi: 10.1016/j.ijrobp.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Hou H, Sucheta A, et al. Implantable resonators - a technique for repeated measurements of oxygen at multiple deep sites with in vivo EPR. Adv Exp Med Biol. 2009 doi: 10.1007/978-1-4419-1241-1_38. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu K, Gast P, Moussavi M. Lithium phthalocyanine: A probe for electron paramagnetic resonance oximetry in viable biological systems. Proc Natl Acad Sci USA. 1993;90:5438–5442. doi: 10.1073/pnas.90.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu K, Gast P, Moussavi M. Lithium phthalocyanine: a probe for electron paramagnetic resonance oximetry in viable biological systems. Proc Natl Acad Sci USA. 1993;90:5438–5442. doi: 10.1073/pnas.90.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swartz HM, Clarkson RB. The measurement of oxygen in vivo using epr techniques. Physics in Medicine & Biology. 1998;43:1957–1975. doi: 10.1088/0031-9155/43/7/017. [DOI] [PubMed] [Google Scholar]
- 7.Smirnov AI, Norby SW, Clarkson RB, et al. Simultaneous multi-site epr spectroscopy in vivo. Magn Reson Med. 1993;30:213–220. doi: 10.1002/mrm.1910300210. [DOI] [PubMed] [Google Scholar]
- 8.Hou H, Grinberg OY, Grinberg SA, et al. Cerebral tissue oxygenation in reversible focal ischemia in rats: multi-site EPR oximetry measurements. Physiol Meas. 2005;26:131–141. doi: 10.1088/0967-3334/26/1/012. [DOI] [PubMed] [Google Scholar]