Abstract
Bisphenol A (BPA) is a man-made endocrine disrupting compound used to manufacture polycarbonate plastics. It is found in plastic bottles, canned food linings, thermal receipts and other commonly used items. Over 93% of people have detectable BPA levels in their urine. Epidemiological studies report correlations between BPA levels during pregnancy and activity, anxiety, and depression in children. We fed female mice control or BPA–containing diets that produced plasma BPA concentrations similar to concentrations in humans. Females were mated and at birth, pups were fostered to control dams to limit BPA exposure to gestation in the first generation. Sibling pairs were bred to the third generation with no further BPA exposure. First (F1) and third (F3) generation juveniles were tested for social recognition and in the open field. Adult F3 mice were tested for olfactory discrimination. In both generations, BPA exposed juvenile mice displayed higher levels of investigation than controls in a social recognition task. In F3 BPA exposed mice, dishabituation to a novel female was impaired. In the open field, no differences were noted in F1 mice, while in F3, BPA lineage mice were more active than controls. No impairments were detected in F3 mice, all were able to discriminate different male urine pools and urine from water. No sex differences were found in any task. These results demonstrate that BPA exposure during gestation has long lasting, transgenerational effects on social recognition and activity in mice. These findings show that BPA exposure has transgenerational actions on behavior and have implications for human neurodevelopmental behavioral disorders.
Keywords: endocrine disrupting chemicals, social recognition, transgenerational inheritance, Attention deficit hyperactivity disorder, Autism, epigenetics
Introduction
Humans encounter a variety of endocrine disrupting compounds (EDCs) on a daily basis (Bergman et al. 2013). Studies with rodents, primates, and other species demonstrate that exposure during development can increase disease risk as adults (Diamanti-Kandarakis et al. 2009; Flint et al. 2012; Kundakovic and Champagne 2011; Skinner et al. 2011; Wolstenholme et al. 2011a). Moreover, some EDCs have long lasting actions. Generations after a prenatal exposure, disease rates continue to be higher in EDC-exposed lineages than controls (Anway and Skinner 2006; Doyle et al. 2013; Jirtle and Skinner 2007; Manikkam et al. 2013; Salian et al. 2009). The World Health Organization (WHO) recently stated that wildlife, laboratory animal, and human studies show a strong likelihood that early life EDC exposure increases risk for reproductive dysfunction, cancers, obesity, diabetes, and behavioral disorders (Bergman et al. 2013). The present study was conducted to determine the long lasting and perhaps permanent effects of one such EDC, Bisphenol A, on juvenile behaviors using a mouse model and exposures within the range typically found in humans.
Bisphenol A (BPA) is a man-made EDC commonly used as a plasticizer. This compound causes heritable transgenerational reductions in sperm quality and changes in behavior and gene expression (Manikkam et al. 2013; Wolstenholme et al. 2012). High prenatal and/or childhood BPA concentrations in urine are correlated with various social and emotional behaviors in children such as increased activity, impulsiveness, aggression, and poor emotional control (Braun et al. 2011; Braun et al. 2009; Harley et al. 2013; Miodovnik et al. 2011). An earlier WHO report on safety of BPA also noted this potential problem (FAO/WHO 2010). In animals, early life exposure to low doses of BPA increases social behavior and social interactions, anxiety and aggression, and causes some cognitive impairments (Cox et al. 2010; Kawai et al. 2003; Kundakovic et al. 2013; Patisaul and Bateman 2008; Porrini et al. 2005; Wolstenholme et al. 2012; Wolstenholme et al. 2011b). BPA also affects maternal behavior (Cox et al. 2010; Della Seta et al. 2005; Kundakovic et al. 2013; Palanza et al. 2002), which can impact offspring behaviors in adulthood (Daxinger and Whitelaw 2012; Skinner et al. 2011). We recently reported that human-relevant BPA exposure during gestation changed behavior in pairs of interacting juvenile mice, and decreased vasopressin (Avp) and oxytocin (Oxt) transcripts in embryonic brain (Wolstenholme et al. 2012). Both neuropeptides are part of the neural circuitry that controls social recognition (Bielsky et al. 2005; Bielsky and Young 2004; Choleris et al. 2003; Lim and Young 2006; Shepard et al. 2009). Moreover, these effects were transgenerational, present in both first (F1) and fourth (F4) generation mice.
In the present study, we exposed mice to the same human-relevant dose of BPA (Wolstenholme et al. 2012) throughout gestation. We tested the F1 generation offspring as juveniles, which were directly exposed to BPA during gestation. To determine if the effects we observed were transgenerational, juvenile mice in the F3 generation, the first generation not directly exposed to BPA, were tested. Changes found in this generation are transmitted via the germline and are likely to be permanent (Anway and Skinner 2006; Jirtle and Skinner 2007). We tested all mice for social recognition, a task regulated by vasopressin and oxytocin. In this task, repeated exposure to the same animal typically produces habituation, a decline in social investigation. When normal mice are exposed to a novel individual; investigation is elevated; this is dishabituation. Changes in social recognition may have many underlying mechanisms. Here, we used the open field to assess the possible contributions of anxiety and locomotor activity in juvenile BPA and control mice. In addition, we examined olfactory discrimination abilities in F3 adults to determine if BPA could have long-term effects on olfactory function.
Methods and Materials
Animals
All procedures were conducted in compliance with the University of Virginia Animal Use and Care Committee. Mice were housed in a 12:12 light cycle (lights off at 1200 EST). All mice used in these studies were C57BL/6 originally purchased form the Jackson Laboratory. Adult female C57BL/6J mice from Jackson Laboratories (Bar Harbor, ME) were randomly assigned to either a phytoestrogen-free chow (n=74, Harlan Teklad TD95092) or the same chow supplemented with 5 mg BPA per kg diet (n=22, Harlan Teklad TD09386). Mice were switched to these diets 7–10 days before pairing with a male for two weeks. All females consumed their assigned diets and water ad libitum. At this BPA dose, we calculated BPA intake to be 20 µg per day (Wolstenholme et al. 2012). Free BPA levels in the plasma of pregnant dams consuming this dose of BPA averages 3.9 ng/ml (Wolstenholme et al. 2012), within the range (0.3 – 4.0 ng/ml) reported in pregnant women (Vandenberg et al. 2007).
Within 12 hours after birth, pups were placed with foster dams (on control diet) that had given birth within the past 24 hours. We did this to limit the offspring’s' BPA exposure to gestation, and because BPA may cause differences in maternal behavior which could effect offspring behavior (Cox et al. 2010; Kundakovic et al. 2013). Foster dams (n=48) retained two biological pups, not included in the study, and received 4 fostered pups from the same litter (control litters n=26, BPA litters n=22). To distinguish the biological from foster pups, we clipped tail tips of the biological pups. All pups (control: n=23 males, n=19 females; BPA: n=22 males, n=20 females) were weaned at postnatal day 21 (PN21) when they were placed on standard chow (Harlan Teklad diet #7912) containing phytoestrogens, group housed (3–5 per cage) by litter and sex, and tested for behaviors. F1 and F2 brother-sister pairs (F1, BPA n=9, control n=9, F2, BPA n=15 control n=15) were used to produce the transgenerational offspring. As adults, males were introduced into the cages of adult females for mating (~2–3 weeks) and removed prior to birth of the pups. All subsequent generations consumed standard mouse chow containing phytoestrogens (Harlan Teklad diet #7912). As adults, the F3 mice used for the olfaction test were briefly placed on control diet during breeding to create F4 offspring for another study. Adults consumed standard chow for at least two weeks prior to testing.
Behavior Tests
Each mouse was tested in only one behavior task. Only one mouse of each sex was tested in each litter to reduce potential litter effects. Social and odor recognition tasks were scored live between 1000 and 1200 hours. Open field activity was conducted in the dark between 1200 and 1800 hours and recorded under red light. Test boxes were cleaned with 10% ethanol and wiped dry between tests. All tests were scored by observers blind to the treatment conditions of the mice.
Juvenile social recognition
On PN21, juvenile mice were singly housed for 20 minutes in a standard mouse cage with bedding but no food or water. A small metal cylinder with a round top (10.16 cm diam. × 13.97 cm) and vertical bars (spaced 1 cm apart) was placed in the cage for 10 minutes. During the habituation phase, the same ovariectomized (OVX) C57BL/6J adult was repeatedly placed under the cylinder for eight 1-minute trials each separated by a 9-minute inter-trial interval. On the ninth trial, a novel OVX female was placed under the cylinder for 1 minute (Imwalle et al. 2002; Tejada and Rissman 2012). The time each juvenile (F1 control: n=15 males, 10 females; F1 BPA: n=14 males, 12 females, F3 control: n=9 males, 8 females; F3 BPA n=7 males, 8 females) spent investigating the cylinder and/or the stimulus female was recorded. Investigation is defined as contact with the head or body of the stimulus mouse at a distance less than 1 cm or directly touching the bars of the cylinder. Ovariectomized females were used as the stimulus mice to reduce possible variability caused by the estrus cycle.
Juvenile open field activity
Juvenile (PN23–24) mice were habituated to the behavioral testing room for one hour. The open field apparatus is a large white Plexiglas box (60 cm × 60 cm × 45 cm) divided evenly into a 25 (5 × 5) square grid. F1 and F3 mice from BPA (F1: control n=8 males, 8 females, F3: n=6 males, 7 females) and control (F1: n=8 males, 9 females, F3: n=6 males, 7 females) lineages were placed in one corner of the apparatus. Locomotor activity was recorded for 5 minutes by video and scored using Noldus Observer (Leesburg, VA). The box was divided into three zones, as described (Imwalle et al. 2002): corner, wall and center. Locomotor activity was scored as the number of grid lines crossed in each zone. The center zone consisted of the inner nine squares, the wall zone consisted of the outer twelve squares, and the corner zone consisted of the remaining four corner squares. Increased time spent in the center zone is interpreted as a low anxiety phenotype, while more time in the corners or next to the wall indicates higher anxiety.
Adult odor discrimination test
To assess olfactory abilities in mice from the BPA and control lineages, adult F3 males and females were tested in an odor habituation-dishabituation task (Yang and Crawley 2009). Urine was collected from two sets of adult male mice (n=4/group), pooled into two groups (pool A and B), aliquots were immediately frozen, and stored at −80°C. On the test day, F3 adult mice from the control (n=5 males and 6 females) and BPA lineages (n=6 males and 6 females) were habituated to a clean cage for 30 minutes then repeatedly exposed to volatile odors from water or urine. Ten µl of water or urine was dropped onto a piece of filter paper taped to a small plastic weigh boat and inverted onto an empty wire cage lid. Mice had three two-minute sessions with water with one-minute intervals between each presentation. This was followed by three presentations of one urine pool. In the third trial, the other urine pool was used. The order of presentation of the two urine pools was counter-balanced between groups; water was always presented first.
Statistical analysis
All data were analyzed using NCSS (Kaysville, Utah 2007). For social recognition and odor discrimination, we used two-way repeated measures ANOVA with diet and sex as main factors and trials as the repeated measure. For the social recognition test, data from trials 1–8 and trials 8–9 were subjected to separate two-way repeated measures ANOVAs with diet and sex as factors and trial as the repeated measure. To analyze open field behavior we used, two-way ANOVA with sex and diet as factors. Significant results were assessed by Fisher Exact post-hoc tests that adjust significance levels to account for multiple comparisons. Effect sizes were calculated with partial eta2 (ηp2). The partial eta2 is an estimate of the percent of the variance associated with each factor and its error. A large effect size is closer to 1, while a small effect size is closer to 0.
Results
BPA exposure increased social investigation and in F3 mice reduced responses to novel females
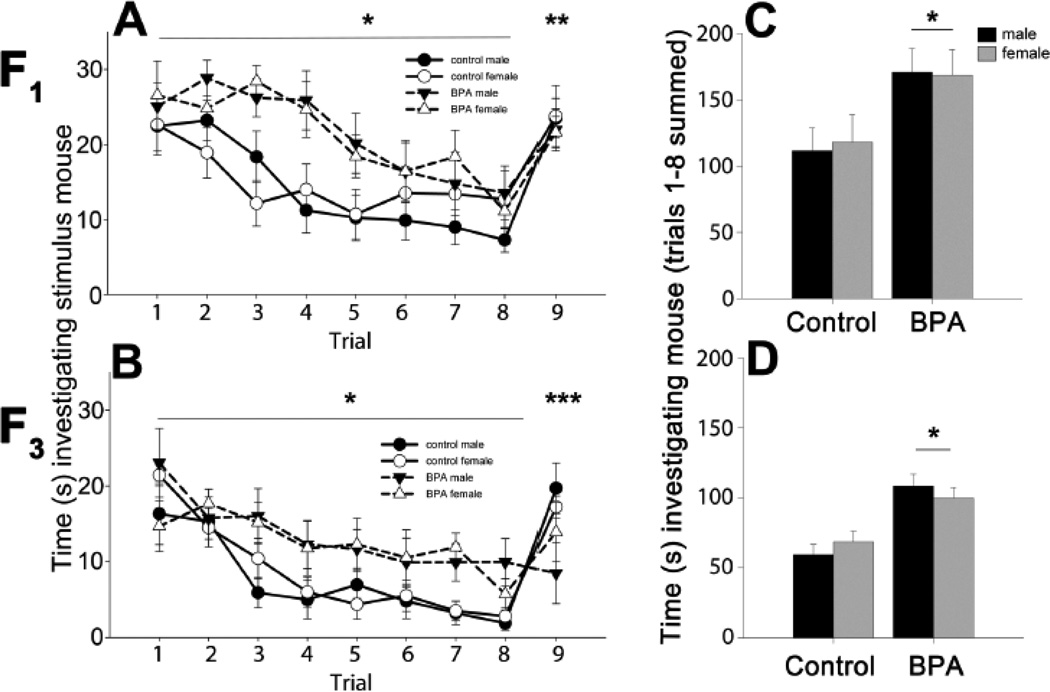
Juvenile mice prenatally exposed to BPA (F1 males and females) spent more time than control mice investigating and interacting with OVX stimulus mice during the first 8 trials (Figure 1). A main effect of diet was noted on trials 1–8 (F(1,47)=8.71, p<0.005, ηp2=0.16) where BPA mice investigated the stimulus female more than the controls investigated. A significant diet by trial interaction effect was present (F(1,7)=2.24, p<0.035, ηp2=0.05). Specifically, BPA exposed, as compared to control, mice spent more time investigating the stimulus mice on trials 2, 3, 4 and 5. When investigation times over trials 1–8 were summed, a main effect of diet (F(1,51)=8.71, p<0.005, ηp2=0.156) was found and BPA exposed mice spent more total time than controls investigating the stimulus mouse. Mice in all groups displayed increased investigation of a novel female on trial 9 (main effect of trial: F(1,47)=67.42, p<0.001, ηp2=0.59). There was no main effect of sex on investigation of the stimulus mouse during habituation (F(1,47)=0.01, ηp2=0.0002) or dishabituation (F(1,47)=0.08, ηp2=0.001).
Figure 1.
Mean +/− SEM time in seconds spent investigating an ovariectomized adult mouse during a social recognition task. In panels A and B, circles and solid lines represent control mice, black for male and white for female data. BPA animals' data are in triangles with dashed lines. As with controls, male symbols are black and female symbols are white. Panels A and B show time spent investigating on each trial, including the last, when a novel stimulus mouse was presented. A) Juvenile (PN22) male and female F1 mice were exposed to control (n=15 males and n=10 females) or BPA supplemented diet during gestation (n=14 males and n=12 females). B) F1 mice were mated to the third generation to create F3 control (n=9 males and n=8 females) and F3 BPA lineage mice (n=7 males and n=8 females). In panels C and D, male data are presented in black and female data are in gray histograms. These histograms show the sum of all time spent investigating the female during the habituation phase in the C) F1 and D) F3 generation juvenile mice. * Significant main effect of diet, p<0.05. ** Significant difference between trial 8 (familiar mouse) and 9 (novel mouse), p<0.05. ***Significant diet effect, only control mice had a significant increase in investigation between trials 8 and 9, p<0.05.
In the third generation, juvenile BPA lineage males and females as compared with control mice, interacted more with stimulus females during the first eight trials (main effect of diet, F(1,28)=26.87, p<0.001, ηp2= 0.49). Again, BPA exposed mice engaged in elevated investigation of the other mouse. A significant diet by trial interaction (F(1,196)=13.07, p<0.001, ηp2=0.31) was caused by differences on nearly every trial (2,3,4,5,7,and 8). When investigation on all trials (1–8) was summed, a main effect of diet was observed; F3 BPA lineage mice interacted more than controls with the familiar female (F(1,32)=14.45 p<0.001, ηp2=0.51). Males and females behaved similarly in the entire task (main effect of sex: F(1,28)=0.001, ηp2<0.001 on trials 1–8). In the final trial, when a novel female was presented, a significant diet by trial interaction was found (F(1,28)=13.19, p<0.001, ηp2=0.32). Only F3 control lineage mice displayed the expected increase in investigation directed toward the novel mouse in trial 9. No effects of diet (F(1,28)=0.19, ηp2=0.007) or sex (F(1,28)=0.001, ηp2<0.001 on trials 8–9) were detected in the dishabituation trial. The three-way interaction indicated a trend for a diet by sex by trial effect (F(1,28)=3.47, p=0.073, ηp2=0.11). Planned comparison tests revealed that the BPA lineage males were significantly different from all other groups (p<0.05) on trials 8 and 9.
In the open field BPA lineage mice displayed higher locomotor activity
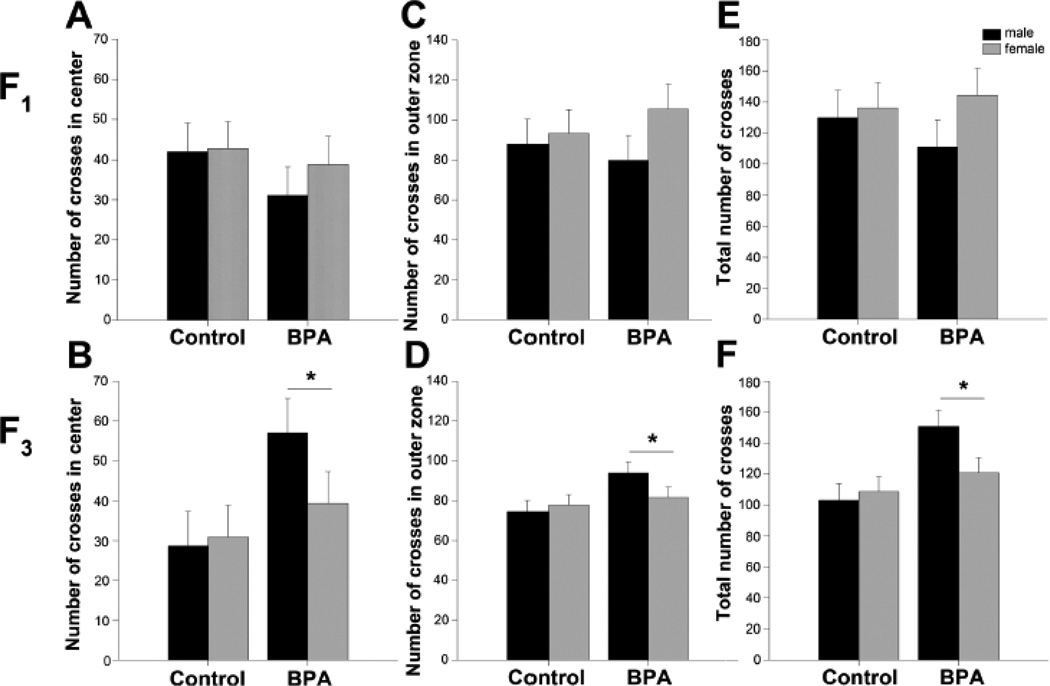
Because BPA exposed mice spent more time than control mice investigating a stimulus mouse during the social recognition task, we tested juvenile F1 and F3 mice for general activity in the open field. We hypothesized that the BPA-exposed mice would be more active than the control mice. In the F1 generation, no significant differences were found between any groups in any measures (Table 1). F1 control and BPA exposed mice did not differ in the time spent in the center (F(1,29)=0.40, ηp2=0.01), near walls (F(1,29)=0.84, ηp2=0.03), or corners (F(1,29)=0.22, ηp2=0.008), nor did they differ in the total number of line crosses (F(1,29)=0.10, ηp2=0.003), line crosses in the center (F(1,29)=1.14, ηp2=0.04) or outer regions (F(1,29)=0.03, ηp2=0.001) of the field. We performed a zone analysis of the open field data by dividing the number of line crosses within each zone (center and outer) by the total number of lines in each region. This is a measurement of the amount of locomotor activity within each zone and not simply the time spent in the zone. A three-way ANOVA with diet, sex and zone as factors revealed that all mice traveled more in the outer zone (by the wall) of the open field as compared to the center (main effect of zone: F(1,65)=99.45, p<0.001, ηp2=.63). Again, no differences in lines crossed within each zone were found between diet groups (F(1,65)=0.05, ηp2=0.0008) or sex (F(1,65)=1.93, ηp2=0.03).
Table 1.
Activity in the open field. Time spent in each section of the open field is presented as mean ± SEM in seconds. No significant differences were found in anxiety measures in this task.
| F1 control male |
F1 control female |
F1 BPA male |
F1 BPA female |
F3 control male |
F3 control female |
F3 BPA male |
F3 BPA female |
|
|---|---|---|---|---|---|---|---|---|
| time in center | 27.1 ± 4.6 | 20.7 ± 3.4 | 21.67 ± 4.1 | 20.7 ± 5.1 | 25.9 ± 7.2 | 27.3 ± 8.0 | 43.8 ± 8.6 | 29.2 ± 6.0 |
| time in corner | 144.4 ± 12.5 | 149.8 ± 7.1 | 137.4 ± 7.9 | 147.7 ± 10.9 | 151.3 ± 13.1 | 130.4 ± 8.8 | 124.7 ± 9.9 | 127.1 ± 7.4 |
| time on wall | 128.5 ± 9.5 | 129.5 ± 5.0 | 140.9 ± 7.0 | 131.7 ± 10.0 | 122.8 ± 12.6 | 142.3 ± 2.6 | 131.1 ± 7.2 | 142.9 ± 5.8 |
| time on outside | 272.9 ± 4.6 | 279.35 ± 3.4 | 278.3 ± 4.1 | 279.4 ± 5.1 | 274.1 ± 7.2 | 272.7 ± 8.0 | 255.8 ± 8.8 | 270.0 ± 6.2 |
In the F3 generation, juvenile BPA lineage mice were more active than controls in the open field. Crosses within the center of the arena (F(1,22)= 4.86, p<0.04, ηp2=0.18), crosses along the outer edge of the arena (F(1,22)=4.44, p<0.05, ηp2=0.17), and the total number of crosses (F(1,22)=8.56, p<0.008, ηp2=0.28) were significantly greater in F3 BPA mice than F3 controls (Figure 2). We did not observe any significant differences between diet groups or between sexes for any of the anxiety measures (Table 1). Control F3 mice spent a similar amount of time in the center (F(1,25)=1.74, ηp2=0.07), along the wall (F(1,25)=0.35, ηp2=0.02) and in the corners (F(1,25)=2.32, ηp2=0.09) of the arena as compared to mice from the BPA lineage. When activity was dissected into center and outer zones main effects of zone (F(1,51)=206.21, p<0.001, ηp2=0.82) and diet (F(1,51)=9.30, p<0.004, ηp2=0.17) were found, but not of sex (F(1,51)=1.93, ηp2=0.03). Again, mice from control and BPA lineages were more active in the outer regions (by the walls) than in the center of the open field. F3 BPA mice crossed more lines in all the zones than control mice. There were no main effects of sex, or interactions between the activity in each zone and diet or sex, indicating that the F3 BPA mice do not differ in anxiety measures, but are more active than control mice.
Figure 2.
Mean +/− SEM number of lines crossed in the open field. Data from males are presented in black histograms and females are in gray. A, C, E) F1 juvenile (PN23–24) control (n=8 males and n=9 females), BPA supplemented diet (n=8 males and n=8 females) mice. B, D, F) F3 juvenile control (n=6 males and n=7 females) and BPA lineage (n=6 males and n=7 females) mice. In panels A and B are numbers of lines crossed in the center zone. In C and D are numbers of lines crossed in the outer zone. Panels E and F show total numbers of line crossed in the entire apparatus. There were no effects of diet or sex in the F1 mice. *Significant difference between BPA and control lineages, p<0.05.
Adult F3 mice from both lineages displayed normal olfactory discrimination
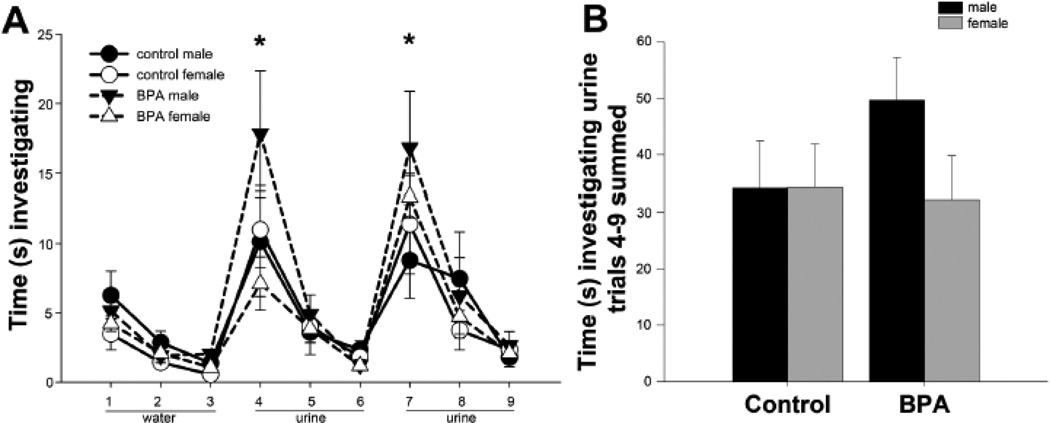
In the F3 BPA lineage, during the dishabituation portion of the social recognition test, investigation was significantly reduced. One possible reason for this could be an olfactory system impairment, and thus, a failure to differentiate between the familiar and novel individuals. To test this we used an odor habituation-dishabituation task. A two-way repeated measure ANOVA revealed no main effect of diet (F(1,206)=0.96, ηp2=0.03) or sex (F(1,206)=1.97, ηp2=0.10), but did reveal a significant effect of trial (Figure 3, F(1,206)=28.24, p<0.001, ηp2=0.31). The trial effect was due to increased time spent investigating male urine upon its first presentation (trials 4 and 7), which was significantly greater than previous and subsequent trials (p<0.05). The total time spent investigating the urine did not different between the groups (F(1,20)=0.75, ηp2=0.04).
Figure 3.
Mean +/− SEM of time spent investigating water and male urine in an odor discrimination task. In panel A, circles and solid lines represent control lineage mice, black for male and white for female data. BPA lineage data are in triangles with dashed lines. As with controls, male symbols are black and female symbols are white. A) Time in seconds spent investigating water (trials 1–3) and male urine from two different pools (trials 4–6 and 7–9). F3 adult mice: control (n=5 males and n=6 females) and BPA lineage mice (n=6 males and n=6 females) were tested. B) Total time investigating male urine summed from trials 4–9 in control and BPA lineage mice. In all groups we noted a significant difference between * trial (trials 4 and 7) as compared to all other trials, p<0.05.
Discussion
Here, we report that prenatal BPA exposure increased social investigation in male and female juvenile mice during a social recognition task. This effect was long lasting and persisted to at least the third generation offspring from BPA mice. Furthermore, F3 juveniles failed to recognize a novel stimulus, perhaps indicating a deficit in their social memory. These results present a small disconnect between investigating the familiar animal more, yet, failing to dishabituate when a novel stimulus animal was presented. Moreover, as adults, F3 mice from both control and BPA lineages discriminated between two pools of male urine, suggesting normal olfactory system function. Our interpretation of these data is that BPA F3 mice do not discriminate the familiar from the novel stimuli for reasons other than olfactory failure. Importantly, the social recognition task uses whole awake stimulus animals. Perhaps, visual, auditory, or other cues are not interpreted correctly by the BPA lineage mice, and despite differences in odor, they fail to recognize the novel mouse.
We did not find any effects of diet on anxiety, in agreement with our previous results using the elevated plus maze (Wolstenholme et al. 2012). Locomotor activity in the open field in F1 mice was equivalent between the groups. However, F3 mice in the BPA lineage displayed increased activity in the open field. These data are in line with the increased activity in the habituation portion of the social recognition task. These data confirm and extend our previous findings that social interactions are modified by gestational exposure to human-relevant levels of BPA (Wolstenholme et al. 2012). Specifically, F4 BPA lineage mice, raised under identical conditions, were more actively engaged with social peers than control mice (Wolstenholme et al. 2012). Here, in the social recognition task, BPA lineage mice again engaged in more social interaction than controls. Thus, the current data extend this finding in a second social task.
It is important to note that in some cases when BPA's effects persisted into the F3 and F4 generation, we found that the effects were more pronounced in these subsequent generations than in the first generation. For example, only F3 BPA mice failed to recognize a novel mouse in a social recognition task, and they were more active than controls in the open field. In our previous report, F4 mice from the BPA lineage were more interactive than controls were with peers, whereas in F1 mice, BPA diet had the reverse effect (Wolstenholme et al. 2012). At doses higher than most human's experience, the fungicide vinclozolin also produced transgenerational changes in mouse behavior, specifically in social preference, anxiety and stress responses (Crews et al. 2007; Skinner et al. 2008). These altered behaviors were coupled with disrupted genomic and epigenomic alterations in sperm and/or brain, suggesting potential targets through which these phenotypes can be passed through the generations. Our data augment these observations by demonstrating that even low doses of EDCs can have transgenerational effects on behavior.
There are many potential reasons why BPA exposure elevated social investigatory behavior. We tested two hypotheses in this set of studies. Hyperactivity could lead to more time investigating another animal. In other studies, low doses of BPA experienced during pre- and postnatal development did not have a consistent effect on locomotor activity in the open field task (Matsuda et al. 2012; Nakamura et al. 2012; Xu et al. 2012). However, spontaneous motor activity assessed over a 72-hour period in home cages, increased locomotor activity in mice exposed to low doses of BPA (Anderson et al. 2013). Our open field data concur with this finding, at least in the F3 generation. Another reason that BPA lineage mice might display both enhanced investigation and no dishabituation in the social recognition task is that BPA affects olfactory capabilities. However, adult F3 BPA and control mice were all equally able to discriminate male urine from water, and between different pools of male urine. Thus, the failure to habituate to familiar females or respond to novel females by F3 BPA mice was not likely due to an olfactory system deficit.
Social recognition is complex, controlled by estrogens, androgens, vasopressin, oxytocin, and their receptors (Bielsky et al. 2005; Bielsky and Young 2004; Choleris et al. 2003; Imwalle et al. 2002; Lim and Young 2006; Shepard et al. 2009; Tejada and Rissman 2012). Vasopressin receptor knock down decreased social recognition (Wacker and Ludwig 2012; Wersinger et al. 2002), while brain infusion of AVP increased social recognition and rescued these deficits in genetic knockouts. Oxytocin knockout mice failed to habituate during social recognition (Ferguson et al. 2000), but oxytocin receptor knockouts have only mild deficits. Oxytocin and vasopressin can bind to each other’s receptors, thus, oxytocin may be acting on vasopressin receptors, which would account for a greater phenotype in oxytocin knockouts as compared with oxytocin receptor knockouts. We hypothesis that vasopressin is the important neuropeptide for social memory since it is required for the retention and recall of social cues (Bielsky and Young 2004).
Estrogen receptor alpha (Esr1) regulates vasopressin and Esr1 knock out mice as compared to wild types, displayed elevated investigation of a familiar mouse (Imwalle et al. 2002). Brains of F1 embryos and mice exposed to BPA in utero, have gene-specific differences in neural estrogen receptor alpha (Esr1), estrogen receptor beta (Esr2), estrogen-related receptor gamma (Esrrg), and vasopressin mRNA expression as compared with controls (Cao et al. 2013; Kundakovic et al. 2013; Wolstenholme et al. 2012). In F4 male embryo brains from a BPA lineage (using the same dose used here), both Oxt and Avp mRNA were depressed as compared with controls, but mRNA levels for estrogen receptors were similar (Wolstenholme et al. 2012). Our present findings could be caused by this transgenerational decrease in Avp and Oxt expression since juveniles exposed to BPA during gestation and their F3 descendants have deficits in social memory indicated by increased time spent investigating a familiar mouse. Studies in rats (Carr et al. 2003) and other mouse strains have shown that BPA effects on social interactions or spatial memory deficits may be mediated through changes in expression and methylation of Esr1 (Kundakovic et al. 2013), Esr2 and/or NMDA signaling (Xu et al. 2010). However, these studies only assessed F1 offspring. Pilot studies done in our laboratory (Wolstenholme and Rissman, unpublished) found changes in methylation of the Esr1 promoter in F1 brains, but no differences in F3. Thus, changes in Esr1 are restricted to F1 and do not explain differences in Avp and Oxt in F3 brains.
Dose may change the direction of behavioral effects. Low dose prenatal BPA increased social interactions in juvenile female (Wolstenholme et al. 2011b) and in adult male mice (Ogi et al. 2013). In contrast, higher BPA exposure in rats and mice reduced juvenile play (Dessi-Fulgheri et al. 2002; Porrini et al. 2005) and reduced sex differences in juvenile interactions (Cox et al. 2010). A recent study using multiple BPA doses showed non-monotonic effects of BPA on social interactions such as huddling, sniffing and other play behaviors in mice (Kundakovic et al. 2013). These data highlight the importance of dose, especially with BPA and suggest non-monotonic actions of EDCs on behavior (Vandenberg et al. 2012). Our study adds to the evidence that early life exposure to EDCs alters juvenile social behavior.
Few studies have investigated BPA's effects on emotional behaviors other than anxiety or hyperactivity (Anderson et al. 2013; Gioiosa et al. 2013). Pre- and postnatal BPA exposure eliminated the normal sex differences in exploring a novel arena in juvenile and adult mice by specifically decreasing exploratory and anxiety behavior in females exposed to BPA (Gioiosa et al. 2013). Interestingly, postnatal BPA exposure appeared to have a stronger effect than prenatal exposure. In general, pre- or perinatal BPA exposure leads to hyperactivity during behavioral tasks (Ishido et al. 2011; Jones and Watson 2012; Kiguchi et al. 2008; Masuo et al. 2004). In these studies, BPA could be acting via an estrogen receptor to increase dopamine signaling (Jones and Miller 2008). In juvenile rats, perinatally exposed to dietary low dose BPA, males were more active than controls, and displayed corresponding changes in dopamine signaling (Zhou et al. 2011). Hallmarks of ADHD include hyperactivity, inattention and impulsiveness, many of which are regulated by dopamine (Ishido et al. 2005; Masuo et al. 2004). Here, we only assessed activity, although the increased investigation time during social recognition could indirectly suggest increased attention, impaired memory formation, impulsivity, and/or perseveration in BPA mice. Other tasks designed to directly test these possibilities are the focus of our future work.
BPA-induced hyperactivity could be related to increased risk for neurobehavioral disorders in humans. In fact, many of the social behavioral changes observed here, in mice directly and transgenerationally exposed to BPA, are similar to recent findings in humans, in which prenatal BPA levels were associated with hyperactivity and changes in social and emotional behaviors in children. Deficits in social memory and recognition are commonly found in disorders such as autism spectrum disorders, ADHD and learning disabilities (Bhaumik et al. 1997; Friedman et al. 2003; Reiersen et al. 2007). Maternal BPA during pregnancy was associated with hyperactivity, increased aggression and anxiety, poor inhibition and emotional control in young girls, but not boys (Braun et al. 2011; Braun et al. 2009; Harley et al. 2013). In school-aged children, gestational BPA exposure was linked with impaired social behaviors on an autism diagnosis subscale (Miodovnik et al. 2011). Based on these data, BPA exposure during critical developmental periods may contribute to the risk for social symptoms commonly associated with ADHD and/or autism spectrum disorders. All the human data reported to date on gestational BPA exposures are in F1, no work has been conducted yet on transgenerational actions of BPA in humans. Our data suggest that social phenotypes are caused by BPA and may be enhanced over generations in mice.
Conclusion
In sum, these data demonstrate that bisphenol A, a ubiquitous man-made environmental endocrine disruptor, has transgenerational actions on social recognition and locomotor activity. The behavior of mice three generations removed from BPA exposure is striking. These data suggest that even after BPA is eliminated from use, many behavioral changes may persist. Ancestral exposure to BPA and other EDCs may be contributing to the increased incidence of developmental neurobehavioral disorders in humans.
Highlights.
A human-relevant gestational exposure to BPA increases social investigation in juvenile mice.
Mice, 3 generations removed from BPA exposure, display elevated social investigation.
Mice, 3 generations away from BPA exposure, display enhanced activity in the open field.
In generation 3, mice from a BPA lineage but not controls, display deficits in social recognition.
Acknowledgment
This work is supported by NIH R01 MH057759 (EFR). JTW is supported by F32 ES019404.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
None of the authors have biomedical financial interests or potential conflicts of interest to report.
References
- Anderson OS, Peterson KE, Sanchez BN, Zhang Z, Mancuso P, Dolinoy DC. Perinatal bisphenol A exposure promotes hyperactivity, lean body composition, and hormonal responses across the murine life course. Faseb J. 2013;27(4):1784–1792. doi: 10.1096/fj.12-223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway MD, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology. 2006;147(6 Suppl):S43–S49. doi: 10.1210/en.2005-1058. [DOI] [PubMed] [Google Scholar]
- Bergman Å, Heindel J, Jobling S, Kidd K, Zoeller R. The State-of-the-Science of Endocrine Disrupting Chemicals – 2012. [Retrieved May 13, 2013];2013 from http://www.who.int/ceh/publications/endocrine/en/index.html.
- Bhaumik S, Branford D, McGrother C, Thorp C. Autistic traits in adults with learning disabilities. Br J Psychiatry. 1997;170:502–506. doi: 10.1192/bjp.170.6.502. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47(4):503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25(9):1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, Lanphear BP. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128(5):873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, Lanphear BP. Prenatal bisphenol A exposure and early childhood behavior. Environmental health perspectives. 2009;117(12):1945–1952. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Rebuli ME, Rogers J, Todd KL, Leyrer SM, Ferguson SA, Patisaul HB. Prenatal bisphenol a exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicol Sci. 2013;133(1):157–173. doi: 10.1093/toxsci/kft035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr R, Bertasi F, Betancourt A, Bowers S, Gandy BS, Ryan P, Willard S. Effect of neonatal rat bisphenol a exposure on performance in the Morris water maze. J Toxicol Environ Health A. 2003;66(21):2077–2088. doi: 10.1080/713853983. [DOI] [PubMed] [Google Scholar]
- Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proc Natl Acad Sci U S A. 2003;100(10):6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K, Gatewood J, Howeth C, Rissman E. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Horm Behav. 2010;58(5):754–761. doi: 10.1016/j.yhbeh.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, Anway MD, Skinner MK. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci U S A. 2007;104(14):5942–5946. doi: 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daxinger L, Whitelaw E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat Rev Genet. 2012;13(3):153–162. doi: 10.1038/nrg3188. [DOI] [PubMed] [Google Scholar]
- Della Seta D, Minder I, Dessi-Fulgheri F, Farabollini F. Bisphenol-A exposure during pregnancy and lactation affects maternal behavior in rats. Brain research bulletin. 2005;65(3):255–260. doi: 10.1016/j.brainresbull.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Dessi-Fulgheri F, Porrini S, Farabollini F. Effects of perinatal exposure to bisphenol A on play behavior of female and male juvenile rats. Environ Health Perspect. 2002;110(Suppl 3):403–407. doi: 10.1289/ehp.110-1241190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle TJ, Bowman JL, Windell VL, McLean DJ, Kim KH. Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol Reprod. 2013;88(5):112. doi: 10.1095/biolreprod.112.106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO Food and Agriculture Organization of the United Nations and the World Health Organization. Toxicological and Health Aspects of Bisphenol A. [Retrieved September 20, 2013];2010 from http://whqlibdoc.who.int/publications/2011/97892141564274_eng.pdf.
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25(3):284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Flint S, Markle T, Thompson S, Wallace E. Bisphenol A exposure, effects, and policy: a wildlife perspective. J Environ Manage. 2012;104:19–34. doi: 10.1016/j.jenvman.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Friedman SR, Rapport LJ, Lumley M, Tzelepis A, VanVoorhis A, Stettner L, Kakaati L. Aspects of social and emotional competence in adult attention-deficit/hyperactivity disorder. Neuropsychology. 2003;17(1):50–58. [PubMed] [Google Scholar]
- Gioiosa L, Parmigiani S, Vom Saal FS, Palanza P. The effects of bisphenol A on emotional behavior depend upon the timing of exposure, age and gender in mice. Hormones and behavior. 2013;63(4):598–605. doi: 10.1016/j.yhbeh.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Harley KG, Gunier RB, Kogut K, Johnson C, Bradman A, Calafat AM, Eskenazi B. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ Res. 2013 doi: 10.1016/j.envres.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imwalle DB, Scordalakes EM, Rissman EF. Estrogen receptor alpha influences socially motivated behaviors. Hormones and behavior. 2002;42(4):484–491. doi: 10.1006/hbeh.2002.1837. [DOI] [PubMed] [Google Scholar]
- Ishido M, Masuo Y, Terasaki M, Morita M. Rat hyperactivity by bisphenol A, but not by its derivatives, 3-hydroxybisphenol A or bisphenol A 3,4-quinone. Toxicol Lett. 2011;206(3):300–305. doi: 10.1016/j.toxlet.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Ishido M, Morita M, Oka S, Masuo Y. Alteration of gene expression of G protein-coupled receptors in endocrine disruptors-caused hyperactive rats. Regul Pept. 2005;126(1–2):145–153. doi: 10.1016/j.regpep.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8(4):253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BA, Watson NV. Perinatal BPA exposure demasculinizes males in measures of affect but has no effect on water maze learning in adulthood. Hormones and behavior. 2012;61(4):605–610. doi: 10.1016/j.yhbeh.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Jones DC, Miller GW. The effects of environmental neurotoxicants on the dopaminergic system: A possible role in drug addiction. Biochem Pharmacol. 2008;76(5):569–581. doi: 10.1016/j.bcp.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Kawai K, Nozaki T, Nishikata H, Aou S, Takii M, Kubo C. Aggressive behavior and serum testosterone concentration during the maturation process of male mice: the effects of fetal exposure to bisphenol. A Environ Health Perspect. 2003;111(2):175–178. doi: 10.1289/ehp.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiguchi M, Fujita S, Oki H, Shimizu N, Cools AR, Koshikawa N. Behavioural characterisation of rats exposed neonatally to bisphenol-A: responses to a novel environment and to methylphenidate challenge in a putative model of attention-deficit hyperactivity disorder. J Neural Transm. 2008;115(7):1079–1085. doi: 10.1007/s00702-008-0044-5. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Curras-Collazo MC. Neuroendocrine actions of organohalogens: thyroid hormones, arginine vasopressin, and neuroplasticity. Front Neuroendocrinol. 2010;31(4):479–496. doi: 10.1016/j.yfrne.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Champagne FA. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav Immun. 2011;25(6):1084–1093. doi: 10.1016/j.bbi.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, Champagne FA. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1214056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Hormones and behavior. 2006;50(4):506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One. 2013;8(1):e55387. doi: 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuo Y, Morita M, Oka S, Ishido M. Motor hyperactivity caused by a deficit in dopaminergic neurons and the effects of endocrine disruptors: a study inspired by the physiological roles of PACAP in the brain. Regul Pept. 2004;123(1–3):225–234. doi: 10.1016/j.regpep.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Matsuzawa D, Ishii D, Tomizawa H, Sutoh C, Nakazawa K, Amano K, Sajiki J, Shimizu E. Effects of perinatal exposure to low dose of bisphenol A on anxiety like behavior and dopamine metabolites in brain. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(2):273–279. doi: 10.1016/j.pnpbp.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, Silva MJ, Calafat AM, Wolff MS. Endocrine disruptors and childhood social impairment. Neurotoxicology. 2011;32(2):261–267. doi: 10.1016/j.neuro.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Itoh K, Dai H, Han L, Wang X, Kato S, Sugimoto T, Fushiki S. Prenatal and lactational exposure to low-doses of bisphenol A alters adult mice behavior. Brain Dev. 2012;34(1):57–63. doi: 10.1016/j.braindev.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Ogi H, Itoh K, Fushiki S. Social behavior is perturbed in mice after exposure to bisphenol A: a novel assessment employing an IntelliCage. Brain Behav. 2013;3(3):223–228. doi: 10.1002/brb3.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanza PL, Howdeshell KL, Parmigiani S, vom Saal FS. Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environmental health perspectives. 2002;110(Suppl 3):415–422. doi: 10.1289/ehp.02110s3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Bateman HL. Neonatal exposure to endocrine active compounds or an ERbeta agonist increases adult anxiety and aggression in gonadally intact male rats. Hormones and behavior. 2008;53(4):580–588. doi: 10.1016/j.yhbeh.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Porrini S, Belloni V, Della Seta D, Farabollini F, Giannelli G, Dessi-Fulgheri F. Early exposure to a low dose of bisphenol A affects socio-sexual behavior of juvenile female rats. Brain Res Bull. 2005;65(3):261–266. doi: 10.1016/j.brainresbull.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Volk HE, Todd RD. Autistic traits in a population-based ADHD twin sample. J Child Psychol Psychiatry. 2007;48(5):464–472. doi: 10.1111/j.1469-7610.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- Salian S, Doshi T, Vanage G. Impairment in protein expression profile of testicular steroid receptor coregulators in male rat offspring perinatally exposed to Bisphenol. A Life Sci. 2009;85(1–2):11–18. doi: 10.1016/j.lfs.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Shepard KN, Michopoulos V, Toufexis DJ, Wilson ME. Genetic, epigenetic and environmental impact on sex differences in social behavior. Physiol Behav. 2009;97(2):157–170. doi: 10.1016/j.physbeh.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS One. 2008;3(11):e3745. doi: 10.1371/journal.pone.0003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of endocrine disruptors. Reprod Toxicol. 2011;31(3):337–343. doi: 10.1016/j.reprotox.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejada LD, Rissman EF. Sex differences in social investigation: effects of androgen receptors, hormones and test partner. J Neuroendocrinol. 2012;24(8):1144–1153. doi: 10.1111/j.1365-2826.2012.02322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, Shioda T, Soto AM, Vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocrine reviews. 2012 doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24(2):139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Calamandrei G, Ricceri L. A social recognition test for female mice reveals behavioral effects of developmental chlorpyrifos exposure. Neurotoxicol Teratol. 2006;28(4):466–471. doi: 10.1016/j.ntt.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Ricceri L, Tait S, Calamandrei G. Sex dimorphic behaviors as markers of neuroendocrine disruption by environmental chemicals: the case of chlorpyrifos. Neurotoxicology. 2012;33(6):1420–1426. doi: 10.1016/j.neuro.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Wacker DW, Ludwig M. Vasopressin, oxytocin, and social odor recognition. Hormones and behavior. 2012;61(3):259–265. doi: 10.1016/j.yhbeh.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Ginns EI, O'Carroll AM, Lolait SJ, Young WS., 3rd Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7(9):975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Wolstenholme JT, Edwards M, Shetty SR, Gatewood JD, Taylor JA, Rissman EF, Connelly JJ. Gestational exposure to bisphenol a produces transgenerational changes in behaviors and gene expression. Endocrinology. 2012;153(8):3828–3838. doi: 10.1210/en.2012-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Rissman EF, Connelly JJ. The role of Bisphenol A in shaping the brain, epigenome and behavior. Hormones and behavior. 2011a;59(3):296–305. doi: 10.1016/j.yhbeh.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Taylor JA, Shetty SR, Edwards M, Connelly JJ, Rissman EF. Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. PLoS One. 2011b;6(9):e25448. doi: 10.1371/journal.pone.0025448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Hong X, Xie L, Li T, Yang Y, Zhang Q, Zhang G, Liu X. Gestational and lactational exposure to bisphenol-A affects anxiety- and depression-like behaviors in mice. Hormones and behavior. 2012;62(4):480–490. doi: 10.1016/j.yhbeh.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Xu XH, Zhang J, Wang YM, Ye YP, Luo QQ. Perinatal exposure to bisphenol-A impairs learning-memory by concomitant down-regulation of N- methyl-D-aspartate receptors of hippocampus in male offspring mice. Hormones and behavior. 2010;58(2):326–333. doi: 10.1016/j.yhbeh.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci. 2009;Chapter 8(Unit 8):24. doi: 10.1002/0471142301.ns0824s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Bai Y, Yang R, Zhu Y, Chi X, Li L, Chen L, Sokabe M. Abnormal synaptic plasticity in basolateral amygdala may account for hyperactivity and attention-deficit in male rat exposed perinatally to low-dose bisphenol-A. Neuropharmacology. 2011;60(5):789–798. doi: 10.1016/j.neuropharm.2011.01.031. [DOI] [PubMed] [Google Scholar]