Abstract
Reproductive success is maximized when female sexual motivation and behavior coincide with the time of optimal fertility. Both processes depend upon coordinated hormonal events, beginning with signaling by the gonadotropin-releasing hormone (GnRH) neuronal system. Two neuropeptidergic systems that lie upstream of GnRH, gonadotropin-inhibitory hormone (GnIH; also known as RFamide related peptide-3) and kisspeptin, are potent inhibitory and excitatory modulators of GnRH, respectively, participate in the timing of the preovulatory luteinizing hormone (LH) surge and ovulation. Whether these neuropeptides serve as neuromodulators to coordinate female sexual behavior with the limited window of fertility has not been thoroughly explored. In the present study, either intact or ovariectomized, hormonetreated female hamsters were implanted for fifteen days with chronic release osmotic pumps filled with GnIH or saline. The effect of GnIH on sexual motivation, vaginal scent marking, and lordosis was examined. Following mating, FOS activation was quantified in brain regions implicated in the regulation of female sexual behavior. Intracerebroventricular administration of GnIH reduced sexual motivation and vaginal scent marking, but not lordosis behavior. GnIH administration altered FOS expression in key neural loci implicated in female reproductive behavior, including the medial preoptic area, medial amygdala and bed nucleus of the stria terminalis, independent of changes in circulating gonadal steroids and kisspeptin cell activation. Together, these data point to GnIH as an important modulator of female proceptive sexual behavior and motivation, independent of downstream alterations in sex steroid production.
Keywords: RFamide related peptide, (RFRP), RFamide neuropeptides, proceptivity, receptivity
Introduction
In order to maximize reproductive success, female sexual motivation and behavior must coordinate with the time of optimal fertility (Nequin et al., 1975; Sarkar et al., 1976). This orchestration is particularly critical in rodents, in which peak fertility lasts for only hours per cycle (Huck et al., 1986). Individuals employ the same neuroendocrine mediators that induce ovulation to regulate female sexual motivation and behavior (Blaustein, 2009). Whereas the neuroendocrine control of female copulatory behavior (e.g., the lordosis reflex) has been well studied, the neural substrates and neurochemical systems regulating female sexual motivation are less well specified. The present studies were designed to further understanding of the neural control of female sexual motivation and performance.
Sex steroid concentrations vary across the ovulatory cycle and are controlled by a neuroendocrine cascade beginning in the hypothalamus with the secretion of gonadotropin-releasing hormone (GnRH) into the hypophyseal portal system. GnRH stimulates the release of the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone, from the anterior pituitary; in turn, LH and follicle-stimulating hormone induce ovarian steroidogenesis and follicle maturation, respectively. For most of the ovulatory cycle, sex steroids feed back to the brain to inhibit GnRH and the gonadotropins secretion (i.e., negative feedback). In contrast, just prior to ovulation, a sustained increase in the concentration of circulating estradiol paired with a signal from the circadian pacemaker initiates the LH surge on the day of proestrus (i.e., positive feedback) (Legan and Karsch, 1975; de la Iglesia and Schwartz, 2006; Kriegsfeld and Silver, 2006; Christian and Moenter, 2010; Mahoney et al., 2004; Chappell, 2005). Following the LH surge, high concentrations of progesterone act in the ventromedial nucleus of the hypothalamus (VMH) to stimulate female sexual receptivity (review in Blaustein and Erskine, 2002). The coincidence of estradiol and circadian signaling ensures the temporal coordination of ovulation with the appropriate time of day for mating. In hamsters, we have implicated two opposing RFamide neuropeptides, gonadotropin-inhibitory hormone (GnIH; also known as RFamiderelated peptide-3 in mammals) and kisspeptin, as key participants in the circadian regulation of the LH surge (Gibson et al., 2008; Williams et al., 2011).
GnIH, first discovered in 2000 in Japanese quail (Tsutsui et al., 2000), is a potent inhibitor of pituitary gonadotropin secretion in birds (for reviews, see Tsutsui, 2009; Tsutsui et al., 2010) and mammals (Ukena et al., 2002; Kriegsfeld et al., 2006; Ubuka et al., 2006; 2009a; 2009b; 2012). Across avian and mammalian species, administration of GnIH suppresses LH secretion (Tsutsui et al., 2000; Osugi et al., 2004; Bentley et al., 2006; Kriegsfeld et al., 2006; Ubuka et al., 2006; Murakami et al., 2008; Sari et al., 2009; Ubuka et al., 2012). In mammals, GnIH acts, at least in part, directly on the GnRH system. GnIH fibers are found in close apposition to GnRH neurons (Kriegsfeld et al., 2006; Johnson et al., 2007; Ubuka et al., 2009a; 2009b; 2012) and a subset of GnRH neurons express the GnIH receptor, GPR147 (Ubuka et al., 2012), and decrease firing rates after direct application of GnIH (Ducret et al., 2009). Additionally, GnIH receptors are observed in the mammalian pituitary (Kriegsfeld et al., 2006; Clarke et al., 2008; Ubuka et al., 2009a; 2009b), suggesting further regulation at this locus. In contrast to GnIH, kisspeptin, is a robust stimulator of GnRH secretion (de Roux et al. 2003, Seminara et al. 2003; Gottsch et al., 2004). As with GnRH neurons, GPR147 is expressed in a subset of kisspeptin neurons (Rizwan, 2012) suggesting that GnIH may inhibit the reproductive axis by acting directly on kisspeptin cells, GnRH cells, or both.
Our previous findings (Gibson et al., 2008) indicate that, at least in part, the timing of ovulation is controlled by the coordinated removal of GnIH inhibition of the GnRH system at the time of the LH surge. The present experiments evaluated the possibility that, in addition to timing ovulation, removal of GnIH inhibition is required for appropriate expression of female sexual motivation and behavior. Support for this hypothesis is seen in birds and rodents. In female white-crowned sparrows, for example, administration of GnIH reduces solicitation behavior (Bentley et al., 2006) and acute GnIH treatment reduces mounts, intromissions and ejaculations in male rats (Johnson et al., 2007). Additionally, food restriction increases GnIH cellular activity and several aspects of female sexual behavior are negatively correlated with GnIH cellular activity in Syrian hamsters (Klingerman et al., 2011).
In the present study we sought to determine if, in addition to decreasing sexual function, GnIH negatively impacts sexual behaviors in female hamsters. To answer this question, the availability of GnIH was chronically increased to determine if improperly timed increases of GnIH disrupt sexual motivation, precopulatory and copulatory behaviors. Because GnIH could affect sexual motivation either through downstream alterations in gonadal steroid secretion or through direct neuromodulation in the central nervous system, we controlled circulating gonadal steroid concentrations to select among these possibilities. Additionally, we used FOS as a marker of neuronal activity to determine putative neural loci, including kisspeptin cells, at which GnIH might act to affect distinct components of female sexuality.
Materials and methods
Animals
Adult (>10 wks of age; n=34) female Syrian hamsters (Mesocricetus auratus; LVG (SYR) obtained from Charles River (Wilmington, MA) were maintained on a 14:10 light:dark (LD) cycle (14 h light/day, lights off at 2000 h Pacific Standard Time (PST)). Tap water and Lab Diet Prolab 5P00 were available ad libitum. Hamsters were singly housed at 23 ± 1 °C in polypropylene cages (48 × 25 × 21 cm) furnished with Tek-Fresh Lab Animal Bedding (Harlan Teklab, Madison, WI). All procedures were approved by the Animal Care and Use Committee of the University of California at Berkeley and conformed to principles enunciated in the NIH guide for the use and care for laboratory animals.
Experimental procedures and design
Upon arrival hamsters were given 2 weeks to acclimate to local conditions during which daily estrus checks were performed. All hamsters displayed 3 consecutive, 4-day estrous cycles prior to the experiment. Estrous cycling was verified through daily, gentle vaginal palpation to detect the sticky vaginal discharge that occurs on the day after vaginal proestrus (Orsini, 1961). Upon verification of regular estrous cycles, half of the animals (n=17) were ovariectomized (OVX) and the remainder left intact (n=17). Intact hamsters maintained normal cyclicity for the duration of the experiment. A week after surgery, a cannula aimed at the lateral ventricle was implanted in all hamsters as described below. Cannula placement was verified by injections of angiotensin II (10 ng in 5μ1 sterile saline) into the lateral ventricle as previously described (Williams et al., 2011). Hamsters with cannulas effectively penetrating the ventricular system display drinking behavior within minutes of injection. Between four and eight days after angiotensin II testing, corresponding to day one of vaginal estrus, hamsters received subcutaneous (s.c.) implants of osmotic pumps in the scapular region (ALZET, Cupertino, CA; model 2002) filled with 200 μ1, connected to brain cannulae to provide 0.5 μ1/h, of constant infusion of either vehicle (0.9% saline) or GnIH [(Rat RFamide-related peptide; ANMEAGTMSHFPSLPQRF; Ukena et al., 2002) 50 ng/μl resulting in 600 ng/day] into the ventricular system for the duration of the experiment. This dose was based on previously published (Kriegsfeld et al., 2006) and unpublished work in our lab to identify a chronic dose of GnIH that is below the threshold that significantly alters peri-ovulatory LH concentrations. Whereas the dose of GnIH selected markedly inhibits peri-ovulatory LH concentrations when injected as a single bolus, when this same dose is spread across the day via osmotic mini pump, LH concentrations are not significantly impacted. Because female sexual behavior is regulated by ovarian hormones, we required a dose that was below the threshold that grossly disrupts the LH surge and estrous cyclicity. Pumps were monitored for patency daily. Hamsters were excluded from all following behavior tests if a pump became non-patent. All OVX females were injected s.c. with estradiol benzoate (5 μg in peanut oil) followed 44 h later with progesterone (500 μg in peanut oil), every 4 days. All injections were dorsal and equidistant between the osmotic pump implanted at the scapula and the flanks of the females. In total, four separate groups were studied: OVX + saline (n=8), OVX+ GnIH (n=9), intact + saline (n=8), intact + GnIH (n=9). Groups did not vary in body mass prior to differential treatment (p>0.05 in all cases).
Behavioral tests commenced on days 3, 6, and 11 post-implant for partner preference, vaginal scent marking, and the expression of lordosis, to test sexual motivation, proceptive, and receptive behaviors, respectively (see Fig. 1 for experimental design and time course). Behavioral tests occurred within two hours before light offset on the day of vaginal proestrus (during sexual receptivity), except for vaginal scent marking tests, which occurred during diestrus II (the day before vaginal proestrus) when the behavior is most strongly expressed (Johnston, 1977). The LH surge, and ovulation, occurs 3-4 hours prior to lights out in hamsters (Gibson et al., 2008) and thus, testing females for sexual behavior within 2 hours of lights off captures periovulatory behavior, when sexual behaviors are expressed strongly.
Fig 1.
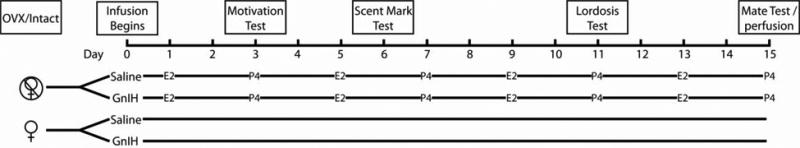
Schematic of the experimental design. All hamsters were cannulated, and half were ovariectomized (OVX) while the other half remained intact. Half of the animals in each of these groups were then implanted with osmotic pumps that infused either saline, or GnIH, into the lateral ventricle for the duration of the experiment. OVX females were then injected with estradiol (E2) and progesterone (P4) on a four day schedule that maintains the expression of reproductive behaviors. Females were tested for sexual motivation, vaginal scent marking, and lordosis. Finally, on day 15, females received 5 intromissions in a mating test, and were perfused 60 minutes later.
To explore potential neural loci on which GnIH acts to impact female copulatory behavior, brains were collected to examine neural activation after copulation. After 15 days of pump implantation, an intact male was placed into the home cage of each female. Upon receipt of 5 intromissions, males were removed and females were left undisturbed in their cage for 60 min before perfusion and brain collection. Just prior to perfusion, females were anesthetized with isoflurane vapors (Baxter Healthcare, Deerfield, IL) and blood was drawn from the retroorbital sinus. Immediately following, hamsters were administered an overdose of pentobarbital (200 mg/kg) intraperitoneally (i.p.) and perfused as described below. Their brains and ovaries were excised and osmotic pumps extracted and inspected for patency. In situ infusion rates of pump delivery were verified as per the manufacturer's instructions (Alzet, Cupertino, CA).
Behavior test procedures
All behavior tests occurred within 3 hours before light offset (1700-2000 PST) and were video-taped and later scored by an observer uninformed about the hamster's treatment. To examine female sexual motivation, partner preference tests were performed. Female hamsters were placed in a clear box (30×25×18 cm) connected on either side to larger, white chambers (60×45×40 cm) in which either a castrated or intact male was housed behind a wire mesh enclosure (34×16.5×10 cm) at the opposite corner of the chamber. The females had visual, chemosensory and auditory contact with the male, but no physical contact. Males were randomly assigned to chambers. Tests lasted 15 minutes and the time spent in each chamber and time spent within the sexual incentive zone (i.e., with both forepaws within 10 cm of the male's enclosure) were recorded.
To assess effects of treatment on female proceptive sexual behavior outside of the period of fertility, vaginal scent marking tests were conducted in a clear Plexiglas box (41×21×21 cm) set above a slanted mirror to facilitate observation of scent marking behavior. Intact male hamsters were housed in the chambers for 2 hours and removed immediately prior to introduction of the female. The number of vaginal scent marks was recorded for 5 minutes. Vaginal marks were scored when a female dragged her vaginal opening against the horizontal substrate with a stereotypical concave curvature of the spine (Takahashi and Lisk, 1983).
Consummatory aspects of female sexual behavior were assessed in 10 min lordosis tests conducted in Plexiglas boxes (41×21×21 cm) set above a slanted mirror. An intact male was housed behind a wire cage-lid in one end of the box that permitted chemosensory, auditory and visual contact but no direct physical contact. To induce lordosis the experimenter provided constant light stimulation of the flanks with two eyelid brushes taped together to stimulate both flanks simultaneously. The latency of lordosis onset and total amount of time spent in lordosis were recorded.
Surgical procedures
Ovariectomies were performed by anesthetizing hamsters with isoflurane vapors (Baxter Healthcare, Deerfield, IL) and excising ovaries through a midline incision in the abdominal cavity. Incisions were closed with sterile sutures and wound clips (Mikron Auto Clip 9mm, Becton Dickinson, Franklin Lakes, NJ). Hamsters were injected s.c. with analgesic (5% buprenorphine (0.2 ml/animal); Hospira Inc., Lake Forest, IL), postoperatively.
Cannulations were performed under deep anesthesia induced by 0.3 ml i.p. ketamine cocktail (21 mg ketamine, 2.4 mg xylazine, and 0.3 mg acepromazine per ml). The head was shaved, prepared for aseptic surgery, and positioned in a stereotaxic device (David Kopf Instruments, Tujanga, CA). Cannulas were aimed at the following coordinates relative to Bregma: 1.1 mm anterior, 1.3 mm lateral, and 3.0 mm ventral from dura mater. Security of cannulas was maintained by three skull screws and dental cement.
Osmotic mini-pumps were implanted as per the manufacturers instructions (ALZET, Cupertino, CA); the hamsters were anesthetized with isoflurane vapors and pumps were implanted s.c. in the scapular region through a small incision at the nape of the neck. Vinyl tubing connected the pumps with the cannulas and was primed overnight at 37 °C so that solution was delivered to the brain immediately upon implantation.
Perfusion and immunohistochemistry
Prior to perfusion, a blood sample was obtained from the retro-orbital sinus from intact females 1 hour after experience with a sexually active male. To collect brain tissue, hamsters were deeply anesthetized with sodium pentobarbital solution (200 mg/kg) and perfused transcardially with 150 ml 0.9% saline followed by 300 ml 4% paraformaldehyde in 0.1 M PBS (PH 7.4). Brains were postfixed for 3 h in 4% paraformaldehyde followed by cryoprotection in 30% sucrose in 0.1 M PBS overnight. Brains were then frozen at -80 °C until processed. 40 μm coronal brain sections were collected on a cryostat at -20 °C. Slices were stored at -20 °C in an ethylene glycol and sucrose based antifreeze until immunohistochemistry was carried out.
To visualize FOS and the colocalization of kisspeptin/FOS, every fourth 40 μm brain slice was washed in PBS, followed by 0.5% hydrogen peroxide. Brain sections were then transferred to PBS and incubated for 1 h in normal donkey serum suspended in 0.1% Triton X-100 (PBT). Sections were incubated for 48 h at 4 °C in a rabbit polyclonal anti-kisspeptin-10 antiserum (1:2000; validated for specificity in Syrian hamsters in Williams et al., 2011). After incubation in the primary antibody, brain sections were washed in PBS followed by 1 h in biotinylated goat-anti-rabbit (1:300 Vector Laboratories, Burlingame, CA), washed in PBT, and incubated in avidin-biotin-horseradish peroxidase complex (ABC Elite Kit, Vector Laboratories). Brains were then washed in PBT followed by biotinylated tyramide solution (0.6%) for 30 min. Cells were labeled with the fluorophore CY-3 conjugated streptavidin (The Jackson Laboratory, Sacramento, CA). Slices were then washed in PBS and incubated in a rabbit anti-FOS primary antibody diluted at 1:2500 in PBT (Santa Cruz Biotechnology) and blocked with normal donkey serum for 48 h. Brain sections were then washed in PBT and labeled with the fluorophore CY-2 donkey-anti-rabbit (The Jackson Laboratory, Sacramento, CA).
Luteinizing Hormone Assay
Blood was collected into non-heparanized tubes and left overnight at 4 °C. Blood was then spun at 3,000 rpm and serum collected which was stored at -80 °C until assayed. Circulating LH concentrations were determined in duplicate 50 and 7 μl aliquots of serum by means of a radioimmunoassay described previously (Legan and Callahan, 1999). The first antibody was CSU120 (kindly provided by Dr. Terry Nett, Colorado State University, Fort Collins, CO), used at a working dilution of 1:10,000 in 1:100 normal rabbit serum (Millipore, St. Charles, MO) in 0.05 M EDTA in phosphate buffered saline (PBS). The tubes were incubated at 4° C for 48 h following addition of 100 μl first antibody and after adding radiolabeled LH (∼20-25,000 cpm/100 μl assay buffer (0.1% gelatin in PBS), and for 72 h after addition of the secondary antibody (200 ul anti-rabbit gamma globulin, 1:50 in gel-PBS; MP Biomedicals, LLC, Santa Ana, CA). Plasma LH concentrations are reported in terms of ng RP-3 per milliliter (National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases, and Dr. Parlow). All samples were assayed in one assay for which the sensitivity (100% - 2 SD of maximum binding) was 0.017 ng/tube, and the intra-assay coefficient of variation was 4.0% for a standard serum reading 3.95 ng/ml at 50% of maximum binding.
Light microscopy
Tissue sections were investigated with a Zeiss Z1 microscope (Carl Zeiss, Thornwood, NY) using the standard wavelengths for CY-2 (488 nm) and CY-3 (568 nm). Every fourth section through the anteroventral periventricular area was investigated for colocalization of kisspeptin and FOS positive staining. Images of these sections were digitally captured using a cooled CCD camera (Zeiss). Each label was captured as a single image without moving the plane of focus and superimposed digitally over the other. Double labeling was then determined using Photoshop software in which the red and green channels could be examined independently or together. The percentage of kisspeptin positive neurons that co-expressed FOS in the nucleus was quantified by an observer blind to the experimental groups.
Total number of FOS-labeled cells was quantified in brain areas implicated in the neural control of female sexual behavior, including the bed nucleus of the stria terminalais (BnST), dorsomedial nucleus of the hypothalamus, lateral habenula, lateral septum, anteroventral medial amygdala (MeAV), anterodorsal medial amygdala, posterodorsal medial amygdala, posteroventral medial amygdala, medial preoptic area (mPOA), nucleus accumbens shell, nucleus accumbens core, and ventromedial hypothalamus, using sampling boxes placed as indicated in Figure 2 [from the Golden Hamster Brain Atlas (Morin and Wood, 2001)]. Boxes were placed conservatively to ensure their placement was completely contained within the nucleus in question (Fig. 2). Total FOS counts were determined with the image J software (National Institute of Health, Bethesda MD) following a thresholding procedure applied to all brain sections.
Fig 2.
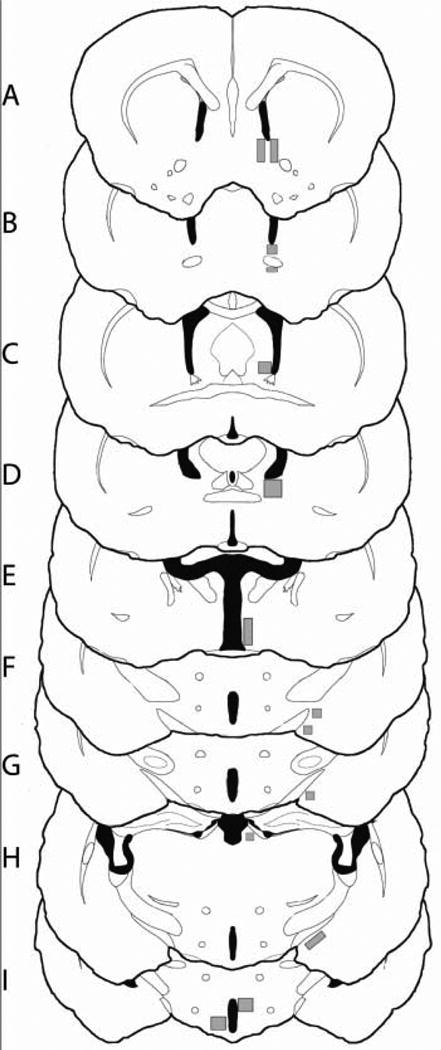
Schematic of sampling boxes for FOS quantification. Slices are presented rostral to caudal (A-I) and sampling boxes are identified as the grey shaded regions with corresponding areas in parentheses. Regions quantified, rostral to caudal, include: nucleus accumbens (core and shell; 0.178mm2 each; A), anterior bed nucleus of the stria terminalis (0.129mm2; B), lateral septum (0.081mm2; C), posteromedial bed nucleus of the stria terminalis (0.195 mm2; D), medial preoptic area (0.244mm2;E), medial amygdala (anteroventral and anterodorsal; 0.081mm2; F), medial amygdala (posteroventral; 0.081mm2; G), medial amygdala (posterodorsal; 0.178mm2; H), dorsomedial hypothalamus (0.226mm2;I), ventromedial hypothalamus (0.226mm2;I).
Statistics
Partner preference tests were analyzed with independent t-tests comparing the total time spent with each male. Scent mark tests, lordosis tests, and cell counts were analyzed using 2 × 2 (pharmacological treatment × ovarian status) ANOVAs. Results were considered significant if p<0.05. All analyses were conducted with R (R 2.12.1; R Foundation for Statistical Computing, Vienna, AT).
Results
Behavior
Examination of the impact of GnIH on sexual motivation
GnIH inhibited sexual motivation in both intact females in behavioral estrus and hormone-treated, OVX females, indicating that the impact of GnIH occurs independently of downstream alterations in hormones of the hypothalamo-pituitary-gonadal (HPG) axis. Specifically, the intact + saline and OVX + saline groups spent significantly more time exploring the chamber containing an intact male (t12=4.49 and t14=2.70, respectively; p<0.05) (Fig. 3A). This preference was abolished by treatment with GnIH, independent of hormone replacement status (t16=1.46 and t16=0.49, respectively; p<0.05) (Fig. 3A). As with chamber preference, both saline infused groups actively investigated the intact male (i.e., spent more time in the sexual incentive zone) significantly more than the castrated male (intact + saline: t12=4.58 and OVX + saline: t14=2.47; p<0.05) (Fig. 3B). This preference was eliminated in hamsters infused with GnIH (intact + GnIH: t16=0.04 and OVX + GnIH: t16=0.57; p >0.05) (Fig. 3B). Consistent with the results observed for female partner preference, there was a significantly reduced incidence of vaginal scent marking in GnIH treated groups (F1,30=14.85; Fig. 4; p<0.05) as well as a main effect of gonadal status in which OVX groups displayed fewer scent marks than intact groups (F1,30=4.30; p<0.05); no interaction was present (F1,30=0.12; p>0.05) (Fig. 4)
Fig 3.
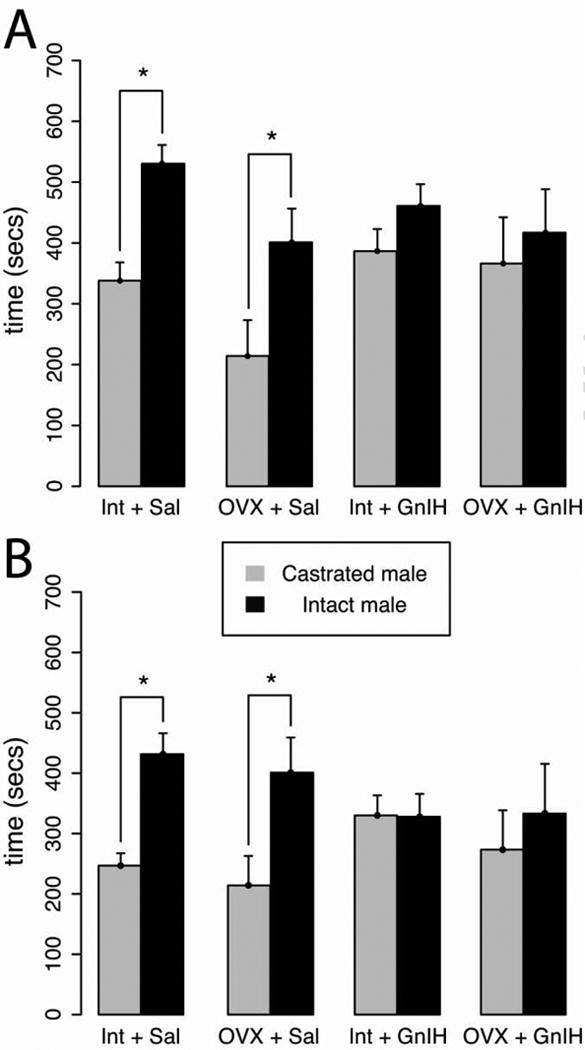
Female's were given a choice to spend time with an intact male, a castrated male, or neither. Tests lasted for 15 mins. Mean ± S.E.M. (A) amount of time spent in each male's chamber, (B) amount of time spent within ten cm of each male's enclosure. * significantly different (p<0.05). Saline infused females spent significantly more time with the intact male while females administered GnIH, regardless of hormonal status, displayed no preference between the intact and castrated males.
Fig 4.
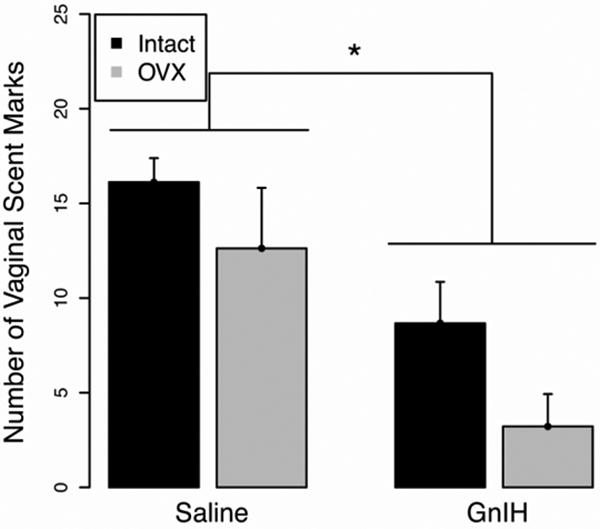
Mean ± S.E.M. total number of vaginal scent marks during a five minute test. * significantly different (p<0.05). GnIH administration reduced the number of vaginal scent marks displayed in response to an intact male's scent.
Examination of the impact of GnIH on copulatory behavior
Contrary to results observed for sexual motivation, the administration of GnIH did not significantly affect female copulatory behavior. There was neither a significant main effect of GnIH infusion (F1,27=0.64; p>0.05) nor gonadal status (F1,27=0.21; p>0.05) and no interaction (F1,27=0.28; p>0.05) on lordosis duration (Fig. 5A). Likewise, there were no significant main effects of GnIH infusion (F1,27=0.35; p>0.05) or gonadal status (F1,27=3.16; p>0.05) and there was no interaction (F1,27=0.26; p>0.05) for lordosis latency (Fig. 5B).
Fig 5.
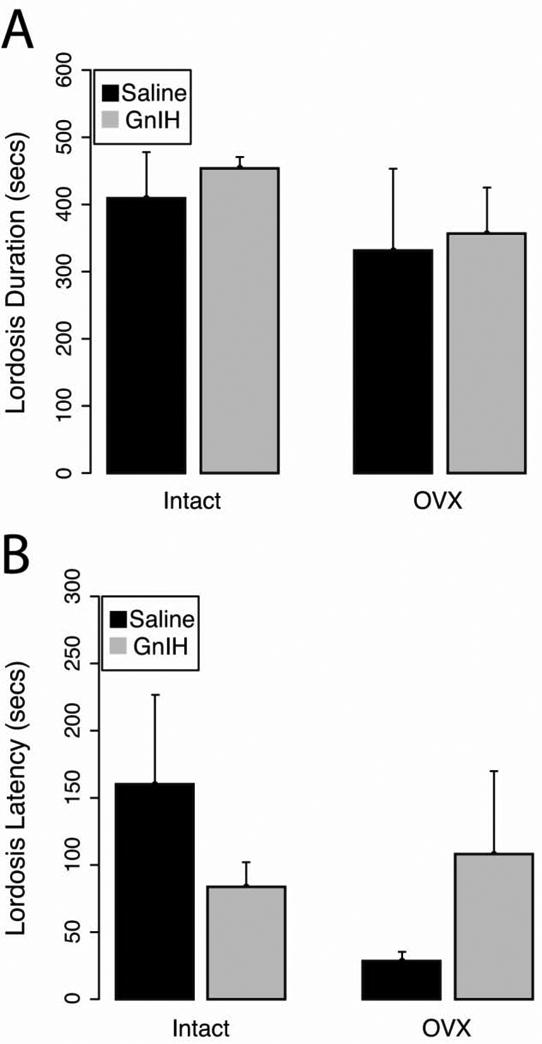
Mean ± S.E.M (A) lordosis duration and (B) lordosis latency. GnIH administration did not alter either the total duration of lordosis or the latency to display lordosis (p>0.05).
Luteinizing Hormone and Immunohistochemistry
Because the onset of female sexual motivation on the day of proestrus is not robust until well after the LH surge, blood collection occurred following behavioral assessment, during the descending limb of the LH surge. As anticipated, based on our pilot work, LH concentrations were not significantly different between groups (Intact + saline = 7.8ng/ml ± 1.76, while Intact + GnIH = 5.38 ± 1.01; t11=1.30; p>0.05). Given that the GnIH treatment was insufficient to reduce circulating LH concentrations, these findings suggest that the neural substrates regulating female sexual motivation and proceptivity are potentially more sensitive to GnIH than those suppressing the positive feedback action of estradiol.
To explore potential neural loci at which GnIH might act directly or indirectly to impact female sexual behavior, FOS expression was quantified in a number of brain regions implicated in the regulation of female sexual behavior and motivation. All females included in analysis of FOS expression received 5 intromissions from a conspecific male 60 min prior to perfusion. Some females either did not receive the requisite number of intromissions or had lost patency of osmotic pumps between the last behavior test and perfusion. As a result, sample sizes were as follows: OVX + Saline (n=6), OVX+ GnIH (n=4), Intact + Saline (n=7), Intact + GnIH (n=8).
In the mPOA, GnIH increased FOS expression, with a significant main effect of drug infusion (F1,21=8.31; p<0.05) but no effect of gonadal status (F1,21=2.38; p>0.05) and no interaction (F1,21=0.02; p>0.05) (Fig. 6). Analogous results were observed in the anterior BnST, with GnIH leading to increased FOS expression (Fig. 7A; F1,20=4.36; p<0.05) whereas there was no effect of gonadal status (F1,20=0.01; p>0.05) and no interaction (F1,20=0.49; p>0.05). A similar trend was observed in the posteromedial BnST (F1,21=3.01; p=0.097) but there was no effect of gonadal status (F1,21=0.75; p>0.05) and no interaction (F1,21=1.01; p>0.05) (Fig. 7B). Finally, in the MeAV, GnIH suppressed FOS expression, with a main effect of drug infusion (F1,21=5.88 p<0.05) but not of gonadal status (F1,21=0.74; p>0.05) and no interaction effect (F1,21=1.64; p>0.05) (Fig. 8). Other brain areas that did not show significant differences among groups are listed in Table 1.
Fig 6.
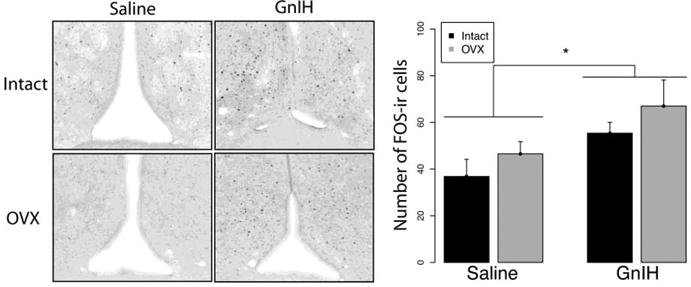
Mean ± S.E.M. FOS expression in the medial preoptic area. * significantly different (p<0.05). FOS expression was higher in females infused with GnIH than with saline.
Fig 7.
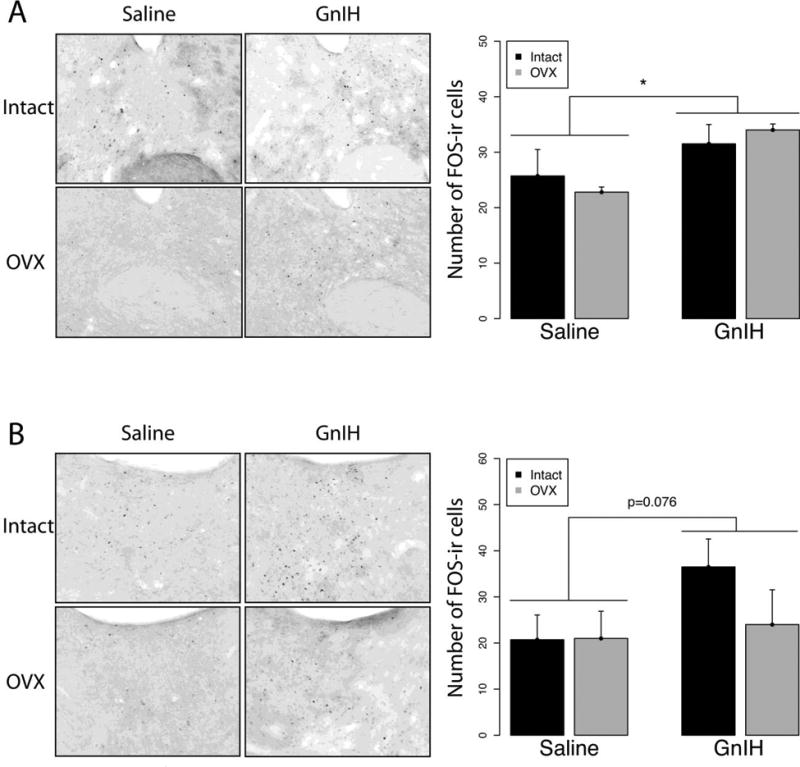
Mean ± S.E.M. FOS expression in the (A) anterior and, (B) posteromedial Bed nucleus of the Stria Terminalis (BnST). * significantly different (p<0.05). GnIH increased FOS expression in the anterior BnST and trended towards significance in the postermedial BnST.
Fig 8.
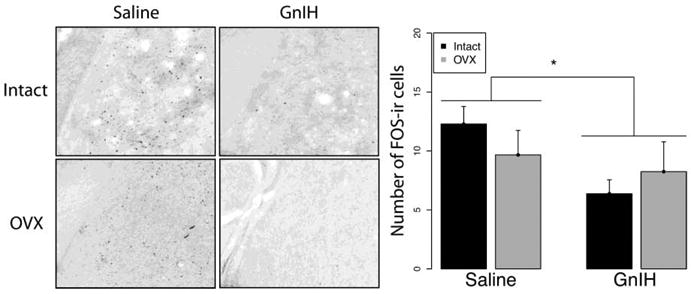
Mean ± S.E.M. FOS expression in the anteroventral medial amygdala. * significantly different (p<0.05). GnIH administration suppressed FOS expression in the anteroventral Medial Amygdala compared to saline controls.
Table 1. Total c-FOS counts for neural nuclei sensitive to mating related stimuli.
| VMH | DMH | Lat Hab | Lat Sept | MeApd | MeApv | MeAD | NaCC Core | NaCC Shell | |
|---|---|---|---|---|---|---|---|---|---|
| Saline Intact | 5.17 ± 0.73 | 16.00 ± 5.30 | 64.14 ± 9.92 | 26.29 ± 6.36 | 35.43 ± 10.24 | 7.50 ± 1.01 | 11.00 ± 3.09 | 22.29 ± 3.11 | 14.29 ± 3.70 |
| Saline OVX | 7.83 ± 0.94 | 30.17 ± 7.52 | 71.00 ± 7.45 | 29.17 ± 7.91 | 26.00 ± 7.74 | 11.17 ± 2.90 | 9.83 ± 2.50 | 17.40 ± 3.27 | 19.20 ± 4.89 |
| GnIH Intact | 3.43 ± 0.76 | 20.86 ± 4.96 | 76.13 ± 12.57 | 28.75 ± 5.73 | 31.63 ± 7.19 | 7.86 ± 1.92 | 7.25 ± 1.47 | 19.88 ± 5.10 | 18.88 ± 3.70 |
| GnIH OVX | 9.50 ± 3.38 | 27.00 ± 6.37 | 45.50 ± 8.01 | 24.00 ± 9.84 | 26.75 ± 6.92 | 8.50 ± 2.25 | 8.75 ± 2.87 | 22.00 ± 3.28 | 20.00 ± 3.97 |
Mean ± S.E.M. Fos expression was not altered in the following brain regions: the lateral septum (Lat Sept), ventromedial hypothalamus (VMH), dorsomedial nucleus of the hypothalamus (DMH), core and shell of the nucleus accumbens (NaCC), lateral habenula (Lat Hab), and subdivision of the medial amygdala: posterodorsal (MeApd), posteroventral (MeApv), and anterodorsal (MeAD).
GnIH does not appear to affect sexual behavior through alterations in anteroventral periventricular nucleus kisspeptin-ir cellular activity as there were no main effects of drug infusion (F1,18=0.00; p>0.05) or gonadal status (F1,18=0.22; p>0.05) and no interaction (F1,18=0.33; p>0.05) on the percentage of anteroventral periventricular kisspeptin-ir cells expressing FOS (Fig. 9).
Fig 9.
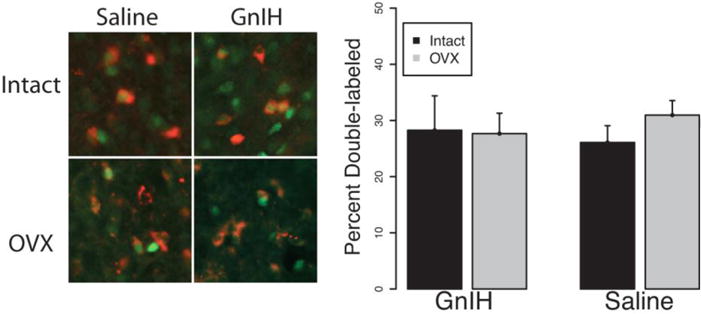
Mean ± S.E.M. Fos expression colocalized with kisspeptin neurons in the anteroventral periventricular nucleus. There were no significant differences between groups in the percentage of kisspeptin neurons colocalizing with FOS after sexual experience.
Discussion
The present study establishes an inhibitory role for GnIH in female sexual motivation and proceptive behavior (appetitive components), without influencing consummatory components of sexual behavior (lordosis). More specifically, central administration of GnIH eliminated the preference for an intact male compared to a castrated male and significantly reduced the incidence of vaginal scent marking. However, neither the duration, nor latency to show lordosis, were affected. Furthermore, FOS-immunoreactivity (-ir) was differentially expressed in key neural loci implicated in female sexual behavior after GnIH administration. The impact of GnIH was independent of circulating steroids and kisspeptin cellular activation, pointing to a neuromodulatory role of GnIH in female sexual motivation rather than downstream impact on the HPG axis. These finding are consistent with observations in female sparrows and male rats (Bentley et al., 2006; Johnson et al., 2007), suggesting that the role of GnIH in sexual behavior is conserved across taxa and the sexes. These data further demonstrate the need to differentiate sexual motivation and precopulatory behaviors from lordosis behavior; reproduction requires the coordination of sexual physiology with sexual behaviors, each of which can be independently regulated. Additionally, that GnIH disrupts sexual behaviors independent of changes in circulating gonadal steroids points to important opportunities for further investigating the neuromodulatory role of this neuropeptide.
In female Syrian hamsters and other rodents, GnIH neurons originate in the dorsomedial hypothalamus but project widely in the brain, including to the anterior hypothalamus, amygdala, BnST, lateral septum, and preoptic area (Kriegsfeld et al., 2006), all of which are involved in female sexual behavior. Many of these nuclei do not express GnRH neurons suggesting that GnIH can affect brain function independent of the HPG axis. Additional studies are necessary to determine if GnIH acts directly or via intermediate neural loci to inhibit female sexual behavior. For example, GnRH projects to multiple nuclei in the basal forebrain and hypothalamus (Boehm et al., 2005) and these projections promote female sexual behavior in hypophysecomized rats (Pfaff, 1973). Although GPR147 is located on kisspeptin neurons (Rizwan et al., 2012), GnIH did not alter FOS expression in this cell population, suggesting impact on female sexual behavior is independent of changes in this cell population.
In rodents, intromissions induce expression of FOS in a number of neural loci implicated in the regulation of female sexual behavior (Erskine, 1993; Joppa et al., 1995; Shelley and Meisel, 2005). We sampled FOS expression in these regions and identified differential regulation in the mPOA, MeA, and BnST. Interestingly, these three subdivisions are part of the extended accessory olfactory system, an extension of the vomeronasal organ and accessory olfactory bulb (AOB) that integrates sexually-relevant odors with hormone signals (Petrulis, 1999; Bressler and Baum, 1996; Wood and Coolen, 1997). The AOB projects directly to the MeAV and BnST, (Mohedano-Moriano et al., 2007) each of which, in turn, projects to other hypothalamic regions, including the VMH and mPOA (Coolen and Wood, 1998). We observed a decrease in FOS-ir in the anteroventral MeA of GnIH treated hamsters, and an increase in the anterior and posteromedial BnST and mPOA. Alterations in FOS expression in these subregions suggest the possibility of altered chemosensory processing. The MeAV has been described as a “chemosensory filter” that recognizes social odors but filters that signal to only relay sexually relevant odors to the posterodorsal medial amygdala (Maras and Petrulis, 2010), where this information is integrated with the internal hormonal milieu. In addition, the BnST, medial amygdala and mPOA regulate sexual and chemosensory dependent behaviors, including sexual motivation and proceptivity.
Lesions of the medial amygdala disrupt vaginal scent marking and preference for male odors (Petrulis and Johnston, 1999). Vaginal scent marking involves smooth muscle contraction and extrusion of specialized fluid stored in glands inside the vagina; retrograde tracing from the vaginal walls identified the mPOA, MeA, and BnST as vaginal wall effectors (Marson and Murphy, 2006). Altered MeA functioning may be reflected in the results of our preference paradigm. GnIH-infused females did not demonstrate a preference for an intact over a castrated male in our tests. It is possible that either GnIH prevents the female from distinguishing intact from castrated male scents or it eliminates sexual motivation. Little is known about the role of the MeA in female sexual motivation, although it has been implicated in the regulation of paced mating in female rats (Erskine and Hanrahan, 1997). Additionally, estradiol binding in the MeA correlates with the induction of proceptive behaviors in olfactory bulbectomized female rats (McGinnis et al., 1985) suggesting that the MeA is involved in sexual motivation. Finally, bilateral lesions of the accessory olfactory bulbs in female rats reduces measures of female sexual behavior and FOS-ir in the MeA and BnST (Dudley and Moss, 1994). Together, these data suggest that the amygdala is involved in the generation of sexual motivation and proceptivity, although more evidence is required to conclusively delineate its precise role.
The medial preoptic area is considered inhibitory to female sexual behavior. Lesions of the mPOA facilitate receptivity in female rats (Powers, 1972), estradiol treatment reduces neuron firing rate (Bueno and Pfaff, 1976), and electrical stimulation reduces lordosis (Malsbury et al., 1980). In the present investigation, FOS-ir in the mPOA is increased without accompanying alterations in lordosis behavior. The mPOA is heterogeneous and has been implicated in regulating the length of the receptive period as well as sexual motivation (Ramos and DeBold, 1999). Female receipt of intromissions decreases the total duration of receptivity (Carter and Schein, 1971) and this decrease in receptivity is blocked when a protein synthesis inhibitor is administered to the mPOA (Ramos and DeBold, 1999). Further, Whitney (1986) found that lesions to the mPOA increase the lordosis response. However, if the female is able to avoid the male, lordosis is not elevated, with females allowing fewer copulatory contacts and displaying blunted sexual motivation. Thus, the present FOS data are consistent with an inhibitory effect of GnIH on sexual motivation and raise the possibility that a rise in GnIH may occur following mating experience to curtail receptivity.
Our experimental design does not permit attribution of alterations in FOS to intromissions, chemosensory input, or a combination of both. Likewise, whereas FOS is a useful marker for uncovering neural loci implicated in a behavioral phenomenon, it can be expressed either in a neuron that is activated or inhibited (Pfaus and Heeb, 1997). Because all females received sexual experience prior to perfusion, it is not possible to determine if differences between groups in FOS expression are due directly to the sexual stimuli, an increase in the basal activation of neurons relative to non-mated females, or a combination of both mechanisms. Whether GnIH increases activity in mating nuclei that then alter mating behavior or whether GnIH alters the response of the brain to mating stimuli represent interesting opportunities for further investigation. Despite these caveats, the present study identifies the MeA, BnST, and mPOA as potential targets for further investigation.
In nature it is likely that all three components of female sexual behavior, sexual motivation, proceptive, and receptive behaviors, are expressed during successful reproduction. Syrian hamsters are solitary creatures that live alone in individual burrows (Gattermann et al., 2001) and both males and females must proactively solicit sexual contact and co-localize before copulation occurs. Vaginal scent marking is one tool female hamsters employ to advertise their impending sexual receptivity. As such, mating likely occurs only when the female is motivated and can display both proceptive and receptive behaviors. In the artificial and confined setting of a research laboratory, females displayed lordosis and copulated without any apparent difficulty despite a lack of sexual motivation and proceptivity. However, in their natural environment, decrements in vaginal scent marking and sexual motivation would likely be catastrophic for reproductive success. Further laboratory experiments compared with studies in natural or seminatural environments will help to clarify the importance of GnIH in female reproduction.
In summary, the present study establishes, for the first time, the inhibitory effect of GnIH on sexual motivation and proceptive behaviors and alters neuronal function in areas of the brain implicated in motivated behavior and chemosensory processing. Furthermore, GnIH appears to act through mechanisms independent of alterations in circulating gonadal steroids and kisspeptin. These data suggest an important role for GnIH in reproduction in this species.
Highlights.
GnIH inhibited sexual motivation and precopulatory behavior in female hamsters.
Despite affecting sexual motivation, GnIH did not have any effect on copulatory behavior.
GnIH appears to act at several, interconnected neural loci to modulate female sexual behavior
Acknowledgments
This work was supported by National Institutes of Health Grant HD050470. We are grateful to Dr. Irving Zucker for helpful comments on a previous version of this manuscript. We thank Dr. Terry Nett for the LH antibody and Dr. A.F. Parlow and the National Hormone and Peptide Program for the LH standard and the highly purified LH for iodination. We also thank Amy Li, Huzaifa Ahmad, and the UC Berkeley Office of Laboratory and Animal Care for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bentley GE, Jensen JP, Kaur GJ, Wacker DW, Tsutsui K, Wingfield JC. Rapid inhibition of female sexual behavior by gonadotropin-inhibitory hormone (GnIH) Horm Behav. 2006;49:550–555. doi: 10.1016/j.yhbeh.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Ubuka T, McGuire NL, Calisi R, Perfito N, Kriegsfeld LJ, Wingfield JC, Tsutsui K. Gonadotrophin-inhibitory hormone: a multifunctional neuropeptide. J Neuroendocrinol. 2009;21:276–281. doi: 10.1111/j.1365-2826.2009.01851.x. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Erskine MS. Feminine Sexual Behavior: Cellular Integration of Hormonal and Afferent Information in the Rodent Forebrain. In: Pfaff DW, et al., editors. Hormones, Brain, and Behavior. Elsevier; San Diego: 2002. [Google Scholar]
- Blaustein JD. Feminine Reproductive Behavior and Physiology in Rodents: Integration of Hormonal, Behavioral, and Environmental Influences. In: Pfaff DW, et al., editors. Hormones, Brain, and Behavior. Elsevier; San Diego: 2009. [Google Scholar]
- Boehm U, Zou Z, Buck LB. Feedback loops link odor and pheromone signaling with reproduction. Cell. 2005;123:683–695. doi: 10.1016/j.cell.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Bressler SC, Baum MJ. Sex comparison of neuronal Fos immunoreactivity in the rat vomeronasal projection circuit after chemosensory stimulation. Neuroscience. 1996;71:1063–1072. doi: 10.1016/0306-4522(95)00493-9. [DOI] [PubMed] [Google Scholar]
- Bueno J, Pfaff DW. Single unit recording in hypothalamus and preoptic area of estrogen-treated and untreated ovariectomized female rats. Brain Res. 1976;101:67–78. doi: 10.1016/0006-8993(76)90988-4. [DOI] [PubMed] [Google Scholar]
- Carter CS, Schein MW. Sexual receptivity and exhaustion in the female golden hamster. Horm Behav. 1971;2:191–200. [Google Scholar]
- Chappell PE. Clocks and the black box: circadian influences on gonadotropin-releasing hormone secretion. J Neuroendocrinol. 2005;17:119–130. doi: 10.1111/j.1365-2826.2005.01270.x. [DOI] [PubMed] [Google Scholar]
- Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev. 2010;31:544–577. doi: 10.1210/er.2009-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke IJ, Sari IP, Qi Y, Smith JT, Parkington HC, Ubuka T, Iqbal J, Li Q, Tilbrook A, Morgan K, Pawson AJ, Tsutsui K, Millar RP, Bentley GE. Potent action of RFamide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology. 2008;149:5811–5821. doi: 10.1210/en.2008-0575. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Schwartz WJ. Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology. 2006;147:1148–1153. doi: 10.1210/en.2005-1311. [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropichypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducret E, Anderson GM, Herbison AE. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology. 2009;150:2799–2804. doi: 10.1210/en.2008-1623. [DOI] [PubMed] [Google Scholar]
- Dudley CA, Moss RL. Lesions of the accessory olfactory bulb decrease lordotic responsiveness and reduce mating-induced c-fos expression in the accessory olfactory system. Brain Res. 1994;642:29–37. doi: 10.1016/0006-8993(94)90902-4. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Mating-induced increases in_FOS_protein in preoptic area and medial amygdala of cycling female rats. Brain Res Bull. 1993;32:447–451. doi: 10.1016/0361-9230(93)90289-n. [DOI] [PubMed] [Google Scholar]
- Erskine MS, Hanrahan SB. Effects of paced mating on c-fos gene expression in the female rat brain. J Neuroendocrinol. 1997;9:903–912. doi: 10.1046/j.1365-2826.1997.00660.x. [DOI] [PubMed] [Google Scholar]
- Gattermann R, Fritzsche P, Neumann K, Al-Hussein I, Kayser A, Abiad M, Yakti R. Notes on the current distribution and the ecology of wild golden hamsters (Mesocricetus auratus) J Zool. 2001;254:359–365. [Google Scholar]
- Gibson EM, Humber SA, Jain S, Williams WP, 3rd, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149:4958–4969. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Huck UW, Lisk RD, Thierjung C. Stimulus requirements for pregnancy initiation in the golden hamster (Mesocricetus auratus) change with time of mating during the receptive period. J Reprod Fertil. 1986;76:449–458. doi: 10.1530/jrf.0.0760449. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav. 2007;51:171–180. doi: 10.1016/j.yhbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RE. The causation of two scent-marking behaviour patterns in female hamsters (Mesocricetus auratus) Anim Behav. 1977;25:317–327. doi: 10.1016/0003-3472(77)90007-0. [DOI] [PubMed] [Google Scholar]
- Joppa MA, Meisel RL, Garber MA. c-Fos expression in female hamster brain following sexual and aggressive behaviors. Neuroscience. 1995;68:783–792. doi: 10.1016/0306-4522(95)00179-m. [DOI] [PubMed] [Google Scholar]
- Klingerman CM, Williams WP, Simberlund J, Brahme N, Prasad A, Schneider JE, Kriegsfeld LJ. Food restriction-induced changes in gonadotropin-inhibiting hormone cells are associated with changes in sexual motivation and food hoarding, but not sexual performance and food intake. Front Endocrinol. 2011;2:101. doi: 10.3389/fendo.2011.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Silver R. The regulation of neuroendocrine function: Timing is everything. Horm Behav. 2006;49:557–574. doi: 10.1016/j.yhbeh.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci USA. 2006;103:2410–2415. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology. 1975;96:57–62. doi: 10.1210/endo-96-1-57. [DOI] [PubMed] [Google Scholar]
- Legan SJ, Callahan WH. Suppression of tonic luteinizing hormone secretion and norepinephrine release near the GnRH neurons by estradiol in ovariectomized rats. Neuroendocrinology. 1999;70:237–245. doi: 10.1159/000054482. [DOI] [PubMed] [Google Scholar]
- Mahoney MM, Sisk C, Ross HE, Smale L. Circadian regulation of gonadotropin-releasing hormone neurons and the preovulatory surge in luteinizing hormone in the diurnal rodent, Arvicanthisniloticus, and in a nocturnal rodent, Rattusnorvegicus. Biol Reprod. 2004;70:1049–1054. doi: 10.1095/biolreprod.103.021360. [DOI] [PubMed] [Google Scholar]
- Malsbury CW, Pfaff DW, Malsbury AM. Suppression of sexual receptivity in the female hamster: neuroanatomical projections from preoptic and anterior hypothalamic electrode sites. Brain Res. 1980;181:267–284. doi: 10.1016/0006-8993(80)90612-5. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. The anterior medial amygdala transmits sexual odor information to the posterior medial amygdala and related forebrain nuclei. Eur J Neurosci. 2010;32:469–482. doi: 10.1111/j.1460-9568.2010.07289.x. [DOI] [PubMed] [Google Scholar]
- Marson L, Murphy AZ. Identification of neural circuits involved in female genital responses in the rat: a dual virus and anterograde tracing study. Am J Physiol Regul Integr Comp Physiol. 2006;291:R419–R428. doi: 10.1152/ajpregu.00864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis MY, Lumia AR, McEwen BS. Increased estrogen receptor binding in amygdala correlates with facilitation of feminine sexual behavior induced by olfactory bulbectomy. Brain Res. 1985;334:19–25. doi: 10.1016/0006-8993(85)90562-1. [DOI] [PubMed] [Google Scholar]
- Mohedano-Moriano A, Pro-Sistiaga P, Ubeda-Bañón I, Crespo C, Insausti R, Martinez-Marcos A. Segregated pathways to the vomeronasal amygdala: differential projections from the anterior and posterior divisions of the accessory olfactory bulb. Eur J Neurosci. 2007;25:2065–2080. doi: 10.1111/j.1460-9568.2007.05472.x. [DOI] [PubMed] [Google Scholar]
- Morin LP, Wood RI. A Stereotaxic Atlas of the Golden Hamster Brain. Academic Press; San Diego: 2001. [Google Scholar]
- Murakami M, Matsuzaki T, Iwasa T, Yasui T, Irahara M, Osugi T, Tsutsui K. Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. J Endocrinol. 2008;199:105–112. doi: 10.1677/JOE-08-0197. [DOI] [PubMed] [Google Scholar]
- Nequin LG, Alvarez J, Schwartz NB. Steroid control of gonadotropin release. J Steroid Biochem. 1975;6:1007–1012. doi: 10.1016/0022-4731(75)90342-8. [DOI] [PubMed] [Google Scholar]
- Orsini MW. The external vaginal phenomena characterizing the stages ofthe estrous cycle, pregnancy, pseudopregnancy, lactation, and the anestrous hamster, Mesocricetus auratus. Proc Anim Care Panel. 1961;11:193–206. [Google Scholar]
- Osugi T, Ukena K, Bentley GE, O'Brien S, Moore IT, Wingfield JC, Tsutsui K. Gonadotropin-inhibitory hormone in Gambel's white-crowned sparrow (Zonotrichia leucophrys gambelii): cDNA identification, transcript localization and functional effects in laboratory and field experiments. J Endocrinol. 2004;182:33–42. doi: 10.1677/joe.0.1820033. [DOI] [PubMed] [Google Scholar]
- Petrulis A, Johnston RE. Lesions centered on the medial amygdala impair scentmarking and sex-odor recognition but spare discrimination of individual odors in female golden hamsters. Behav Neurosci. 1999;113:345–357. doi: 10.1037//0735-7044.113.2.345. [DOI] [PubMed] [Google Scholar]
- Pfaff DW. Luteinizing hormone-releasing factor potentiates lordosis behavior in hypophysectomizedovariectomized female rats. Science. 1973;182:1148–9. doi: 10.1126/science.182.4117.1148. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Heeb MM. Implications of immediate-early gene induction in the brain following sexual stimulation of female and male rodents. Brain Res Bull. 1997;44:397–407. doi: 10.1016/s0361-9230(97)00219-0. [DOI] [PubMed] [Google Scholar]
- Powers B, Valenstein ES. Sexual receptivity: facilitation by medial preoptic lesions in female rats. Science. 1972;175:1003–1005. doi: 10.1126/science.175.4025.1003. [DOI] [PubMed] [Google Scholar]
- Ramos SM, DeBold JF. Protein synthesis in the medial preoptic area is important for the mating-induced decrease in estrus duration in hamsters. Horm Behav. 1999;35:177–85. doi: 10.1006/hbeh.1998.1510. [DOI] [PubMed] [Google Scholar]
- Rizwan MZ, Poling MC, Corr M, Cornes PA, Augustine RA, Quennell JH, Kauffman AS, Anderson GM. RFamide-related peptide-3 receptor gene expression in GnRH and kisspeptin neurons and GnRH-dependent mechanism of action. Endocrinology. 2012;153:3770–3779. doi: 10.1210/en.2012-1133. [DOI] [PubMed] [Google Scholar]
- Sari IP, Rao A, Smith JT, Tilbrook AJ, Clarke IJ. Effect of RF-amide-related peptide-3 on luteinizing hormone and follicle-stimulating hormone synthesis and secretion in ovine pituitary gonadotropes. Endocrinology. 2009;150:5549–5556. doi: 10.1210/en.2009-0775. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Chiappa SA, Fink G, Sherwood NM. Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature. 1976;264:461–463. doi: 10.1038/264461a0. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Shelley DN, Meisel RL. The effects of mating stimulation on c-Fos immunoreactivity in the female hamster medial amygdala are region and context dependent. Horm Behav. 2005;47:212–22. doi: 10.1016/j.yhbeh.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Lisk RD. Organization and expression of agonistic and socio-sexual behavior in golden hamsters over the estrous cycle and after ovariectomy. Physiol Behav. 1983;31:477–482. doi: 10.1016/0031-9384(83)90069-0. [DOI] [PubMed] [Google Scholar]
- Tsutsui K. A new key neurohormone controlling reproduction, gonadotropin-inhibitory hormone (GnIH): Biosynthesis, mode of action and functional significance. Prog Neurobiol. 2009;88:76–88. doi: 10.1016/j.pneurobio.2009.02.003. Review. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Bentley GE, Bedecarrats G, Osugi T, Ubuka T, Kriegsfeld LJ. Gonadotropin-inhibitory hormone (GnIH) and its control of central and peripheral reproductive function. Front Neuroendocrinol. 2010;31:284–295. doi: 10.1016/j.yfrne.2010.03.001. Review. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun. 2000;275:661–667. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- Ubuka T, Inoue K, Fukuda Y, Mizuno T, Ukena K, Kriegsfeld LJ, Tsutsui K. Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone. Endocrinology. 2012;153:373–385. doi: 10.1210/en.2011-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubuka T, Lai H, Kitani M, Suzuuchi A, Pham V, Cadigan PA, Wang A, Chowdhury VS, Tsutsui K, Bentley GE. Gonadotropin-inhibitory hormone identification, cDNA cloning, and distribution in rhesus macaque brain. J Comp Neurol. 2009a;517:841–856. doi: 10.1002/cne.22191. [DOI] [PubMed] [Google Scholar]
- Ubuka T, Morgan K, Pawson AJ, Osugi T, Chowdhury VS, Minakata H, Tsutsui K, Millar RP, Bentley GE. Identification of human GnIH homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis. PLoS ONE. 2009b;4:e8400. doi: 10.1371/journal.pone.0008400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubuka T, Ukena K, Sharp PJ, Bentley GE, Tsutsui K. Gonadotropin-inhibitory hormone inhibits gonadal development and maintenance by decreasing gonadotropin synthesis and release in male quail. Endocrinology. 2006;147:1187–1194. doi: 10.1210/en.2005-1178. [DOI] [PubMed] [Google Scholar]
- Ukena K, Iwakoshi E, Minakata H, Tsutsui K. A novel rat hypothalamic RFamide-related peptide identified by immunoaffinity chromatography and mass spectrometry. FEBS Lett. 2002;512:255–258. doi: 10.1016/s0014-5793(02)02275-5. [DOI] [PubMed] [Google Scholar]
- Whitney JF. Effect of medial preoptic lesions on sexual behavior of female rats is determined by test situation. Behav Neurosci. 1986;100:230–235. doi: 10.1037//0735-7044.100.2.230. [DOI] [PubMed] [Google Scholar]
- Williams WP, 3rd, Jarjisian SG, Mikkelsen JD, Kriegsfeld LJ. Circadian control of kisspeptin and a gated GnRH response mediate the preovulatory luteinizing hormone surge. Endocrinology. 2011;152:595–606. doi: 10.1210/en.2010-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, Coolen LM. Integration of chemosensory and hormonal cues is essential for sexual behaviour in the male Syrian hamster: role of the medial amygdaloid nucleus. Neuroscience. 1997;78:1027–1035. doi: 10.1016/s0306-4522(96)00629-x. [DOI] [PubMed] [Google Scholar]


