Abstract
Optical insulation plays a critical role in the fine visual acuity of the Drosophila compound eye. Screening pigments expressed by a number of cell types contribute to this phenomenon. They provide optical insulation that prevents extraneous light rays from inappropriately activating the photoreceptors. This optical insulation can be divided into two categories; the insulation of the individual ommatidia, and the insulation of the compound eye as a whole. The whole-eye insulation is provided by two sources. The sides of the eye are optically insulated by the pigment rim, a band of pigment cells that circumscribes the eye. The base of the eye is insulated by the subretinal pigment layer; a thick layer of pigment that lies directly underneath the retina. How this subretinal pigment layer is generated has not been clearly described. Here, experiments that manipulate pigment expression during eye development suggest that the subretinal pigment layer is directly derived from pigment cells in the overlying retina.
INDEXING TERMS: optical insulation, visual acuity, screening pigment
The visual ability of the fly depends upon the exquisite cellular architecture of its compound eye. The eye is made from hundreds of ommatidia, each constructed in a stereotypical manner (for a full review of the structural organization of the Drosophila ommatidium, see Wolff and Ready, 1993). The roles of the various cells that make up each ommatidium can be roughly divided into three functions. First, at the heart of the structure lie the photoreceptors, the eight photosensitive sensory neurons that have synaptic targets in the first two ganglia of the underlying optic lobe. Second, above the photoreceptors lies the dioptric apparatus; the lens unit. This unit functions to receive the light rays and focus them down onto the underlying photoreceptors. Third, and germane to this paper, are the pigment cells. These provide a sleeve of insulating pigment that runs from the exterior of the eye to the basal lamina, and ensure that light rays that enter through the lens remain within the ommatidium. This prevents lateral transfer of light rays through the eye, and the photoreceptors of an ommatidium are activated only by photons entering through its lens (Burnet et al., 1967). The pigments that perform this function are known as “screening pigments” to distinguish them from the photosensitive pigments (rhodopsins) found in the photoreceptors.
There are two types of screening pigments. The red pigments are of the pteridine class, and the browns are ommochromes (Ziegler, 1961). With the exception of the bristles, all cells of the eye express one or both of these pigments. The photoreceptors, cone cells, and primary pigment cells contain only ommochromes. The secondary and tertiary pigment cells contain both ommochromes and pteridines. The ommochromes of the photoreceptors are involved in the photoreceptor “pupil” mechanism; in bright light the pigment granules migrate to the photosensitive membranes (the rhabdomeres), and reduce rhodopsin light absorption (Kirschfeld and Franceschini, 1969). The pigment in all the other cells appears to be devoted to the general purpose of optical insulation.
The primary pigment cells ensheath the lens unit and provide an ommochrome-rich cylinder of insulation (Fig. 1C). The cone cells have thin transparent cell bodies that lie immediately below the lens, but unlike the primary pigment cells, which are restricted to the lens region, the cone cells have slender projections that course through the underlying photoreceptors and terminate in a club-shaped foot at the base of the ommatidium (Waddington and Perry, 1963) (Fig. 1C). The ommochrome granules of the cone cells are localized to the feet, which likely serves two functions: it removes pigment from the cone cell bodies, providing transparency below the lens, and it provides a basal pigment screen to prevent ectopic light access to the photoreceptors. The secondary and tertiary pigment cells surround the whole ommatidial unit from surface to base, and enswathe the lens and photoreceptor array in a deep coating of pteridine and ommochrome screening pigments (Fig. 1C).
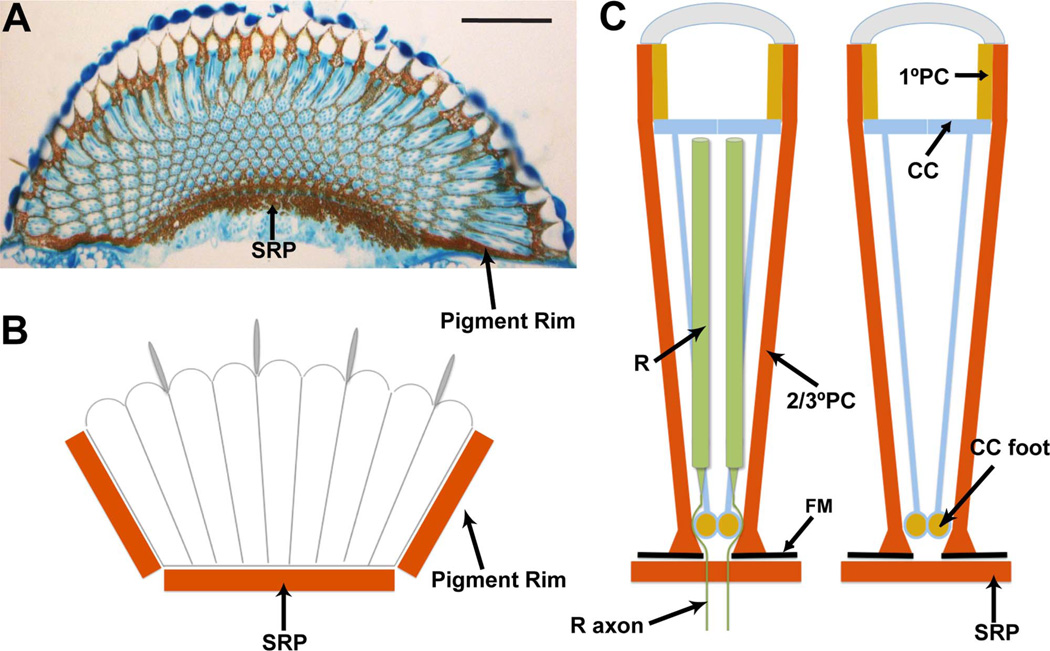
Figure 1.
Summary of the screening pigments in the Drosophila eye. A: A longitudinal section through an eye stained with toluidine blue. Arrows point to the subretinal pigment layer (SRP) and the pigment rim. B: Schematic image of a longitudinal section showing how the eye sits in a pigmented cup made basally by the subretinal pigment, and on the sides by the pigment rim. C: Schematic summary of the screening pigments of individual ommatidia. Two images are shown; the one to the right has the photoreceptors (R) removed to allow a clear view of how the cone cells (CC) send fibers to the base, where ommochrome pigment accumulates in their feet. The primary pigment cells (1°PC) surround the lens unit. The secondary and tertiary pigment cells (2/3°PC) extend the depth of the eye and end basally in enlarged feet that sit directly on the fenestrated membrane (FM). The photoreceptors extend much of the retina and then send axons through a hole in the fenestrated membrane. Immediately below the fenestrated membrane lies the subretinal pigment layer (SRP). Scale bar = 50 µm in A. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In addition to the individual ommatidia, the entire eye is also optically insulated. It sits in a cup-shaped structure rich in pteridine and ommochrome (Fig. 1A,B). The side of the cup is formed from the pigment rim of the retina. The pigment rim is a thick band of pigment cells that circumscribes the eye and runs from the surface to the base. During pupal development the outermost ring of ommatidia dies (Wolff and Ready, 1991), and their associated secondary and tertiary pigment cells coalesce to form the pigment rim (Tomlinson, 2003). The base of the pigment cup is made from the subretinal pigment layer, which lies immediately below the basal lamina of the retina (Cagan and Ready, 1989).
The basal lamina of the retina has exit holes under each ommatidium through which the photoreceptor axons pass on their way to their synaptic targets in the optic lobe neuropils. These holes give the name “fenestrated membrane” to this basal lamina, and they are a potential route for ectopic light entry to the ommatidia. However, a combination of the feet of the pigment cells and cone cells provides an extensive layer of pigment to prevent this. The feet of the secondary and tertiary pigment cells spread out along the basal lamina, and the feet of the cone cells plug the holes (Cagan and Ready, 1989). To escape the retina, the photoreceptor axons “squeeze” between the feet of the cone cells and the secondary and tertiary pigment cells, and, accordingly, there is little ability for light to gain access to photoreceptors through the basal holes (Cagan and Ready, 1989).
Thus, in the basal regions of the eye there are two distinct pigment layers that straddle the fenestrated membrane. Above, there is the basal retinal pigment layer made from the feet of the cone cells and secondary and tertiary pigment cells, and below, there is the subretinal pigment layer. Both of these layers likely function to optically insulate the base of the retina.
There are a number of contrasting descriptions of the cellular organization and pigmentation of the subretinal region. Cagan and Ready (1989) describe a population of dispersed subretinal cells that appear similar to secondary and tertiary pigment cells. They noted that clones lacking pigment in the eyes overlie subretinal regions also lacking pigment, and inferred that cells that generate the subretinal pigment delaminate from the local presumptive retina and move below the incipient fenestrated membrane during the third larval instar. In contrast, Kretzschmar et al. (2000) describe pigmented glia lying below the fenestrated membrane (and presumably providing the subretinal pigment). A population of glial cells lying close to the fenestrated membrane was described by Winberg et al. (1992), but any expression of pigment was not mentioned. Finally, Edwards and Meinertzhagen (2010), referring to the work of Saint Marie and Carlson (1983) in Musca, note that there is a population of unpigmented glia closely opposed to the retinal basal lamina called the fenestrated glia (which likely correspond to the glia population described by Winberg et al., 1992 in Drosophila). Thus, there appear to be contrasting descriptions of the subretinal region, including whether glia or pigment cells provide the subretinal pigment.
This article explores the origins of the subretinal pigment layer. Manipulations of pigment within the retina itself argue that a subretinal population of cells providing the subretinal pigment does not exist. Instead, this pigment layer is provided by pigment cells in the overlying retina.
MATERIALS AND METHODS
Preparation of adult eyes
Heads were dissected in phosphate-buffered saline (PBS), and most of the central brains were removed. They were fixed in 2% glutaraldehyde/PBS for 45 minutes, dehydrated through a graded alcohol series (30%, 50%, 70%, 80%, 90% and 100%, 5 minutes each) followed by propylene oxide (5 minutes) and then propylene oxide/Durcupan Resin (50/50 mix) for 1 hour (or overnight). The heads were then transferred to pure resin and further dissected to allow the eyes to be laid flat in coffin-shaped embedding molds to allow for longitudinal sectioning.
Transgene preparation
The white transgene used was a hybrid between the cDNA and the genomic sequence. The 5′ end of the cDNA until the Apa1 site was fused to the genomic 3′ end of the gene. This was then placed downstream of the GMR repeats with the minimal hsp70 promoter (Hay et al., 1994), and cloned into a variant of the Carnegie 20 transformation vector before injection and subsequent establishment of the transformant line. The UAS-w+ line was established in the same manner, substituting the UAS repeats for the GMR repeats.
The tub>y+>Gal4 stock was a gift from Gary Struhl (Columbia University, New York, NY).
Sectioning and microscopy
Eyes were serially sectioned (3–5 µm thick) and examined with brightfield and phase contrast microscopy.
Immunostaining of eye discs
Eye discs were fixed in 2% formaldehyde (PBS, pH 7.2), and antibody incubations were performed in PBT (PBS/0.1% Triton X-100). Antibodies used were anti-green fluorescent protein (GFP; Molecular Probes, Eugene, OR) and anti-Repo (Campbell et al., 1994).
Antibody characterization
Rabbit polyclonal anti-GFP (A6455; Molecular Probes) was raised against GFP isolated from the jellyfish Aequorea victoria. The specificity of this antibody was confirmed by experiments in which UAS-GFP was driven by various Gal4 driver lines. The antibody specifically labeled the tissue in which Gal4 was active. The Repo antibody (RK2–5′) was raised against a fusion protein containing RK2 amino acid sequences from 25 (K) to 123 (Q), and was shown to specifically label the nuclei of glia (Campbell et al., 1994).
Photomicrograph production
Photographs of adult and larval eyes were captured by using Improvision (Emeryville, CA) OpenLab software. The brightness, contrast, and color balance of the images were manipulated by using Adobe Photoshop and Adobe Illustrator (Adobe Systems, San Jose, CA).
RESULTS
Subretinal pigmentation is rescued by retinal-specific expression of the white gene
The introductory section reviews the ambiguity that exists in regard to the origin of the subretinal pigment. In one description, presumptive pigment cells delaminate from the retina during the third larval instar, and come to lie under the fenestrated membrane (Cagan and Ready, 1989); in another, pigmented glia are considered to be located immediately below the fenestrated membrane (Kretzschmar et al., 2000). The glia are thought to be derived from the central nervous system, and to migrate up the optic stalk to populate the subretinal region (Choi and Benzer, 1994). Thus, if the subretinal pigment is supplied by glia, those cells would not be expected to originate from the retina.
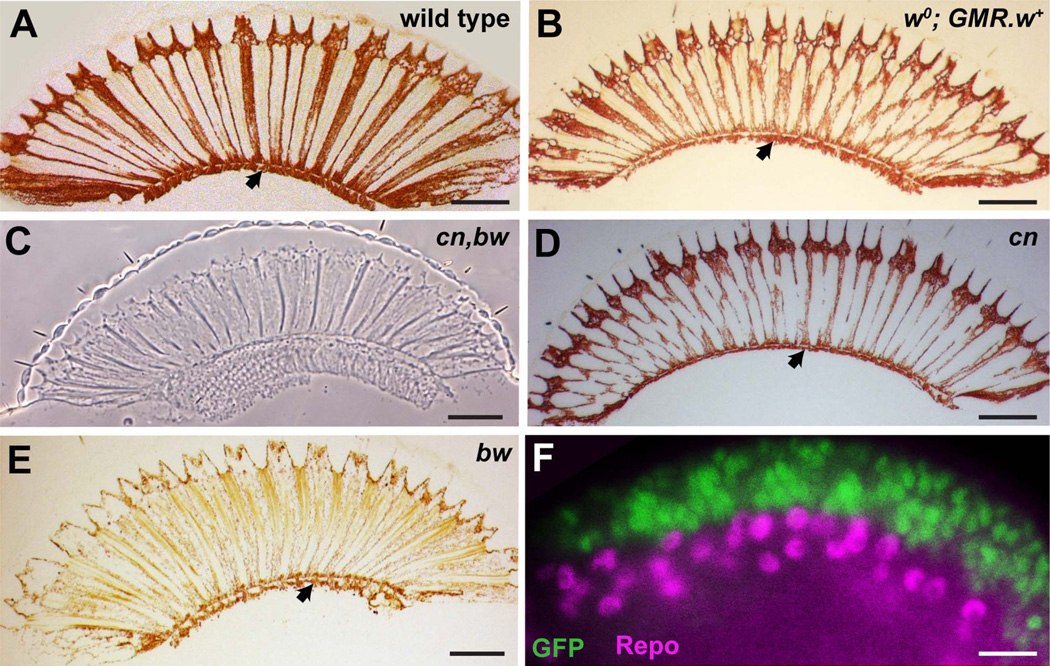
The white gene (w) is essential for the biosynthesis of the ommochromes and pteridines, and in its absence the eye is characteristically white in color; specifically lacking the screening pigments (including those of the subretinal region). To test whether the subretinal pigment was of retinal origin, the wild-type white gene (w+) gene was restored only to the retina of an otherwise white mutant (w0) fly. GMR is an eye-specific enhancer element that drives transcription only in the retina (Hay et al., 1994) (Fig. 2F). It begins expression immediately behind the morphogenetic furrow in the third instar, and maintains expression thereafter throughout development. A transgene in which w+ was expressed under GMR transcriptional control (GMR-w+) was crossed in to a w0 background (w0; GMR-w+). In these eyes, only the cells expressing GMR will have the ability to make pigment. Sectioning and examination of these eyes showed the restoration of all eye pigmentation, including the subretinal pigment (Fig. 2B). Because glia do not express GMR, this result suggests that the subretinal pigment is not derived from glia but from cells of a retinal origin.
Figure 2.
A–E: The presence of the subretinal pigment layer in various genetic backgrounds that manipulate pigment expression. A: A brightfield image through a section of a wild-type eye shows the characteristic subretinal pigment layer lying directly beneath the retina (arrowhead). B: A brightfield image of a white mutant eye (w0) in the presence of a transgene driving expression of w+ in the presumptive retina (GMR-w+), which creates a normally pigmented eye, with a normal subretinal pigment layer (arrowhead). C: A phase contrast image of a section through a cn,bw mutant eye in which pigment is absent from the entire eye including the subretinal region. D: A brightfield image of a section through a cn mutant eye in which a general reduction in pigment levels in the eye is seen, and a reduced subretinal pigment layer is still present (arrowhead). E: A brightfield image of a section through a bw mutant eye in which a general reduction in pigment levels in the eye is seen, and a reduced subretinal pigment layer is still present (arrowhead). F: Fluorescence microscopy image of a late third-instar GMR-Gal4; UAS-GFP eye disc stained for GFP (green) and Repo (magenta) a pan-glia marker. The GMR-expressing cells overlie the glia, and there is no overlap in expression. The image is taken from the region of the disc that rolls laterally and allows the upper and lower layers of the tissue to be viewed in cross section. Scale bar = 50 µm in A–E; 10 µm in F.
The subretinal pigment appears similar to that of the secondary and tertiary pigment cells
As noted by Cagan and Ready (1989), the subretinal pigment appears similar to that found in the secondary and tertiary pigment cells. These cells are characterized by the co-expression of ommochromes and pteridines. To demonstrate that the subretinal pigment did indeed contain ommochromes and pteridines, mutations that selectively remove each pigment were examined.
Brown is a protein required for pteridine biosynthesis, and in its absence (bw) the lack of the red pigments leaves the eye with a characteristic brown color. In contrast, Cinnabar is required for ommochrome biosynthesis, and in its absence (cn) the lack of the brown pigments leaves the eye with a bright red appearance. Eyes of both these genotypes were sectioned. In the bw mutant, the eye contained only brown pigments (including in the subretinal pigment; Fig. 2E), and in the cn mutant, the eyes contained only red pigments (including in the subretinal pigment; Fig. 2D). In the double mutant (cn,bw) all pigment was lost from the eye (including the subretinal region; Fig. 2C). Thus, the subretinal pigment layer shows the same pigment profile (co-expression of ommochromes and pteridines) as the secondary and tertiary pigment cells.
The subretinal pigment appears to be derived directly from the overlying secondary and tertiary pigment cells
The experiments so far had suggested that the subretinal pigment was of retinal origin, and appeared by composition to be of the secondary/tertiary pigment cell type. To examine the origin of this pigment layer further, the ability to make pigment (restoration of w+ function) was supplied to eyes in a mosaic manner (some cells expressing w+ and other not), at various stages of development, and any correlation between expression in the retina and corresponding expression in the subretinal region was scrutinized.
A flip-out system was used to generate a random array of individual cells expressing w+ in an otherwise w0 animal. Flies of the genotype w0,hs-flip; tub>y+>Gal4, UAS-w+ were heat shocked at various stages of development to drive expression of the flipase gene (under expression of the hsp70 promoter: hs-flip). The Flipase protein then excised the y+ flip-out cassette from tub>y+>Gal4 and activated the tubulin promoter-driven expression of the Gal4 gene. The expression of Gal4 protein then drove expression of the UAS-w+ transgene, thereby restoring white gene function to that cell. By controlling the time and the extent of the heat shock, variable numbers of cells expressing pigment could be restored to the retina at different times of development.
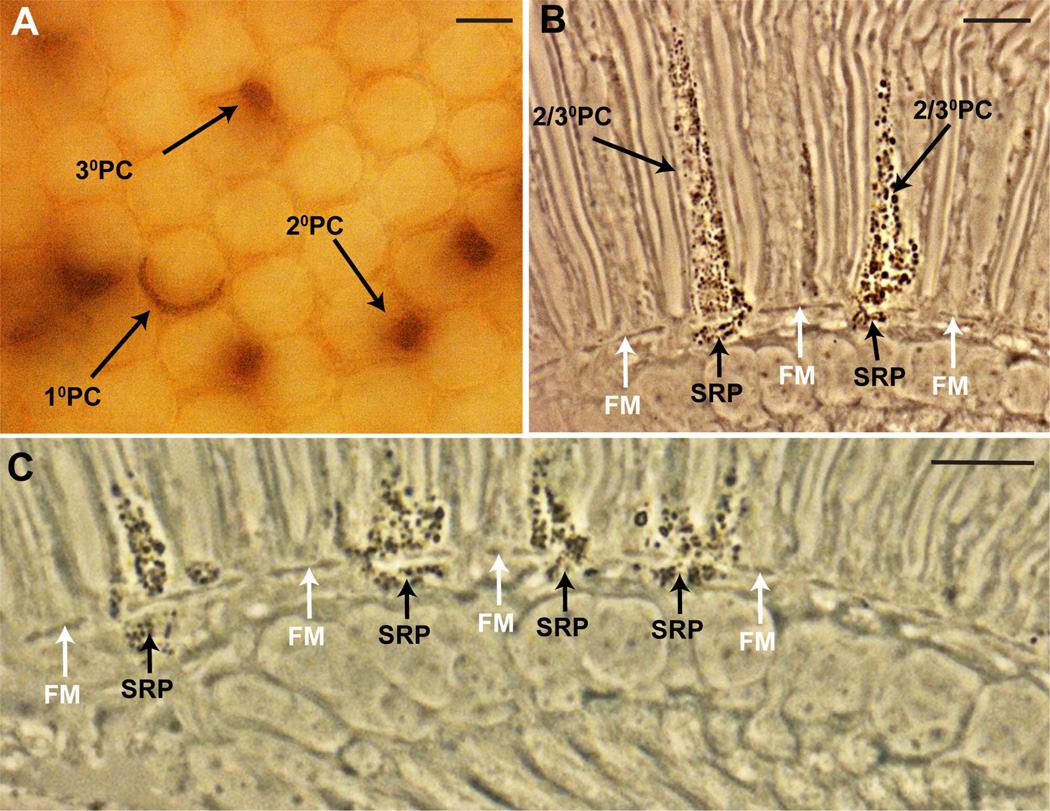
Before eyes were embedded for sectioning, they were examined by using brightfield microscopy to assess the extent and types of mosaicism induced. Figure 3A shows an example; primary, secondary, and tertiary pigment cells are all labeled, and there appears to be a random restoration of pigment to the cells of the retina by this method.
Figure 3.
The presence of pigment in the secondary/tertiary pigment cells corresponds with pigment in the underlying subretinal pigment layer. All images are from flies of the genotype w0; tub>y+>Gal4, UAS-w+ heat shocked in the first 2 days of pupation to produce a random array of cells expressing pigment. A: Cross-sectional brightfield image from a whole mount eye (before embedding for sectioning) focused to the level of the lenses. A random array of pigment cell types is evident. Primary pigment cells (1°PC) appear as crescent shaped, and surround half of a lens unit. The secondary pigment cells (2°PC) are located between two adjacent ommatidia, whereas the tertiary pigment cells (3°PC) are located at the vertices between three ommatidia. B: Longitudinal section through an eye similar to the one shown in A viewed with phase contrast microscopy. Pigment is evident as dark granules. Two cells of the secondary/tertiary pigment cell class (2/3°PC) express pigment, and each has a corresponding patch of subretinal pigment (SRP) beneath the fenestrated membrane (FM; white arrows). C: An image similar to B, but enlarged in the basal region to show that whenever subretinal pigment is present there is pigment in the overlying pigment cells of the retina. Scale bar = 10 µm in A–C. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
These eyes were then sectioned and examined by using phase contrast microcopy (Fig. 3B,C). Mosaics induced during the first 2 days of pupation showed a clear correlation between the presence of pigment in the secondary/tertiary pigment cells, and the subretinal pigment. This is most striking in eyes in which only a few cells had pigment restored; when the cell with restored pigment was a secondary/tertiary type, then a corresponding patch of pigment was observed in the directly underlying subretinal region (Fig. 3B). Furthermore, whenever pigment was detected in the subretinal layer, there was always corresponding pigment in the overlying pigment cells of the retina (Fig. 3C).
These results are not consistent with the model hypothesizing that presumptive pigment cells delaminate from the retina during the third larval instar and populate the subretinal region. The heat shocks that restore pigmentation were performed in the pupal phase, after the time that the cells would have delaminated. Because the method randomly labels individual cells, then delaminated cells would appear as individually pigmented cells beneath the fenestrated membrane, and they would not show any correlation with pigment in the overlying retina. The strict association of subretinal pigment with overlying pigment cells argues otherwise: that the subretinal pigment is directly supplied by the pigment cells of the retina.
The defining distinction between the secondary and tertiary pigment cells is their position in the ommatidial array: secondary pigment cells lie between two adjacent ommatidia, whereas tertiary pigment cells are found at alternate vertices between three ommatidia (Ready et al., 1976). In longitudinal section these cell types cannot be distinguished, and the question arose as to whether one or both of these were responsible for supplying the subretinal pigment. Some pigment cells of the retina showed considerably more subretinal pigment than others. However, whether this variation correlates with the secondary/tertiary distinction remains unclear.
The pigment rim is made from secondary/tertiary-like pigment cells that encircle the whole eye. Scrutiny of individually labeled cells in the pigment rim showed no associated subretinal pigment (not shown). This finding suggests that the subretinal pigment layer is derived from the secondary/tertiary pigment cells of the ommatidia, without a contribution from the pigment rim cells.
When heat shocks were performed later than 48 hours after pupation, pigmentation was no longer restored in the emerging adult eyes, frustrating efforts to determine whether there was a time in later pupation when the subretinal pigment no longer correlated with the overlying secondary/tertiary pigment cells. Thus there is a time window in the retina after which restoration of white gene function cannot restore the ability of the cells to make pigment. This phenomenon was first described by Steller and Pirrotta (1985).
DISCUSSION
The data presented in this paper collectively argue that the subretinal pigment of the Drosophila eye is derived from the overlying secondary and tertiary pigment cells of the retina proper. First, rescue of the pigmentation pathway (restoration of white gene function in the retina alone (w0; GMR-w+) restores pigment to the subretinal region. Because the white gene is cell autonomous (Beadle and Ephrussi, 1936), it can be inferred that the subretinal pigment is (at some point) derived from the retina. Second, the pigment composition of the subretinal layer (ommochromes and pteridines) is the same as that found in the secondary and tertiary pigment cells, and not other cells of the retina. Third, restoration of pigment biosynthesis to secondary and tertiary pigment cells during the pupal phase correspondingly rescues pigmentation in the directly underlying subretinal layer.
A number of scenarios can be considered for how the subretinal pigment becomes deposited. In one scenario, the pigment is deposited during the pupal phase and is not replenished during adult life. Here, the pigment cell feet may project below the fenestrated membrane during pupal life and then become pinched off, separating them from their parent cell and leaving them as isolated islands of pigment. A second scenario envisages a mechanism whereby the pigment cells actively “pump” the pigment below the fenestrated membrane to populate the subretinal region. This may occur only in the pupal stage without any pigment replenishment throughout later life, or, it may continue throughout the adult phase. A third scenario depicts the pigment in the retina and its subretinal counterpart as contained within a single cell. That is, the 2°/3° pigment cells have thin projections that pass through the fenestra connecting the distinct parts of the cells. However, the descriptions of Cagan and Ready (1989) show the fenestra tightly plugged with cone cell feet and the photoreceptor axons, and if there are any direct connections between the pigment cells and the subretinal pigment layer, then they will likely be extremely slender.
ACKNOWLEDGMENTS
I thank Yannis Mavromatakis for comments on the manuscript and assistance with figure production, and Hina Patel for comments on the manuscript.
Grant sponsor: National Institutes of Health; Grant number: R01 EY 012536.
LITERATURE CITED
- Beadle GW, Ephrussi B. The differentiation of eye pigments in Drosophila as studied by transplantation. Genetics. 1936;21:225–247. doi: 10.1093/genetics/21.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet B, Conolly K, Beck J. Phenogenetic studies on visual acuity in Drosophila melanogaster. J Insect Physiol. 1967;14:855–860. [Google Scholar]
- Cagan RL, Ready DF. The emergence of order in the Drosophila pupal retina. Dev Biol. 1989;136:346–362. doi: 10.1016/0012-1606(89)90261-3. [DOI] [PubMed] [Google Scholar]
- Campbell G, Goring H, Lin T, Spana E, Andersson S, Doe CQ, Tomlinson A. RK2, a glial-specific homeodomain protein required for embryonic nerve cord condensation and viability in Drosophila. Development. 1994;120:2957–2966. doi: 10.1242/dev.120.10.2957. [DOI] [PubMed] [Google Scholar]
- Choi KW, Benzer S. Migration of glia along photoreceptor axons in the developing Drosophila eye. Neuron. 1994;12:423–431. doi: 10.1016/0896-6273(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Edwards TN, Meinertzhagen IA. The functional organisation of glia in the adult brain of Drosophila and other insects. Progress in neurobiology. 2010;90:471–497. doi: 10.1016/j.pneurobio.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay BA, Wolff T, Rubin GM. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994;120:2121–2129. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- Kirschfeld K, Franceschini N. A mechanism for the control of the light flow in the rhabdomeres of the complex eye of Musca. Kybernetik. 1969;6:13–22. doi: 10.1007/BF00288624. [DOI] [PubMed] [Google Scholar]
- Kretzschmar D, Poeck B, Roth H, Ernst R, Keller A, Porsch M, Strauss R, Pflugfelder GO. Defective pigment granule biogenesis and aberrant behavior caused by mutations in the Drosophila AP-3beta adaptin gene ruby. Genetics. 2000;155:213–223. doi: 10.1093/genetics/155.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready DF, Hanson TE, Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. 1976;53:217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Carlson SD. The fine structure of neuroglia in the lamina ganglionaris of the housefly, Musca domestica L. Journal of neurocytology. 1983;12:213–241. doi: 10.1007/BF01148463. [DOI] [PubMed] [Google Scholar]
- Steller H, Pirrotta V. Expression of the Drosophila white gene under the control of the hsp70 heat shock promoter. EMBO J. 1985;4:3765–3772. doi: 10.1002/j.1460-2075.1985.tb04146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson A. Patterning the peripheral retina of the fly: decoding a gradient. Dev Cell. 2003;5:799–809. doi: 10.1016/s1534-5807(03)00326-5. [DOI] [PubMed] [Google Scholar]
- Waddington CH, Perry MM. Inter-retinular fibres in the eyes of Drosophila. J Insect Physiol. 1963;9:475–478. [Google Scholar]
- Winberg ML, Perez SE, Steller H. Generation and early differentiation of glial cells in the first optic ganglion of Drosophila melanogaster. Development. 1992;115:903–911. doi: 10.1242/dev.115.4.903. [DOI] [PubMed] [Google Scholar]
- Wolff T, Ready DF. Cell death in normal and rough eye mutants of Drosophila. Development. 1991;113:825–839. doi: 10.1242/dev.113.3.825. [DOI] [PubMed] [Google Scholar]
- Wolff T, Ready DF. Pattern formation in the Drosophila retina. In: Arias MBaAM., editor. The development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 1277–1325. [Google Scholar]
- Ziegler I. Genetic aspects of ommochrome and pterin pigments. Adv Genet. 1961;10:349–403. [Google Scholar]