Abstract
Foxp3+ regulatory T cells (Tregs) have a well-characterized role in limiting autoimmunity and dampening deleterious immune responses. However, a potential consequence of the immunosuppressive function of Tregs can be the limitation of protective immunity to infectious pathogens. Parasitic infections are a potent stimulus for the generation of Treg responses, which may be beneficial to both the parasite and host by promoting persistence of infection and limiting immune-mediated pathology, respectively. In the present study, we explore the functional role of Tregs following low-dose infection with the intestinal helminth parasite Trichuris muris, which yields a chronic infection due to inefficient induction of Th2 responses. Early Treg depletion after infection resulted in expedited worm clearance, and was associated with reduced Th1-mediated inflammation of the intestinal environment. Interestingly, this protective immunity was lost, and worm burden enhanced, if Tregs were depleted later once the infection was established. Early and late Treg depletion resulted in enhanced Th2 and reduced Th1 cytokine and humoral responses. Blockade of the Th2 cytokine IL-4 resulted in a moderate increase in Th1, but had no effect on worm burden. Our findings suggest that Tregs preferentially limit Th2 cell expansion, which can impact infections where clear immune polarity has not been established. Thus, the impact of Treg depletion is context and time dependent, and can be beneficial to the host in situations where Th1 responses should be limited in favor of Th2 responses.
Keywords: Th1/Th2 cells, Parasitic-Helminth, Regulatory T Cells, Mucosa
Introduction
Intestinal helminth parasites are large multicellular organisms that can establish long term chronic infections lasting for years. Resistance to infection is typically mediated by a T-helper-2 (Th2) response that is characterized by the production of cytokines such as IL-4, IL-5 and IL-13(1). It has been suggested that a key part of the survival strategy of helminth is immune evasion through induction of immune suppression via multiple mechanisms(2). The role of Foxp3+ regulatory T cells (Tregs) during parasitic infection has generated considerable interest since Treg populations expand following parasite infection and exposure to parasite excretory/secretory (E/S) antigens(3–9), leading to the hypothesis that helminths directly drive Treg induction to avoid immune-mediated parasite expulsion(10). Helminth-mediated Treg induction may also have beneficial effects for the host, such as reduced immunopathology(11, 12).
Studies investigating the functional role of Tregs in helminth immunity and inflammation in the intestine have used various methods of Treg depletion, including antibody-mediated depletion and a genetic model to specifically ablate Tregs by administration of diphtheria toxin(11, 12). However, the approaches used led to incomplete or transient Treg ablation leading to somewhat conflicting results regarding the benefits of Tregs in helminth infections and their influence on immunopathology. Antibody-mediated depletion studies with the filarial parasite Litomosoides sigmodontis have shown that Treg depletion can expedite parasite clearance and amplify Th2 cytokine production(13–15). These studies utilized high doses of parasite infection that promote robust Th2 responses, and thus the effect of Treg depletion on the polarization of the immune response is less clear. Recently, it has been shown that Treg depletion can skew the T helper polarization preferentially towards the Th2 lineage, increasing the ratio of IL-4:IFNγ producing cells(16). A Th2 cell response predominates due to a preferential ability of Tregs to control Th2 cell expansion through the induction of apoptosis. This result was obtained independent of a disease setting and thus the physiological and functional ramifications of a Treg depletion-mediated shift in T helper cell responses in a disease setting are currently unknown.
Trichuris muris is a well-characterized murine helminth infection model that is closely related to the human whipworm Trichuris trichuria(17). During T. muris infection, the polarization of the T helper cell response is critical to the infection outcome as a Th2-cell dominated response confers resistance while a Th1-cell dominated response confers susceptibility to chronic infection(18, 19). While most mouse strains are resistant to high doses of T. muris infection, mice given a low dose of T. muris exhibit a chronic infection due to a more Th1 polarized response(20, 21). Low dose infection models may more closely mirror the human infection patterns(21), thus determining the role of Tregs in this context is critical. In the present study, we explore whether Treg deletion during a low dose T. muris infection is beneficial to the host, via induction of a more robust Th2 cell response and expedited worm clearance, or more detrimental, due to immune hyper-activation and increased Th1 or Th2 cytokine-mediated pathology. Our results demonstrate that Tregs preferentially block effector Th2 responses during T. muris infection and thus Treg ablation protects the host from worm-driven intestinal pathology. Our study also identifies for the first time that the outcome of Treg depletion is temporal, and in this model, confers beneficial effects to the host (reduced worm burden and histopathology) only when Tregs are targeted early during the onset of infection, while Treg depletion later in infection can enhance parasite burden and immune pathology.
Materials and Methods
Mice and parasites
Foxp3DTR-GFP mice (100% C57BL/6 based on 96 marker SNP analysis) were provided by A.Y. Rudensky (Sloan-Kettering Institute)(22). C57BL/6 mice were purchased from The Jackson laboratory (Bar Harbor, Maine). Mice were 8–10 weeks of age at the time of infection. T. muris embryonated eggs and excretory/secretory antigen were generated as previously described(23). For the low dose infection, embryonated eggs were individually counted using a dissecting microscope and aliquotted. Mice were infected by oral gavage with 30 embryonated eggs in a volume of 200µl (low-dose infection). All experiments were performed in American Association for the Accreditation of Laboratory Animal Care-accredited, specific-pathogen-free, MNV-free and Helicobacter-free facilities at St. Jude Animal Resource Center in accordance with federal, state and institutional guidelines and all protocols were approved by the St. Jude Animal Care and Use Committee.
Experimental design
For all experiments, mice were infected with 30 embryonated T. muris eggs via oral gavage on day 0, followed by Treg depletion (Early versus Late) by five intra-peritoneal (i.p.) injections of 10µg/kg diphtheria toxin (DT) (Sigma-Aldrich, St. Louis, MO) in 200µl of sterile PBS or sterile PBS alone, and harvested on day 35. For the “Early DT” experiments, mice were administered DT or PBS on days 0, 2, 4, 6 and 8 while for the “Late DT” experiments, DT or PBS was administered on days 9, 11, 13, 15 and 17. Optimal dosage of DT was determined by in vivo titration to ensure maximal Treg depletion in Foxp3DTR-GFP mice with no apparent toxicity in C57BL/6 mice. For experiments involving IL-4 neutralization, mice received three i.p. injections of 0.5mg anti-mouse-IL-4 antibody (Clone: 11B11, BioXCell) or isotype control (Rat IgG1) on days 2, 5 and 8 (1.5 mg/mouse). For all the experiments, Tregs were allowed to recover and mice harvested on day 35 post-infection for assessment of lumenal adult worm presence as well as other inflammation parameters (cytokine and humoral responses and cecum pathology). For worm counting, the cecum was extracted and opened with scissors. Fecal matter was shaken out in PBS and worms were picked out under a dissecting microscope with fine-tipped forceps.
Histology
Mouse ceca were harvested, flushed with PBS, fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned and stained with hematoxylin and eosin (H&E). All sections were scored by a blinded observer. Sections were scored for inflammation severity (0 = Absent; 1 = Minimal; 2 = Mild; 3 = Moderate; 4 = Marked; 5 = Severe), Ulceration (0 = normal; 1 = mild); 2 = moderate; 3 = severe), Hyperplasia (0 = normal; 1 = mild; 2 = moderate; 3 = severe) and Extent (0 = normal; 1 = minimal; 2 = mild; 3 = moderate; 4 = severe). Total inflammatory score was calculated as (Inflammation severity + Ulceration + Hyperplasia) × Extent. Submucosal edema was scored separately (0 = normal; 1 = minimal; 2 = mild; 3 = moderate; 4 = severe).
Flow cytometry
For Foxp3 staining, cells were surface stained with anti-CD4-Pacific Blue (clone GK1.5; Biolegend, San Diego, CA) and intracellularly stained with anti-Foxp3-PE (clone FJK-16s, eBioscience). For intracellular cytokine staining, cells were surface stained, fixed, and intracellularly stained with anti-IL-4-PE (clone 11B11; Biolegend), anti-IFNγ-PE/Cy7 (clone XMG1.2; Biolegend), anti-IL-17A PerCP/Cy5.5 (clone TC11-18H10.1; BioLegend) and anti-IL-13 eFluor660 (clone eBio13A, eBioscience) using Cytofix/Cytoperm kit (BD Biosciences, San Jose, CA) following the manufacturer’s instructions.
Cytokine analysis
Draining (Mesenteric) and Non-draining (Axillary and Brachial) lymph nodes were removed and mashed over 70µm cell strainers. For intracellular cytokine staining, cells were stimulated at 5×106 cells/ml in DMEM media with PMA/Ionomycin (100ng/ml and 500ng/ml, respectively; Sigma-Aldrich) or 5µg/ml of Trichuris E/S antigen for 12–16 hours in the presence of Monensin (1:1000; eBioscience).
Antibody ELISAs
For T. muris antigen specific IgG1 and IgG2a ELISAs, microtiter plates were coated with 5µg/ml T. muris excretory/secretory antigen. Plates were then blocked in 1% BSA. Serially diluted serum samples were incubated at room temperature starting with an initial serum dilution of 1/10 in PBS + 1% BSA. Antigen-specific IgG1 and IgG2a were detected with biotinylated anti-mouse IgG1 and IgG2a (clones A85-1 and R19-15, respectively; BD Biosciences) followed by incubation with streptavidin-HRP and developed with TMB substrate. The reaction was terminated with 1N H2SO4 and OD450 was determined with a spectrophotometer. Total serum IgE was determined using the Mouse IgE ELISA MAX kit (Biolegend) following the manufacturer’s instructions.
Statistical analysis
All results are expressed as the mean ± SEM. Statistical analysis was performed using GraphPad Prism software using unpaired Student’s t-test or 2way ANOVA analysis. p values were categorized into the following levels: *p<0.05, **p<0.01, ***p<0.001, NS, non-significant.
Results
Early Treg depletion following infection expedites T. muris clearance
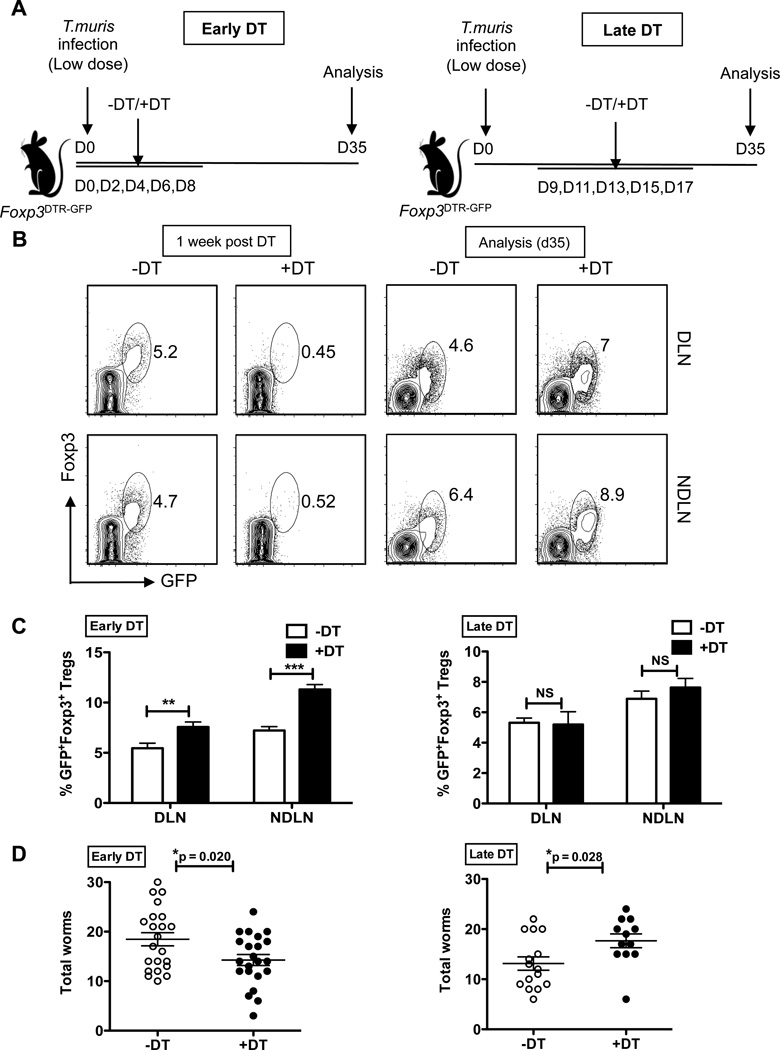
During a low dose infection, the ability of a highly resistant strain (such as BALB/K) versus a less resistant strain (C57BL/6) to clear T. muris infection can be clearly delineated at 35 days post-infection(18). Thus, we chose this time point to analyze the ability of Treg depleted mice to clear T. muris infection. Foxp3DTR-GFP mice can sustain near complete Treg depletion for up to two weeks, at which point significant mortality is seen due to the ensuing autoimmunity(22). Therefore, our experimental design involved DT administration for a maximum of 10 days to avoid increased mortality associated with long-term treatments. To gain a comprehensive assessment of the role of Tregs during immunity to this intestinal helminth parasite, we used two approaches for Treg deletion – “Early DT” involved DT administration during the initial stages of infection (day 0 – day 8), while “Late DT” involved Treg depletion once the infection was established (day 9 – day 17) (Figure 1A). To validate that Tregs could effectively be depleted following DT administration, we infected separate cohorts of mice with a low (30) dose of T. muris eggs, performed early versus late DT treatments, and analyzed the mice one week after the last DT treatment. We observed a near complete absence of Foxp3+ cells within the CD4+ T cell fraction from both the gut-draining mesenteric lymph node (MLN) and the non-gut-draining axillary and brachial lymph nodes (NDLN) (Figure 1B). However, by day 35, Tregs showed a robust recovery with both DT treatment approaches (Figures 1B and 1C). Interestingly, early Treg depletion resulted in a modest but significantly increased ability to clear T. muris infection by day 35, while late Treg depletion reduced worm clearance (Figure 1D). These data reveal a differential role for Tregs at different time points during infection on worm burden.
Figure 1. Early Treg depletion accelerates clearance of low dose T. muris infection.
(A) Schematic of the Early (left) versus Late (right) DT treatment regimens for depletion of Tregs following low dose T. muris infection. Mice were infected via oral gavage with 30 embryonated T. muris eggs on day 0, DT was administered five times every other day using one of the two approaches (Early DT: d0–d8 or Late DT: d9–d17), and mice analyzed at d35.
(B) Representative flow cytometric plots depicting Foxp3+ Treg depletion and recovery following treatment with DT (+DT) or PBS (−DT). MLN and NDLN cells isolated from infected Foxp3DTR-GFP mice, and surface stained for CD4 and intracellular Foxp3. Cells gated on CD4+ T cells and analyzed for percentage of GFP+Foxp3+ Tregs one-week after the last DT treatment (left) and at the time of analysis, d35 (right).
(C) Treg recovery assessed by the percentages of GFP+Foxp3+ Tregs at day 35 for Early DT (left) and Late DT (right) treatment approaches. Data averaged from two independent experiments (n=8–10 mice per group).
(D) Total worm burden at day 35 from the cecum of mice infected with low dose T. muris as in (A) and treated with Early and Late DT approaches. Each data point on the scatter plots represents an individual mouse with mean indicated. Data averaged from 4 independent experiments for Early DT approach (n=20–22 mice per group) and 3 independent experiments for Late DT approach (n=12–15 mice per group).
*p<0.05, **p<0.01, ***p<0.001, NS, not significant (Unpaired Student’s t-test).
Differential T. muris-mediated immunopathology following early versus late Treg depletion
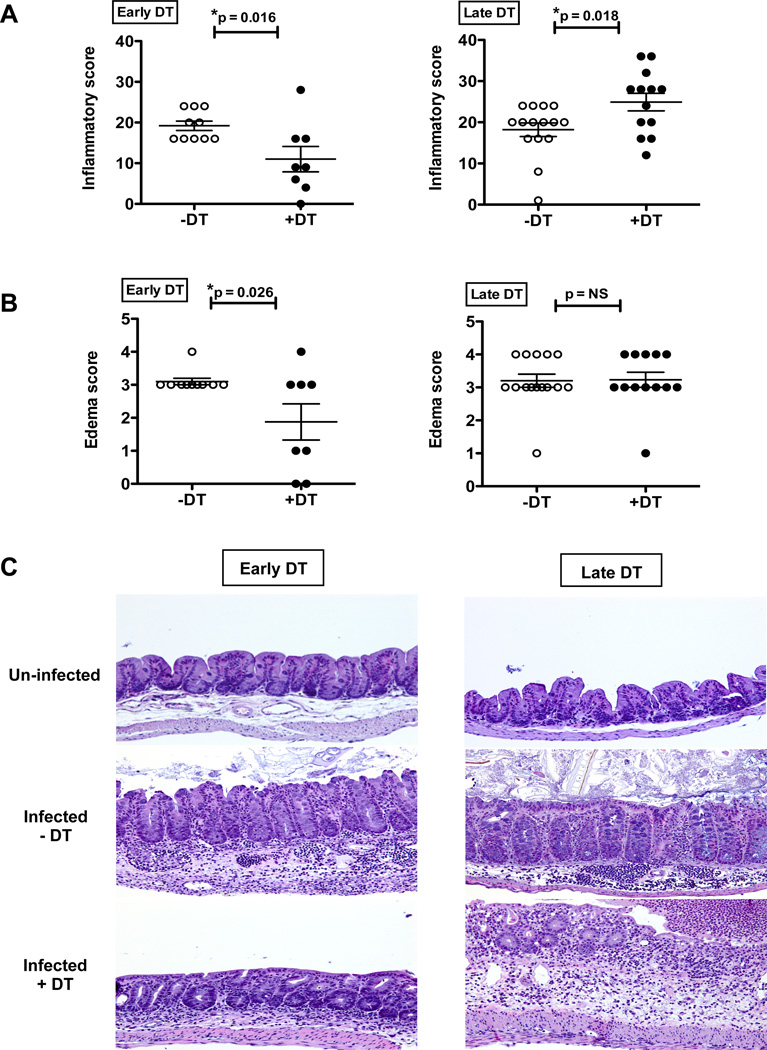
We next assessed the impact of Treg depletion on Trichuris infection-induced intestinal inflammation. Since low dose T. muris infection in C57BL/6 mice results in Th1 cytokine-mediated chronic infection and intestinal inflammation, we hypothesized that Treg depletion in this model would exacerbate tissue pathology consistent with previous studies using different models of helminth infection(11, 12). Surprisingly, histological analysis of intestinal sections revealed that total inflammation scores were significantly lower in the cecum of T. muris-infected mice treated with early DT compared with control mice (Figure 2A–C). Although the numbers, distribution, and composition of inflammatory cell infiltrates in the cecal mucosa were similar in both treatment groups (+DT, −DT), higher overall inflammation severity scores were assigned to the untreated mice (−DT) largely due to the much greater extent and severity of submucosal edema (Figure 2B). In contrast to the effects with early DT, the late DT treatments in T.muris infected mice increased intestinal histopathology (Figures 2A–C). The most notable difference between the mice receiving late DT and not treated with DT (−DT) was the lower number of intraepithelial globule leukocytes (also known as mucosal mast cells) and lymphocytes in the mucosa of late DT-treated mice compared to untreated mice (data not shown). Overall, the histopathology results were concordant with the worm burden data for early versus late DT treatments (Figure 1D), suggesting a differential effect of Tregs on parasite burden and intestinal inflammation during the early versus late stages of infection.
Figure 2. Diminished intestinal pathology upon early Treg depletion in low dose T.muris infection.
(A) Inflammatory score as assessed by H&E staining of cecal sections of low dose T. muris infected mice treated with Early DT (left) and Late DT (right) approaches and analyzed at day 35.
(B) Submucosal edema score from mice treated as in (A).
(C) Representative H&E stained cecal sections from mice treated as in (A). Cecal sections from un-infected mice used as controls.
Each data point on scatter plots represents an individual mouse with mean indicated. Data represents two independent experiments for Early DT (n=8–10 mice per group) and three independent experiments for Late DT (n=12–15 mice per group).
*p<0.05, NS, not significant (Unpaired Student’s t-test).
Treg depletion during T. muris infection leads to induction of potent Th2 responses
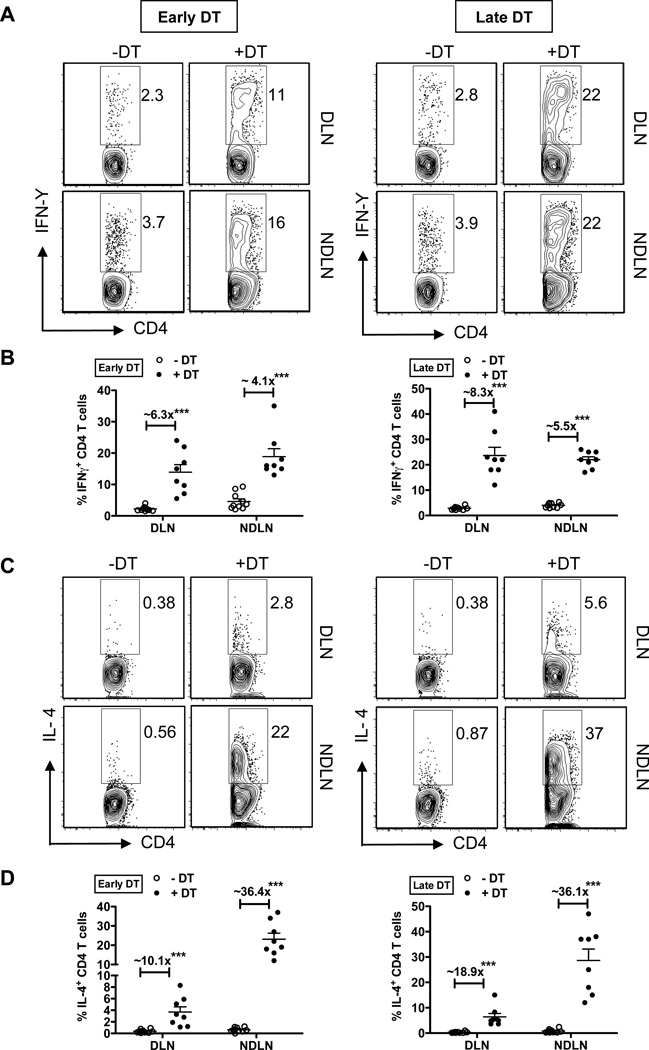
Since tissue pathology and host susceptibility are associated with the balance between a protective Th2 cytokine response and a detrimental Th1 cytokine response, we assessed alterations in the polarity of the immune response following Treg depletion in low dose T.muris-infected mice. It was recently reported that Treg depletion results in a preferential expansion of Th2 cells over Th1 cells(16). We hypothesized that this skewed response following Treg depletion could impact disease outcome following low dose T. muris infection. Treg cells are known to curtail excessive Th1, Th2 and Th17 effector responses to maintain immune homeostasis(24, 25). Consistent with published reports, there was a significant expansion of both IFNγ and IL-4 producing CD4+ T cells in the gut-draining (DLN) and non-gut-draining axillary and brachial lymph nodes (NDLN) following Treg depletion (Figure 3A–D). The overall fold-induction of IFNγ+ CD4+ T cells (as assessed by the ratio of +DT/−DT) was higher in the DLN (~6.3 fold for early DT and ~ 8.3 fold for late DT) relative to the NDLN, consistent with the increased Th1 polarization in the gut following low dose T. muris infection (Figure 3A and 3B). The percentages of IL-4 producing CD4+ T cells were strikingly high in the NDLNs, compared to DLNs for both the DT treatment approaches (Figure 3C and 3D). This could be attributed to the counter-regulation to Th2 induction mediated by the increased Th1 responses at the site of infection (DLN), while Th2 responses are unrestrained in the NDLNs following Treg depletion. Importantly, however, the fold induction of IL-4+ CD4+ T cells (ratio of +DT/−DT) is higher (~10–20 fold in DLN and ~36 fold in NDLN) relative to the fold induction of IFN+ CD4+ T cells (less than 10-fold at both DLN and NDLN) with the two DT treatments (Figure 3B and 3D). IL-17+ CD4 T cells were also increased following early and late Treg depletion (Supplementary Figure 1A and 1B), although the overall induction was lower than that of IFNγ and IL-4 producing CD4+ T cells. These results highlight the preferential induction of potent Th2 responses following Treg depletion that can affect the disease outcome in scenarios where clear immune polarity is not established. Thus, in the low dose T. muris infection setting, Tregs limit Th2 effector responses far more strongly than Th1 responses, thereby inadvertently restraining protective anti-helminthic immunity. Importantly, while comparable Th2 responses were induced following both early versus late Treg depletion, these responses have a favorable outcome to the host (reduced worm burden and parasite-driven intestinal pathology) only when unleashed during the initial stages of infection, but fail to have an effect once the infection is well established.
Figure 3. Preferential Th2 expansion upon Treg depletion.
(A) Representative flow plots gated on CD4+ T cells stained for the Th1 cytokine, IFNγ. DLN and NDLN cells were isolated from T. muris infected mice treated with Early DT (left) or Late DT (right) approaches and analyzed at day 35. Cells were stimulated with PMA and Ionomycin for 12–16 hours in the presence of Monensin for cytokine secretion analysis.
(B) Percentages of IFN+ CD4 T cells in the DLN and NDLNs following Treg depletion in T.muris infected mice as discussed in (A). Fold induction of IFNγ+ CD4 T cells (ratio of +DT/−DT) indicated.
(C) Representative flow plots gated on CD4+ T cells stained for the Th2 cytokine, IL-4. DLN and NDLN cells were isolated from T. muris infected mice as discussed in (A).
(D) Percentages of IL-4+ CD4 T cells in the DLN and NDLN following Treg depletion in T. muris infected mice as discussed in (A). Fold induction of IL-4+ CD4 T cells (ratio of +DT/−DT) indicated.
Each data point on the scatter plots represents an individual mouse with mean indicated. Data represents two independent experiments for both Early and Late DT treatments (n=8–10 mice per group).
***p<0.001; NS, not significant (Unpaired Student’s t-test).
Treg depletion alters T. muris-specific Th1/Th2 cell polarization and humoral responses
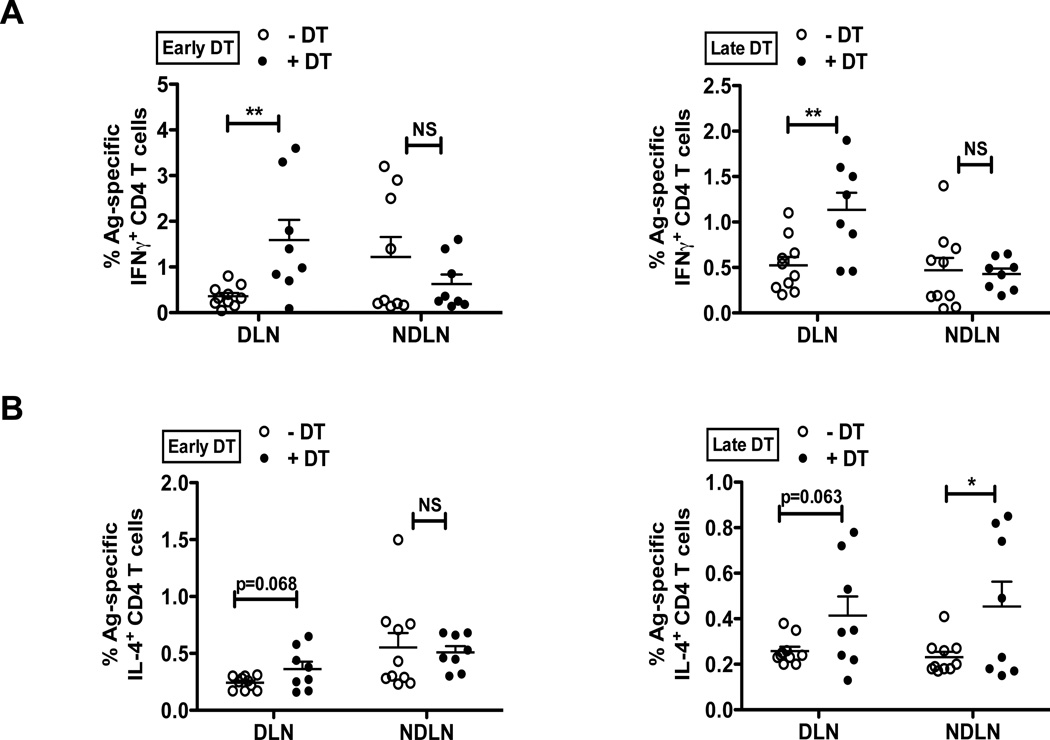
To determine if T. muris-specific immune parameters were altered following Treg depletion in low dose T. muris-infected mice, we next assessed the percentages of Ag-specific IFNγ+ and IL-4+ CD4 T cells (Figure 4). The overall Th1/Th2 trend in the DLN and NDLN following both early versus late Treg depletion was comparable to the effects observed following polyclonal stimulation (Figure 3), however, the magnitude of response was lower as anticipated. Consistent with the induction of chronic Th1 response following low dose T. muris infection, Ag-specific IFNγ+ CD4+ T cells were significantly elevated in the mesenteric lymph nodes (DLN, site of infection) following Treg depletion, compared to the NDLNs (Figure 4A). While there was a trend towards increased percentage of Ag-specific IL-4+ CD4+ T cells following DT treatment, the effects did not reach significance (Figure 4B), thus further corroborating that only the Th1 induction in this model was T. muris-specific, while the potent Th2 responses noted following polyclonal stimulation (Figure 3C and 3D) were attributed primarily to Treg ablation.
Figure 4. Treg depletion alters T. muris-specific Th1/Th2 cell polarization.
(A) Percentages of Ag-specific IFNγ+ CD4 T cells in the DLN and NDLNs following Treg depletion by Early DT (left) and Late DT (right) approaches in T.muris infected mice and analyzed at day 35. Cells were stimulated with T. muris antigen for 12–16 hours in the presence of Monensin for cytokine secretion analysis.
(B) Percentages of Ag-specific IL-4+ CD4 T cells in the DLN and NDLNs following Treg depletion as discussed in (A).
Each data point on the scatter plots represents an individual mouse with mean indicated. Data represents two independent experiments for both Early and Late DT treatments (n=8–10 mice per group).
*p<0.05, **p<0.01; NS, not significant (Unpaired Student’s t-test).
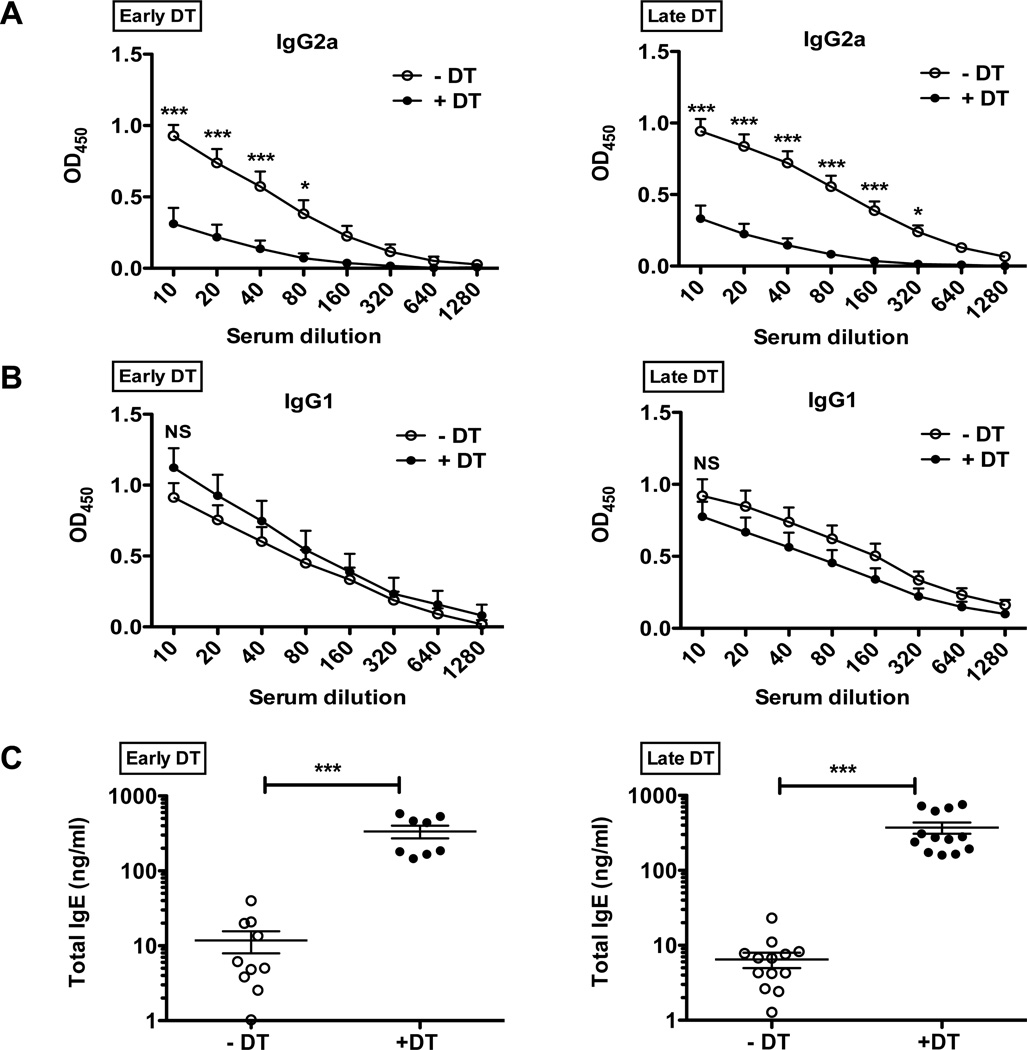
Parasite-specific serum IgG1 and IgG2a titers correlated with Th2 IL-4-dependent IgG1 class switching and Th1 IFN-dependent IgG2a class switching, respectively(26). While low dose T. muris infection of non-DT treated (−DT) Foxp3DTR-GFP mice resulted in robust Ag-specific IgG2a titers, parasite-specific IgG2a was nearly undetectable in both early and late Treg-depleted mice (Figure 5A), consistent with potent induction of Th2 responses and a shift away from Th1 with Treg depletion. Antigen-specific IgG1 titers, however, did not show a significant alteration following Treg depletion at either time point (Figure 5B). Elevation in total serum IgE is another characteristic response to helminth infection and is associated with a Th2 cytokine response(27). Treg depletion causes a substantial increase in total IgE (Figure 5C), consistent with Tregs preferentially curbing Th2 responses. Again, comparable humoral responses are induced with both the early and late DT treatment approaches, reinforcing the observation that the enhanced Th2 cytokine and humoral responses following Treg depletion can mediate effective parasite clearance only during the onset of infection but not once it becomes established. Indeed, enhanced Th2 responses during the latter stages of T. muris infection appear to negatively impact worm burden and inflammation.
Figure 5. Antibody isotype responses shift during T. muris infection upon Treg depletion.
(A) Antigen-specific IgG2a as assessed by ELISA. Serum was obtained from T. muris infected mice following Treg depletion by Early DT (left) and Late DT (right) approaches and analyzed at day 35. Serial dilution of serum was performed starting with 1/10 initial dilution.
(B) Antigen-specific IgG1 as assessed by ELISA, for mice treated similar to (A).
(C) Total serum IgE as assessed by ELISA, for mice treated as in (A). Each data point on scatter plots represents an individual mouse with mean indicated.
Data represents two independent experiments for Early DT (n=8–10 mice per group) and three independent experiments for Late DT (n=12–15 mice per group).
For (A) and (B), *p<0.05, ***p<0.001, NS, not significant (Two-way ANOVA),
For (C), ***p<0.001 (Unpaired Student’s t-test).
Th2 responses unleashed following Treg depletion contribute to anti-helminthic immunity
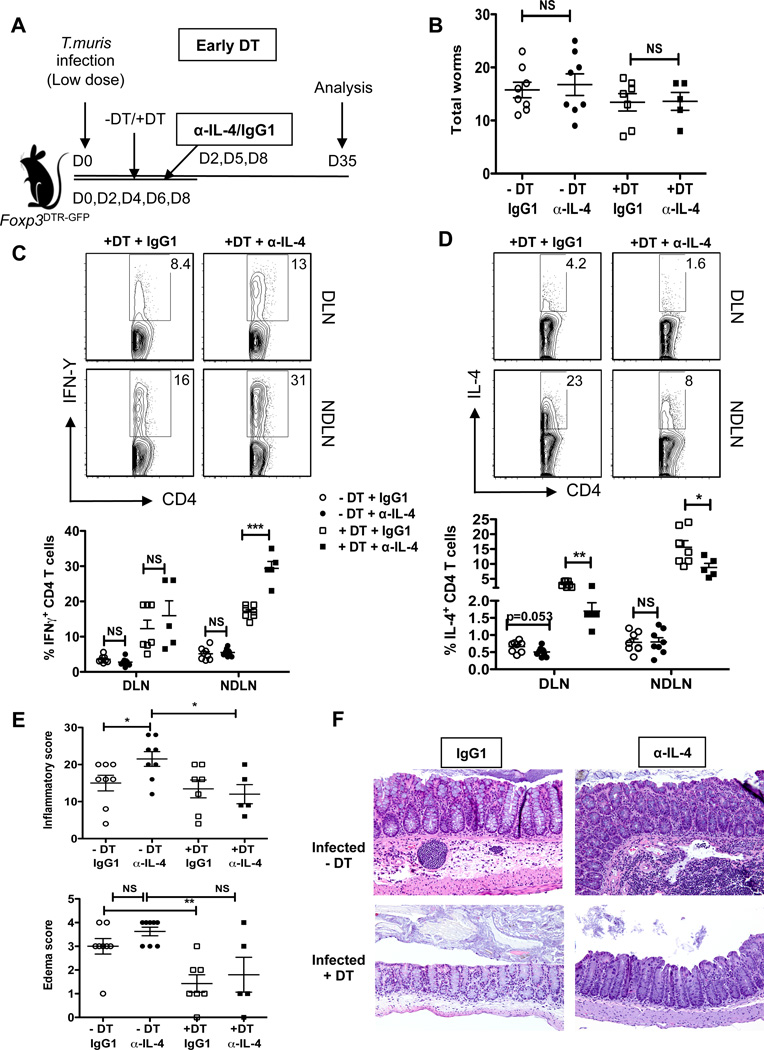
To provide further mechanistic insight, we assessed whether the increased Th2 responses, specifically IL-4, following early Treg depletion contributed to enhanced worm clearance and reduced intestinal pathology. Mice received three injections (1.5 mg/mouse total) on days 2, 5, 8 of either IL-4 neutralizing antibody (11B11) or isotype control (IgG1) during the early DT treatment regimen (d0 – d8), and worm burden and inflammation parameters assessed at d35 post-infection (Figure 6A). Treg recovery was not affected by the antibody treatments, as percentages of Foxp3+ Tregs were either comparable or augmented following DT treatment at both the DLN and NDLNs (Supplementary Figure 2A). While we observed a trend towards reduced worm burden in the DT treatment groups (+DT+IgG1 and +DT+α-IL-4) compared to untreated groups (−DT+IgG1 and −DT+α-IL-4), IL-4 neutralization did not alter worm burden (Figure 6B). Analysis of the Th1/Th2 cytokine responses demonstrated a significant reduction in the percentage of IL-4+ CD4+ T and a reciprocal increase in the percentage of IFNγ+ CD4+ T cells following IL-4 neutralization in the DT treatment groups (+DT+IgG1 and +DT+α-IL-4) (Figure 6C and 6D). Antibody treatments did not lead to any major alterations in the Th1/Th2 response in the non-DT groups (−DT+IgG1 and −DT+α-IL-4). T muris-specific IgG1 and total IgE trended towards a moderate decrease following IL-4 neutralization, however, we did not observe any increase in T. muris-specific IgG2a (Supplementary Figure 2B). Interestingly, while we did not see an increase in the worm burden following IL-4 neutralization, histological examination revealed significant increase in the overall inflammation severity and increased edema score in mice receiving the IL-4 blocking antibody (Figure 6E and 6F). Early Treg depletion resulted in reduced overall intestinal inflammation in the DT groups as expected; however, IL-4 neutralization in the DT groups did not exacerbate the pathology. Thus, while IL-4 blockade led to increased Th1 polarization and concomitantly increased immune pathology, it did not reverse the protective effects of Treg depletion. This could be due to incomplete neutralization of IL-4 (IL-4+ CD4 T cells following DT treatment were still observed in the DLN and NDLNs) and/or compensatory effects driven by other Th2 cytokines (e.g. IL-13), which was also increased following Treg depletion (Supplementary Figure 2C). It is also possible that T cell hyper-activation due to Treg depletion, rather then increased IL-4, is sufficient to reduce worm burden. Studies to further dissect these processes are warranted.
Figure 6. Treg-depletion mediated Th2 induction limits T. muris driven intestinal pathology.
(A) Schematic of IL-4 neutralization in low dose T. muris infected mice following Treg depletion by the Early DT approach. Infected mice received α-IL-4 (11B11) neutralizing antibody or isotype (IgG1) control on days 2, 5 and 8, alongside the early DT treatments (d0–d8) and were analyzed at day 35. Thus, the four treatment groups included: (−DT+IgG1, −DT+α-IL-4, +DT+IgG1 and +DT+α-IL-4).
(B) Total worm burden from ceca of T. muris infected mice as treated in (A). Worms counted at day 35 for the four treatment groups.
(C) Representative flow plots gated on CD4+ T cells stained for the Th1 cytokine, IFNγ. DLN and NDLN cells were isolated from T. muris infected mice treated with IL-4 neutralizing antibody or IgG1 isotype alongside early Treg depletion and analyzed at day 35. Cells stimulated with PMA and Ionomycin for 12–16 hours in the presence of Monensin. Data represented for only the two DT treatment groups (+DT+IgG1 and +DT+ α-IL-4).
(D) Representative flow plots gated on CD4+ T cells stained for the Th2 cytokine, IL-4. DLN and NDLN cells were isolated and treated as in (C).
(E) Inflammation and edema scores as assessed by H&E staining of day 35 cecal sections for the four treatment groups as treated in (A).
(F) Representative H&E stained cecal sections for the four treatment groups as treated in (A).
Each data point on scatter plots represents an individual mouse with mean indicated. Data represents two independent experiments (n=5–8 mice per group)
*p<0.05, **p<0.01, ***p<0.001, NS, not significant (Unpaired Student’s t-test).
Discussion
In the present study we sought to address the functional role of regulatory T cells in immune polarization in a physiological setting where the balance of polarity is critical in disease susceptibility versus resistance(18, 20). Therefore, we chose to utilize the T. muris low dose infection model. T. muris is a natural co-evolved pathogen of mice that closely parallels T. trichuria infection in humans(21). Low dose infection results in a mixed Th1/Th2 response in C57BL/6 mice associated with chronic parasitic infection and intestinal pathology.
Past studies have evaluated the role of Tregs in intestinal helminth infection through Treg depletion strategies. However, these studies have had limitations in the evaluation of the effect of Tregs on the polarization of the immune response. Firstly, the Treg depletion strategies utilizing the CD25 depleting antibody PC61 or DEREG mice have not allowed for complete or sustained Foxp3+ Treg depletion, respectively(11, 12). These shortcomings of previous Treg depletion studies have made interpretation of results difficult. Secondly, these models have utilized high doses of helminth infection that drive a strong Th2 polarization and would typically not reflect most natural human parasite infections. This high antigenic load has likely masked the subtle change in immune polarization, which, as we show, has functional ramifications in the low dose T. muris model.
Since low dose T. muris infection results in elevated Th1 mediated pathology relative to high dose infection, it was unclear to us whether Treg depletion would amplify that pathology or reduce it through the promotion of a productive Th2 response. Our data would support the interpretation that through a shift in immune polarization toward Th2, the detrimental Th1 pathology is limited following Treg ablation in this model. Despite the Treg depletion-mediated shift toward a Th2 response to T. muris low dose infection, the parasite was not completely cleared at 35 days post infection, while strongly Th2 inducing high dose infections mediate parasite clearance within 21 days. This indicates that despite the elevated Th2 response, resistance to infection was incomplete.
Physiologically a “trickle infection”, in which low levels of parasite infectious agents are ingested over time, may be more relevant to human disease than the high dose infection models that are often employed when studying parasitic infection. Studies of trickle infection with T. muris have shown that initially a Th1 response predominates in normally susceptible mice(21). However, as the antigenic load increases with increased infection, a threshold is reached that results in the establishment of a Th2 response that confers resistance. Our data would suggest that during low dose infections and trickle infections, it may be clinically relevant to target immune regulation to direct the polarity of the immune response more strongly toward Th2 and expedite worm clearance. New efforts to understand how to maximize Th2 responsiveness are warranted as parasites have proven to be highly resistant to sterilizing immunity.
It is intriguing that the Th2 response induced following Treg depletion is insufficient for complete parasite expulsion. It is possible that the strength and/or ‘quality’ of the Th2 response is insufficient. Alternatively, it is possible that other regulatory mechanisms, such as suppressive cytokines or inhibitory receptors, may compensate for the absence of Tregs. Further studies will be required to address these possibilities in more detail. Another intriguing finding is the differential impact of Tregs on parasite burden and immunopathology. While the reduced worm burden due to early Treg depletion could be mediated by increased Th2 response, it is unclear why late Treg depletion causes increased worm burden and immunopathology. While it is possible these two events are linked, further studies will be required to dissect this relationship in more detail.
Our data may also provide a new interpretation on why parasites have developed strategies to expand Tregs early during the course of infection. As has recently been demonstrated, Heligmosomoides polygyrus E/S products alone can directly induce Treg generation via the presence of a molecule that mimics the bioactivity of TGF-β(10). Instead of a broad and non-specific dampening of immunity, parasites may drive Tregs to specifically limit Th2 immunity to prolong their survival.
Supplementary Material
Acknowledgements
We would like to thank Alexander Rudensky for providing the Foxp3DTR-GFP mice. We are also very grateful to Karen Forbes, Ashley Castellaw and Amy McKenna for maintenance, breeding and genotyping of mouse colonies; Meenu Pillai and Prajwal Gurung for assistance with the generation of Trichuris antigen and eggs; and the staff of the St. Jude Animal Resource Center for animal husbandry.
This work was supported by the US National Institutes of Health (R01 AI091977 to D.A.A.V. [including a sub-contract to D.A.]), the National Cancer Institute Comprehensive Cancer Center (CA21765 to D.A.A.V.), the American Lebanese Syrian Associated Charities (D.A.A.V.) and the Australian National Health and Medical Research Council Overseas Biomedical Fellowship 613718 (P.R.G).
Abbreviations used in the paper
- Tregs
Regulatory T cells
- DT
Diphtheria toxin
- E/S
Excretory/secretory
- DLN
draining lymph node
- NDLN
non-draining lymph node
- T. muris
Trichuris muris
References
- 1.Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. 2011;11:375–388. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 2.Taylor MD, van der Werf N, Maizels RM. T cells in helminth infection: the regulators and the regulated. Trends Immunol. 2012;33:181–189. doi: 10.1016/j.it.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF, Jr, Weinstock JV. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur J Immunol. 2004;34:2690–2698. doi: 10.1002/eji.200324833. [DOI] [PubMed] [Google Scholar]
- 4.Gillan V, Devaney E. Regulatory T cells modulate Th2 responses induced by Brugia pahangi third-stage larvae. Infect Immun. 2005;73:4034–4042. doi: 10.1128/IAI.73.7.4034-4042.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J Immunol. 2006;176:3248–3256. doi: 10.4049/jimmunol.176.5.3248. [DOI] [PubMed] [Google Scholar]
- 6.Taylor JJ, Mohrs M, Pearce EJ. Regulatory T cell responses develop in parallel to Th responses and control the magnitude and phenotype of the Th effector population. J Immunol. 2006;176:5839–5847. doi: 10.4049/jimmunol.176.10.5839. [DOI] [PubMed] [Google Scholar]
- 7.Persaud R, Wang A, Reardon C, McKay DM. Characterization of the immuno-regulatory response to the tapeworm Hymenolepis diminuta in the non-permissive mouse host. Int J Parasitol. 2007;37:393–403. doi: 10.1016/j.ijpara.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Finney CA, Taylor MD, Wilson MS, Maizels RM. Expansion and activation of CD4(+)CD25(+) regulatory T cells in Heligmosomoides polygyrus infection. Eur J Immunol. 2007;37:1874–1886. doi: 10.1002/eji.200636751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McSorley HJ, Harcus YM, Murray J, Taylor MD, Maizels RM. Expansion of Foxp3+ regulatory T cells in mice infected with the filarial parasite Brugia malayi. J Immunol. 2008;181:6456–6466. doi: 10.4049/jimmunol.181.9.6456. [DOI] [PubMed] [Google Scholar]
- 10.Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, Finney CA, Greenwood EJ, Knox DP, Wilson MS, Belkaid Y, Rudensky AY, Maizels RM. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-beta pathway. J Exp Med. 2010;207:2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Elia R, Behnke JM, Bradley JE, Else KJ. Regulatory T cells: a role in the control of helminth-driven intestinal pathology and worm survival. J Immunol. 2009;182:2340–2348. doi: 10.4049/jimmunol.0802767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rausch S, Huehn J, Loddenkemper C, Hepworth MR, Klotz C, Sparwasser T, Hamann A, Lucius R, Hartmann S. Establishment of nematode infection despite increased Th2 responses and immunopathology after selective depletion of Foxp3+ cells. Eur J Immunol. 2009;39:3066–3077. doi: 10.1002/eji.200939644. [DOI] [PubMed] [Google Scholar]
- 13.Taylor MD, Harris A, Babayan SA, Bain O, Culshaw A, Allen JE, Maizels RM. CTLA-4 and CD4+ CD25+ regulatory T cells inhibit protective immunity to filarial parasites in vivo. J Immunol. 2007;179:4626–4634. doi: 10.4049/jimmunol.179.7.4626. [DOI] [PubMed] [Google Scholar]
- 14.Taylor MD, LeGoff L, Harris A, Malone E, Allen JE, Maizels RM. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J Immunol. 2005;174:4924–4933. doi: 10.4049/jimmunol.174.8.4924. [DOI] [PubMed] [Google Scholar]
- 15.Taylor MD, van der Werf N, Harris A, Graham AL, Bain O, Allen JE, Maizels RM. Early recruitment of natural CD4+ Foxp3+ Treg cells by infective larvae determines the outcome of filarial infection. Eur J Immunol. 2009;39:192–206. doi: 10.1002/eji.200838727. [DOI] [PubMed] [Google Scholar]
- 16.Tian L, Altin JA, Makaroff LE, Franckaert D, Cook MC, Goodnow CC, Dooley J, Liston A. Foxp3(+) regulatory T cells exert asymmetric control over murine helper responses by inducing Th2 cell apoptosis. Blood. 2011;118:1845–1853. doi: 10.1182/blood-2011-04-346056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cliffe LJ, Grencis RK. The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection. Adv Parasitol. 2004;57:255–307. doi: 10.1016/S0065-308X(04)57004-5. [DOI] [PubMed] [Google Scholar]
- 18.Else KJ, Hultner L, Grencis RK. Cellular immune responses to the murine nematode parasite Trichuris muris. II. Differential induction of TH-cell subsets in resistant versus susceptible mice. Immunology. 1992;75:232–237. [PMC free article] [PubMed] [Google Scholar]
- 19.Else KJ, Grencis RK. Cellular immune responses to the murine nematode parasite Trichuris muris. I. Differential cytokine production during acute or chronic infection. Immunology. 1991;72:508–513. [PMC free article] [PubMed] [Google Scholar]
- 20.Else KJ, Finkelman FD, Maliszewski CR, Grencis RK. Cytokine-mediated regulation of chronic intestinal helminth infection. J Exp Med. 1994;179:347–351. doi: 10.1084/jem.179.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bancroft AJ, Else KJ, Humphreys NE, Grencis RK. The effect of challenge and trickle Trichuris muris infections on the polarisation of the immune response. Int J Parasitol. 2001;31:1627–1637. doi: 10.1016/s0020-7519(01)00281-8. [DOI] [PubMed] [Google Scholar]
- 22.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 23.Artis D, Villarino A, Silverman M, He W, Thornton EM, Mu S, Summer S, Covey TM, Huang E, Yoshida H, Koretzky G, Goldschmidt M, Wu GD, de Sauvage F, Miller HR, Saris CJ, Scott P, Hunter CA. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. 2004;173:5626–5634. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- 24.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 25.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 26.Finkelman FD, Holmes J, Katona IM, Urban JF, Jr, Beckmann MP, Park LS, Schooley KA, Coffman RL, Mosmann TR, Paul WE. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 27.Anthony RM, Rutitzky LI, Urban JF, Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.