Abstract
Neuropsychological deficits have been associated with major depression (MD) and persist in some individuals even after symptom remission. However, it is unclear if the deficits are a consequence of MD or are pre-existing and reflect MD vulnerability. We addressed this issue by studying 117 twins from monozygotic (MZ) pairs discordant for lifetime history of DSM-III-R defined MD and 41 twins from MZ pairs in which neither twin had experienced MD. Our assessment included a structured clinical interview and measures from the WMS-III and WAIS-III. The “unaffected” twins from discordant pairs showed the same pattern of performance as their affected cotwins on measures of attention, working memory, verbal memory, and visuo-spatial processing. Compared to twins from pairs with no MD history, twins in discordant pairs had lower performance in the domains of attention, memory, visuo-spatial processing, and general knowledge. However, after adjusting for sex and age, the groups differed only on attention and general knowledge. The similar performance of twins in pairs discordant for MD suggests that familial risk for MD has a greater influence on neuropsychological functioning than individual MD history. Findings of impairment in individuals euthymic for MD are more consistent with pre-existing deficits than scarring effects of MD.
Keywords: major depression, neuropsychological impairment, risk factors, genetics, scarring effects, cognitive functioning, twins
1. Introduction
Major depression (MD) is consistently associated with attentional deficits, including selective and sustained attention, effortful processing, set-shifting and various attentional biases (Hammar et al., 2003; Lyche et al., 2010; Paelecke-Habermann et al., 2005; Weiland-Fiedler et al., 2004). MD has also been associated with other neuropsychological deficits, including impairments in memory (Hinkelmann et al., 2009) and executive functioning (Douglas and Porter, 2009), and at times verbal fluency (e.g., Gohier et al., 2009). These deficits in attention are associated with increased risk for suicidal behavior in individuals with MD (Keilp et al., 2012, 2008). Findings vary regarding the severity, duration, pattern, and persistence of the deficits after improvement or remission of active MD symptomatology (e.g., Halvorsen et al., 2012; Paelecke-Habermann et al., 2005; Weiland-Fiedler et al., 2004; Wekking et al., 2012; Xu et al., 2012). However, studies to date have not measured premorbid cognition and cannot address whether such deficits are a consequence of experiencing MD (a scarring effect) or existed prior to depression, either as a prodromal symptom or as a risk factor.
Studying identical (or monozygotic, MZ) twin pairs discordant for an outcome (e.g., MD) is a powerful method for examining the etiological relationship between the outcome and associated features (e.g., neuropsychological deficits; McGue et al., 2010). MZ pairs are matched for early family environmental and genetic factors, allowing for a clearer identification of direct mechanisms (i.e., MD causes deficits) versus indirect mechanisms (e.g., genetic factors increase risk both for depression and deficits). In the discordant pair design, the neuropsychological performance of the twin “exposed” to MD (i.e., proband), is compared to the performance of his or her cotwin who has not experienced MD. The discordant twin design has been used to study the relation of neuropsychological deficits to the etiology of schizophrenia (e.g., Cannon et al., 2000; Goldberg et al., 1990).
Figure 1 portrays the expected results from a MZ discordant pair design under three hypothesized models for the etiological association between MD and neuropsychological deficits. Under the MD History model, neuropsychological impairments are caused by MD or other processes related to having MD. Thus, twins who have experienced MD are expected to have cognitive impairments, but their cotwins who have not experienced MD will not be impaired.
Figure 1. Expected Patterns of Deficits in Discordant Cotwin-Control Studies Under Alternative Models for the Etiological Relation between MD and Neuropsychological Deficits.
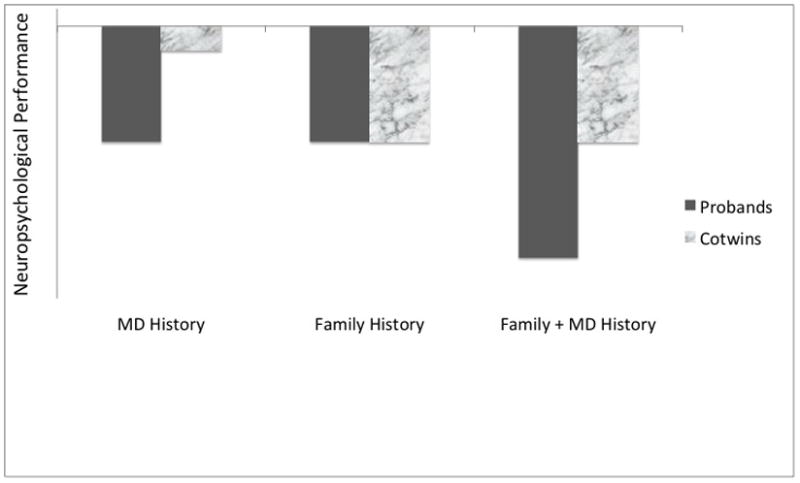
MD History Model: Deficits are associated with clinical history of MD, Probands but not unaffected Cotwins show deficits relative to Controls; Family History Model: Deficits are associated with family history of MD, Probands and unaffected Cotwins show equivalent deficits relative to Controls; Family + MD History Model: Deficits are associated with both sources, as Cotwins and Probands show deficits arising from familial vulnerability and Probands have additional deficits associated with clinical history of MD.
The Family History model represents the situation in which the MD-neuropsychological deficit association arises from overlapping risk factors that have a familial origin (either heritable or family environmental factors). Here, the degree of impairment (relative to a control group) is not expected to be related to personal clinical history. In the context of discordant twin pairs, individuals without MD would show neuropsychological impairments to the same degree as their affected cotwins.
The FamHist+MDHist model combines these two scenarios; individuals with a family history of MD are expected to have neuropsychological deficits associated with this familial risk, and individuals who have had MD are predicted to have additional deficits.
In addition to studying discordant pairs, useful information comes from also studying twin pairs who have never experienced MD. Including unaffected pairs provides a comparison group for assessing the degree of deficits of the “unaffected” members of pairs discordant for MD. This control group can serve as an anchor point for judging the impact of familial risk and MD history; the ability to distinguish between the MDHist and FamHist+MDHist models is contingent upon having a comparison group. The use of all three groups (i.e., affected probands and unaffected cotwins from MD discordant twin pairs, and healthy control pairs) allows for the clearest interpretation of study results.
The goal of this research study was to clarify the relation between MD and neuropsychological deficits, using an MZ twin design to account for the impact of family environment and genetic risk. To our knowledge, this is the first study to apply the discordant twin pair design to examine this issue. A prior study of Danish twins (Christensen et al., 2006) evaluated the cognitive performance of 94 non-depressed cotwins of individuals with MD compared to a control group. However, this study did not include the depressed probands for within-pair comparisons.
Our study was designed to answer three questions related to the three models portrayed in Figure 1: First, do probands with a history of depression show neuropsychological deficits relative to their unaffected cotwins? Second, do individuals with an MD history show neuropsychological deficits relative to controls from unaffected twin pairs? For these two questions, we also examined what clinical variables might be associated with these possible group differences. Third, assuming some differences are detected between probands and the other groups, it is of interest to know if the unaffected cotwins of probands differ from controls.
We assessed attention, working memory, verbal memory, visuo-spatial processing and general knowledge among 158 twins from MZ pairs previously studied for personal and family history of MD. We hypothesized that attention and working memory would be consistent with the MDHist+FamHist model. That is, we predicted both probands and their unaffected MZ cotwins would perform more poorly on these measures than non-depressed controls and that probands would perform more poorly than their unaffected cotwins on these measures due to the “scarring” influence of depression. Longitudinal study of individuals currently with, and remitted from, MD suggest that impairments in attention and frontal function are MD state-independent (Ardal and Hammar, 2011; Douglas and Porter, 2009; Hammar et al., 2003; Xu et al., 2012). Given evidence of attentional impairment from the limited literature on neuropsychological performance in families at risk for MD (Belleau et al., 2013; Christensen et al., 2006), these deficits may exist prior to MD onset. We also predicted that the differences between probands and their unaffected cotwins would be associated with the severity of proband depressive history. Depression severity has been found to impact neuropsychological performance (Austin et al., 1992; Keilp et al., 2012; Naismith et al., 2003; Paelecke-Habermann et al., 2005) though this finding is not always consistent (c.f., Lampe et al., 2004; Wekking et al., 2012). Given the uncertainty in the literature regarding the association between MD and verbal memory (e.g., Austin et al., 1999; MacQueen et al., 2002; Christensen et al., 2006; Smith et al., 2006; Simons et al., 2009), we expected deficits in probands but not did hypothesize whether they would reflect the FamHist model (i.e., also present in unaffected cotwins) or the MDHist model (i.e., related to clinical history). Finally, we hypothesized that the depression-related deficits would be specific to attention and memory; while other domains of cognitive functioning have at times shown impairment in MD, attention and memory are the domains consistently impacted in individuals with MD (Hasselbalch et al., 2011; Landro et al., 2001; McClintock et al., 2010; Murrough et al., 2011; Zakzanis et al., 1998). Therefore we expected no differences among probands, MZ cotwins and controls on measures indexing general knowledge and visuo-spatial processing.
2. Methods
2.1. Subjects
Participants are from the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (VATSPSUD), a longitudinal population-based study of genetic and environmental risk factors for psychopathology (Kendler and Prescott, 2006). The sample was originally identified through the Virginia Twin Registry1, and includes Caucasian twins born in Virginia between 1934 and 1974. The current sample comprises twins from two smaller sub-studies from the VATSPSUD who had neuropsychological testing. Prior to participation in this study, all subjects had been assessed with structured clinical interviews and were known to be competent. For twins in male pairs, assessments were conducted twice across a period of 2–3 years. Female pairs were assessed two to four times over a period of 8–10 years. Potential participants were mailed information letters and consent forms and then contacted to request participation. All pairs in both groups were MZ, determined by a combination of questionnaire responses and photographs, the validity of which had been previously confirmed by genotyping (Kendler and Prescott, 2006). No dizygotic pairs (DZ) were available for this study. All participants were native English speakers. Approval of this human subjects research was given by Virginia Commonwealth University’s Institutional Review Board (IRB).
Eligibility for the Discordant for Affective Disorder (DAD) study was defined by three criteria as assessed in 2000 (Kendler and Gardner, 2001): (i) the affected member (proband) of the pair must have met criteria for one or more episodes of DSM-III-R defined lifetime MD on at least two assessments; (ii) the unaffected member (cotwin) must have denied a lifetime history of episodes of MD on all assessments; and (iii) the age of the unaffected twin at last interview (when found free of a history of MD) was at least 10 years older than the age of onset of MD in his/her cotwin. In addition, neither twin had a history of mania. The sample used in the current analyses consisted of 117 twins from discordant pairs (64 females and 53 males). These included 40 complete pairs and 37 unpaired twins. Of the unpaired twins, 30 were MD probands and 7 unaffected cotwins in the original sample (Kendler and Gardner, 2001). Of the 37 missing twins, 7 probands did not participate in the DAD study. Another 30 cotwins reported some history of MD when reassessed for the DAD interview so were ineligible for analyses here. Thus, our MD Proband group includes 70 individuals; the Cotwin group includes 47.
A Control group was drawn from MZ twin pairs participating in a separate VATSPSUD pilot study which included the same set of neuropsychological measures. This project investigated psychophysiological responses to affective stimuli and participants were recruited on the basis of living in the greater Richmond metropolitan area. They were unselected for clinical history but were required to be under age 50. Thus group membership is confounded with age in our study.
The original pilot study sample included 77 twins. For our Control group, we selected from the pilot study 41 twins from 21 pairs in which neither twin had experienced MD, as verified by multiple clinical interviews. The current analyses utilizing the Control group are thus from 20 complete pairs and 1 twin whose cotwin did not participate.
2.2. Measures & procedures
2.2.1. Demographic variables and group codes
In the analyses, sex was coded 0=female, 1=male; and age was centered at 40. Both sex and age are pair-level variables: MZ pairs are always the same sex, and, as both studies were conducted within a short time-span, twins are nearly perfectly correlated for age at assessment. A binary variable called Mode was coded 0 for participants assessed in person and 1 for participants assessed by telephone. Participants assessed by telephone (and thus missing Block Design (BD) and Digit Symbol-Coding (DSS) scores) did not differ significantly from those tested in person on any of the cognitive measures (all p>0.17).
2.2.2. Neuropsychological measures
Our assessment included four measures from the WAIS-III (Wechsler, 1997a) and Verbal Paired Associates from the WMS-III (Wechsler, 1997b). As age and sex were included as regression model covariates, we analyzed raw scores rather than scaled scores adjusted for age and sex.
Digit Symbol-Coding (DSS) assesses visuo-motor speed, selective and sustained attention, and set shifting. Subjects have 90 seconds to complete up to 133 items.
Letter-Number Sequencing (LNS) assesses working memory and attention. Trials consist of combinations of letters and numbers in a jumbled order, which participants rearrange into digits in numerical order, then letters in alphabetical order. The maximum score is 21.
Block Design (BD) assesses visuo-spatial processing and constructional ability. Subjects are asked to arrange blocks within a time limit to reproduce colored designs. The maximum score is 68.
Vocabulary (VO) was included as an index of pre-morbid verbal cognition and general cognitive ability. The maximum score is 66.
Verbal Paired-Associates (VPA) taps short- and long-term verbal memory. Participants are read a list of 8 word pairs. Immediate recall (VPA-I) is assessed immediately after each of four presentations of the list and has a maximum score of 32 (8 pairs × 4 trials). Delayed recall (VPA-D) is assessed on a single trial 30 minutes after the last VPA-I trial and has a maximum score of 8.
2.2.3. Clinical variables
Assessment of lifetime and past-year MD was conducted at each interview by clinically-trained interviewers using structured interviews adapted from the SCID (for the DSM-III-R) (Spitzer et al., 1985). Twins within a pair were interviewed separately by different interviewers who were unaware of the clinical status of the other twin.
For the analyses reported here, MD was coded as a binary variable: 0=Never met criteria, 1=one or more DSM-III-R defined episodes. We created a severity variable (WorstSev) that was the number of DSM-III-R defined MD criteria met by the individual during their self-defined worst episode of depression, and ranged from 5 to 9. We created an indicator of early onset (Early) as a binary variable coded 1 if MD onset age was younger than 18 years; and 0 otherwise. Number of MD episodes was correlated with early onset (tetrachoric correlation=0.48) and in preliminary multiple regression analyses its inclusion did not improve prediction of neuropsychological scores, so we do not include it in our primary results reported here (although it was included in exploratory analyses). Current depression symptomatology was assessed using the depression symptom subscale from the SCL-90-R (Derogatis, 1975), which contains 10 items scored 0–3. In exploratory analyses we include MDSCL as the average score on these items. 81 individuals are missing this scale because the measure was added midway through these studies.
None of the twins met criteria for history of mania. These samples were not otherwise screened for history of other Axis I or Axis II disorders.
2.2.4. Family history
Paternal and maternal lifetime histories of MD were obtained from twins using Family History-Research Diagnostic Criteria (Andreasen et al., 1977). Individuals were coded as positive if informants reported the target parent experienced five or more FH-RDC criteria (which must have included depressed mood or anhedonia) or sought treatment for depression. Based on this definition, all pairs of twins in the analyses agreed perfectly on presence or absence of parental MD so these variables were treated as family-level covariates. Each parental MD variable was coded as 1 if present and 0 if not.
2.2.5. Procedures
Each participant was assessed separately during a single session by interviewers with a Master’s degree or a Bachelor’s plus at least 2 years clinical experience. The neuropsychological and clinical assessments were supervised by a Master’s level psychologist with more than 10 years of experience in psychological assessment (LJH). In DAD, the assessment consisted of the first portion of a clinical interview, the neuropsychological assessment, followed by the remainder of the clinical interview. Twenty-nine (24.8%) DAD participants were assessed by telephone, usually because they lived at a remote distance. For these participants, Digit Symbol-Coding and Block Design were omitted. All Control group participants were assessed in person by interviewers who were a subset of those working on the DAD study. They had a 2-hour battery of psychophysiological and affective tasks, with neuropsychological assessment mid-way through the battery.
2.3. Statistical analyses
We tested for differences in neuropsychological performance between affected Probands and their unaffected Cotwins from complete twin pairs using paired-sample t-tests (SAS version 9.2, SAS Institute, Cary, NC). We employed one-tailed tests, reflecting our strong a priori hypotheses, that probands are expected to have worse performance than their cotwins and that individuals with a positive family history of MD would perform worse than the comparison group.
Analyses comparing the Control group to the Proband or Cotwin groups were conducted using multilevel regression (MLR) to account for the twin-pair structure of the data (i.e., complete pairs in the Control group). This method allowed us to estimate simultaneously the group difference, adjusted for covariates at the family level (sex, age) and individual level (testing mode). MLR was implemented as a structural equation model using Mplus (version 6.12) (Muthén and Muthén, 2011). Corresponding parameters for the two twins (means, variances, regression weights, and covariances among predictors) were equated across twins within a pair. Covariances between the residuals of the dependent variables (i.e., cognitive scores of twin 1 and twin 2) were freely estimated. This structure produces the same parameter estimates as if the twins were analyzed as individuals, but corrects the standard errors for the nested structure of the data (twins within pairs). All individuals with data on the dependent variable were included in the analyses, regardless of whether data were available for both members of the pair. Clinical and demographic variables were available for all individuals with cognitive data.
3. Results
3.1. Descriptives
Table 1 shows sample characteristics and means, standard deviations, and range of performance on the neuropsychological measures for Probands and Cotwins from discordant pairs and the Control group. As noted above, due to age inclusion criteria, Controls tended to be younger than twins from discordant pairs. Among Probands, the average number of DSM-III-R criteria (of 9) reported for their worst episode was 6.5 (SD=2.3, range=5–9), and 27 (38.5%) reported their initial MD episode began before age 18.
Table 1.
Summary of Group Performance on Each Measure
| DAD Probands (N=70) | DAD Unaff. Cotwins (N=47) | Controls (N=41) | Test of Group Differences
|
||
|---|---|---|---|---|---|
| MD History (Probands vs Cotwins) | Familial Risk (Cotwins vs Controls) | ||||
| Age | |||||
| Mean (SD) | 45.0 (8.2) | 45.4 (7.6) | 33.0 (8.4) | b (se) | b(se) |
| Percent Female | 57.1% | 51.1% | 75.6% | P | P |
|
| |||||
| Digit Symbol Substitution (DSS) 1 | |||||
| Mean (SD) | 74.7 (16.9) | 69.7 (12.5) | 85.5 (12.8) | −10.6 (3.9)a | −15.6 (3.3)a,b |
| Range | 40–116 | 50–96 | 58–113 | 0.006 | <0.001 |
| Letter-Number Sequencing (LNS) | |||||
| Mean (SD) | 10.5 (2.5) | 10.0 (2.2) | 11.9 (2.9) | −1.4 (0.6)a | −1.9 (0.6)a |
| Range | 6–16 | 5–15 | 7–18 | 0.011 | 0.001 |
| Block Design (BD) 1 | |||||
| Mean (SD) | 38.2 (12.3) | 38.5 (10.6) | 45.1 (9.8) | −6.8 (2.8)a | −6.6 (2.6)a |
| Range | 12–61 | 12–60 | 21–63 | 0.007 | 0.006 |
| Verbal Paired Associates – Initial (VPA-I) | |||||
| Mean (SD) | 17.6 (7.7) | 18.1 (7.4) | 20.8 (6.8) | −3.3 (1.7)a | −2.8 (1.8) |
| Range | 3–31 | 2–32 | 6–32 | 0.029 | 0.058 |
| Verbal Paired Associates – Delayed (VPA-D) | |||||
| Mean (SD) | 6.1 (2.3) | 6.0 (2.2) | 6.7 (1.8) | −0.7 (0.5) | −0.7 (0.5) |
| Range | 0–9 | 0–8 | 2–8 | 0.087 | 0.080 |
| Vocabulary (VO) | |||||
| Mean (SD) | 46.7 (10.5) | 43.1 (10.7) | 48.1 (9.7) | −1.5 (2.4) | −5.1 (2.6)a,b |
| Range | 13–65 | 21–62 | 21–63 | 0.267 | 0.025 |
Notes:
N’s for these measures are: Probands=53 and Cotwins=35
Group comparisons based on 1-tailed test (P<.05).
group means differ after adjusting for sex;
group means differ after adjusting for sex and age.
3.2. Impact of MD history: within-pair analyses
Our first aim was to test whether history of MD would distinguish the neuropsychological performance of MD Probands and their unaffected Cotwins – groups that are matched for age, sex, genetic liability to depression, and early upbringing. As depicted in Figure 2, Cotwins did not perform significantly better than their Probands on any of the neuropsychological measures. The largest difference observed was for Vocabulary and this was in the opposite direction expected, with Probands scoring on average 2.5 points higher than Cotwins.
Figure 2. Within Pair Differences (Proband - Cotwin) on Neuropsychological Measures Based on Discordant Twin Pairs.
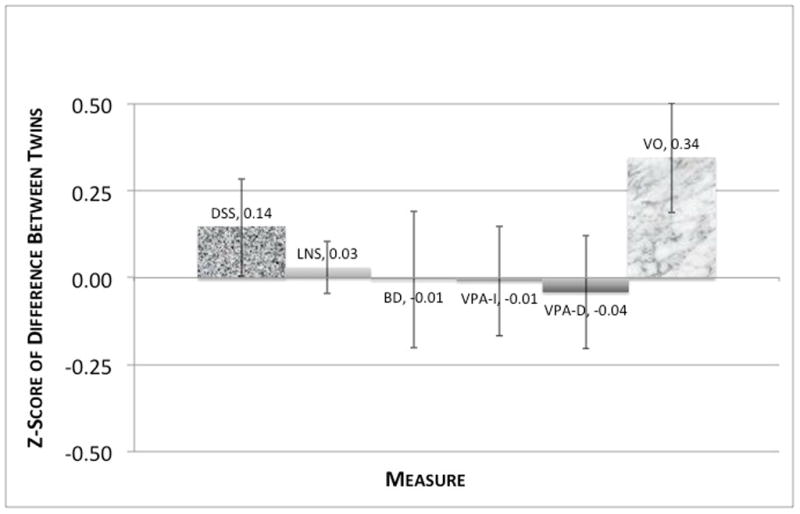
Scores are in SD-units and based on complete MZ pairs with confirmed discordance for MD. DSS=Digit Symbol Substitution; LNS=Letter Number Sequencing; BD=Block Design; VPA-I=Verbal Paired Associates Initial Recall; VPA-D=Verbal Paired Associates Delayed Recall; VO=Vocabulary. Sample Sizes (pairs): DSS=25, LNS=37, BD=26, VPA-I=39, VPA-D=37, VO=39.
3.2.1. Exploration of within-pair differences
Although there were no systematic differences between Probands and their Cotwins on neuropsychological performance, it is possible that the degree to which the twins differ is associated with aspects of the clinical history of the proband. We conducted exploratory analyses to see if within-pair differences in test performance were related to demographic variables (age, sex), testing mode, clinical severity variables, and parental MD history. We do not interpret significance, but focus on effect sizes and general trends across variables.
Results are summarized in Table 2. These variables accounted for 20–30% of the difference in scores between twins in a pair. A consistent finding was that Probands with an MD onset before age 18 tended to have lower scores than their Cotwins (on all measures but VO). Because sex, age, and parental MD are perfectly correlated for twins within a pair, these variables represent effects associated with the magnitude of pair difference. Thus, the parental MD effect for DSS means parental MD is associated with smaller differences between the twins in a pair. There was also a trend for greater differences within male than within female pairs.
Table 2.
Results from Exploratory Regression Analyses Predicting Within-Pair Differences in Neuropsychological Performance Between MD Probands and their Unaffected Cotwins
| Digit Symbol Substitution | Letter-Number Sequencing | Block Design | Verbal Paired Associates – Immediate | Verbal Paired Associates – Delayed | Vocabulary | |
|---|---|---|---|---|---|---|
| N Pairs | 25 | 38 | 26 | 40 | 38 | 40 |
| Pair Difference M | 1.44 | 0.05 | −0.04 | −0.08 | −0.13 | 2.48 |
| (SD) | 9.98 | 1.80 | 7.49 | 8.21 | 3.16 | 7.19 |
|
| ||||||
| Parameter Estimates (SE)
| ||||||
| Intercept | 2.8 (16.2) | 3.5 (2.1) | −7.7 (11.6) | −0.7 (9.0) | 1.5 (3.8) | 5.0 (8.7) |
| Age | −0.06 (0.3) | −0.07 (0.04) | −0.02 (0.2) | −0.06 (0.2) | −0.02 (0.07) | 0.01 (0.2) |
| Sex | −0.3 (4.5) | −0.7 (0.7) | −2.4 (3.2) | −4.5 (2.8) | −0.7 (1.1) | 4.9 (2.7) |
| Mode-Diff a | -- | −0.01 (0.6) | -- | 3.7 (2.4) | 0.9 (1.1) | −1.8 (2.3) |
| Early Onset | −10.7 (5.9) | −0.5 (0.8) | −4.2 (4.0) | −1.8 (3.3) | −0.1 (1.4) | 4.6 (3.2) |
| WorstSev | 0.2 (1.4) | 0.1 (0.2) | 1.7 (1.0) | 0.9 (0.7) | −0.1 (0.3) | −1.0 (0.6) |
| Mother MD | 5.3 (5.3) | −1.5 (0.7) | −4.3 (3.6) | −4.2 (2.8) | −1.1 (1.2) | −1.6 (2.7) |
| Father MD | 2.2 (7.7) | −0.5 (0.8) | 0.4 (5.2) | 8.8 (3.5) | 3.5 (1.4) | 1.0 (3.3) |
| Model R2 (%) | 20.8 | 26.2 | 26.2 | 32.8 | 28.1 | 19.2 |
Sex coded as Male=1. Mode= 1 if telephone assessment; Worst severity = # symptoms during worst episode. Early Onset=first episode before 18. Negative coefficients are in direction of worse performance by the Proband.
not applicable for BD and DSS; all twins with data were tested in person
3.3. Impact of MD history: between-group analyses
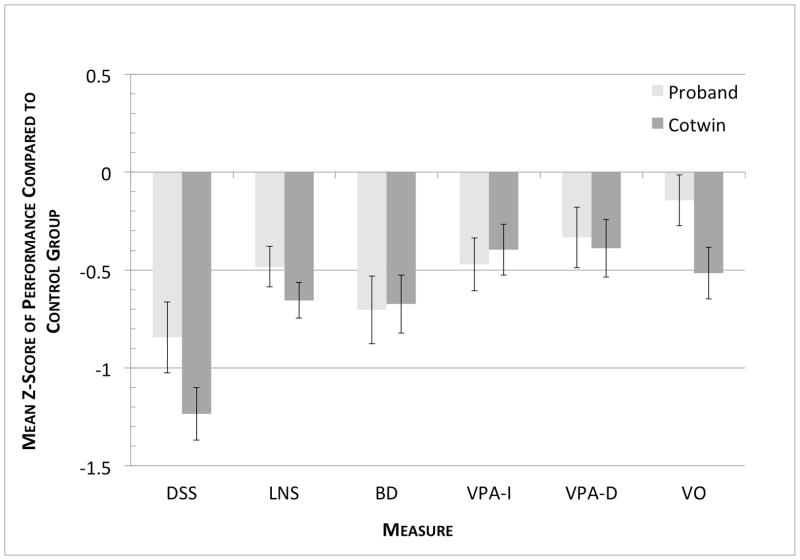
Our second aim was to test whether history of MD was associated with neuropsychological deficits. Probands from discordant pairs were compared to Controls on each of the six outcomes (depicted in Figure 3). Probands had significantly lower scores than Controls on DSS, LNS, BD, and VPA-I, but not VO or VPA-D (Table 1). Group status accounted for 8.1% of the variance in DSS (b=−10.6, SE=3.9, P<0.005), 4.3% of LNS (b=−1.4, SE=0.6, P<0.05), 6.5% of BD (b=−6.8, SE=2.8, P<0.01), and 3.4% of VPA-I (b=−3.3, SE=1.7, P<0.05). Adjusting for sex and age resulted in a substantial reduction of the MD history effect. The MD history effect after accounting for sex and age was DSS: group status b=−1.3, SE=4.1, P>0.05, LNS: group status b=−0.1, SE=0.7, P>0.05, BD: group status b=−3.1, SE=3.1, P>0.05, and VPA-I: group status b=−1.4, SE=2.0, P>0.05.
Figure 3. Discordant Proband and Cotwin Neuropsychological Performance Relative to Control Group.
Group scores are in z-score units and plotted relative to control group means and standard deviations. DSS=Digit Symbol Substitution; LNS=Letter Number Sequencing; BD=Block Design; VPA-I=Verbal Paired Associates Initial Recall; VPA-D=Verbal Paired Associates Delayed Recall; VO=Vocabulary.
3.3.1. Influence of current mood/depression symptoms
We studied the association between current depressed mood and neuropsychological performance among the 77 participants who had scores on the SCL MD scale. SCL score was not predictive of performance on any of the measures (r from 0.005 to 0.12, all P>0.27).
3.3.2. Exploration of MD history effect with clinical and demographic variables
We explored whether clinical and demographic variables were associated with variation in performance among all Probands (i.e., including probands from concordant twin pairs) on the measures for which we observed differences from Controls (DSS, LNS, and BD). Predictors included sex, age, number of symptoms during the individual’s worst episode, number of lifetime episodes of MD, MD onset age < 18, and parental and cotwin MD history. We used multiple regression analyses in SAS with a backward elimination criterion of P=0.15 to identify a wider range of factors possibly relevant to neuropsychological performance but for which our sample did not have sufficient power to evaluate. This reduces Type II error at the likely cost of increasing Type I error, so we do not interpret “significance” and consider these results descriptive and exploratory. Table 3 shows the multiple regression results for the set of predictors that met the threshold for any measure.
Table 3.
Parameter Estimates from Exploratory Regression Analyses Predicting Variation in Neuropsychological Performance Among MD Probands
| Digit Symbol Substitution | Letter-Number Sequencing | Block Design | Verbal Paired Associates – Immediate | Verbal Paired Associates – Delayed | Vocabulary | |
|---|---|---|---|---|---|---|
| Parameter Estimate (se)
| ||||||
| N | 48 | 62 | 48 | 63 | 62 | 63 |
| Intercept | 130.5 (23.7) | 15.1 (3.5) | 76.6 (17.8) | 37.1 (10.7) | 11.2 (3.4) | 55.3 (15.2) |
| Age (per year) | −0.6 (0.3) | −0.07 (0.05) | −0.6 (0.2) | −0.2 (0.1) | −0.06 (0.04) | −0.1 (0.2) |
| Sex | −3.5 (4.9) | −0.1 (0.7) | 3.9 (3.7) | −4.3 (2.1) | −0.9 (0.7) | 0.03 (3.0) |
| Mode a | -- | −0.2 (0.8) | -- | 4.8 (2.5) | 1.1 (0.8) | 7.6 (3.6) |
| Early Onset | −2.5 (5.5) | 0.4 (0.8) | −6.8 (4.1) | −3.2 (2.5) | −0.7 (0.8) | −0.6 (3.5) |
| WorstSev (per sx) | −4.7 (2.1) | −0.3 (0.3) | −1.7 (1.6) | −0.9 (0.9) | −0.3 (0.3) | −0.9 (3.2) |
| Cotwin MD | −0.9 (5.6) | 1.0 (0.7) | −0.7 (4.2) | −4.3 (2.3) | −0.04 (0.7) | 0.9 (1.3) |
| Mother MD | 12.1 (5.3) | 0.7 (0.7) | 3.6 (3.9) | 2.6 (2.2) | 0.2 (0.7) | 5.2 (3.1) |
| Father MD | 4.9 (6.0) | −0.1 (0.9) | −3.2 (4.5) | 4.1 (2.6) | 1.3 (0.8) | 2.2 (3.8) |
|
| ||||||
| Model R2 (%) | 29.6 | 18.5 | 25.6 | 19.2 | 14.2 | 17.0 |
Sex coded as 1=male. Mode= 1 if telephone assessment; Worst severity = # symptoms during worst episode. Early Onset=first episode before 18. Includes all Probands, regardless of cotwin participation or MD status.
not applicable for BD and DSS; all twins with data were tested in person
For DSS, age at assessment, symptoms during the worst episode, and maternal MD history were retained in the final model, explaining 27.0% of the variance. Older age and more symptoms were associated with worse DSS performance, while (in the context of these other variables) maternal MD history was unexpectedly associated with higher scores. As with DSS, predictors of lower LNS performance were older age, more symptoms during the worst episode and presence of maternal MD history (R2=15.3%). For BD, older age and early onset of MD were associated with decreased performance (R2=18.7%).
3.4. Impact of family history of MD
Our third aim was to test whether the familial liability to MD would be associated with deficits among individuals who had themselves never been depressed. Cotwins had lower performance than Controls on DSS, LNS, BD, and VO, but not on VPA-I or VPA-D (see Figure 3). Family history accounted for 25.0% of the variance in DSS (b=−15.6, SE=3.3, P<0.001), 11.0% of LNS (b=−1.9, SE =0.6, P<0.005), 8.3% of BD (b=−6.6, SE=2.6, P<0.01), and 5.1% of VO (b=−5.1, SE=2.6, P<0.05). Adjusting for sex and age substantially reduced group differences for LNS and BD but the differences remained significant for DSS and VO (Table 1). The family history effect after accounting for sex and age was: DSS: family history b=−6.7, SE=3.6, P<0.05, LNS: family history b=−1.2, SE=0.8, P>0.05, BD: family history b=−3.6, SE=3.0, P>0.05, and VO: family history b=−6.5, SE=3.3, P<0.05.
4. Discussion
Substantial evidence indicates MD is associated with some types of neuropsychological deficits but the etiological basis for this association is unclear. This study used a design never before used in this particular area of research, the discordant MZ twin pair design, to investigate the etiological basis for the association between MD and neuropsychological deficits. The discordant twin pairs are nearly perfectly matched on genes as well as early family/shared environment, allowing for a much clearer interpretation of any differences between groups as being associated with MD history. Other studies have considered familial liability by examining at-risk populations for MD, including children of depressed and/or anxious individuals (Micco et al., 2009) or studied the unaffected twins in pairs discordant for an affective disorder (Christensen et al., 2006), but no prior study has the design advantages of comparing a group with history of MD, a group matched on genetic and early environmental factors, and an unaffected comparison sample assessed at multiple waves.
4.1. Etiology of MD-associated deficits
Our first aim was to compare the MZ Probands with a history of MD and their unaffected Cotwins to assess the role of MD history on neuropsychological performance. Probands and their unaffected Cotwins did not significantly differ in the domains of attention, working memory, visuo-spatial processing or verbal memory.
The findings are consistent with our hypothesis that attention and working memory are related to familial risk father than clinical history. We expected Probands might show some deficits in visuo-spatial processing and verbal memory associated with clinical status but Probands and Cotwins performed similarly in these domains.
An obvious issue for interpreting these results is that failure to reject the alternative hypothesis (probands and cotwins differ) is not equivalent to proving the null hypothesis (probands and cotwins do not differ). Using a paired-sample t-test with a one-tailed alpha of 0.05, our sample sizes provide 80% power to detect effect sizes of d=0.28 to d=0.35. The within-pair differences we observed were near zero for four measures and in the opposite direction as predicted (Probands performing better than Cotwins) for Digit Symbol and Vocabulary. Thus, the absence of Proband deficits was not attributable to low power, but instead points to the importance of family history over clinical history in impacting neuropsychological performance.
Our other study goals involved placing the deficits of the Probands and Cotwins in perspective by comparing them to performance among a Control group of MZ twin pairs without a history of MD. We hypothesized that Probands and their Cotwins would perform worse than unaffected twin pairs on measures of attention and working memory but not on measures that index general knowledge and visuo-spatial processing.
Our hypotheses were partially supported: as depicted in Figure 3, both Probands and Cotwins from MZ twin pairs discordant for lifetime MD had significantly worse performance than twins from pairs with no history of MD on measures requiring selective and sustained attention (Digit Symbol Substitution) and general knowledge (as indexed by Vocabulary). Group differences were also observed for working memory (Letter Number Sequencing), verbal memory (Verbal Paired Associates – Initial), and visuo-spatial processing (Block Design) but these were confounded with sex and age differences between the groups.
These results are similar to those reported by Christiansen et al. (2006), in which before adjusting for age and sex, the clinically unaffected MZ and DZ cotwins of individuals with MD showed deficits in selective and sustained attention, executive function, and working memory relative to controls without a family history of affective disorder.
4.2. Correlates of MD-associated deficits
We also hypothesized that impairments in the domains of attention and working memory would be associated with clinical severity of MD. In exploratory analyses conducted within the Proband group (Table 3), we found some evidence that stronger familial liability (indicated by parental MD) and depression severity (indicated by early age of MD onset) were associated with neuropsychological performance. Early MD onset was also associated with poorer performance in exploratory analyses comparing Probands and unaffected Cotwins (Table 2), suggesting it may be an independent factor and not reflecting some aspect of familial liability shared with the cotwin. These results are tentative, but merit further study.
4.3. Study limitations
Our sample of Caucasian Virginia-born twins may limit generalizability of the findings to individuals from other ethnic groups and geographical regions. Studying MZ twin pairs allowed us separate the impact of clinical history from factors that predispose to the development of MD and negatively impact neuropsychological functioning due to inherited and family environmental factors. However, the absence of DZ pairs in our design means we cannot distinguish heritable versus shared environment factors that underlie MD liability. Our sample also limits the generalizability of our results to individuals experiencing MD without psychotic features, as the neuropsychological profile of individuals diagnosed with MD with psychotic features significantly differs from those without psychotic features (for a comprehensive review and meta-analysis, see Fleming et al., 2004).
It is possible that with longer follow-up some unaffected individuals will develop MD. However, the effects of these changes in group status would not alter the implications regarding the absence of differences in neuropsychological performance between members of the discordant pairs, nor the findings regarding early MD onset. In addition, while our definition of case is reliable (based on multiple interviews), these cases may not be particularly severe, as we did not require medication or hospitalization of our cases. This less severe definition could account for the lower impairment seen in our cases relative to their cotwins and explain the discrepancy in findings with prior studies that use samples identified from psychiatric hospitalization records (e.g., Christensen et al., 2006; Hasselbalch et al., 2012).
We do not have information on current antidepressant medication usage so we cannot explicitly account for their usage and impact on cognitive functioning in this study. However, the usage of commonly used antidepressants, particularly SSRIs, has been found to have little impact on cognition and neuropsychological performance (Kessing, 1998; Neu et al., 2005; Paradiso et al., 1997; Peretti et al., 2000; Preiss et al., 2009).
Our attribution of differences between twins focuses on differences in clinical history but factors not assessed in our study (e.g., head trauma) could have contributed to the development of both MD and neuropsychological deficits in some individuals. We are not able to address this possibility but it does not alter our findings or their implications.
A substantial minority of DAD study participants did not complete the Digit Symbol Substitution and Block Design subtests due to being assessed by telephone. There were no differences between DAD individuals who completed these subtests and those who did not in terms of demographic or clinical characteristics, but the smaller sample size undoubtedly reduced the power to detect small effects associated with family and clinical status. We also note Digit Symbol Substitution as a test of attention, though cognitive speed/speed of information processing may underlie part of the variance in performance on this task. However, other timed tasks that involve cognitive speed (e.g., Block Design) do not show the same pattern of results as Digit Symbol Substitution, suggesting that the element of cognitive speed/speed of information processing is not necessarily the primary determinant of variance in Digit Symbol Substitution.
We did not include education as a covariate in our analyses. Typically, education is used as an indicator of socioeconomic status. Because twins within a pair are matched for socioeconomic level during their upbringing, adjusting for educational level is not needed for this purpose. More importantly, lower educational attainment could be an outcome of depression rather than a factor to “correct” for.
4.4. Strengths and future directions
Our design is informative, novel, and of merit in a number of ways. In our study, we were able to compare a high-risk sample (unaffected cotwins in MD discordant twin pairs) with an affected sample (probands in MD discordant pairs) that was closely matched on genes, early family/shared environment, and demographics. As these samples did not significantly differ in neuropsychological functioning, observed deficits in the literature may purely be driven by familial risk. This research study also utilized a more homogenous at-risk sample than previous studies, with individuals included solely on the basis of risk for MD, rather than allowing subjects who might have only been at risk for panic disorder (Micco et al., 2009) or bipolar disorder (Christensen et al., 2006).
The twin pairs were drawn from a larger, population-based sample and thus their depression history is representative of affected individuals in the population rather than selected for those seeking treatment. Unlike many prior studies conducted in clinical settings, our affected group was defined by having a past history of MD and most members were not currently depressed. These sample differences may explain why we observed smaller differences between the affected group and controls than observed in some other studies.
Another measurement strength is our multiple clinical assessments across several years, adding to the confidence in our diagnostic assignments. Our analyses also considered onset age and symptom severity as well as personal and family history of MD.
The results of our study support the Family History etiological model of neuropsychological deficits associated with MD. One implication of the results is that prior findings of impaired attention and working memory among individuals with MD may not reflect state effects or “scarring” from MD. Rather, these impairments may be present before the onset of MD and are associated with familial risk for MD. Munn et al. (2007) found no differences in amygdala volume within twin pairs discordant for MD, supporting the hypothesis that familial or genetic influences may play a larger role than depression status in impacting cortical volume. To the extent our differences reflect true deficits (rather than age effects), they are sizable and of practical significance.
These findings add to prior literature regarding MD and cognitive functioning and raise important questions that are worth further study. Particularly, the mechanisms through which familial/genetic influences impact attentional systems independent of the experience of MD are relatively unexplored. Twin studies of depression indicate that familiality of MD is primarily due to genetic rather than to shared environmental factors (Sullivan et al., 2000). Thus, it seems likely that neuropsychological deficits associated with familial risk are also genetically transmitted. A possible mechanism is suggested by a study by Peterson and colleagues (2009) that found the association between familial risk for MD and inattention was statistically mediated by degree of cortical thinning, particularly in the right hemisphere and portions of the frontal gyri. Of note, the association between inattention and cortical thickness/white matter volumes for these regions are significant not only for the individuals with a family history of MD, but individuals without a family history of MD as well (Peterson and Weissman, 2011). Both cortical volume reduction and deficits in cognitive functioning have been identified as candidate endophenotypes for MD, demonstrating some degree of heritability and familial association (see Hasler et al., 2004 for a comprehensive review). Moreover, deficits in attention, working memory, and cortical volume are top-ranked endophenotype candidates for recurrent MD (Glahn et al., 2012). Our results support this finding that attention and working memory are cognitive domains significantly related to familial risk for MD. Working memory capacity has also been found to be closely related to selective attention (i.e., distractor inhibition) and both may be mediated by a common underlying factor/ability (Ester et al., 2012). More research is needed to clarify how attentional capabilities are associated with depression severity and course (McClintock et al., 2010).
Acknowledgments
Data collection was supported by a Senior Investigator award from the National Alliance for Research on Schizophrenia and Affective Disorders (to KSK). Preparation of this paper was supported by a Fellowship from USC Graduate School (to KJH).
Footnotes
Now part of the Mid-Atlantic Twin Registry.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria: reliability and validity. Archives of General Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Ardal G, Hammar A. Is impairment in cognitive inhibition in the acute phase of major depression irreversible? Results from a 10-year follow-up study. Psychology and Psychotherapy: Theory, Research and Practice. 2011;84:141–150. doi: 10.1348/147608310X502328. [DOI] [PubMed] [Google Scholar]
- Austin MP, Mitchell P, Wilhelm K, Parker G, Hickie I, Brodaty H, Chan J, Eyers K, Milic M, Hadzi-Pavlovic D. Cognitive function in depression: a distinct pattern of frontal impairment in melancholia? Psychological Medicine. 1999;29:73–85. doi: 10.1017/s0033291798007788. [DOI] [PubMed] [Google Scholar]
- Austin MP, Ross M, Murray C, O’Carroll RE, Ebmeier KP, Goodwin GM. Cognitive function in major depression. Journal of Affective Disorders. 1992;25:21–29. doi: 10.1016/0165-0327(92)90089-o. [DOI] [PubMed] [Google Scholar]
- Belleau EL, Phillips ML, Birmaher B, Axelson DA, Ladouceur CD. Aberrant executive attention in unaffected youth at familial risk for mood disorders. Journal of Affective Disorders. 2013;147:397–400. doi: 10.1016/j.jad.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Huttunen MO, Lonnqvist J, Tuulio-Henriksson A, Pirkola T, Glahn D, Finkelstein J, Hietanen M, Kapiro J, Koskenvuo M. The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. American Journal of Human Genetics. 2000;67:369–283. doi: 10.1086/303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen MV, Kyvik KO, Kessing LV. Cognitive Function in Unaffected Twins Discordant for Affective Disorder. Psychological Medicine. 2006;36:1119–1129. doi: 10.1017/S0033291706007896. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. The SCL-90-R. Clinical Psychometric Research; Baltimore, MD: 1975. [Google Scholar]
- Douglas KM, Porter RJ. Longitudinal assessment of neuropsychological function in major depression. Australian and New Zealand Journal of Psychiatry. 2009;43:1105–1117. doi: 10.3109/00048670903279887. [DOI] [PubMed] [Google Scholar]
- Ester EF, Vogel EK, Awh E. Discrete Resource Limits in Attention and Working Memory. In: Posner MI, editor. Cognitive Neuroscience of Attention. The Guilford Press; New York, NY: 2012. pp. 99–110. [Google Scholar]
- Fleming SK, Blasey C, Schatzberg AF. Neuropsychological correlates of psychotic features in major depressive disorders: a review and meta-analysis. Journal of Psychiatric Research. 2004;38:27–35. doi: 10.1016/s0022-3956(03)00100-6. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Curran JE, Winkler AM, Carless MA, Kent JW, Charlesworth JC, Johnson MP, Göring HHH, Cole SA, Dyer TD, Moses EK, Olvera RL, Kochunov P, Duggirala R, Fox PT, Almasy L, Blangero J. High Dimensional Endophenotype Ranking in the Search for Major Depression Risk Genes. Biological Psychiatry. 2012;71:6–14. doi: 10.1016/j.biopsych.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohier B, Ferracci L, Surguladze SA, Lawrence E, El Hage W, Kefi MZ, Allain P, Garre JB, Le Gall D. Cognitive inhibition and working memory in unipolar depression. Journal of Affective Disorders. 2009;116:100–105. doi: 10.1016/j.jad.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Ragland JD, Torrey EF, Gold JM, Bigelow LB, Weinberger DR. Neuropsychological assessment of monozygotic twins discordant for schizophrenia. Archives of General Psychiatry. 1990;47:1066–1072. doi: 10.1001/archpsyc.1990.01810230082013. [DOI] [PubMed] [Google Scholar]
- Halvorsen M, Høifødt RS, Myrbakk IN, Wang CEA, Sundet K, Eisemann M, Waterloo K. Cognitive function in unipolar major depression: A comparison of currently depressed, previously depressed, and never depressed individuals. Journal of Clinical and Experimental Neuropsychology. 2012;34:782–790. doi: 10.1080/13803395.2012.683853. [DOI] [PubMed] [Google Scholar]
- Hammar AA, Lund A, Hugdahl K. Long-lasting cognitive impairment in unipolar major depression: a 6-month follow-up study. Psychiatry Research. 2003;118:189–196. doi: 10.1016/s0165-1781(03)00075-1. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering Endophenotypes for Major Depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Hasselbalch BJ, Knorr U, Hasselbalch SG, Gade A, Kessing LV. Cognitive deficits in the remitted state of unipolar depressive disorder. Neuropsychology. 2012;26:642–651. doi: 10.1037/a0029301. [DOI] [PubMed] [Google Scholar]
- Hasselbalch BJ, Knorr U, Kessing LV. Cognitive impairment in the remitted state of unipolar depressive disorder: A systematic review. Journal of Affective Disorders. 2011;134:20–31. doi: 10.1016/j.jad.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Hinkelmann K, Moritz S, Botzenhardt J, Riedesel K, Wiedemann K, Kellner M, Otte C. Cognitive impairment in major depression: association with salivary cortisol. Biological Psychiatry. 2009;66:879–885. doi: 10.1016/j.biopsych.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Gorlyn M, Oquendo MA, Burke AK, Mann JJ. Attention deficit in depressed suicide attempters. Psychiatry Research. 2008;159:7–17. doi: 10.1016/j.psychres.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilp JG, Gorlyn M, Russell M, Oquendo MA, Burke AK, Harkavy-Friedman J, Mann JJ. Neuropsychological function and suicidal behavior: attention control, memory and executive dysfunction in suicide attempt. Psychological Medicine. 2012;43:539–551. doi: 10.1017/S0033291712001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO. Monozygotic twins discordant for major depression: A preliminary exploration of the role of environmental experiences in the aetiology and course of illness. Psychological Medicine. 2001;31:411–423. doi: 10.1017/s0033291701003622. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, environment, and psychopathology: Understanding the causes of psychiatric and substance use disorders. The Guilford Press; New York, NY: 2006. [Google Scholar]
- Kessing LV. Cognitive impairment in the euthymic phase of affective disorder. Psychological medicine. 1998;28:1027–1038. doi: 10.1017/s0033291798006862. [DOI] [PubMed] [Google Scholar]
- Lampe IK, Sitskoorn MM, Heeren TJ. Effects of recurrent major depressive disorder on behavior and cognitive function in female depressed patients. Psychiatry Research. 2004;125:73–79. doi: 10.1016/j.psychres.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Landro NI, Stiles TC, Sletvold H. Neuropsychological function in nonpsychotic unipolar major depression. Cognitive and Behavioral Neurology. 2001;14:233–240. [PubMed] [Google Scholar]
- Lyche P, Jonassen R, Stiles TC, Ulleberg P, Landro NI. Attentional Functions in Major Depressive Disorders With and Without Comorbid Anxiety. Archives of Clinical Neuropsychology. 2010;26:38–47. doi: 10.1093/arclin/acq095. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Galway TM, Hay J, Young LT, Joffe RT. Recollection Memory Deficits in Patients with Major Depressive Disorder Predicted by Past Depressions but Not Current Mood State or Treatment Status. Psychological Medicine. 2002;32:251–258. doi: 10.1017/s0033291701004834. [DOI] [PubMed] [Google Scholar]
- McClintock SM, Husain MM, Greer TL, Cullum CM. Association between depression severity and neurocognitive function in major depressive disorder: A review and synthesis. Neuropsychology. 2010;24:9–34. doi: 10.1037/a0017336. [DOI] [PubMed] [Google Scholar]
- McGue M, Osler M, Christensen K. Causal Inference and Observational Research The Utility of Twins. Perspectives on Psychological Science. 2010;5:546–556. doi: 10.1177/1745691610383511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micco JA, Henin A, Biederman J, Rosenbaum JF, Petty C, Rindlaub LA, Murphy M, Hirshfeld-Becker DR. Executive functioning in offspring at risk for depression and anxiety. Depression and Anxiety. 2009;26:780–790. doi: 10.1002/da.20573. [DOI] [PubMed] [Google Scholar]
- Munn MA, Alexopoulos J, Nishino T, Babb CM, Flake LA, Singer T, Ratnanather JT, Huang H, Todd RD, Miller MI, Botteron KN. Amygdala Volume Analysis in Female Twins with Major Depression. Biological Psychiatry. 2007;62:415–422. doi: 10.1016/j.biopsych.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Iacoviello B, Neumeister A, Charney DS, Iosifescu DV. Cognitive dysfunction in depression: Neurocircuitry and new therapeutic strategies. Neurobiology of Learning and Memory. 2011;96:553–563. doi: 10.1016/j.nlm.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 6. Muthén & Muthén; Los Angeles, CA: 2011. [Google Scholar]
- Naismith SL, Hickie IB, Turner K, Little CL, Winter V, Ward PB, Wilhelm K, Mitchell P, Parker G. Neuropsychological Performance in Patients With Depression is Associated With Clinical, Etiological and Genetic Risk Factors. Journal of Clinical and Experimental Neuropsychology (Neuropsychology, Development and Cognition: Section A) 2003;25:866–877. doi: 10.1076/jcen.25.6.866.16472. [DOI] [PubMed] [Google Scholar]
- Neu P, Bajbouj M, Schilling A, Godemann F, Berman RM, Schlattmann P. Cognitive function over the treatment course of depression in middle-aged patients: correlation with brain MRI signal hyperintensities. Journal of Psychiatric Research. 2005;39:129–135. doi: 10.1016/j.jpsychires.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Paelecke-Habermann Y, Pohl J, Leplow B. Attention and executive functions in remitted major depression patients. Journal of Affective Disorders. 2005;89:125–135. doi: 10.1016/j.jad.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Lamberty GJ, Garvey MJ, Robinson RG. Cognitive impairment in the euthymic phase of chronic unipolar depression. The Journal of nervous and mental disease. 1997;185:748–754. doi: 10.1097/00005053-199712000-00005. [DOI] [PubMed] [Google Scholar]
- Peretti S, Judge R, Hindmarch I. Safety and tolerability considerations: tricyclic antidepressants vs. selective serotonin reuptake inhibitors. Acta Psychiatrica Scandinavica. 2000;101:17–25. doi: 10.1111/j.1600-0447.2000.tb10944.x. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Warner V, Bansal R, Zhu H, Hao X, Liu J, Durkin K, Adams PB, Wickramaratne P, Weissman MM. Cortical thinning in persons at increased familial risk for major depression. Proceedings of the National Academy of Sciences. 2009;106:6273–6278. doi: 10.1073/pnas.0805311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Weissman MM. A Brain-Based Endophenotype for Major Depressive Disorder. Annual Review of Medicine. 2011;62:461–474. doi: 10.1146/annurev-med-010510-095632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss M, Kucerova H, Lukavsky J, Stepankova H, Sos P, Kawaciukova R. Cognitive deficits in the euthymic phase of unipolar depression. Psychiatry Research. 2009;169:235–239. doi: 10.1016/j.psychres.2008.06.042. [DOI] [PubMed] [Google Scholar]
- Simons CJP, Jacobs N, Derom C, Thiery E, Jolles J, Van Os J, Krabbendam L. Cognition as predictor of current and follow-up depressive symptoms in the general population. Acta Psychiatrica Scandinavica. 2009;120:45–52. doi: 10.1111/j.1600-0447.2008.01339.x. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Muir WJ, Blackwood DH. Neurocognitive impairment in euthymic young adults with bipolar spectrum disorder and recurrent major depressive disorder. Bipolar Disorders. 2006;8:40–46. doi: 10.1111/j.1399-5618.2006.00275.x. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Gibbon M, Williams JB. Instruction manual for structured clinical interview for DSM-III-R (SCID) New York State Psychiatric Institute; 1985. [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. American Journal of Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: Psychological Corporation; 1997a. (WAIS-III) [Google Scholar]
- Wechsler D. WMS-III: Wechsler Memory Scale. Psychological Corporation; 1997b. [Google Scholar]
- Weiland-Fiedler P, Erickson K, Waldeck T, Luckenbaugh DA, Pike D, Bonne O, Charney DS, Neumeister A. Evidence for continuing neuropsychological impairments in depression. Journal of Affective Disorders. 2004;82:253–258. doi: 10.1016/j.jad.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Wekking EM, Bockting CLH, Koeter MWJ, Schene AH. Cognitive functioning in euthymic recurrently depressed patients: Relationship with future relapses and prior course of disease. Journal of Affective Disorders. 2012;141:300–307. doi: 10.1016/j.jad.2012.03.034. [DOI] [PubMed] [Google Scholar]
- Xu G, Lin K, Rao D, Dang Y, Ouyang H, Guo Y, Ma J, Chen J. Neuropsychological performance in bipolar I, bipolar II and unipolar depression patients: A longitudinal, naturalistic study. Journal of Affective Disorders. 2012;136:328–339. doi: 10.1016/j.jad.2011.11.029. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Leach L, Kaplan E. On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry, Neuropsychology, & Behavioral Neurology. 1998:111–119. [PubMed] [Google Scholar]