1933 – Boivin A, Mesrobeanu I and Mesrobeanu L describe a toxic factor enclosed in the outer membrane of Gram-negative bacteria – nowadays known as endotoxin (C.R. Soc. Biol. Paris 1933;114:307). Ever since, researchers have tried to employ mechanisms to neutralize this endotoxin and thus limit the progression of disease in patients with sepsis due to Gram-negative bacilli. Meanwhile, the use of antibiotics over the past decades has lead to the emergence of resistance, multidrug resistance, extensive resistance and pan resistance.
One particular antibiotic is known to display anti-endotoxin activity by binding to the lipid A component of the bacterial lipopolysaccharide. This old antibiotic, colistin, is now reevaluated against multidrug resistant Gram-negative germs.
The research on polymyxins A through E started back in 1947 and this class currently includes only two drugs of human use: polymyxin A and polymyxin E (colistin). First isolated and identified in Japan in 1949 as a product of Bacillus polymyxa var. colistinus, colistin was used until the '80s, when it was replaced by other drugs, because of its adverse reaction profile. A few decades later, during 2003-2005, colistin reentered clinical practice after a reevaluation of its nephrotoxicity (Falagas et al. Clin Infect Dis. 2005;40:1333-41).
Colistin has brought 3 C's to the medical world: Confusion of terms, Complexity of pharmacology, Contradiction of posology.
Confusion of terms: there are two forms of colistin available: colistin sulfate for topical and digestive use, and colistimethate sodium (CMS), for parenteral use; these are not interchangeable.
Complexity of pharmacology: CMS is a prodrug that undergoes hydrolysis to active colistin and sulfomethylated derivatives in aqueous environments. While CMS is eliminated through the kidney, its main byproduct –colistin– undergoes tubular reabsorption and is eliminated through non-renal mechanisms.
Contradiction of posology: there are different nomenclatures for units and labeling conventions. Labels present different dosage information, for either the prodrug (CMS) or the active substance (colistin base), with the doses differing by almost 2.5-fold. Also, different measurement units are used: milligrams for CMS and for colistin base versus million international units (IU) for biological standardization. In 2011 the US Institute for Safe Medication Practices issued a warning on the risk of fatal error with confusion between CMS versus colistin base doses. A hypothetical correspondence between differently labeled doses thus becomes an important clinical and theoretical instrument.
 |
(Falagas et al., Antimicrob Agents Chemother. 2006;50(6):2274-5).
To complicate the issue even further, different guidelines calculate and recommend different doses based on body weight, renal function, and other parameters. However, despite these contradictions, the past decade has made it possible to study the pharmacokinetic properties of colistin within a mathematical model for preventing the emergence of bacterial resistance.
A first important aspect is the need for a loading dose: the administered quantity of CMS needs to ensure a circulating concentration of active colistin above the MIC of the pathogenic agent. In 2009 Plachouras et al. have shown that the optimal loading dose is 9 million IU (Antimicrob Agents Chemother. 2009;53:3430-6).
Second, the half-life of colistin, the area under the curve, and the time needed to reach a steady state also plead for the use of a loading dose, to decrease the time span when bacteria are exposed to suboptimal antibiotic concentrations and undergo selective resistance pressure.
Last but not least, the in vitro study of colistin administration once, twice, or three times per day has shown that the bactericidal rate can be sustained with drug administration at 8/12/24 hours, but that the Q8h administration regimen appears to be more effective in preventing emergence of resistance (Bergen et al., J Antimicrob Chemother. 2008;61:636-42).
When it comes to administering colistin in a critical patient, a new approach is needed: it is essential to avoid subtherapeutic concentrations in the first hours of therapy to reduce the odds of therapeutic failure or induction of resistance (Plachouras, Antimicrob Agents Chemother. 2009;53:3430-6).
Therefore, colistin should be administered with a loading dose of 9 million IU as 2-hour infusion, followed by two administrations of 3 million IU at 12 hours and then by 3 million IU maintenance doses every 8 hours. To avoid the risk of overdosing or nephrotoxicity, in obese patients the doses are calculated based on ideal body weight and new data come to show that the loading dose can be adjusted from 9 to 6 million IU based on body weight (above and below 60 kg) – see Figure.
Figure. Colistin administration regimen.
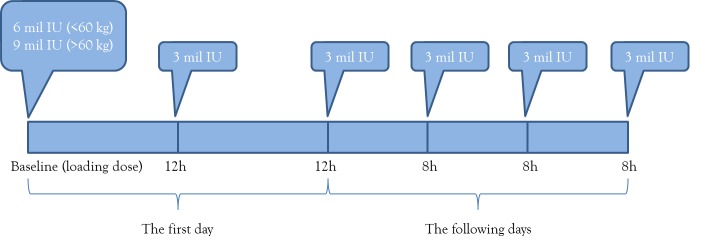
Notably, colistin should never be used as monotherapy, but rather it should be associated with potent drugs, active on Gram-negative bacilli (e.g. carbapenems), particularly in combinations that display documented synergy, as is the case with colistin increasing the susceptibility to meropenem.
In conclusion, this old drug has been reevaluated and reintroduced into clinical practice, particularly in the management of severe infections with multidrug resistant Gram-negative bacilli.


