SUMMARY
Self-reactive T cell clones that escape negative selection are either deleted or rendered functionally unresponsive (anergic), thus preventing them from propagating host tissue damage. By using an in vivo model, we investigated molecular mechanisms for T cell tolerance, finding that despite a characteristic inability to generate effector cytokine proteins, self-reactive T cells express large amounts of cytokine mRNAs. This disconnect between cytokine message and protein was not observed in T cells mounting productive responses to foreign antigens but, instead, was seen only in those responding to self, where the block in protein translation was shown to involve conserved AU-rich elements within cytokine 3′UTRs. These studies reveal that translation of abundant cytokine mRNAs is limited in self-reactive T cells and, thus, identify posttranscriptional silencing of antigen-driven gene expression as a key mechanism underlying the anergic phenotype of self-reactive T cells.
INTRODUCTION
Self-reactive clones make up a considerable fraction of the postthymic T cell repertoire and are a driving force in many clinically relevant inflammatory disorders, including arthritis, diabetes, and multiple sclerosis. However, despite the constant threat of self-reactivity, autoimmune disease is rare, in large part because these potentially dangerous clones are kept at bay through a process known as peripheral T cell tolerance (Mueller, 2010; Schwartz, 2003). The current paradigm holds that, under homeostatic conditions, self-reactive T cells encounter cognate antigen in the absence of overt inflammation, such that they receive a T cell receptor (TCR) trigger in the context of low-level costimulation and few proinflammatory cytokines. This suboptimal stimulus is interpreted as a “tolerogenic” signal and, in turn, self-reactive T cells are either deleted (apoptosis) or rendered functionally unresponsive (anergy), thereby preventing host tissue damage (Fathman and Lineberry, 2007; Schwartz, 2003). Alternatively, a subset of self-reactive T cells can become peripheral T regulatory (Treg) cells which, despite having critical immunosuppressive functions, also display at least one defining feature of T cell anergy—the inability to produce effector cytokines in response to antigenic stimulation (Bluestone and Abbas, 2003).
Although it has long been known that anergic, self-reactive T cells can persist for long periods (Pape et al., 1998), the molecular mechanisms underlying T cell anergy remain poorly defined. One key unresolved issue is whether self-reactive T cells fail to produce effector cytokines because of a direct block in cytokine gene expression or because of their general inability to respond to antigen. Regarding the former, there is evidence that cytokine transcription is suppressed in anergic T cells, with epigenetic silencing, induction of regulatory factors, and suppression of inducing factors all having been proposed as mechanisms (Fathman and Lineberry, 2007; Schwartz, 2003). However, though cytokine transcription may be impaired, it is also known that cytokine mRNAs can be detected within the “transcriptome” of anergic T cells, which suggests that transcriptional inhibition alone may not account for their cytokine null phenotype (Knoechel et al., 2006; Macian et al., 2002). Regarding the latter, it is well established that cytokine production is strongly influenced by the TCR and, because TCR-derived signals are known to be depressed in anergic T cells, some have proposed that the cytokine phenotype is secondary to their overall unresponsive state. Consistent with this idea, numerous studies have shown that, once self-reactive T cells are rendered anergic, they become refractory to stimulation with cognate antigen, even when presented in a highly immunogenic context (Schwartz, 2003). Moreover, when the molecules thought to underlie “TCR desensitization” are deleted or blocked, it typically leads to a break in peripheral T cell tolerance and attendant immunopathology, as in mice lacking cytotoxic T-lymphocyte-associated protein 4 (CTLA4), programmed cell death 1 (PD1), or Casitas B-lineage lymphoma b (Cbl-b), among others, and mice engineered to have constitutive or enhanced TCR signaling (Fathman and Lineberry, 2007; Mueller, 2010; Sonnenberg et al., 2010). However, despite a wealth of data indicating that anergic cells have a block in TCR signaling, it is also recognized that antigenic stimulation is necessary for the induction of T cell anergy and that, when removed from persistent antigen, anergic T cells can recover the ability to produce effector cytokines which, paradoxically, suggests that TCR-derived signals are required to maintain the unresponsive state (Rocha et al., 1993; Schwartz, 2003). Accordingly, when T cells are deprived of “tolerogenic” TCR signals it also leads to a break in peripheral T cell tolerance, as in mice with loss-of-function mutations in the TCR signaling molecule LAT, mice that have been depleted of tolerogenic dendritic cells and mice with T cell-specific deletion of calcium sensors (Mingueneau et al., 2009; Oh-Hora et al., 2008; Ohnmacht et al., 2009).
By using an in vivo model, we addressed the issue of why self-reactive T cells fail to produce effector cytokines. We demonstrate that, in response to high-affinity antigen, self-reactive T cells can express high amounts of cytokine mRNA but do not generate the corresponding proteins because of a cytokine-specific block in mRNA translation. Our studies also reveal that the disconnect between message and protein is mediated by conserved sequences within the 3′UTR of cytokine mRNAs, particularly AU-rich elements. Based on these data, we propose that posttranscriptional silencing of effector cytokines underlies the anergic phenotype of self-reactive T cells and that, in general, posttranscriptional control of antigen-driven gene expression is critical for T cell tolerance.
RESULTS
Self-Reactive T Cells Fail to Produce Effector Cytokines in Response to Antigenic Stimulation
To study cell-intrinsic mechanisms of peripheral tolerance, we have developed a mouse model where T cells bearing a highaffinity TCR specific for chicken ovalbumin (DO11) are adoptively transferred into mice that express ovalbumin as a soluble protein in the bloodstream, representing a neo-self antigen (sOva) (Lohr et al., 2004). As our basic experimental set-up, donor CD4+ T cells were purified from DO11 TCR transgenic mice crossed onto a Rag2-deficient background, thus yielding a starting population that is monoclonal and devoid of memory or regulatory lymphocytes (Figure S1 available online). These were adoptively transferred into sOva hosts, where they encountered antigen in a tolerogenic setting; WT hosts, where they did not encounter antigen; or WT hosts that were then immunized with Ova-pulsed dendritic cells (Ova-DC), where they encountered antigen in an inflammatory setting. At various times posttransfer, lymph nodes (LNs) and spleens were dissected from recipient mice and donor T cells were visualized with a monoclonal antibody that specifically recognizes the DO11 TCR (Figure S1). Consistent with previous reports (Lohr et al., 2004), we found that DO11 T cells exhibited a short bust of antigen-driven proliferation followed by a steep contraction phase when transferred into sOva hosts. A similar pattern was observed in the Ova-DC-immunized group, though the initial expansion was greater and subsequent decline less dramatic (Figure 1A; Figure S1). There was also a difference in the site of donor T cell proliferation such that, in sOva hosts, accumulation was greater in the LNs whereas, in Ova-DC hosts, it was greater in the spleen. Given that naive T cells homed to LNs in unimmunized WT mice, and that sOva mice express high amounts of antigen within the LNs, we conclude that the preferential expansion in sOva hosts is a function of where antigen was first encountered and not a fundamental biological feature of the tolerogenic response. According to that logic, it is also likely that preferential expansion in Ova-DC hosts is due to migration of the immunizing DCs, which are known to accumulate in the spleen upon intravenous administration. Accordingly, we found similar quantitative changes in the expression of cell-surface activation markers, including CD25, CD44, CD69, and CD62L, when comparing the LNs of sOva hosts to the spleens of immunized Ova-DC hosts (Figure 1A; Figure S1; data not shown).
Figure 1. Self-Reactive T Cells Fail to Poduce Effector Cytokines during Ex Vivo Restimulation.
(A) DO11 T cells were transferred into WT (naive), sOva (anergic), or WT mice, which were then immunized with Ova-pulsed DCs (effector). At 5–10 days post-transfer (P.T.), LNs and spleens were dissected from recipient mice and the percentage of total or activated (CD44hi) donor T cells was measured by flow cytometry. Total numbers were calculated by multiplying the percentage of CD4+DO11+ events by the number of viable cells recovered. Data are compiled from five experiments. Error bars indicate standard deviation between individual experiments, and asterisks denote significant differences between the effector (Ova-DCs) and anergic (sOva) groups (p < 0.05).
(B) CD4+ cells from recipient mice were restimulated and cytokine production measured by flow cytometry (day 5). Only CD4+DO11+ events are shown and percentages denote the cytokine-positive fraction within each quadrant.
(C) Flow cytometry data are compiled from four to five experiments. Error bars indicate standard deviation between individual experiments, and asterisks denote significant differences between the effector and anergic groups (p < 0.05).
(D) CD4+DO11+ donor T cells were purified from recipient mice (day 5) and restimulated, and cytokine production measured by ELISA. Data are representative of three experiments and error bars indicate the standard deviation between experimental replicates.
See Figure S1 for supplemental data on donor T cell behavior in sOva hosts.
To measure effector cytokine production, CD4+ cells were purified from recipient mice, restimulated overnight with Ova-pulsed dendritic cells, then fixed and stained for intracellular flow cytometry (IFC). As expected, there were few IL-2+, IL-4+, IL-13+, or IFN-γ+ donor T cells in sOva hosts although these were all readily detected in immunized Ova-DC hosts (Figures 1B and 1C). We did observe a small number of IL-17+ donor T cells in sOva hosts, though the percentage and amount of cyto-kine produced per cell were both lower than in Ova-DC hosts, leading us to conclude that, like Th1 and Th2 cell, Th17 cell differentiation is restricted in anergic cells. Consistent with this latter point, we could not detect IL-2, IL-4, IL-17A, or IFN-γ in culture supernatants from sOva hosts but could in those from Ova-DC hosts (Figure 1D). We also noted that donor T cell cytokine production was equally depressed in the LNs and spleens of sOva mice, which confirms that there is no site-specific influence on T cell anergy in this model (Figure S1 and data not shown).
In some models of T cell tolerance, anergic T cells can produce effector cytokines when exposed to polyclonal mitogens that bypass the TCR, such as PMA or ConA (Schwartz, 2003). To ask whether this applies to sOva hosts, we performed adoptive transfers, then measured donor T cell cytokine production in response to the combination of PMA and ionomycin. Consistent with previous reports (Macian et al., 2002; Teague et al., 2008), we found that donor T cells could produce IL-2 when exposed to this mitogenic cocktail, which confirms that TCR-dependent mechanisms can impact cytokine production in the context of T cell tolerance (Figure S1). However, production of lineage-restricted cytokines, like IFN-γ, IL-4, and IL-17, was not “released” under these conditions, which suggests that TCR-dependent mechanisms alone may not account for the general cytokine-null phenotype of anergic T cells (Figure S1). Collectively, the data presented in this section also indicate that, despite bearing an “activated” surface phenotype, self-reactive T cells are indeed limited in their capacity to produce effector cytokines and, thus, are functionally impaired.
Cytokine Transcripts Are Abundant in Self-Reactive T Cells
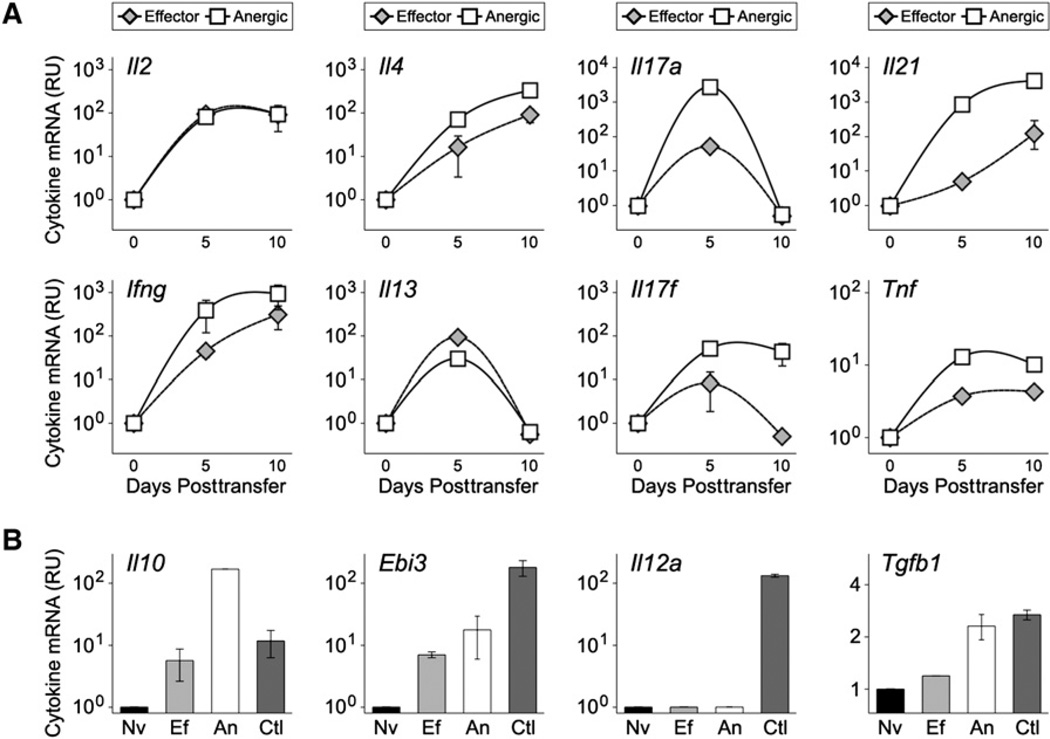
To further investigate the cytokine-null phenotype of self-reactive T cells, we performed adoptive transfers, then purified antigen-experienced (CD4+CD44hi DO11) donor cells and used real-time PCR to measure cytokine mRNAs (Figure S2). Surprisingly, we found that, despite their inability to generate effector cytokine proteins, donor T cells from sOva mice had an abundance of cytokine mRNAs. In fact, transcript levels for IL-2, IL-4, IL-13, IL-17A, IL-17F, IL-21, IFN-γ, and TNF-α were comparable to and often greater than those seen in donor T cells from Ova-DC-immunized hosts, which suggests that antigen-driven transcription of these genes is a common feature of T cells responding to both self and foreign antigens (Figure 2A). Consistent with published reports (Grogan et al., 2001), we also found that naive T cells (from donor DO11 mice) had detectable levels of some cytokine transcripts but, in most cases, these were orders of magnitude lower than in the other experimental groups which, again, suggests that TCR-derived signals are key for driving cytokine transcription (data not shown). In addition, though recent studies have shown that some “inducible” genes constitutively produce low levels of unspliced transcripts (Hargreaves et al., 2009), the majority of IFN-γ and IL-4 mRNAs were fully spliced in donor T cells from sOva and Ova-DC hosts, making it unlikely that differences in mRNA processing account for their distinct protein outputs (Figure S3).
Figure 2. Cytokine mRNAs Are Abundant in Self-Reactive T Cells.
(A) Adoptive transfers were performed as in Figure 1. At 5–10 days posttransfer, CD4+DO11.10+ CD44hi donor cells were purified from pooled LNs and spleens of recipient mice and cytokine mRNAs measured by PCR (Figure S2). Data are representative of five experiments and are presented as the fold increase (X > 1) or decrease (X < 1) relative to naive controls (Nv = 1). Error bars indicate the standard deviation between experimental replicates.
(B) Cytokine mRNAs were measured on day 5 posttransfer. LPS-activated DCs are included as a positive control (Ctl). Data are representative of three experiments and error bars indicate the standard deviation between experimental replicates.
See Figures S2 and S3 for supplemental data on donors T cell purification, PCR controls, and measurement of cytokine mRNA splicing.
Similar to effector cytokines like IL-4 and IFN-γ, we also found that mRNA expression for IL-10, a regulatory cytokine, was higher in donor T cells from sOva hosts than in those from Ova-DC hosts (Figure 2B). However, as above, we could detect IL-10 protein only in the immunized Ova-DC group, which suggests that the translational block associated with T cell anergy is not limited to overtly proinflammatory factors (data not shown). mRNA for TGF-β, another regulatory cytokine, was also slightly enriched but, in this case, expression was also high in naive controls and the difference between sOva hosts and the other groups was less than 2-fold. EBI3, a component of the regulatory cytokine IL-35, was strongly induced in both sOva and Ova-DC hosts but its partner in this heterodimer, IL-12p35, was not detected in any of the experimental groups (Figure 2B).
To visualize cytokine transcription directly ex vivo and at the single-cell level, we crossed DO11 donors with “cytokine reporter” mice that express either YFP (Yeti) or GFP (4get) downstream of the endogenous IFN-γ and IL-4 loci, respectively (Mohrs et al., 2001; Stetson et al., 2003). The resulting doubletransgenic offspring were not on a Rag2-deficient background so, to ensure that only naive, antigen-specific cells were used, CD4+CD25−YFP− or GFP− DO11 cells were sorted prior to transfer (Figure S4). As with our PCR studies, we saw little expression of YFP or GFP in WT Balb/c hosts but found clear induction in sOva and Ova-DC hosts. For both, fluorescence was linked to increased expression of CD44, meaning that it was dependent on antigenic stimulation and was heterogeneous, with some cells “brighter” than others (Figures 3A and 3B). To ask whether transgene expression was associated with protein expression, we restimulated YFP- and GFP-positive donor cells from sOva and Ova-DC hosts, then quantified IFN-γ and IL-4 productionby ELISPOT, whichisconsidered among the most sensitive metrics. Consistent with our flow cytometry studies (Figure 1), we found that YFP- and GFP-positive donor cells from sOva hosts could not produce IFN-γ or IL-4 whereas those from Ova-DC hosts could (Figures 3C and 3D). These data indicate that, even when actively transcribing effector cytokine loci, anergic T cells cannot generate the corresponding cytokine proteins.
Figure 3. Translation of Effector Cytokines Is Limited in Self-Reactive T Cells.
(A and B)Naive donor T cells were purified from (A) Yeti-DO11or (B) 4get-DO11 cytokine reporter mice (Figure S4). These were transferred into recipient mice and, 5 days later, YFP and GFP fluorescence was measured by flow cytometry. Only CD4+DO11+ events are shown. Right: Data are compiled from four to five experiments and asterisks denote significant differences between the indicated group and naive controls (p > 0.05).
(C and D) YFP+ or GFP+ donor cells were purified from recipient mice, and production of IFN-γ or IL-4 was measured by ELISPOT (day 5). Left: Data are compiled from two to three experiments. Error bars indicate standard deviation between individual experiments, and asterisks denote significant differences between the indicated group and naive controls (p < 0.05). See Figure S4 for supplemental data on donor T cell purification from cytokine reporter mice.
To confirm that increased expression of cytokine mRNAs is a general feature of self-reactive T cells, and not unique to our adoptive transfer system, we crossed 4get-DO11 mice with antigen-expressing sOva mice, thereby generating every combination of single-, double-, and triple-transgenic offspring. Predictably, we found very few CD4+DO11+ cells in either 4get or 4get-sOva mice whereas these were abundant in 4get-DO11 mice, comprising nearly 30% of all lymphocytes and 10% of all splenocytes (Figure 4A and data not shown). Because of negative selection, the percentage of CD4+ DO11 cells was dramatically reduced in 4get-DO11-sOva triple-transgenic mice but, as with the adoptive transfer system, there also remained an anergic population, here characterized by reduced TCR levels and an inability to produce IL-2 (Figures 4A and 4B and data not shown). A substantial percentage of these CD4+ DO11 cells also expressed GFP in triple-transgenic mice, which confirms that some are undergoing posttranscriptional silencing of abundant IL-4 transcripts (Figure 4C). Together with our adoptive transfer studies, these data indicate that, beyond deletion of self-reactive clones, peripheral T cell tolerance is enforced by posttranscriptional mechanisms that limit translation of abundant cytokine transcripts.
Figure 4. Expression of Cytokine mRNAs in Self-Reactive T Cells that Escape Negative Selection.
(A) LNs were dissected from 4get, 4get-sOva, 4get-DO11, and 4get-DO11-sOva mice. Shown is the percentage of CD4+DO11+ cells within the total lymphocyte population. Data are representative of four experiments.
(B) Lymphocytes from double- and triple-transgenic mice were restimulated and IL-2 production measured by flow cytometry. Only CD4+ events are shown. Right: Data are compiled from three experiments.
(C) Flow cytometry was performed as in (A). Only CD4+DO11+ events are shown and percentages represent the fraction of GFP+ cells. Right: Data are compiled from four experiments.
(B and C) Asterisks denote significant differences between the indicated group and either DO11 or 4get-DO11 controls (p < 0.05).
Mechanisms for Posttranscriptional Control of T Cell Cytokine Production
Previous work has shown that the integrated stress response (ISR), which typically curtails protein synthesis under conditions of stress, can also limit cytokine production during helper T cell differentiation (Scheu et al., 2006). To ask whether the ISR has a similar effect in the context of T cell tolerance, we purified donor cells from sOva and Ova-DC hosts and measured expression of ATF4, BiP, and CHOP, three critical components of the ISR. We found that, although these genes were active in all experimental groups (including naive controls), there was no specific enrichment in the sOva group, leading us to conclude that preferential induction of the ISR is unlikely to account for the observed differences in cytokine output (Figure S5).
Numerous studies have shown that cytokine mRNA translation can be influenced by sequences within the 3′UTR and, specifically, that AU-rich elements (AREs) can impact both half-life and translational efficiency (Anderson, 2010; Kruys et al., 1989). To ask whether UTR-mediated silencing can influence T cell cytokine production, we engineered a series of “UTR sensors” where cytokine (or control) UTRs were fused to GFP, thus allowing for protein levels to be measured by GFP fluorescence. These were delivered into primary mouse T cells by means of an MSCV-based vector that, upon integration, uses its own viral promoter to drive transcription, thereby assuring that each construct had similar output and that none would be subject to transcriptional silencing (as is the case for the endogenous cytokine loci). It should also be noted that naive T cells could not be transduced with these vectors so, for the subsequent studies, all T cells were activated with TCR and CD28 antibodies prior to retroviral (RV) infection. To confirm that this in vitro manipulation would not affect T cell behavior in vivo, DO11 T cells were transduced with a control GFP construct, transferred into sOva hosts and, 5 days later, their ability to produce effector cytokines was measured. As with our naive cell transfers (Figure 1), we found that RV-infected donors could not produce IL-2, IL-4, or IFN-γ when restimulated ex vivo, which demonstrates that neither “preactivation” nor RV infection had much impact on the anergic phenotype in this model (Figure S7 and data not shown).
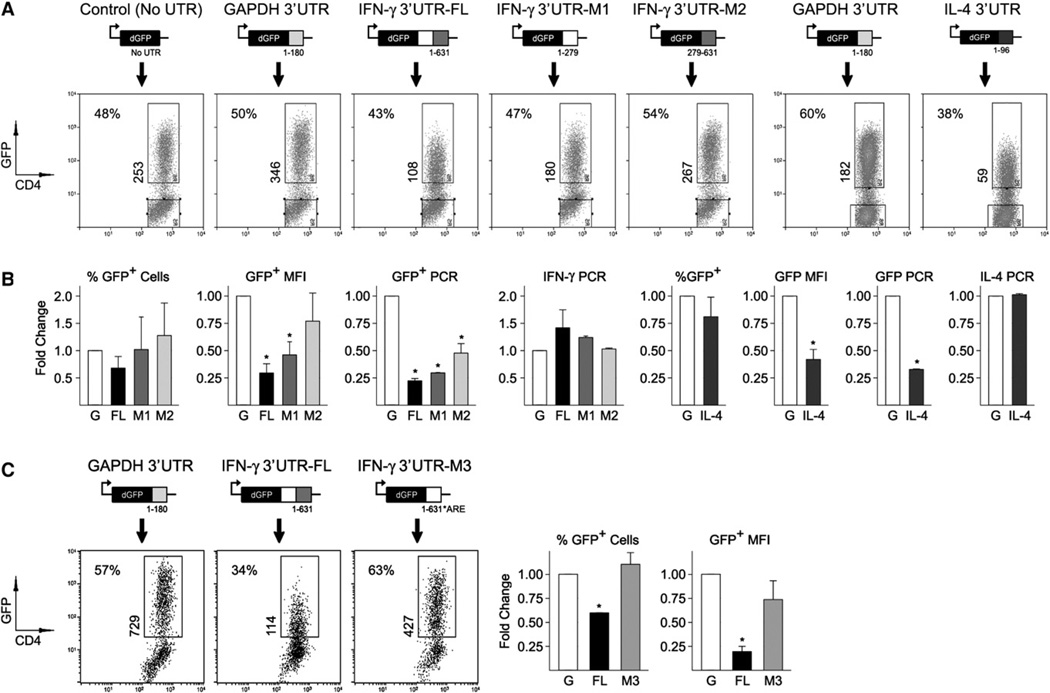
As our first test case, we chose IFN-γ, which has a relatively long 3′UTR (631 bp) and a clear stretch of AU-rich sequence proximal to the stop codon. We generated a full-length construct (FL), which has the entire 3′UTR sequence downstream of GFP, and two truncated mutants, one containing the AREs (M1, bases 1–279) and one lacking them (M2, bases 279–631). As an additional control, we also generated a construct with the 3′UTR of GAPDH, which is relatively short (200 bp) and not known to undergo posttranscriptional silencing (Figure S6). We found that T cells could be readily transduced by all of these constructs, with each yielding a comparable percentage of GFP+ cells, but we also noted that the full-length and M1 constructs had lower mean fluorescence intensity (MFI) (Figures 5A and 5B). To further investigate this effect, we purified GFP+ cells from all groups and used PCR to quantify GFP transcripts. As with our protein measurements, we found that less GFP mRNA could be detected in cells transduced with the FL and M1 constructs than in those transduced with the GAPDH or M2 constructs, which suggests that, beyond translational effects, the IFN-γ UTR may also prompt mRNA decay (Figure 5B). Consistent with these findings, and the idea that multiple cytokines are influenced by UTR-dependent mechanisms, GFP protein and message were also depressed in cells transduced with a full-length IL-4 3′UTR construct, which is short (95 bp) and contains a prominent AU-rich region (Figures 5A and 5B; Figure S6).
Figure 5. 3′UTR-Dependent Mechanisms Limit Translation of Ifng and Il4 mRNAs.
(A) CD4+ T cells were transduced with UTR-sensor constructs and GFP fluorescence measured by flow cytometry. Shown is the percentage of GFP+ cells (top left) and the mean fluorescence intensity of those GFP+ events (vertical).
(B) Flow cytometry and PCR data are compiled from three to four in vitro experiments. For PCR experiments, GFP+ cells were purified by high-speed cell sorting and assayed as in Figure 2. Fold change was calculated by dividing experimental group measurements by those of GAPDH controls (G = 1).
(C) CD4+ T cells were transduced with the GAPDH, IFN-γ (FL), or IFN-γ M3 (ARE mutant) 3′UTR constructs and GFP fluorescence measured as in (A). Right: Data are compiled from two experiments.
(B and C) Error bars indicate standard deviation between individual experiments, and asterisks denote significant differences between the indicated group and GAPDH controls (p < 0.05).
See Figures S5 and S6 for supplemental PCR data, information about UTR-sensor construct design, and bioinformatic analysis of cytokine 3′UTRs.
To directly address the role of AREs in limiting T cell cytokine production, we started with a computational approach. Sequence analysis revealed that, indeed, the cytokine mRNAs that we found to be enriched in anergic T cells, like IL-4, IL-21, and IFN-γ, all had predicted AREs within their 3′UTRs (Table 1). In most cases, the AREs were located within highly conserved regions of the 3′UTR, with IL-17A and IL-17F being the exceptions and IFN-γ the most representative, exhibiting a high degree of conservation in the proximal AU-rich region (corresponding to the M1 construct) and much less in the distal “AU-poor” region (Figure S6). To test the biological impact of these conserved AREs, we mutated our full-length IFN-γ 3′UTR construct such that predicted AREs were substituted with GC-rich sequence (Figure S6). As before, the construct was packaged into retro-virus (along with controls) and transduced into primary mouse T cells and, 24 hr later, GFP fluorescence was measured. We found that, compared to the WT construct (IFN-γ 3′UTR-FL), GFP fluorescence was substantially higher in the ARE mutant (Figure 5C). Taken together with our truncation mutant studies (Figure 5A), these data support the hypothesis that 3′UTR-dependent mechanisms curb T cell cytokine production and identify AREs as key mediators in that process.
Table 1.
Sequence and Position of AU-Rich Elements within Cytokine 3′UTRs
| Gene | Accession | UTR Length | AU-Rich Element | Sequence | Position | ARED Cluster |
|---|---|---|---|---|---|---|
| Ifng | NM008337 | 631 | yes | TGTTATTTATAAT | 188–200 | 5 |
| Tnf | NM013693 | 754 | yes | ATTTATTTATTTG | 458–470 | 3 |
| Il2 | NM008366 | 382 | yes | CTATTTATTTAAA | 35–47 | 4 |
| Il4 | NM021283 | 96 | yes | ATTTATATATTTA | 53–65 | 3 |
| Il10 | NM010548 | 702 | yes | AAATATTTATTAC | 125–37 | 5 |
| Il13 | NM008355 | 741 | yes | TCTTATTTATTAT | 304–16 | 5 |
| Il17a | NM010552 | 637 | yes | ATTTGTTTATTTA | 472–84 | 3 |
| Il17f | NM145856 | 618 | yes | ATATATTTATGTT | 504–16 | 5 |
| Il21 | NM021782 | 2578 | yes | GATTATTTATTAT | 2491–503 | 5 |
| Tgfb1 | NM011577 | 54 | no | |||
| Ebi3 | NM015766 | 245 | no | |||
| Ifnb1 | NM010510 | 201 | no | |||
| Gapdh | NM008084 | 180 | No |
ARED Cluster refers to the type of AU-rich element, as defined by the AU-Rich Element mRNA Database (ARED, http://brp.kfshrc.edu.sa/ARED).
To test whether UTR- and ARE-mediated silencing are relevant in the setting of T cell tolerance, we transduced DO11 cells with UTR-sensor constructs, then purified the GFP-positive fraction and transferred them into sOva hosts. We found that, compared to GAPDH, the FL and M1 constructs had greatly reduced fluorescence both in terms of MFI and in terms of percent GFP+ cells, which suggests that many were suppressed to below the limit of detection (Figures 6A and 6B). By comparison, the M2 construct was only slightly lower than the GAPDH control, though it should be noted that the M1 construct was not as dim as the FL construct, which implies that beyond the AU-rich M1 region, there may be a contribution from the more distal M2 region. As with our in vitro experiments (Figure 5C), we found that GFP fluorescence was greater in the ARE mutant than in the wild-type IFN-γ construct, which provides direct evidence for the involvement of AREs in the context of T cell anergy (Figure 6C). Also, like our in vitro experiments, GFP fluorescence was strongly depressed in cells transduced with the IL-4 3′UTR construct which, again, demonstrates that multiple cytokines may be subject to 3′UTR-dependent regulation (Figures 6A and 6B).
Figure 6. AU-Rich Elements Limit Translation of Cytokine mRNAs in Self-Reactive Cells.
(A) DO11 T cells were transduced as in Figure 5. CD4+DO11+GFP+ cells were then purified by high-speed cell sorting (top) and transferred into sOva hosts (bottom). 3–5 days later, CD4+ cells were purified from pooled LNs and spleens of recipient mice and GFP fluorescence was measured by flow cytometry. Only CD4+DO11+ events are displayed. Shown is the percentage of GFP+ cells (top left) and the mean fluorescence intensity of those GFP+ events (vertical).
(B) Flow cytometry data are compiled from three to four experiments. Fold change was calculated by dividing experimental group measurements by those of GAPDH controls (G = 1).
(C) DO11 T cells were transduced with the GAPDH, IFN-γ (FL), or IFN-γ M3 (ARE mutant) 3′UTR constructs. Adoptive transfers and flow cytometry were then performed as in (A). Right: Data are compiled from two experiments.
(D) Donor T cells were transduced and sorted as in (A). These were then transferred into sOva Rag2−/− recipients and, 5 days later, GFP fluorescence was measured. Right: Flow cytometry data are compiled from three experiments.
(B–D) Error bars indicate standard deviation between individual experiments, and asterisks denote significant differences between the indicated group and GAPDH controls (p < 0.05).
See Figure S7 for supplemental data on effector cytokine production in RV-transduced donor T cells.
Previous work has shown that, when DO11 T cells are transferred into lymphopenic sOva mice (crossed onto a Rag2-deficient background), they do not become anergic and, instead, generate a robust effector response characterized by unrestrained IFN-y production (Caretto et al., 2010). To test whether UTR-mediated inhibition can be relieved under these permissive conditions, we transduced DO11 T cells with UTR-sensor constructs and transferred them into sOva Rag2−/− mice. Unlike “intact” sOva hosts, where there was robust inhibition of the full-length and M1 constructs, we found that the percent of GFP+ cells was similar for all groups in lymphopenic hosts (Figure 6D). Taken together with our studies in “lymphoreplete” hosts (sOva Rag2+/+), these data argue that 3′UTR- and ARE-dependent mechanisms can prohibit cytokine translation in a “tolerogenic” setting but not in a lymphopenic or “autoimmune” setting, which suggests that posttranscriptional silencing is either shut down or overcome when T cells are primed under proinflammatory conditions.
DISCUSSION
The studies presented here establish that, in response to high-affinity antigen, self-reactive T cells can express cytokine mRNAs but fail to generate the corresponding proteins because of a block in translation that involves AU-rich elements within cytokine 3′UTRs. A similar disconnect between protein and message has been observed in other cell types, including NK cells, NKT cells, neutrophils, and basophils but, in these lineages, it has generally been viewed as a proinflammatory event, an indication that they are poised for rapid cytokine production (Gessner et al., 2005; Stetson et al., 2003). It is also known that, when helper T cells become activated, cytokine transcription is rapidly induced while translation lags behind, thereby creating a circumstance where cytokine message is abundant but protein is scarce. In those studies, it was revealed that anti-genic stimulation triggers the integrated stress response (ISR) and that this is responsible for the lag in cytokine production during the early stages of differentiation (Scheu et al., 2006). Though we did not find that the ISR was selectively active in anergic T cells (compared to naive or effectors), the 3′UTR-dependent mechanisms described here are likely to work in concert with the ISR, which is 5′ cap dependent, and with other 5′UTR-mediated processes (Anderson, 2010; Ben-Asouli et al., 2002; Mazumder et al., 2010). Therefore, based on current and past findings, we propose that multiple 5′ and 3′UTR-dependent pathways cooperate to limit translation of effector cytokine mRNAs in anergic, self-reactive T cells. We also put forth that, although numerous cytokines may be affected, this translational block is cytokine specific and does not represent a global shutdown in protein synthesis, as evidenced by our own studies, which demonstrate that cell-surface markers like CD25, CD44, and CD69 are strongly induced in anergic T cells, and by numerous others which have shown that certain molecules are selectively expressed under tolerogenic conditions (Fathman and Lineberry, 2007; Schwartz, 2003).
It is well established that the 3′UTR can act as a platform for posttranscriptional regulation of cytokine mRNAs (Anderson, 2010). Accordingly, we hypothesize that AREs within the 3′UTR of cytokine mRNAs limit translation in self-reactive T cells, a conclusion supported by numerous in vitro studies demonstrating that AREs promote decay and/or translational arrest of cytokine transcripts, and by the phenotypes of mice lacking AREs in the UTRs of TNF-α and GM-CSF, which exhibit aberrant cytokine production and severe autoimmune disease (Carballo et al., 1998; Garcia-Sanz and Lenig, 1996; Han et al., 1990; Houzet et al., 2001; Kontoyiannis et al., 1999; Kruys et al., 1989; Taylor et al., 1996). However, it should be noted that ARE-independent mechanisms are also likely to influence cytokine production in this setting. For instance, there is a growing list of RNA-binding proteins (RBPs), including Roquin, 4E-BP1, Zc3h12a, and Zcchc11, that are known to affect cytokine production, and many of these could be relevant in helper T cells (Colina et al., 2008; Jones et al., 2009; Matsushita et al., 2009; Yu et al., 2007). Another class of regulatory factor that could impact cytokine production are microRNAs, short (20–25 nt) single-stranded RNAs, which target complementary sequences within 3′UTRs, thereby silencing the associated transcripts. Consistent with this latter point, T cells lacking Dicer, an enzyme necessary for the biogenesis of microRNAs, exhibit aberrant cytokine production, and an increasing number of individual microRNAs have been shown to directly interact with cytokine mRNAs (Jones et al., 2009; Matsushita et al., 2009; Muljo et al., 2005; Sharma et al., 2009; Zhou et al., 2008).
Based on our data, which demonstrate that posttranscriptional mechanisms limit cytokine production in the context of T cell tolerance, and studies showing that naive T cells can express cytokine mRNAs (Grogan et al., 2001), we propose that there is a translational threshold that prevents T cells from generating effector cytokines until they encounter requisite inflammatory cues, such as would be provided during acute infection or immunization. Consistent with this hypothesis, we demonstrate that cytokine translation is repressed under tolerogenic conditions (sOva mice) and derepressed under autoimmune conditions (sOva Rag2−/− mice), which confirms that the translational threshold can be overcome when T cells are primed in a permissive environment. The mechanism(s) for that effect remains unknown but several theories can be proposed. One possibility is that, when T cells are exposed to polarizing stimuli, they generate enough cytokine mRNAs to overwhelm the regulatory machinery, thus allowing some translation to proceed. It is also possible that the regulatory machinery is shut down when T cells become activated, which would lead to increased translation without a vast excess of cytokine message, or that protein synthesis is turned up, which would lead to increased translation of all mRNAs, not just those encoding cytokines. These three ideas are not mutually exclusive and do not represent an exhaustive list, but there is already some evidence for the second, with recent studies demonstrating that mRNAs from activated T cells tend to have shorter UTRs than those from naive cells, making them less susceptible to the type of ARE-mediated regulation described here (Sandberg et al., 2008).
By using an in vivo model, we demonstrate that self-reactive T cells express cytokine mRNAs and that, on a per cell basis, the amount is similar to that observed in “cytokine-competent” effector T cells. Although this seems to be at odds with the idea that cytokine transcription is depressed under tolerogenic conditions (Macian et al., 2002), it should be noted that previous work employed mostly in vitro models, which are known to induce a different type of anergy, often termed “clonal anergy” (Schwartz, 2003), and that despite this difference in methodology, they also offer some evidence that anergic T cells can express cytokine mRNAs (albeit at lower amounts than effector counterparts). Thus, we propose that pre- and posttranscriptional mechanisms cooperate to limit cytokine production in the context of T cell tolerance. In addition, given the widespread use of gene arrays (and PCR) to measure T cell responses, we also suggest that cytokine transcripts do not always equate to protein and that, as a result, mRNA data must be interpreted with care and, whenever possible, confirmed by means of protein-based measurements.
Numerous posttranscriptional mechanisms are known to impact T cell tolerance, including RBPs, phosphatases, kinases, and ubiquitin ligases, and it is certain that others will emerge, particularly in the field of microRNAs where the discovery of immunologically relevant targets has only just begun (Fathman and Lineberry, 2007; Mueller, 2010; Schwartz, 2003). Our work builds on this idea and, though we have considered only a select group of factors (cytokines) within only one cell type (CD4+ helper T cells), we hypothesize that 3′UTR-mediated silencing may be a general paradigm for limiting the expression of inflammatory gene products, affecting multiple classes of proteins (i.e., costimulatory molecules, cytolytic molecules) and multiple immune cell lineages (i.e., NK cells, dendritic cells), an idea bolstered by recent work demonstrating that granzyme-B (in CD8+ T cells) and IL-10 (in NKT cells) are also subject to posttranscriptional control (Fehniger et al., 2007; Maroof et al., 2008). Thus, based on current and past evidence, we propose that the components of this regulatory machinery, such as those involved in UTR- and ARE-dependent inhibition, are relevant therapeutic targets in the setting of autoimmune disease.
EXPERIMENTAL PROCEDURES
Animals
For donors, DO11.10 TCR transgenics (from K. Murphy, Washington University, St. Louis, MO) were crossed with Rag2−/− mice (Jackson Laboratories, Bar Harbor, ME) or Yeti/4get cytokine reporter mice (from R. Locksley, UCSF, San Francisco, CA) (Mohrs et al., 2001; Stetson et al., 2003). For recipients, WT Balb/c mice were purchased from Jackson Labs. sOva-transgenic mice were generated as described and, where indicated, bred onto a Rag2-deficient background (Lohr et al., 2004). For some studies, sOva and 4get DO11.10 mice were crossed, thereby generating every combination of single-, double-, and triple-transgenic offspring. Genotyping was done by PCR. All animals were maintained in specific-pathogen-free housing at the University of California, San Francisco, CA, and experiments were carried out according to guidelines set by the Institutional Animal Care and Use Committee.
Adoptive Transfers and Immunizations
LNs and spleens were pooled from 4- to 6-week-old DO11 Rag2−/− mice and CD4+ cells purified (>96% purity) by positive selection with magnetic beads (Dynal Beads: Invitrogen, Carlsbad, CA) (Figure S1). For cytokine reporters, LNs were dissected from Yeti DO11.10 or 4get DO11.10 mice and stained directly ex vivo with fluorochrome-conjugated CD4, DO11.10 (KJ1-26), and CD25 antibodies (eBioscience, San Diego, CA). Naive CD4+ DO11.10 TCR+, CD25−, YFP/GFP− cells were then purified by high-speed cell sorting (>99% purity) (Figure S4). For all adoptive transfers, 2–5 × 105 donor cells were intravenously injected into age- and sex-matched recipients (in 400 ml PBS). For immunizations, donor T cells were transferred into WT Balb/c mice and, 24 hr later, these were intravenously injected with 1–5 × 105 bone marrow-derived dendritic cells (BM-DCs) that had been activated with LPS and loaded with Ova peptide (1 mg/ml each; Sigma; St. Louis, MO) (Figure S1).
Ex Vivo Cytokine Production
CD4+ cells were purified from LNs and spleens of recipient mice and stimulated overnight with LPS/Ova-pulsed BM-DCs (5:1 T cell to DC ratio). Cultures were then treated with Brefeldin A (10 mg/ml) for 2–3 hr, fixed (4% paraformaldehyde), permeabilized (0.25% Saponin), and stained with fluorochrome-labeled CD4 and KJ1-26 antibodies in combination with anti-IFN-γ, anti-IL-17A, anti-IL-17F, anti-TNF-α, anti-IL-2, anti-IL-4, and/or anti-IL-13 (eBioscience). Where indicated, purified CD4+ cells were also restimulated with a combination of Phorbol 12-myristate 13-acetate (PMA 50 ng/ml) and ionomycin (500 ng/ml; 4 hr total with BFA for the final 2 hr), then stained as above (Figure S1). All 4-color flow cytometry was performed on a FACScalibur instrument and analyzed with CellQuest Pro Software (Becton Dickinson; Franklin Lakes, NJ). for ELISA and ELISPOT, CD4+DO11.10TCR+, YFP/GFP+ donor T cells were purified from recipient mice by high-speed cell sorting (1–5 × 104 cells/well for ELISA and 2–10 × 103 for ELISPOT). These were restimulated overnight (1:2 T cell to DC ratio) and then assayed for cytokine production with standard protocols. For all studies, cells were maintained in supplemented tissue culture medium (RPMI-1640 with 10% fetal calf serum, 1% sodium pyruvate, 1% nonessential amino acids, 0.1% β-Mercaptoethanol, 100 U/ml penicillin, 100 µg/ml /streptomycin; GIBCO/Invitrogen) at a density of 2–4 × 106 cells/ml in round-bottomed 96-well plates (200 µl/ well; Sigma/Costar).
Ex Vivo PCR
CD4+CD44hi DO11 cells from recipient mice, along with naive controls (CD4+ CD25 −CD44lo DO11 cells from DO11.10 mice), were purified by high-speed cell sorting (2–10 × 104 per group) (Figure S2). Total RNA was then extracted and converted to cDNA by means of oligo-dT priming and SuperScript III reverse transcriptase (100–250 ng RNA per reaction; Invitrogen, Carlsbad, CA). PCR amplification was performed with SYBR green master mix (5–10 ng cDNA per reaction; Applied Biosystems, Foster City, CA) via an iQ5 Real-Time PCR thermal cycler (BioRad, Hercules, CA). Primer sequences and relevant information are provided in Table S1. Reactions were performed in duplicate, Ct values were normalized to β -actin levels, and fold induction (n > 1) or reduction (n < 1) calculated ( ΔΔCt) with respect to the indicated controls (n = 1) (Figure S2).
Retroviral Gene Transduction
Mouse IFN-γ (FL), IL-4, and GAPDH 3′UTRs were PCR amplified with high-fidelity polymerase and a cDNA library derived from activated, Balb/c CD4+ T cells (Easy A Polymerase; Stratagene, Cedar Creek, TX) (Figure S6). PCR products were then digested and ligated into a modified MIG-R1 vector (directly downstream of a destabilized GFP; d4GFP from Clonetech, Mountain View, CA; restriction enzymes and T4 ligase from NEB, Ipswich, MA). Only recombinant clones with ~100% sequence homology to the NCBI GenBank mRNA entry were selected for further amplification (PCR sequencing by Sequentech, Mountain View, CA; Mini- and Maxi-prep kits from QIAGEN, Germantown, MD). IFN-γ M1 and M2 constructs were PCR amplified (with the full-length construct as template), then cloned into the MIG-R1-d4GFP vector, sequenced, and amplified. IFN-γ M3 was generated by site-directed mutagenesis within the full-length IFN-γ MigR1 vector (mutagenesis by GenScript, Piscataway, NJ) (Figure S6). All 3′UTR vectors (or “empty” controls) were transfected into Phoenix packaging cells (together with pCL-Eco helper plasmid) and the resulting culture supernatants used to infect DO11 CD4+ T cells (Rag2+/+). These were cultured with plate-bound anti-CD3s (1 µg/ml, Clone: 17A2) and soluble anti-CD28 (0.5 µg/ml, Clone: 37.51) for 48 hr, exposed to viral supernatant for 1 hr (at 2200 rpm, 19°C) and cultured for an additional 24 hr before flow cytometry or high-speed cell sorting. For adoptive transfers, purified CD4+GFP+ DO11 cells were intravenously injected into recipient mice (2–10 × 104 per group) (Figure 6A). 3–5 days later, LNs and spleens were pooled and CD4+ cells were purified and 1 × 107 stained for flow cytometry. All contour plots are gated on CD4+ DO11 donor cells and contain >100 events.
Statistics
Paired Student’s t test (two-tailed) was used to quantify statistical deviation between experimental groups. In all figures, an asterisk represents significant differences (p < 0.05).
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank members of the A.K.A., Anderson, Bluestone, Krummel, M.T.M., and Tang laboratories for helpful discussions. We also thank M. Krummel for scientific discussions and C. Benitez for expert animal husbandry. This work was supported by NIH grants PO1-AI35297 and RO1-AI64677 to A.K.A., a minority postdoctoral supplement to A.V.V. (PA-05-015), a Ruth L. Kirschstein National Research Service Award to S.D.K. (F32-AI077199), an NIH grant (R01-DA026065) and W.M. Keck Award to M.T.M, and support from the UCSF Program in Breakthrough Biomedical Research.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures and seven figures and can be found with this article online at doi:10.1016/j.immuni.2010.12.014.
The authors have no conflicting financial interests.
REFERENCES
- Anderson P. Post-transcriptional regulons coordinate the initiation and resolution of inflammation. Nat. Rev. Immunol. 2010;10:24–35. doi: 10.1038/nri2685. [DOI] [PubMed] [Google Scholar]
- Ben-Asouli Y, Banai Y, Pel-Or Y, Shir A, Kaempfer R. Human interferon-gamma mRNA autoregulates its translation through a pseudoknot that activates the interferon-inducible protein kinase PKR. Cell. 2002;108:221–232. doi: 10.1016/s0092-8674(02)00616-5. [DOI] [PubMed] [Google Scholar]
- Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998;281:1001–1005. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- Caretto D, Katzman SD, Villarino AV, Gallo E, Abbas AK. Cutting edge: The Th1 response inhibits the generation of peripheral regulatory T cells. J. Immunol. 2010;184:30–34. doi: 10.4049/jimmunol.0903412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colina R, Costa-Mattioli M, Dowling RJ, Jaramillo M, Tai LH, Breitbach CJ, Martineau Y, Larsson O, Rong L, Svitkin YV, et al. Translational control of the innate immune response through IRF-7. Nature. 2008;452:323–328. doi: 10.1038/nature06730. [DOI] [PubMed] [Google Scholar]
- Fathman CG, Lineberry NB. Molecular mechanisms of CD4+ T-cell anergy. Nat. Rev. Immunol. 2007;7:599–609. doi: 10.1038/nri2131. [DOI] [PubMed] [Google Scholar]
- Fehniger TA, Cai SF, Cao X, Bredemeyer AJ, Presti RM, French AR, Ley TJ. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 2007;26:798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Garcia-Sanz JA, Lenig D. Translational control of interleukin 2 messenger RNA as a molecular mechanism of T cell anergy. J. Exp. Med. 1996;184:159–164. doi: 10.1084/jem.184.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J. Immunol. 2005;174:1063–1072. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- Grogan JL, Mohrs M, Harmon B, Lacy DA, Sedat JW, Locksley RM. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity. 2001;14:205–215. doi: 10.1016/s1074-7613(01)00103-0. [DOI] [PubMed] [Google Scholar]
- Han J, Brown T, Beutler B. Endotoxin-responsive sequences control cachectin/tumor necrosis factor biosynthesis at the translational level. J. Exp. Med. 1990;171:465–475. doi: 10.1084/jem.171.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves DC, Horng T, Medzhitov R. Control of inducible gene expression by signal-dependent transcriptional elongation. Cell. 2009;138:129–145. doi: 10.1016/j.cell.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houzet L, Morello D, Defrance P, Mercier P, Huez G, Kruys V. Regulated control by granulocyte-macrophage colony-stimulating factor AU-rich element during mouse embryogenesis. Blood. 2001;98:1281–1288. doi: 10.1182/blood.v98.5.1281. [DOI] [PubMed] [Google Scholar]
- Jones MR, Quinton LJ, Blahna MT, Neilson JR, Fu S, Ivanov AR, Wolf DA, Mizgerd JP. Zcchc11-dependent uridylation of microRNA directs cytokine expression. Nat. Cell Biol. 2009;11:1157–1163. doi: 10.1038/ncb1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoechel B, Lohr J, Zhu S, Wong L, Hu D, Ausubel L, Abbas AK. Functional and molecular comparison of anergic and regulatory T lymphocytes. J. Immunol. 2006;176:6473–6483. doi: 10.4049/jimmunol.176.11.6473. [DOI] [PubMed] [Google Scholar]
- Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: Implications for joint and gut-associated immunopathol-ogies. Immunity. 1999;10:387–398. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- Kruys V, Marinx O, Shaw G, Deschamps J, Huez G. Translational blockade imposed by cytokine-derived UA-rich sequences. Science. 1989;245:852–855. doi: 10.1126/science.2672333. [DOI] [PubMed] [Google Scholar]
- Lohr J, Knoechel B, Kahn EC, Abbas AK. Role of B7 in T cell tolerance. J. Immunol. 2004;173:5028–5035. doi: 10.4049/jimmunol.173.8.5028. [DOI] [PubMed] [Google Scholar]
- Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- Maroof A, Beattie L, Zubairi S, Svensson M, Stager S, Kaye PM. Posttranscriptional regulation of II10 gene expression allows natural killer cells to express immunoregulatory function. Immunity. 2008;29:295–305. doi: 10.1016/j.immuni.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Takeuchi O, Standley DM, Kumagai Y, Kawagoe T, Miyake T, Satoh T, Kato H, Tsujimura T, Nakamura H, Akira S. Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature. 2009;458:1185–1190. doi: 10.1038/nature07924. [DOI] [PubMed] [Google Scholar]
- Mazumder B, Li X, Barik S. Translation control: A multifaceted regulator of inflammatory response. J. Immunol. 2010;184:3311–3319. doi: 10.4049/jimmunol.0903778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingueneau M, Roncagalli R, Gregoire C, Kissenpfennig A, Miazek A, Archambaud C, Wang Y, Perrin P, Bertosio E, Sansoni A, et al. Loss of the LAT adaptor converts antigen-responsive T cells into pathogenic effectors that function independently of the T cell receptor. Immunity. 2009;31:197–208. doi: 10.1016/j.immuni.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysisof type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- Mueller DL. Mechanisms maintaining peripheral tolerance. Nat. Immunol. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- Muljo SA, Ansel KM, Kanellopoulou C, Livingston DM, Rao A, Rajewsky K. Aberrant T cell differentiation in the absence of Dicer. J. Exp. Med. 2005;202:261–269. doi: 10.1084/jem.20050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endo-plasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat. Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J. Exp. Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape KA, Merica R, Mondino A, Khoruts A, Jenkins MK. Direct evidence that functionally impaired CD4+ T cells persist in vivo following induction of peripheral tolerance. J. Immunol. 1998;160:4719–4729. [PubMed] [Google Scholar]
- Rocha B, Tanchot C, Von Boehmer H. Clonal anergy blocks in vivo growth of mature T cells and can be reversed in the absence of antigen. J. Exp. Med. 1993;177:1517–1521. doi: 10.1084/jem.177.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg R, Neilson JR, Sarma A, Sharp PA, Burge CB. Proliferating cells express mRNAs with shortened 3’ untranslated regions and fewer microRNA target sites. Science. 2008;320:1643–1647. doi: 10.1126/science.1155390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheu S, Stetson DB, Reinhardt RL, Leber JH, Mohrs M, Locksley RM. Activation of the integrated stress response during T helper cell differentiation. Nat. Immunol. 2006;7:644–651. doi: 10.1038/ni1338. [DOI] [PubMed] [Google Scholar]
- Schwartz RH. T cell anergy. Annu. Rev. Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kumar M, Aich J, Hariharan M, Brahmachari SK, Agrawal A, Ghosh B. Posttranscriptional regulation of interleukin-10 expression by hsa-miR-106a. Proc. Natl. Acad. Sci. USA. 2009;106:5761–5766. doi: 10.1073/pnas.0808743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Mangan PR, Bezman NA, Sekiguchi DR, Luning Prak ET, Erikson J, Maltzman JS, Jordan MS, Koretzky GA. Mislocalization of SLP-76 leads to aberrant inflammatory cytokine and autoantibody production. Blood. 2010;115:2186–2195. doi: 10.1182/blood-2009-08-237438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, Locksley RM. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GA, Carballo E, Lee DM, Lai WS, Thompson MJ, Patel DD, Schenkman DI, Gilkeson GS, Broxmeyer HE, Haynes BF, Blackshear PJ. A pathogenetic role for TNF alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity. 1996;4:445–454. doi: 10.1016/s1074-7613(00)80411-2. [DOI] [PubMed] [Google Scholar]
- Teague RM, Greenberg PD, Fowler C, Huang MZ, Tan X, Morimoto J, Dossett ML, Huseby ES, Ohlen C. Peripheral CD8+ T cell tolerance to self-proteins is regulated proximally at the T cell receptor. Immunity. 2008;28:662–674. doi: 10.1016/j.immuni.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Tan AH, Hu X, Athanasopoulos V, Simpson N, Silva DG, Hutloff A, Giles KM, Leedman PJ, Lam KP, et al. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature. 2007;450:299–303. doi: 10.1038/nature06253. [DOI] [PubMed] [Google Scholar]
- Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT, Bluestone JA. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J. Exp. Med. 2008;205:1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.