Abstract
AIM: To investigate cancer cell absence or presence in wide excision after biopsy of squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) patients.
METHODS: 200 patients (100 BCC and 100 SCC) from the same dermatology clinic, who had positive margin upon biopsy, were selected from a computer generated randomized report. All selected patients had wide excision following biopsy. To determine the correlation of gender, age distribution and cancer absence, BCC and SCC cases were separated based on excision-cancer absent or present after wide excision. χ2 tests, Fisher’s exact tests were used to analyze the ratio of male to female between excision-cancer absent and excision-cancer present patients, while Mann-Whitney U test were used to compare the age distribution in the two groups. Statistical analyses were performed using SPSS version 16.0 for Windows.
RESULTS: Our retrospective chart review of the patients showed that cancer cells were absent in 49% of BCC patients (n = 100) and 64% of SCC patients (n = 100) who had previously had positive margins upon biopsy. Gender analysis showed the ratio of male to female (M/F) in the BCC arm was significantly higher compared with the SCC arm in those with excision-cancer absent (2.06 vs 0.66; P = 0.004; χ2 test). But M/F of excision-cancer absent and excision-cancer present in neither BCC nor SCC patients was statistically significant. Age adjustment showed no significant difference between excision-cancer absent and excision-cancer present in BCC and SCC patients. Nevertheless, in excision-cancer absent cases, the age distribution showed that the BCC patients were younger than SCC patients (average age 67 vs 74; P < 0.001; Mann-Whitney U test). In addition, our data also indicated that in the patient group of 71-80 years old, there were more SCC patients who showed excision-cancer absence (67.6% vs 39.4%; P = 0.02; χ2 test).
CONCLUSION: Our study indicates that approximately 50% or more of BCC and SCC patients with positive margins found on biopsies did not have cancer cells present at the time of wide excisions.
Keywords: Biopsy, Histology, Positive margin, Skin cancer, Wide excision
Core tip: Wide excisions are performed for skin cancers when malignant cells extend to the margins of biopsy. It is expected that cancer cells will appear in the excised tissue at the time of wide excision. However, an analysis of wide excision tissue samples from 200 patients revealed that approximately 50% or more basal and squamous cell carcinoma patients with cancer cells that extended to the margins in biopsy did not have cancer cells present on wide excision. This finding suggests that the wound caused by the biopsy itself may trigger a body response to eliminate cancer cells.
INTRODUCTION
The incidence and mortality rates of skin cancer are increasing in the United States and many other countries[1]. Of these skin cancers basal cell carcinoma (BCC) is the most common type followed closely by squamous cell carcinoma (SCC)[2]. The incidence of BCC is 200/100000 in men and 100/100000 in women[3]. BCC incidence increases with age; the median age for diagnosis is 68 years old[4]. SCC incidence is 100/100000 in men and 50/100000 in women[3]. Clinically if skin cancer is suspected, a biopsy is taken. The biopsy shows a small sample of the lesion, which can be looked at microscopically by a pathologist. The pathology report will describe the type of cancer and if the cancer extends to the border (positive margin) or is completely excised (negative margin) in the tissue sample[5] (Figure 1). If the lesion is completely removed, no further surgery is needed. For lesions with a positive margin, a wide excision surgery is done to remove the remaining cancer. This wide excision is a common and essential way to treat skin cancer, and incomplete excision may lead to cancer recurrence[6-8]. Theoretically, all the tissue samples from wide excision should contain cancer cells. However, pathological analyses of tissue samples from wide excision in our clinic often only show evidence of scar with no cancer cells remaining. All lesions had photos to ensure the correct lesion was excised. This observation has not been reported. In this study, we systematically analyzed the cancer cells absence or presence in wide excision after biopsy by subtype as well as sex and age from 100 SCC and 100 BCC patients. Our results will provide guidance for future determination of potential mechanism that leads to the disappearance of cancer cells in surrounding tissues of biopsies.
Figure 1.
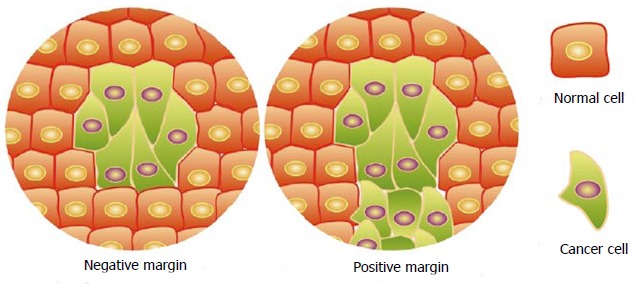
Appearances of negative and positive margins in biopsies.
MATERIALS AND METHODS
Patients’ data extraction
Chart review of 200 patients (100 BCC and 100 SCC) was obtained from a single dermatology office. The patients were selected from a computer generated randomized report of those who had SCC and a separate list for those who had BCC. Only patients who had a positive margin at the time of biopsy were included. Patients were excluded if pathology was unavailable for either biopsy or wide excision. In cases where patients with more than one biopsy or wide excision, the first lesion from the list was chosen, this was the case unless there was no corresponding wide excision; or location of biopsy and wide excision did not match. There was no exclusion of patients who had SCC and BCC.
Statistical analysis
In this chart review, χ2 tests were used to compare the difference of gender distribution and excision-cancer absent percentage in assigned groups. When the sample size was less than 40, Fisher’s exact tests were used instead. All the difference of age distribution was evaluated by Mann-Whitney U test. P < 0.05 was considered as statistically significant. These statistical analyses were performed using SPSS version 16.0 for Windows.
RESULTS
Clinical characteristics
First, the characteristics of the selected patients were analyzed (Table 1). Among the 200 cases that were reviewed, including 100 BCC and 100 SCC patients, SCC was more common than BCC among female patients (57% vs 38%; P = 0.007; χ2 test) while BCC was more common than SCC among male patients (62% vs 43%; P = 0.007; χ2 test). Despite a similar age range, the average age of SCC patients were 8 years older than BCC patients (77 years vs 69 years old; P = 0.003; Mann-Whitney U test). All skin cancer patients showed a positive margins upon biopsy. However, cancer cells were found to be absent in excised tissue of 49% of the BCC patients and 64% of the SCC patients. In addition, excision-cancer absent percentage in SCC patients was significantly greater than that in BCC patients (64% vs 49%; P = 0.032; χ2 test). Analyses of the excision-cancer absent in the different subtypes of BCC and SCC indicated that there was no statistic significant difference in percentage among the analyzed subtypes of BCC or SCC (Table 2).
Table 1.
Patients characteristics
| BCC | SCC | Total patients | |
| No. of cases | 100 | 100 | 200 |
| Sex | |||
| Male | 62 | 43 | 105 |
| Female | 38 | 57 | 95 |
| Male/female | 1.63 | 0.75 | 1.111 |
| Median age (range) | 69 (42-92) | 77 (45-95) | 73 (42-95)2 |
| Biopsy margins positive | 100 | 100 | 200 |
| Excision-cancer absent | 49 | 64 | 1133 |
P value (BCC vs SCC):
P = 0.007;
P = 0.003;
P = 0.032. BCC: Basal cell carcinoma; SCC: Squamous cell carcinoma.
Table 2.
Skin cancer subtype distribution n (%)
| Subtype | No. of patients | Excision-cancer absent | |
| BCC | Nodular | 53 | 26 (49.1) |
| Infiltrative | 11 | 6 (54.5) | |
| Nodular and superficial | 25 | 15 (60) | |
| Nodular and infiltrative | 10 | 2 (20) | |
| Infiltrative and superficial | 1 | 0 (0) | |
| SCC | In situ | 45 | 26 (57.8) |
| Keratoacanthoma type | 12 | 9 (75) | |
| Moderately-differentiated | 4 | 3 (75) | |
| Well-differentiated | 16 | 12 (75) | |
| Invasive | 12 | 6 (50) | |
| Other types1 | 11 | 6 (54.5) | |
Include following complex subtypes, intraepidermal epithelioma pattern, in situ-intraepidermal epithelioma pattern, in situ and invasive, in situ- well differentiated, moderately to poor differentiated, in situ acantholytic and focally invasive, in situ and focally superficial invasive, keratinizing well differentiated, and unknown types. BCC: Basal cell carcinoma; SCC: Squamous cell carcinoma.
Gender distribution of excision-cancer absence with positive margins
To evaluate if cancer cells absence or presence after wide excision was associated with gender, BCC and SCC patients were grouped based on excision-cancer absence/presence and then the ratio of male to female was calculated respectively (Table 3). Despite the fact that a higher ratio of male to female was found in excision-cancer absent BCC patients than that in excision-cancer present BCC patients, the difference was not statistically significant (2.06 vs 1.32; P = 0.28; χ2 test). A close ratio of male to female SCC patients was observed in excision-cancer absent and present groups (0.66 vs 0.95; P = 0.382; χ2 test). However, in excision-cancer absent cases, the ratio of male to female in BCC was significantly higher compared with SCC (2.06 vs 0.66; P = 0.004; χ2 test).
Table 3.
Gender distribution between excision-cancer absent and excision-cancer present cases of basal cell carcinoma and squamous cell carcinoma n (%)
| M | F | Total | M/F | P value | ||
| BCC | Excision-cancer absent | 33 (67.3) | 16 (32.7) | 49 | 2.06 | 0.2801 |
| Excision-cancer present | 29 (56.9) | 22 (43.1) | 51 | 1.32 | ||
| SCC | Excision-cancer absent | 25 (39.7) | 38 (60.3) | 63 | 0.66 | 0.3821 |
| Excision-cancer present | 18 (48.6) | 19 (51.4) | 37 | 0.95 | ||
| Excision- cancer absent cases | BCC | 33 (67.3) | 16 (32.7) | 49 | 2.06 | 0.0042 |
| SCC | 25 (39.7) | 38 (60.3) | 63 | 0.66 | ||
P value:
In BCC or SCC cases, the percentage of male (female) patients in excision-cancer absent vs present groups;
In excision-cancer absent cases, the percentage of male (female) patients in BCC vs SCC. BCC: Basal cell carcinoma; SCC: Squamous cell carcinoma; M: Male; F: Female.
Age distribution of excision-cancer absence with positive margins
To evaluate if the malignancy presence after excision was associated with age, BCC and SCC patients were grouped based on cancer presence/absence, and then the median and average ages for the patients in each group were calculated (Table 4). Our data indicated that while there was no statistically significant difference in ages for excision malignancy present and absent patients in both BCC (P = 0.191) and SCC (P = 0.534), the patients’ ages of excision-cancer absent cases for SCC were 8 years older than that for BCC (74 years vs 67 years old; P < 0.001; Mann-Whitney U test), which is similar to the difference between average age of SCC and BCC patients (Table 2). Further evaluation of the percentage of excision-cancer absence in BCC and SCC based on age distributions (< 60, 61-70, 71-80 and > 80 years old) revealed that while the percentage of excision-cancer absence cases among the elderly SCC patients was increased, the percentage among the elderly BCC patients was decreased (Figure 2). Our data indicated that in the patient group of 71-80 years old, SCC showed significantly higher percentage compared with BCC (67.6% vs 39.4%; P = 0.02; χ2 test).
Table 4.
Age distribution between excision-cancer absent and excision-cancer present cases of basal cell carcinoma and squamous cell carcinoma
| Median age (range), yr | Average age, yr | P Value | ||
| BCC | Excision-cancer absent | 66 (45-92) | 67 | 0.1911 |
| Excision-cancer present | 72 (42-90) | 70 | ||
| SCC | Excision-cancer absent | 76 (48-95) | 74 | 0.5341 |
| Excision-cancer present | 78 (45-91) | 75 | ||
| Excision- cancer absent | BCC | 66 (45-92) | 67 | < 0.0012 |
| SCC | 76 (48-95) | 74 | ||
P value:
In BCC or SCC cases, age distribution of excision-cancer absent vs present cases;
In excision-cancer absent cases, age distribution of BCC vs SCC. BCC: Basal cell carcinoma; SCC: Squamous cell carcinoma.
Figure 2.
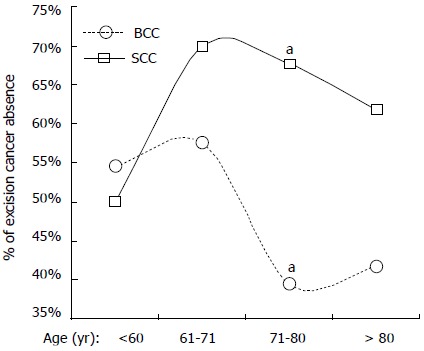
Age distribution and percentage of excision-cancer absence in basal cell carcinoma and squamous cell carcinoma. Basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) cases were divided into four groups based on age, including cases younger than 60, 61-70, 71-80 and older than 80 years old. The percentage of excision-cancer absence in each group is shown as squares (SCC) and circles (BCC). aP = 0.02, percentage of excision-cancer absence in BCC vs SCC in 71-80 years old group.
DISCUSSION
While currently there are limited reports linking local immune response and cutaneous carcinoma regression after biopsy, it is noteworthy that the local immune cells might contribute to eliminating residual skin cancer cells. For instance, dendritic cells (DCs), the typical antigen-presenting immune cells which consist dermal DCs, Langerhans cells and plasmacytoid DCs, are abundant in both epidermal and dermal tissues and play central role in initiating immune response[9-11].
Despite DCs being present in human carcinomas, the potential to initiate an immune response is largely diminished by tumor environment[12,13]. Tumors suppress the function of DCs significantly by exploiting different cytokines, including interleukin-6 (IL-6), macrophage colony-stimulating factor, IL-10 and IL-13[14-16]. It was reported that the number of Langerhans cells decreased in SCC, though the mechanism is not fully understood[17-19]. In addition, SCC-associated DCs have much lower potential to stimulate proliferation of T-cells compared with DCs derived from normal tissue[20]. In view of this tumor mediated suppression of DCs, stimulating their function becomes a promising way to fight against skin cancer, as a limited number of DCs are sufficient to induce an immune response[21,22].
During the original biopsy of the SCC or BCC, a substantial part of the cancer tissue, as well as adjacent tumor-associated DCs and macrophages, were removed. This “tissue injury” would cause inflammation, accompanied with both innate and adaptive immune responses in the wound healing process[23-25]. Neutrophils, followed by monocytes, infiltrate the wound where monocytes would differentiate into DCs or macrophages. On the one hand, the immune cells which were either suppressed by the tumor or benefit the tumor growth were removed so that local immune suppression is relieved. Fresh healthy immune cells would then enter the wound area to “clean” the environment. Macrophages are the most abundant cells before fibroblast proliferation, which induced cancer cell apoptosis by producing nitric oxide or inducing nitric oxide production in tumor cells[26-30]. It is possible that the residual cancer cells were killed by these immune responses during the initial wound healing, leading to excision-cancer absence. Further research is needed to understand the underlying mechanism.
In a conclusion, wide excisions from nearly 50% or more BCC and SCC patients with positive margins at biopsy appeared to be absent of cancer cells. The excision-cancer absence is more frequently observed in SCC than BCC patients. No significant correlation between gender and cancer absence is found. However, while the percentage of excision-cancer absence was increased in elder SCC patients, it was decreased in elder BCC patients. These findings might provide evidence for a study on the specific mechanism of cancer cell absence resulting from a biopsy induced immune response.
ACKNOWLEDGMENTS
The authors would like to thank Mr. Rui Zhang (Nanjing University) for his assistance in statistic analysis.
COMMENTS
Background
When examining patients for suspected cancer, biopsies are performed to confirm that the lesion is in fact cancer, which type of cancer, as well as to determine if the cancer is confined to the biopsy or extends to the margins (positive margins). For biopsies which show positive margins, wide excision will be performed. It is expected that cancer cells will appear in the excised tissues of wide excisions. However, clinically this is not always the case.
Research frontiers
As the classic treatment for skin cancer, the wide excision is necessary after the positive margin is confirmed in biopsy. Biopsy is indispensable to define the risk factors of skin cancer. The research hotspot in this field is how to determine the excision margins of different types of skin cancer based on result of biopsy.
Innovations and breakthroughs
This retrospective chart review provided evidences that 49% basal cell carcinoma (BCC) and 64% squamous cell carcinoma (SCC) patients did not have cancer cells left in the original location where the positive margin was reported in biopsy. Although the correlation between excision-cancer absence and gender or age distribution was excluded in both SCC and BCC patients, the analysis revealed that in excision-cancer absent cases, there were more male patients in BCC than that in SCC while SCC patients were older than BCC patients.
Applications
The data provided in this study suggests a potential role of immune response caused by biopsy in removing residual cancer cells. The high percentage of excision-cancer absence might cushion the SCC and BCC patients against the fear of biopsy and promote compliance of them with biopsy demanded by dermatologists.
Terminology
Excision-cancer present SCC or BCC patient means that cancer cells were found in the widely excised tissue which contained positive margin which had been reported previously in biopsy. Excision-cancer absent SCC or BCC means no cancer cells were found in wide excision containing previously defined positive margin.
Peer review
The work is well written and focuses on an interesting aspect of skin cancer surgery.
Footnotes
P- Reviewers: LadoyanniE, Negosanti L S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
Supported by NIH RO1CA086928 to Wu S; and Graduate assistantship to Yuan Y from the Department of Chemistry and Biochemistry, Ohio University
References
- 1.Marks R. An overview of skin cancers. Incidence and causation. Cancer. 1995;75:607–612. doi: 10.1002/1097-0142(19950115)75:2+<607::aid-cncr2820751402>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Dubas LE, Ingraffea A. Nonmelanoma skin cancer. Facial Plast Surg Clin North Am. 2013;21:43–53. doi: 10.1016/j.fsc.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Sterry W, Paus R, Burgdirf W. Dermatology (Thieme Clinical Companions). 1 ed. New York, NY: Thieme; 2006. pp. 419–433. [Google Scholar]
- 4.Bolognia JL, Jorizzo JL, Schaffer JV, Callen JP, Cerroni L, Heymann WR, Hruza GJ. Dermatology. 3 ed. Philadelphia, PA: Saunders; 2012. p. Chapter 108. [Google Scholar]
- 5.Weinstein MC, Brodell RT, Bordeaux J, Honda K. The art and science of surgical margins for the dermatopathologist. Am J Dermatopathol. 2012;34:737–745. doi: 10.1097/DAD.0b013e31823347cb. [DOI] [PubMed] [Google Scholar]
- 6.Tan PY, Ek E, Su S, Giorlando F, Dieu T. Incomplete excision of squamous cell carcinoma of the skin: a prospective observational study. Plast Reconstr Surg. 2007;120:910–916. doi: 10.1097/01.prs.0000277655.89728.9f. [DOI] [PubMed] [Google Scholar]
- 7.Pugliano-Mauro M, Goldman G. Mohs surgery is effective for high-risk cutaneous squamous cell carcinoma. Dermatol Surg. 2010;36:1544–1553. doi: 10.1111/j.1524-4725.2010.01576.x. [DOI] [PubMed] [Google Scholar]
- 8.Leibovitch I, Huilgol SC, Richards S, Paver R, Selva D. Scalp tumors treated with Mohs micrographic surgery: clinical features and surgical outcome. Dermatol Surg. 2006;32:1369–1374. doi: 10.1111/j.1524-4725.2006.32308.x. [DOI] [PubMed] [Google Scholar]
- 9.Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol. 2005;17:273–283. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Zaba LC, Krueger JG, Lowes MA. Resident and “inflammatory” dendritic cells in human skin. J Invest Dermatol. 2009;129:302–308. doi: 10.1038/jid.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunger RE, Sieling PA, Ochoa MT, Sugaya M, Burdick AE, Rea TH, Brennan PJ, Belisle JT, Blauvelt A, Porcelli SA, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. 2004;113:701–708. doi: 10.1172/JCI19655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 13.Vicari AP, Caux C, Trinchieri G. Tumour escape from immune surveillance through dendritic cell inactivation. Semin Cancer Biol. 2002;12:33–42. doi: 10.1006/scbi.2001.0400. [DOI] [PubMed] [Google Scholar]
- 14.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1:510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 15.Steinbrink K, Wölfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- 16.Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, Marches F, Banchereau J, Palucka AK. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galan A, Ko CJ. Langerhans cells in squamous cell carcinoma vs. pseudoepitheliomatous hyperplasia of the skin. J Cutan Pathol. 2007;34:950–952. doi: 10.1111/j.1600-0560.2007.00741.x. [DOI] [PubMed] [Google Scholar]
- 18.Gibson GE, O’Grady A, Kay EW, Leader M, Murphy GM. Langerhans cells in benign, premalignant and malignant skin lesions of renal transplant recipients and the effect of retinoid therapy. J Eur Acad Dermatol Venereol. 1998;10:130–136. [PubMed] [Google Scholar]
- 19.Gatter KC, Morris HB, Roach B, Mortimer P, Fleming KA, Mason DY. Langerhans’ cells and T cells in human skin tumours: an immunohistological study. Histopathology. 1984;8:229–244. doi: 10.1111/j.1365-2559.1984.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 20.Bluth MJ, Zaba LC, Moussai D, Suárez-Fariñas M, Kaporis H, Fan L, Pierson KC, White TR, Pitts-Kiefer A, Fuentes-Duculan J, et al. Myeloid dendritic cells from human cutaneous squamous cell carcinoma are poor stimulators of T-cell proliferation. J Invest Dermatol. 2009;129:2451–2462. doi: 10.1038/jid.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yanofsky VR, Mitsui H, Felsen D, Carucci JA. Understanding dendritic cells and their role in cutaneous carcinoma and cancer immunotherapy. Clin Dev Immunol. 2013;2013:624123. doi: 10.1155/2013/624123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 23.Tsirogianni AK, Moutsopoulos NM, Moutsopoulos HM. Wound healing: immunological aspects. Injury. 2006;37 Suppl 1:S5–S12. doi: 10.1016/j.injury.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 24.DiPietro LA. Wound healing: the role of the macrophage and other immune cells. Shock. 1995;4:233–240. [PubMed] [Google Scholar]
- 25.Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg. 2004;187:11S–16S. doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 26.Rieder J, Jahnke R, Schloesser M, Seibel M, Czechowski M, Marth C, Hoffmann G. Nitric oxide-dependent apoptosis in ovarian carcinoma cell lines. Gynecol Oncol. 2001;82:172–176. doi: 10.1006/gyno.2001.6242. [DOI] [PubMed] [Google Scholar]
- 27.Binder C, Schulz M, Hiddemann W, Oellerich M. Induction of inducible nitric oxide synthase is an essential part of tumor necrosis factor-alpha-induced apoptosis in MCF-7 and other epithelial tumor cells. Lab Invest. 1999;79:1703–1712. [PubMed] [Google Scholar]
- 28.Reveneau S, Arnould L, Jolimoy G, Hilpert S, Lejeune P, Saint-Giorgio V, Belichard C, Jeannin JF. Nitric oxide synthase in human breast cancer is associated with tumor grade, proliferation rate, and expression of progesterone receptors. Lab Invest. 1999;79:1215–1225. [PubMed] [Google Scholar]
- 29.Binder C, Schulz M, Hiddemann W, Oellerich M. Caspase-activation and induction of inducible nitric oxide-synthase during TNF alpha-triggered apoptosis. Anticancer Res. 1999;19:1715–1720. [PubMed] [Google Scholar]
- 30.Kwak JY, Han MK, Choi KS, Park IH, Park SY, Sohn MH, Kim UH, McGregor JR, Samlowski WE, Yim CY. Cytokines secreted by lymphokine-activated killer cells induce endogenous nitric oxide synthesis and apoptosis in DLD-1 colon cancer cells. Cell Immunol. 2000;203:84–94. doi: 10.1006/cimm.2000.1682. [DOI] [PubMed] [Google Scholar]


