Abstract
The aim of the study was to compare the efficacy of pulmonary vein antrum isolation (PVAI), extended PVAI and ganglionic plexi (GP) ablation in persistent AF.
Methods
One hundred and twenty patients 56.2 ± 8.8 years old were randomized into three groups: GP ablation (n = 37), PVAI (n = 42), and extended PVAI (n = 41). The following parameters were studied: sinus rhythm restoration, vagal reactions, fluoroscopy time, procedure duration, lesion surface area. In 16 months after the procedure, echocardiography and Holter monitoring were performed.
Results
Significant differences were found in the amount of X-ray exposure, procedure duration, lesion surface area and vagal reactions. In all the cases, atrial mechanical function worsened after the procedure. However, there were no significant differences between the groups. Sixteen months after the procedure, sinus rhythm without antiarrhythmic therapy was maintained in 38% of patients in GP group, in 56% of patients in PVAI group and in 69% of patients in extended PVAI group.
Conclusions
In persistent AF, the extended PVAI is more effective than PVAI and GP ablation. After the PVAI or extended PVAI, abnormalities of the left atrial mechanical function occurred if the lesion area exceeded 25% of the total LA surface area.
Keywords: atrial fibrillation, critical mass, radiofrequency ablation
Introduction
The leading mechanism of paroxysmal atrial fibrillation (AF) is a spontaneous electrical activity of the triggers located mostly in the pulmonary veins (PV) ostia. Thereafter, radiofrequency pulmonary vein antrum isolation (PVAI) is a standard interventional approach of paroxysmal AF treatment [1, 2]. In the forefront of a persistent AF pathogenesis, there is not only trigger activity but also the ability of atrial contractile myocardium to maintain AF due to refractoriness and conduction anisotropy. In recent years, the literature has paid considerable attention to the development of methods of the AF substrate identification and its elimination or modification.
For this purpose, the methods of left atrial (LA) fragmentation by making linear lesions on posterior wall, roof and mitral isthmus are mostly used [3]. This approach improves the efficacy of the procedure but is associated with such complications as postprocedural tachycardias and atrio-esophageal fistulae [4, 5].
The search for the method which would eliminate the risk of these complications has led to the development of procedures correcting one of the major AF inducers – the sympathetic-vagal disbalance. Pappone et al. have identified the nerve fiber areas by vagal reflexes observed during radiofrequency delivery and attempted to eliminate these areas [6]. Later, the anatomical approach of ganglionic plexi (GP) ablation was proposed by Pokushalov et al. [7]. Unfortunately, this technique while minimizing the risk of the above-mentioned complications has the disadvantage of keeping the PV triggers unimpaired.
Therefore, researchers are continuing to develop methods which would combine the advantages of well-known approaches, while minimizing their disadvantages. In this regard, the concept of myocardial mass ‘electrical exclusion’ based on experimental models seems to be promising [8]. The basic principle of this approach is the creation of homogeneous radiofrequency lesions in the LA to extend the cumulative area of radiofrequency lesions exceeding 25% of the total LA surface area, avoiding damaging the LA posterior wall and mitral isthmus. Because the PV triggers were kept unimpaired (the disadvantage similar to that of GP ablation), we combined this procedure with PVAI. By its design, such a procedure (we call it ‘extended PVAI’) is similar to simultaneous GP ablation and PVAI differing from latter by the following:
wider lesion creation;
the criterion of procedure termination is attaining of the defined cumulative lesion area;
the main left-side lesion is extended not towards the LA posterior wall but toward the lateral ridge and LA appendage thereby avoiding the risk of esophageal damage.
The clinical efficacy of extended PVAI and its influence on intracardiac hemodynamics are unclear particularly in terms of LA contractile function and ‘left atrial stiffness’ due to the large area of myocardial damage [9].
The aim of the study was to estimate the relationship between LA contractile function and cumulative lesion surface area, and to compare the clinical efficacy of PVAI, extended PVAI and GP ablation.
Methods
One hundred and twenty consecutive patients, 56.2 ± 8.8 years old (72 males, 48 females) with persistent AF, were enrolled in the study. All of them received warfarin before and 3 months after the procedure (target INR: 2.0–3.0), amiodarone (200–400 mg/day), and AT1-angiotensin-II receptor inhibitors in the individual doses. A day before the procedure, transesophageal echocardiography was done to exclude intracardiac thrombosis, spontaneous echo contrast, and to determine the LA appendage outflow peak velocity.
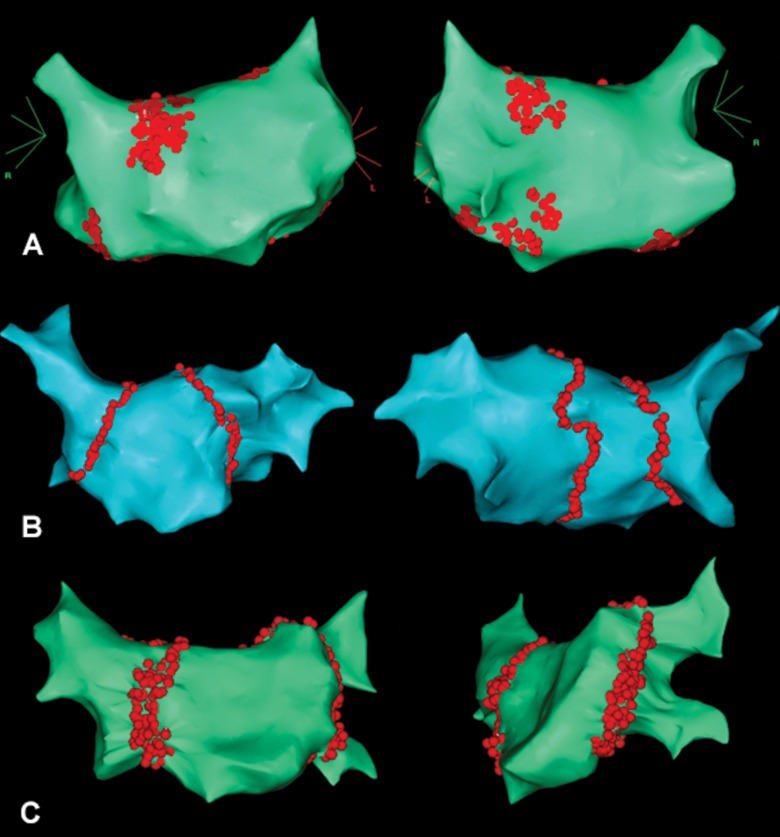
All the patients were randomized into three groups by procedure type (Fig. 1): GP ablation (Fig. 1A) in group I (n = 37), PVAI (Fig. 1B) in group II (n = 42), extended PVAI (Fig. 1C) in group III (n = 41). The groups did not have any significant statistical differences in most clinical parameters (Table I).
Fig. 1.
Electroanatomical maps illustrating the designs of radiofrequency ablation: A – ablation of ganglionic plexi; B – pulmonary vein antrum isolation; C – extended pulmonary vein antrum isolation
Table I.
Clinical characteristics of the studied patients
| Characteristic | GP ablation (n = 37) |
PVAI (n = 42) |
Extended PVAI (n = 41) |
p |
|---|---|---|---|---|
| Age, years | 55.8 ± 9.2 | 56.3 ± 8.1 | 57.5 ± 7.7 | 0.11 |
| Long-persistent AF (n, %) | 9, 24% | 9, 21% | 11, 27% | 0.848 |
| History of myocardial infarction (n, %) | 4, 11% | 5, 12% | 4, 10% | 0.952 |
| Arrhythmic history, months | 28.7 ± 8.4 | 25.2 ± 8.1 | 30.0 ± 8.8 | 0.61 |
| LA diameter in long-axis parasternal view, mm | 45.3 ± 4.1 | 47.1 ± 4.8 | 46.1 ± 4.5 | 0.64 |
| LA appendage outflow peak velocity, cm/s | 25.7 ± 5.7 | 24.5 ± 6.3 | 25.4 ± 5.3 | 0.57 |
| Systolic pressure in the right ventricle, mmHg | 19.2 ± 6.6 | 17.8 ± 6.3 | 20.0 ± 4.7 | 0.61 |
| Left ventricular end-diastolic diameter by Simpson-biplane, mm |
50.7 ± 6.4 | 51.5 ± 7.1 | 52.1 ± 5.9 | 0.51 |
| Left ventricular end-systolic diameter by Simpson-biplane, mm |
32.6 ± 3.4 | 31.8 ± 3.7 | 32.8 ± 4.0 | 0.63 |
| Left ventricular ejection fraction by Simpson-biplane, % |
46.6 ± 3.8 | 45.8 ± 4.1 | 45.5 ± 4.4 | 0.57 |
| All data are presented as absolute values (n) and percentages (%), as well as means (M) and standard deviation (σ) | ||||
The Biotok (Biotok, Russia) electrophysiology station and Carto 3 navigation system (Biosense Webster, Israel) were used to carry out electroanatomic maps. Radiofrequency ablation was performed with EZ Steer Thermocool NAV catheters (Biosense Webster, USA) with the power of 30–40 W and the temperature of 42–45 °C.
All maps were performed in the FAM mode with the reconstruction of left atrium including its appendage and pulmonary veins. The design of extended PVAI consists of the following stages:
PV antrum isolation by two separate lesions for right and left PVs (Fig. 1B);
isolation lesions extend anteriorly towards interatrial septum and lateral ridge in the form of homogeneous nonlinear lesions (Fig. 1C);
the criterion of procedure termination is a consummation of the cumulative lesion area exceeding 25% of the total LA surface area.
In all the cases of PVAI and extended PVAI, the entrance and exit blocks were confirmed electrophysiologically without the adenosine test.
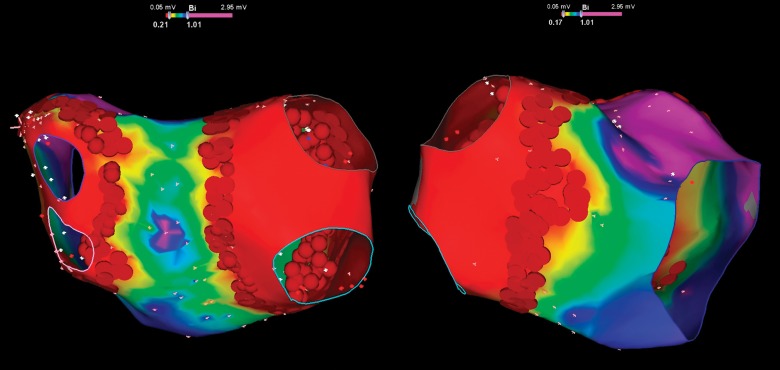
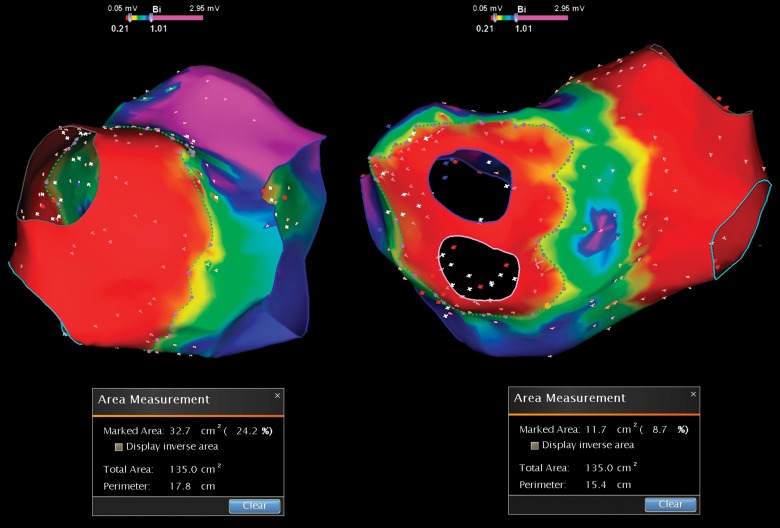
At the end of the procedure, the ratio of cumulative lesion surface area to LA total area in percentage was determined by the navigation system software. This phase consisted of the following steps. Firstly, we performed a bipolar amplitude remapping (Fig. 2). In order to correctly determine the areas, pulmonary veins were erased from the map, and their ostia and the mitral valve annulus were marked by the ‘anatomical structure’ tool. The relationship of lesions localization and signal amplitude data was performed during each procedure (Fig. 3). In the event of their mismatch, additional ablation was performed until the loss of potentials. After that, the measurements were done (Fig. 4). The ratio of cumulative lesion surface area to LA total area in the overall group of patients was 25.8 ± 4.1%.
Fig. 2.
Bipolar amplitude remapping at the end of procedure. The low voltage areas correspond isolated antrums in combination with homogeneous lesions in the septum and lateral ridge
Fig. 3.
In order to determine correctly the lesion and LA surface areas pulmonary veins were erased from the map, and their ostia and the mitral valve annulus were marked by the ‘anatomical structure’ tool. Then, the relationship of lesions localization and signal amplitude data were determined
Fig. 4.
Measurement of the lesion surface area to LA total area ratio in percentage determined by the navigation system software
During the procedure, the following parameters were studied: sinus rhythm at the end of the procedure, ‘stop-and-restart’ phenomenon, vagal reactions as pauses or transient AV block, fluoroscopy time, procedure duration, number of ablation points, cumulative lesion surface area, and its relation to the total LA area.
Immediately before and after the ablation, transesophageal echocardiography was performed in those patients of the PVAI and extended PVAI groups who had sinus rhythm. The following parameters were assessed:
anteroposterior LA diameter (LA AP);
left atrial volume (V LA);left atrial ejection fraction (LA EF);
peak velocity of transmitral blood flow during passive left ventricular filling (Peak E), active left atrial contraction (Peak A) and ratio of the forenamed velocities (E/A);
velocity-time integral of transmitral blood flow during active left atrial contraction (VTI A);
velocity-time integral of total transmitral blood flow (VTI);
atrial filling fraction (AFF or VTI A/VTI);
peak velocity of pulmonary venous blood flow during left ventricular systole (Peak S), passive left ventricular filling (Peak D) and active left atrial contraction/retrograde phase (Peak Ar);
duration of pulmonary venous blood flow during active left atrial contraction retrograde phase (T Ar);
velocity-time integral of pulmonary venous blood flow during the total cardiac cycle (VTI PV).
In short-term and long-term follow-up periods, all the patients underwent echocardiography and 24-h Holter monitoring. The efficacy of the procedure was defined as the complete absence of AF episodes on daily ECG recording and symptomatic arrhythmias according to the survey.
The variables were presented as absolute values, percentages of the overall group quantity or median and quartiles in case of non-normal distribution. All the statistical calculations were done with the Statistica 7.0 software package (Statsoft, USA) using the chi-square test, Kruskal–Wallis H-test and the analysis of variance. The correlation between parameters was evaluated by the Spearman’s R-test. The dynamics of arrhythmia recurrence was assessed using the Kaplan–Meier estimate.
Results
The intraprocedural parameters are presented in Table II. As expected, significant differences were found in the amount of X-ray exposure, procedure duration, cumulative lesion surface area, and sympathetic-vagal balance modification.
Table II.
Clinical characteristics of the studied patients
| Characteristics | GP ablation (n = 37) |
PVAI (n = 42) |
Extended PVAI (n = 41) |
p |
|---|---|---|---|---|
| Sinus rhythm at the end of the procedure (n, %) | 17 (45%) | 17 (40%) | 19 (46%) | 0.836 |
| Stop-and-restart phenomenon (n, %) | 14.38% | 11.25% | 14.35% | 0.524 |
| Vagal reactions (n, %) | 11.30% | 5.12% | 10.25% | 0.138 |
| Fluoroscopy time, min | 33.2 ± 6.7 | 38.6 ± 4.4 | 40.1 ± 6.6 | 0.08 |
| Procedure duration, min | 142.5 ± 16.6 | 153.0 ± 18.1 | 166.5 ± 13.5 | 0.06 |
| Overall duration of the RF energy delivery, min | 44.6 ± 6.7 | 53.1 ± 8.2 | 64.3 ± 11.7 | 0.03 |
| Ratio of cumulative lesion surface area to the total LA area, % | 21.4 ± 1.7 | 23.3 ± 2.6 | 27.9 ± 2.53 | 0.001 |
| All data are presented as absolute values (n) and percentages (%), as well as means (M) and standard deviation (σ) | ||||
In all, the patients’ mechanical function of the LA and PVs worsened after the procedure, which was confirmed by the changes of transmitral and pulmonary venous flow pattern (Table III). Typical changes in intracardiac hemodynamics were ‘pseudorestrictive’ type of LV filling and reduced LA ejection fraction primarily during the passive phase. However, there were no statistically significant differences between the groups in all of the parameters studied.
Table III.
Echocardiographic characteristics before and after the PVAI
| Characteristics | Extended PVAI (n =
36) |
PVAI (n =
30) |
||
|---|---|---|---|---|
| Before RFA | After RFA | Before RFA | After RFA | |
| LA AP, cm | 4.2 (4; 4.4) | 4.3 (4.1; 4.5) | 4.3 (4; 4.5) | 4.4 (4; 4.5) |
| V LA, mL | 71.5 (68.3; 85) | 80 (64.3; 85.5)* | 74.1 (66.4; 87) | 78 (66; 88)* |
| LA EF, % | 40 (19.5; 45.8) | 22 (18.3; 34.5)* | 40.3 (21; 46.2) | 23.8 (19; 33)* |
| Peak E, cm/s | 68 (60; 76) | 83 (70; 97)* | 63 (57; 70) | 84 (71; 101)* |
| Peak A, cm/s | 61 (48; 76) | 53 (39; 60)* | 58 (45; 67) | 54 (36; 59)* |
| E/A, % | 122 (90; 144) | 174 (119; 263)* | 110 (89; 138) | 165 (123; 181)* |
| VTI, cm | 30 (26; 40) | 22.3 (20; 31.3)* | 31 (27.5; 39) | 24 (20.2; 29.6)* |
| VTI A, cm | 10 (8.1; 13) | 10.5 (7.3; 13) | 12.1 (8.4; 13.1) | 11.9 (8.3; 14) |
| AFF, % | 29.6 (26.8; 44.9) | 37.5 (24.4; 48.1)* | 32.1 (27; 44.2) | 42.8 (29; 46.1)* |
| Peak S, cm/s | 50 (44; 56) | 45 (38; 47) | 47.1 (42.5; 54.4) | 46.4 (41.1; 53.5) |
| Peak D, cm/s | 48 (36; 64) | 55 (46; 59) | 46 (37.7; 62.9) | 52.5 (44; 60)* |
| Peak Ar, cm/s | 23 (14; 25) | 15 (0; 23) | 22.4 (14; 24.2) | 12.9 (0; 24)* |
| T Ar, ms | 167 (141; 175) | 198 (181; 204) | 154 (144; 169) | 189 (150; 202)* |
| VTI PV, cm | 24.5 (20.5; 26) | 24 (22.3; 31) | 26 (22; 26.5) | 25.8 (21.6; 29.4) |
| All the data are presented as median (lower quartile; upper quartile). *p < 0.05 in intragroup comparison | ||||
Table IV shows the correlation between overall lesion surface area, LA volume measured by Carto and changes in echocardiographic parameters in the form of Spearman’s R-test.
Table IV.
Correlation between LA volume measured by Carto, the overall lesion surface area and changes in echocardiographic parameters
| Indicator | Overall lesion surface area measured by Carto |
LA volume measured by Carto |
|---|---|---|
| LA AP, cm | 0.081978 | 0.357188 |
| V LA, mL | –0.030168 | 0.101659 |
| LA EF, % | –0.048946 | 0.239824 |
| Peak E, cm/s | 0.162014 | 0.269617 |
| Peak A, cm/s | –0.220093 | 0.759021 |
| E/A, % | –0.042225 | 0.397802 |
| VTI, cm | 0.094171 | –0.250564 |
| VTI A, cm | –0.291450 | 0.655666 |
| AFF, % | –0.191333 | –0.138614 |
| Peak S, cm/s | 0.154624 | –0.169417 |
| Peak D, cm/s | –0.640233 | 0.278457 |
| Peak Ar, cm/s | 0.044447 | 0.520879 |
| T Ar, ms | –0.214694 | –0.294830 |
| VTI PV, cm | –0.186678 | –0.424176 |
| The correlations that are significant in p < 0.05 are marked in bold | ||
Thus, Spearman’s R-test of the correlation between LA volume and lesion surface area did not show any significant results, i.e., with a larger amount of LA volume, the injured area of the myocardium did not increase. Also, there were no correlations between LA volumes measured by echocardiography and by Carto. The results showed a negative correlation between the peak D and myocardial injury, reflecting a reduction in passive LA emptying. However, the scatterplot of this relationship shows that a significant peak D decrease is observed only when the lesion area exceeds 25% (Fig. 5). Meanwhile, the degree of LA contractility change (peak A and its integral) depended on the LA volume but not on the lesion surface area.
Fig. 5.
Scatterplot of the correlation between cumulative lesion surface area and peak D of the PV flow pattern. The solid line is polynomial fitting; the dashed line is its 95% confidence interval
Long-term results were followed during 16 months after the procedure. At a specified time after the intervention, the efficacy reached statistical significance between the groups (Fig. 6): sinus rhythm without antiarrhythmic therapy was maintained in 38% of patients in group I (GP), in 56% of patients in group II (PVAI), and in 69% of patients in group III (extended PVAI). According to the Kaplan–Meier estimate, there were differences in the dynamics of AF recurrence testifying favorability of extended PVAI.
Fig. 6.
The dynamics of symptomatic AF recurrence between 3 (‘blinded’ period) and 16 months after the procedure by the Kaplan–Meier analysis
Discussion
The pathogenesis of AF is complex, and most researchers are working on the development of the efficient RFA design, eliminating some mechanisms (trigger activity of pulmonary veins, re-entry/rotor circles, anisotropy of the electrical properties of cardiomyocytes, autonomic imbalance, etc.) simultaneously minimizing the lesion surface, i.e., myocardial trauma. This has led to the development of more than ten different RFA designs and their modifications suggested by different research groups to treat AF. However, none of them has proved its advantages over the others. Analyzing the possible reasons for the failure of all the proposed procedures and the results of our own research over the last few years, we have come to the conclusion that it is necessary to refuse the selective approaches.
Today, we are forced to turn to the research of the 1960s, namely, the theory of the critical myocardial mass (for the atrial approach the term would rather be a ‘critical area’), which is necessary to persist AF. Moe and Abildskov theoretically calculated that its value was 4 cm2 [10]. West and Landa later experimentally confirmed the role of a critical mass in maintaining the atrial re-entry arrhythmias [11].
Based on the critical mass theory, Cox developed the ‘Maze’ procedure, which is the most effective of all the proposed interventions to date [11]. Long history of the successful use of the ‘Maze’ procedure in various modifications has proved the fidelity of the pathogenetic approach to the theory of critical mass.
Our own experimental and clinical studies [8] demonstrated the high efficacy of the method based on the ‘electrical exclusion’ of a critical mass of the atrial myocardium. These results suggested that the main determinant of AF elimination cannot be the design of RF lesion, but its overall area. This work is a pilot study undertaken to test this hypothesis in a clinical trial. The presented results demonstrated that the extended PVAI was more effective as compared to PVAI and GP ablation due to the ‘electrical exclusion’ of wider LA surface area, which was responsible for maintaining the arrhythmia.
As control groups, PVAI and GP ablation designs were selected because the first design is the ‘gold standard’, and the second has the lowest risk of atrial-esophageal fistulas and post-ablative atrial tachycardia.
Interestingly, Caponi et al. in one of their papers [13] also demonstrated the ablation methodology, which had many similarities with the extended PVAI. The authors did not focus on this fact, pointing out that 12 months after the intervention, sinus rhythm was maintained in 54% of patients with paroxysmal AF and 43% with persistent disease without antiarrhythmic therapy.
However, good results of the ‘critical mass exclusion’ in some cases might be achieved at the cost of the ‘atrial stiffness’ syndrome associated with massive RF exposure in LA, which undoubtedly plays an important role in the ‘pseudorestriction’ of transmitral bloodflow. It should be mentioned that ‘pseudorestriction’ is a common finding in patients after the restoration of sinus rhythm not only by radiofrequency ablation [14, 15]. It is possible that one of the main mechanisms, which could also be involved in such hemodynamic disorder, is the changes in PV contractility due to their sleeves damage. This study found that PVAI or extended PVAI increased the LA ‘stiffness’. The development of this phenomenon depended mainly on LA volume, but not on the lesion area. PVAI ‘extension’ to a certain limit (less than 25% of the total LA surface area) did not affect the atrial hemodynamics, but once this limit has exceeded, the abnormalities of passive LA stretching occur.
Conclusions
In persistent AF, the extended PVAI was more effective than conventional PVAI and GP ablation due to the ‘electrical exclusion’ of wider LA surface area, which was responsible for maintaining of the arrhythmia. After performing PVAI or extended PVAI, the LA mechanical dysfunction occurred if the overall lesion area exceeded the 25% of the total LA surface area.
Funding Statement
Funding sources: Nothing declared.
Footnotes
Author's Contributions: SEM has performed about 25% of ablation procedures, designed and wrote the article; INM has performed echocardiography; EAK has performed about 75% of ablation procedures and made all intraprocedural calculations; NSB has assisted as electrtophysiologist and ‘Carto’ workstation operator, made a statistical analysis of intraprocedural data; DAS has collected all follow-up data and made its statistical analysis. All authors had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest: The authors declare no conflict of interest.
Contributor Information
Sergey E. Mamchur,
Irina N. Mamchur,
Egor A. Khomenko,
Nikita S. Bokhan,
Diana A. Scherbinina,
References
- 1.Oral H, Chugh A, Good E, Sankaran S, Reich SS, Igic P, Elmouchi D, Tschopp D, Crawford T, Dey S, Wimmer A, Lemola K, Jongnarangsin K, Bogun F, Pelosi F, Jr., Morady F. A tailored approach to catheter ablation of paroxysmal atrial fibrillation. Circulation. 2006 Apr 18;113(15):1824–1831. doi: 10.1161/CIRCULATIONAHA.105.601898. [DOI] [PubMed] [Google Scholar]
- 2.Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, Salvati A, Dicandia C, Mazzone P, Santinelli V, Gulletta S, Chierchia S. Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation. 2000 Nov 21;102(21):2619–2628. doi: 10.1161/01.cir.102.21.2619. [DOI] [PubMed] [Google Scholar]
- 3.Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F, Jr., Bates ER, Lehmann MH, Vicedomini G, Augello G, Agricola E, Sala S, Santinelli V, Morady F. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006 Mar 2;354(9):934–941. doi: 10.1056/NEJMoa050955. [DOI] [PubMed] [Google Scholar]
- 4.Hazell W, Heaven D, Kazemi A, Fourie D. Atrio-oesophageal fistula: an emergent complication of radiofrequency ablation. Emerg Med Australas. 2009 Aug;21(4):329–332. doi: 10.1111/j.1742-6723.2009.01205.x. [DOI] [PubMed] [Google Scholar]
- 5.Siegel MO, Parenti DM, Simon GL. Atrial-esophageal fistula after atrial radiofrequency catheter ablation. Clin Infect Dis. 2010 Jul 1;51(1):73–76. doi: 10.1086/653425. [DOI] [PubMed] [Google Scholar]
- 6.Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, Mazzone P, Tortoriello V, Landoni G, Zangrillo A, Lang C, Tomita T, Mesas C, Mastella E, Alfieri O. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004 Jan 27;109(3):327–334. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 7.Pokushalov E, Romanov A, Shugayev P, Artyomenko S, Shirokova N, Turov A, Katritsis DG. Selective ganglionated plexi ablation for paroxysmal atrial fibrillation. Heart Rhythm. 2009 Sep;6(9):1257–1264. doi: 10.1016/j.hrthm.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Oferkin AI. Sufficient area and localization of lesions in atrial fibrillation ablation. Functional critical surface of atria. Vestnik Aritmologii. 2008;39(Suppl):A40. (In Russian) [Google Scholar]
- 9.Gibson DN, Di Biase L, Mohanty P, Patel JD, Bai R, Sanchez J, Burkhardt JD, Heywood JT, Johnson AD, Rubenson DS, Horton R, Gallinghouse GJ, Beheiry S, Curtis GP, Cohen DN, Lee MY, Smith MR, Gopinath D, Lewis WR, Natale A. Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm. 2011 Sep;8(9):1364–1371. doi: 10.1016/j.hrthm.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 10.Moe GK, Abildskov JA. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am Heart J. 1959 Jul;58(1):59–70. doi: 10.1016/0002-8703(59)90274-1. [DOI] [PubMed] [Google Scholar]
- 11.West TC, Landa JF. Minimal mass required for induction of a sustained arrhythmia in isolated atrial segments. Am J Physiol. 1962 Feb;202:232–236. doi: 10.1152/ajplegacy.1962.202.2.232. [DOI] [PubMed] [Google Scholar]
- 12.Shen J, Bailey MS, Damiano RJ., Jr. The surgical treatment of atrial fibrillation. Heart Rhythm. 2009 Aug;6(8 Suppl):S45–S50. doi: 10.1016/j.hrthm.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caponi D, Corleto A, Scaglione M, Blandino A, Biasco L, Cristoforetti Y, Cerrato N, Toso E, Morello M, Gaita F. Ablation of atrial fibrillation: does the addition of three-dimensional magnetic resonance imaging of the left atrium to electroanatomic mapping improve the clinical outcome? A randomized comparison of Carto-Merge vs. Carto-XP three-dimensional mapping ablation in patients with paroxysmal and persistent atrial fibrillation. Europace. 2010 Aug;12(8):1098–1104. doi: 10.1093/europace/euq107. [DOI] [PubMed] [Google Scholar]
- 14.Kelley GP, Dalati GA, Helmcke FR, Jain N, Al-Bataineh M, Glancy DL, Kerut EK. Atrial stunning masquerading as restrictive Doppler flow pattern: a case of mitral inflow "pseudorestriction". Echocardiography. 2006 Feb;23(2):172–175. doi: 10.1111/j.1540-8175.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- 15.Yamada H, Donal E, Kim YJ, Agler DA, Zhang Y, Greenberg NL, Mazgalev TN, Thomas JD, Grimm RA. The pseudorestrictive pattern of transmitral Doppler flow pattern after conversion of atrial fibrillation to sinus rhythm: is atrial or ventricular dysfunction to blame? J Am Soc Echocardiogr. 2004 Aug;17(8):813–818. doi: 10.1016/j.echo.2004.04.021. [DOI] [PubMed] [Google Scholar]