Abstract
Background and Objectives
In the United States, use of oral opioid analgesics has been associated with increasing rates of addiction, abuse, and diversion. However, little is known about recent national use of non-illicit prescription opioid analgesics (those prescribed in a doctor-patient relationship), the primary source of these drugs for the general US population. Our primary objective was to examine trends in the use of prescription opioid analgesics in the United States and to identify defining characteristics of patient users of prescribed opioids from 2000 to 2010.
Methods
We used the nationally representative Medical Expenditure Panel Survey to examine trends in prescription oral opioid analgesic use from 2000 to 2010. We used survey design methods to make national estimates of adults (18 years and older) who reported receiving an opioid analgesic prescription (referred to as opioid users) and used logistic regression to examine predictors of opioid analgesic use. Our primary outcome measures were national estimates of total users of prescription opioid analgesics and total number of prescriptions. Our secondary outcome was that of observing changes in the disability and health of the users.
Results
The estimated total number of opioid analgesic prescriptions in the United States increased by 104%, from 43.8 million in 2000 to 89.2 million in 2010. In 2000, an estimated 7.4% (95% CI, 6.9–7.9) of adult Americans were prescription opioid users compared with 11.8% (95% CI, 11.2–12.4) in 2010. Based on estimates adjusted for changes in the general population, each year was associated with a 6% increase in the likelihood of receiving an opioid prescription from 2000 to 2010. Despite the apparent increase in use, there were no demonstrable improvements in the age- or sex-adjusted disability and health status measures of opioid users.
Conclusions
The use of prescription opioid analgesics among adult Americans has increased in recent years, and this increase does not appear to be associated with improvements in disability and health status among users. On a public health level, these data suggest that there may be an opportunity to reduce the prescribing of opioid analgesics without worsening of population health metrics.
INTRODUCTION
The United States (U.S.) Department of Health and Human Services has declared oral opioid analgesic diversion, addiction, and overdose an epidemic and the fastest-growing drug problem in the United States.1,2 More recently, the Centers for Disease Control and Prevention (CDC) reported that total deaths in the United States from oral prescription opioids has exceeded deaths from cocaine and heroin combined.3 For every death from a prescription opioid analgesic there are 10 treatment admissions for abuse and 32 emergency room visits for misuse.4
Available information regarding prescription oral opioid use is primarily limited either to voluntary data provided by the private sector or health and behavior surveys that seek specifically to describe illicit use. For instance, IMS Health, a company that tracks pharmaceutical sales data,5 reports that hydrocodone/acetaminophen was the most prescribed medication of any category for at least the last 5 years.6 Internationally, hydrocodone was prescribed 136.7 million times in 2011 and oral opioids overall 238 million times in 2011.7 Despite being originally marketed and designed for chronic cancer pain, IMS Health also reports that the annual number of prescriptions for oxycodone hydrochloride controlled release (Oxycontin) for noncancer pain, an off-label use, increased tenfold, from about 670,000 in 1997 to about 6.2 million in 2002. Coinciding with this increase in Oxycontin, the Drug Abuse Warning Network (DAWN) reported that the misuse of Oxycontin alone rose from 221,000 persons in 1997 to 3,176,800 in 2004.8
Given that prescription opioid analgesics are the primary source of such drugs for the general U.S. population,9 a better understanding of utilization patterns, as well as characteristics of prescription opioid analgesic users, would provide important information for both health care providers and policy makers as they confront the growing opioid-related health care crisis. Therefore, we used the nationally representative Medical Expenditure Panel Survey (MEPS) to examine recent trends in the use of prescription opioid analgesics from 2000 to 2010. More specifically, we sought to determine whether use of prescription opioid analgesics is changing over time and, if so, what characteristics may explain such changes. As potential increases in the use of prescription opioid analgesics may, in fact, improve health and functional status among Americans in need of these medications, we also examined disability and general health status of users over time.
METHODS
We used a serial cross-sectional study design to examine recent national trends in the use of prescription opioid analgesics. For all analyses, we used data from the MEPS, which is a nationally representative health survey conducted annually by the U.S. Agency for Healthcare Research and Quality and the National Center for Health Statistics. The MEPS is a well-known source of national data on U.S. health service use and expenditures and is comprised of both household and insurance components.10,11 As this study used only de-identified and publically available data, it was deemed exempt from institutional board review by the Dartmouth College Committee for the Protection of Human Subjects.
Sample
The MEPS Household Component distributes questionnaires to individual household members to collect nationally representative data on sociodemographic characteristics, health conditions, health status, use of health care services, charges and payments, access to care, satisfaction with care, and health insurance coverage.11,12 The MEPS uses the previous year’s National Health Interview Survey as a sampling frame and uses an overlapping panel design involving 5 rounds of interviews over a 2½-year period. Telephone interviews and record abstractions from health care providers, hospitals, and home health caregivers and from pharmacies provide further utilization and expenditure data.
We analyzed data from all study participants to the MEPS Household Component surveys from 2000 through 2010 who were 18 years and older. In 2010, there were 23,694 adult MEPS study participants, representing, when weighted, a national estimate of approximately 233.8 million adults.
Identification of prescription opioid analgesic use
We used the National Drug Code (NDC) classification system to identify the use of prescription opioid analgesics among adult MEPS study participants. The Drug Listing Act of 1972 requires registered drug establishments to provide the U.S. Food and Drug Administration (FDA) with a current list of all drugs manufactured. The NDC serves as a universal product identifier for drugs, with the FDA publically maintaining up to date information. All analyses were restricted specifically to oral prescription opioid analgesics. We operationally defined a prescription opioid analgesic as any oral prescription that had a NDC code that fit within the classification of “narcotic analgesic” or “narcotic analgesic combination;” the former category captured pure agonists like oxycodone and the later captured combination drugs such as oxycodone/acetaminophen (eg, Percocet). We identified 3,080 unique prescription types for opioid analgesics, each consisting of some varying aspect of manufacturer, dose, or formulary. A participant was classified as an “opioid user” if there was at least one reported prescription opioid analgesic during the last year. In addition, we also counted the number of prescriptions for each participant as an additional measure of opioid consumption.
Disability and health status
Because we anticipated the potential changes in the use of opioid analgesics might be associated with changes in functional status among users, we examined disability and health status measures according to oral opioid user status. We calculated the percentage of respondents who reported any limitations in: physical functioning, (eg, walking, climbing stairs, lifting, bending, standing); social functioning; cognitive functioning; work related to pain, and work, school, or home activities. We also calculated the percentage of respondents who reported any limitations at all during the entire year. Self-reported general health status was dichotomized into “good to excellent” vs “ fair to poor.” These responses to general health status were distinct from the 12-Item Short Form Health Survey that we also examined from 2000 to 2010 (both physical and mental components). We converted 2000–2002 version 1 scores to the version 2 equivalents, because version 2 was administered after 2002.13
Sociodemographic data
We analyzed data related to age, sex, race/ethnicity, education, and type of insurance coverage (“any private insurance,” “public only coverage,” or “uninsured”), total income, and U.S. Census region. Race/ethnicity were determined through respondent self-report using categories defined by the U.S. Census Bureau and were aggregated into “Hispanic,” “non-Hispanic white,” “non-Hispanic black,” and “other/multiple.” We compared sociodemographic characteristics of opioid users in 2000 versus 2010 to identify potential characteristics that might be associated with changes in opioid use over this time period.
Statistical Analyses
The MEPS uses stratified random probability sampling method that allows the generation of national estimates. All national estimates were generated by using established survey design methods in Stata version 12.0 (StataCorp, College Station, Texas) that account for the probability of study inclusion and stratified sampling design.10,11 To compare differences between opioid users and non-opioid users in 2010, as well as 2010 opioid users vs 2000 opioid users, we used a chi-squared test for categorical variables and an unpaired t-test for continuous measures. For all analyses we set the P-value for statistical significance to 0.05 (2-sided).
To determine whether the disability and health status of opioid users changed over the 11-year time period, we calculated estimates adjusted for age and sex using logistic regression models, and bootstrap resampling methods to obtain 95% confidence intervals (CI). We constructed a logistic regression model to examine associations between study participant characteristics and opioid use over time. The variable for year in this model was explored both as a continuous variable as well as indicator variables with 2000 as the reference category.
RESULTS
National Trends in the Use of Prescription Opioid Analgesics
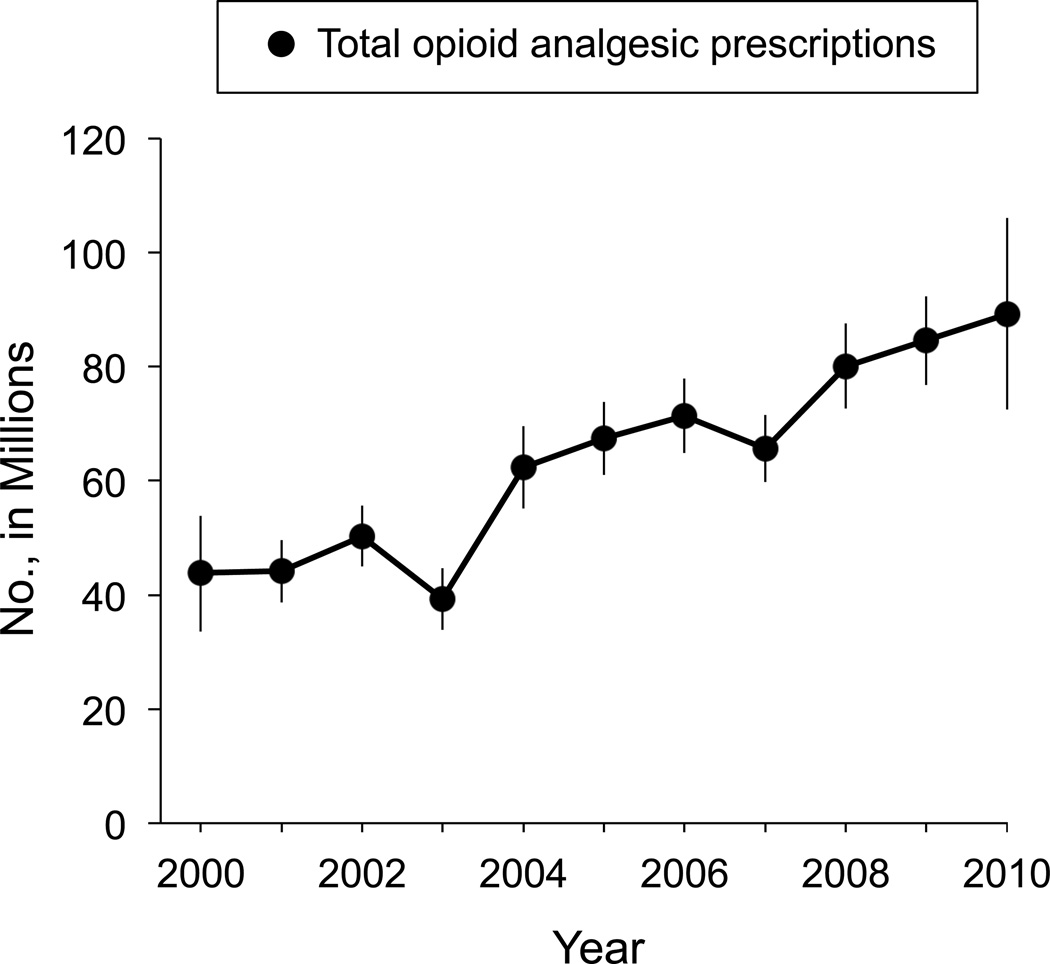
According to our estimates, the total number of prescriptions of opioid analgesics increased by 104%; from 43.8 million in 2000 to 89.2 million in 2010, a net increase of 45.4 million prescriptions (95% CI, 38.8–52.0) (Figure 1).
Figure 1.
Estimated total oral opioid prescriptions, 2000–2010. Line graph depicts point estimates with 95% confidence intervals.
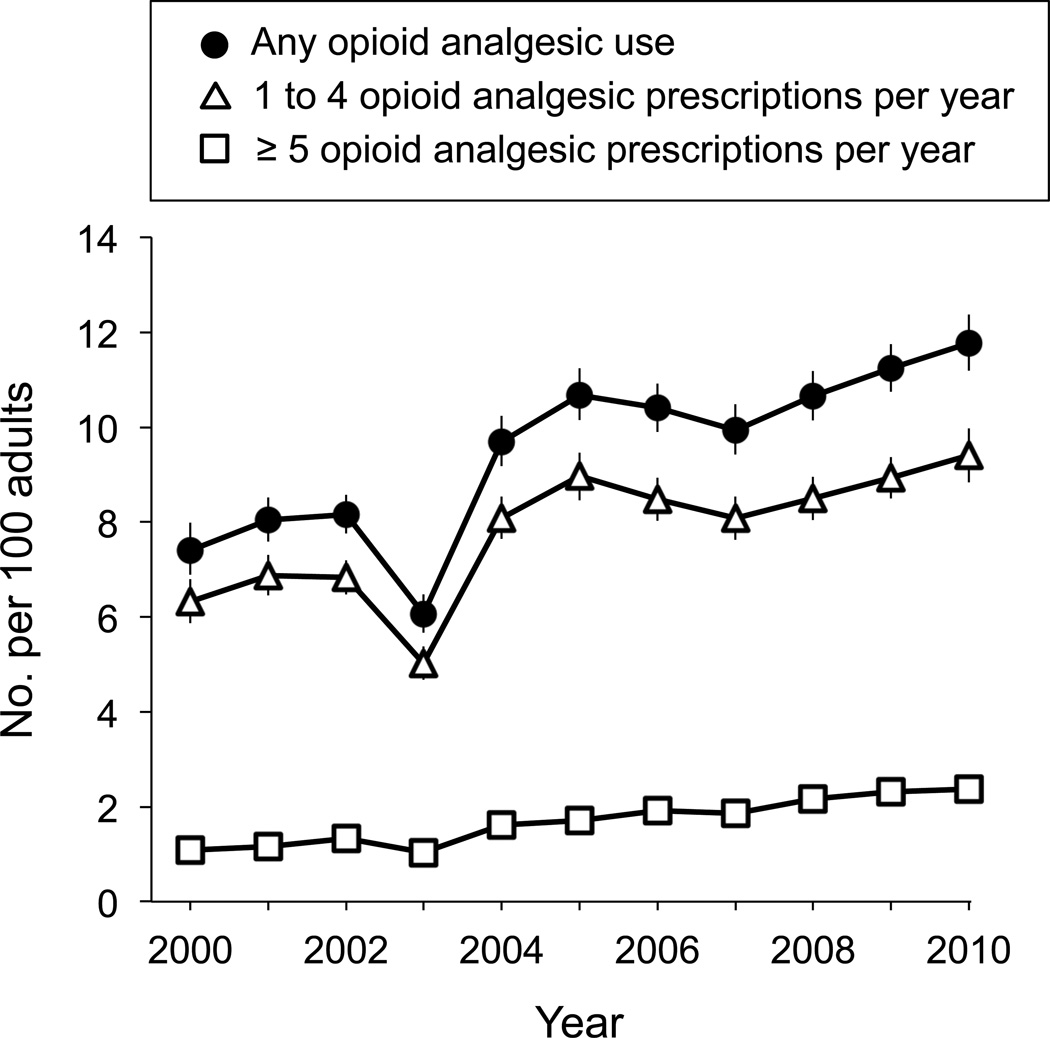
The total number of adult Americans who received a prescription for an opioid analgesic rose approximately 80% from 15.3 million (95% CI, 14.2–16.4) in 2000 to 27.5 million (95% CI, 26.1–28.9) in 2010 (Table 1). As a percentage of all adult Americans, an estimated 7.4% adults (95% CI, 6.9–8.0) were opioid users in 2000 and 11.8% (95% CI, 11.2–12.4) in 2010. According to the rate of opioid analgesic prescriptions per 100 adults, we observed a persistent increase in those adults receiving 5 or more prescriptions per year (Figure 2). Among all opioid users, the mean number of opioid analgesic prescriptions per user did not statistically change over the 11-year time period: the mean number of prescriptions per user was 3.0 (95% CI, 2.9–3.1).
Table 1.
Respondent characteristics associated with oral opioid use, Medical Expenditure Panel Survey 2000 to 2010 a b
| % receiving opioids | Odds Ratio | 95% CI | P-value | |
|---|---|---|---|---|
| Year c | 9.5 | 1.06 | (1.05, 1.06) | <0.001 |
| Age | ||||
| Young adult (18–30) | 7.2 | 1.0 (reference) | ||
| Adult (30–64) | 9.8 | 0.87 | (0.82, 0.92) | <0.001 |
| Older adult (65+) | 11.3 | 0.59 | (0.55, 0.65) | <0.001 |
| Sex | ||||
| Female | 11.1 | 1.0 (reference) | ||
| Male | 7.7 | 0.75 | (0.72, 0.79) | <0.001 |
| Race/ethnicity | ||||
| White | 10.4 | 1.0 (reference) | ||
| Hispanic | 6.2 | 0.64 | (0.59, 0.68) | <0.001 |
| Black | 9.5 | 0.84 | (0.79, 0.89) | <0.001 |
| Other | 6.4 | 0.62 | (0.56, 0.68) | <0.001 |
| PCS health score d | ||||
| First quartile, (<44) | 20.2 | 1.0 (reference) | ||
| Second quartile, (44–53) | 9.1 | 0.42 | (0.40, 0.44) | <0.001 |
| Third quartile, (54–57) | 5.8 | 0.28 | (0.27, 0.29) | <0.001 |
| Fourth quartile, (>57) | 5.2 | 0.23 | (0.21, 0.24) | <0.001 |
| MCS health score d | ||||
| First quartile, (<45) | 14.9 | 1.0 (reference) | ||
| Second quartile, (45–53) | 9.7 | 0.79 | (0.76, 0.84) | <0.001 |
| Third quartile, (54–58) | 7.4 | 0.69 | (0.65, 0.73) | <0.001 |
| Fourth quartile, (>58) | 8.0 | 0.56 | (0.56, 0.64) | <0.001 |
| Current Smoking | ||||
| No | 8.9 | 1.0 (reference) | ||
| Yes | 13.1 | 1.39 | (1.32, 1.45) | <0.001 |
| Obesity | ||||
| No | 8.4 | 1.0 (reference) | ||
| Yes | 12.7 | 1.20 | (1.15, 1.25) | <0.001 |
| Marital Status | ||||
| Married | 9.4 | 1.0 (reference) | ||
| Previously married | 12.6 | 1.07 | (1.02, 1.13) | <0.001 |
| Never married | 7.2 | 0.91 | (0.87, 0.97) | 0.002 |
| Insurance | ||||
| Any private | 9.5 | 1.0 (reference) | ||
| Public only | 13.3 | 1.00 | (0.95, 1.06) | 0.001 |
| Uninsured | 5.3 | 0.53 | (0.49, 0.56) | <0.001 |
| Census Region | ||||
| Northeast | 8.2 | 1.0 (reference) | ||
| Midwest | 10.5 | 1.23 | (1.14, 1.33) | <0.001 |
| South | 10.1 | 1.20 | (1.12, 1.29) | <0.001 |
| West | 8.9 | 1.20 | (1.12, 1.29) | <0.001 |
Abbreviations: CI, confidence interval; BMI, body mass index; PCS, physical composite summary; MCS, mental composite summary
All estimates based on weighted sample using survey design methods for adults 18 and older.
Odds ratios were generated from the multivariable logistic regression model predicting opioid use. All covariates are represented except education and income that were not statistically significant.
All years (2000–2010) included for analysis. Odds ratio for year represents the test of trend across 11 years.
PCS and MCS scores range from 0–100, with a higher score indicating better functioning
Figure 2.
Estimated total oral opioid users per 100 U.S. Adults. Line graphs depict varying prescription quantities with point estimates and 95% confidence intervals.
Predictors of Opioid Analgesic Use
After accounting for changes in population characteristics and health measures over the 11 years, each new year was associated with a statistically significant increase of 6% in the likelihood of being an opioid user (OR test of trend 1.06; 95% CI, 1.05–1.06) (Table 2). In a similar model, year 2010 versus 2000 was associated with a higher likelihood of being a prescription oral opioid user (OR 1.76; 95% CI, 1.58–1.95). Table 1 reveals additional independent predictors of being an opioid user. The characteristics of poor physical health, poor mental health, young, obese, uninsured, smoking, white, and female were all associated with higher likelihood of opioid use.
Table 2.
Self-reported health status and disability measures for adult opioid users, age and sex adjusted, MEPS 2000–2010a,b
| Measure | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimated No. of adult opioid users, millions | 15.3 | 16.9 | 17.7 | 13.3 | 21.2 | 23.8 | 23.4 | 22.5 | 24.6 | 25.9 | 27.4 |
| Summary score, meanc | |||||||||||
| PCS | 43.5(38.8, 48.2) | 43.5(39.9, 47.1) | 43.9(40.5, 47.4) | 41.9(37.5, 46.4) | 43.5(40.1, 46.8) | 42.3(38.6, 45.9) | 42.8(39.4, 46.2) | 42.3(38.3, 46.2) | 41.8(37.9, 45.8) | 42.1(38.5, 45.7) | 41.7(37.3, 45.7) |
| MCS | 48.5(43.7, 53.2) | 48.4 (44.6, 52.2) | 48.2(44.3, 52.1) | 47.9(42.8, 52.9) | 47.6(43.8, 51.4) | 48.0(44.4, 51.7) | 48.3(44.7, 51.9) | 48.1(44.3, 51.9) | 47.8 (44.2, 51.6) | 47.8(43.9, 51.7) | 47.6(52.3, 42.9) |
| Any limitation in physical function, % yes | 29.2(29.0, 29.5) | 29.1(28.9, 29.3) | 29.1(28.8, 29.3) | 28.9(28.7, 29.2) | 28.9(28.7, 29.0) | 28.8(28.7, 29.0) | 28.7(28.4, 29.0) | 28.6(28.4, 28.8) | 28.5(28.3, 28.8) | 28.4(28.2, 28.6) | 28.4(28.1, 28.6) |
| Any work, home, or school limitation, % yes | 24.1 (23.9, 24.3) | 23.9(23.8, 24.2) | 23.8(23.6, 23.9) | 23.6(23.4, 23.8) | 23.4(23.3, 23.6) | 23.2(23.1, 23.4) | 23.1(22.9, 23.3) | 22.9(22.8, 23.0) | 22.7 (22.6, 22.8) | 22.5(22.4, 22.7) | 22.4(22.2, 22.5) |
| Excellent to good health status, % yes | 71.8(71.7, 71.9) | 71.8 (71.7, 71.9) | 71.9(71.7, 72.0) | 71.9(71.7, 72.0) | 71.9(71.7, 72.0) | 71.9(71.8, 72.0) | 71.9(71.7, 72.0) | 71.9(71.8, 72.0) | 71.9(71.7, 72.0) | 71.9(71.8, 72.0) | 71.9 (71.8, 72.0) |
| Excellent to good mental health status, % yes | 87.3(87.2, 87.3) | 86.9(86.9, 87.0) | 86.7(86.7, 86.8) | 86.5(86.4, 86.5) | 86.2(86.1, 86.2) | 85.9(85.9, 86.0) | 85.7(85.6, 85.7) | 85.4(85.3, 85.4) | 85.1(85.0, 85.1) | 84.8(84.8, 84.8) | 84.5(84.4, 84.5) |
| Any cognitive limitation, % yes | 8.5(8.4, 8.5) | 8.7(8.6, 8.67) | 8.8(8.9, 9.0) | 9.0(8.9, 9.1) | 9.2(9.1, 9.2) | 9.3(9.3, 9.4) | 9.5(9.4, 9.6) | 9.6(9.6, 9.8) | 9.9(9.8, 9.9) | 10.1(10.0, 10.3) | 10.2(10.2, 10.3) |
| Any social limitation, % yes | 14.6(14.6, 14.7) | 14.5(14.4, 14.5) | 14.4(14.3, 14.5) | 14.3(14.4, 14.4) | 14.2(14.1, 14.3) | 14.0(13.9, 14.1) | 13.9(13.8, 14.0) | 13.8(13.7, 13.9) | 13.7(13.6, 13.8) | 13.6(13.5, 13.7) | 13.5(13.4, 13.6) |
| Any limitation during year, % yes | 49.1(48.8, 49.3) | 48.9(48.7, 49.2) | 48.8 (48.6, 49.1) | 48.7(48.6, 48.9) | 48.6(48.4, 48.8) | 48.5(48.2, 48.7) | 48.4(48.1, 48.6) | 48.2(47.9, 48.5) | 48.1 (47.9, 48.4) | 47.9(47.7, 48.1) | 47.9(47.6, 48.1) |
| Any limitation in work from pain, % yes | 69.4(69.2, 69.6) | 69.6(69.5, 69.8) | 69.8(69.6, 70.0) | 70.0(69.9, 70.2) | 70.3(70.1, 70.4) | 70.5(70.3, 70.6) | 70.7(70.6, 70.8) | 70.9(70.7, 71.1) | 71.1(71.0, 71.2) | 71.3(71.1, 71.5) | 71.5(71.3, 71.7) |
| Moderate to vigorous physical activity, % yesd | 47.7(47.6, 47.8) | 48.0(47.9, 48.1) | 48.3(48.2, 48.4) | 48.6(48.5, 48.7) | 48.9(48.7, 49.0) | 49.2 (49.0, 49.3) | 49.4(49.3, 49.8) | 49.7 (49.6, 49.8) | 50.0(49.9, 50.1) | 50.3(50.2, 50.4) | 50.6(50.5, 50.7) |
Abbreviations: MEPS, Medical Expenditure Panel Survey; CI, confidence interval; MCS, Mental Composite Summary; PCS, Physical Composite Summary; adult, 18 and older.
All estimates based on weighted sample using survey design methods.
Values expressed as either count, mean (95% CI) or proportion answering “yes” (95% CI)
PCS and MCS scores range from 0–100, with a higher score indicating better functioning.
Frequency defined as three times per week
Disability and Health Status of Opioid Users Over Time
From 2000 to 2010, we found few changes in age and sex adjusted disability and general health status measures among opioid users (Table 2). Compared with 2000, opioid consumers in 2010 were more likely to report a poor mental health status (OR 1.39; 95% CI, 1.12–1.74). Additionally, opioid users in 2010 were more likely to report cognitive limitations compared to 2000 (OR 1.52; 95% CI, 1.12–2.07). The mean Physical Component Summary and Mental Component Summary scores for the SF-12 did not appreciably change. Additionally, there were no appreciable changes in self-reported physical health status, physical limitations; home, work, or school limitations; social limitations; physical activity, or any limitations.
Characteristics of Opioid Users
Compared with 2000, adult opioid users in 2010 were older, more likely to receive public health insurance, more likely to be unemployed, more likely to have higher education, more likely to be obese, and more likely to be in a low-income category (Table 3). In 2010, adult opioid users compared with non-users were older and had a higher proportion who were non-Hispanic white, female, covered by public insurance, unemployed, obese, current smokers, and in a lower income category (Table 3). Additionally, in 2010, adult opioid users compared to non-users, reported overall poorer mental health (4.0 lower Mental Component Summary scores; 95% CI −4.6, −3.4) and physical health (8.9 points lower Physical Component Summary scores; 95% CI, −9.6–8.1).
Table 3.
Characteristics of US adult population by opioid status, Medical Expenditure Panel Survey 2000 and 2010
| 2000 a | 2010 a | P-value for difference between groups b | ||||
|---|---|---|---|---|---|---|
| Variable | Opioid user | Non-opioid user | Opioid user | Non-opioid user | Opioid users and non-users, 2010 |
Opioid users, 2000 vs. 2010 |
| No. sampled (respondents) | 1,277 | 16,481 | 2,542 | 21,152 | ||
| Estimated No. adults in US population, millions | 15.3 | 190.7 | 27.5 | 206.3 | ||
| Age | 46.4 (45.0, 47.7) | 44.9 (44.5, 45.5) | 50.1 (49.0, 51.2) | 45.9 (45.5, 46.4) | <0.001 | <0.001 |
| Men, % | 39.8 (36.7, 43.1) | 48.5 (47.9, 49.2) | 40.2 (38.2, 42.3) | 49.6 (48.9, 50.2) | <0.001 | 0.19 |
| Employment c, % | 50.3 (46.7, 53.9) | 61.2 (59.9, 62.5) | 44.3 (41.5, 47.2) | 57.3 (56.6, 58.6) | <0.001 | 0.01 |
| Race /ethnicity, % | <0.001 | 0.01 | ||||
| hispanic | 6.7 (4.9, .9) | 11.0 (9.3, 13.0) | 10.3 (8.6, 12.2) | 14.6 (13.1, 16.3) | ||
| white | 80.2 (77.8, 82.5) | 73.4 (71.2, 75.6) | 73.6 (71.5, 75.5) | 66.9 (64.8, 68.9) | ||
| black | 10.0 (8.1, 12.3) | 11.6 (9.8, 13.7) | 12.1 (10.6, 13.7) | 11.5 (10.3, 12.7) | ||
| other | 3.1 (2.1, 4.7) | 4.0 (3.4, 4.8) | 4.1 (3.4, 5.1) | 7.1 (6.0, 8.3) | ||
| College or advanced degree d, % | 24.9 (22.4, 27.5) | 29.9 (28.4, 31.5) | 31.9 (29.0, 34.9) | 37.3 (36.2, 38.5) | <0.001 | <0.001 |
| Marriage, % | <0.001 | 0.02 | ||||
| current married | 58.6 (54.8, 62.3) | 55.5 (54.1, 56.9) | 52.1 (49.8, 54.4) | 53.2 (51.7, 54.6) | ||
| never married | 16.9 (14.1, 20.3) | 25.0 (23.9, 26.2) | 19.5 (17.7, 21.5) | 28.2 (27.2, 29.3) | ||
| previously married | 24.4 (21.7, 27.3) | 19.5 (18.6, 20.4) | 28.4 (26.2, 30.1) | 18.6 (17.7, 19.5) | ||
| Low income, % | 39.1 (36.2, 42.1) | 32.9 (31.8, 34.2) | 35.3 (33.0, 37.7) | 30.2 (29.2, 31.1) | <0.001 | 0.05 |
| Insurance, % | <0.001 | <0.001 | ||||
| private | 74.8 (71.6, 77.7) | 73.8 (72.3, 75.2) | 62.1 (59.6, 64.5) | 67.9 (66.6, 69.1) | ||
| public | 17.9 (15.3, 20.8) | 13.3 (12.3, 14.4) | 28.1 (25.7, 30.1) | 16.0 (15.2, 16.9) | ||
| uninsured | 7.3 (12.1, 13.9) | 12.9 (5.8, 9.2) | 9.8 (8.7, 11.1) | 16.1 (15.1, 17.1) | ||
| Census region, % | 0.003 | 0.51 | ||||
| Northeast | 15.0 (10.6, 20.8) | 19.4 (16.2, 23.1) | 17.7 (14.0, 22.2) | 18.6 (16.2, 21.2) | ||
| Midwest | 24.7 (19.4, 30.8) | 22.9 (18.9, 27.3) | 24.9 (20.4, 29.6) | 21.3 (17.9, 25.2) | ||
| South | 36.2 (29.5, 43.5) | 35.4 (29.7, 41.6) | 38.3 (34.2, 42.5) | 36.5 (33.3, 39.7) | ||
| West | 24.1 (17.0, 32.9) | 22.3 (17.5, 27.9) | 19.4 (16.1, 23.0) | 23.7 (20.6, 27.0) | ||
| Obesitye, % | 27.8 (27.4, 28.3) | 26.8 (26.6, 26.9) | 29.2 (28.8, 29.5) | 27.6 (27.4, 27.7) | <0.001 | <0.001 |
| Current smoker, % | 31.4 (27.9, 35.1) | 22.4 (21.5, 23.4) | 27.7 (25.5, 30.0) | 16.6 (15.7, 17.6) | <0.001 | 0.08 |
| Summary health scoref, mean | ||||||
| PCS | 43.5 (42.7, 44.4) | 50.8 (50.5, 51.0) | 41.6 (40.9, 42.4)) | 50.5 (50.3, 50.7) | <0.001 | 0.001 |
| MCS | 48.4 (47.6, 49.3) | 51.2 (50.9, 51.4) | 47.6 (47.0, 48.2) | 51.6 (51.4, 51.8) | <0.001 | 0.11 |
Abbreviations: Abbreviations: CI, confidence interval; BMI, body mass index; PCS, physical composite summary; MCS, mental composite summary
Values express as either proportion answering “yes” or mean (95% CI)
Categorical data compared using chi-square, continuous data compared using two-sample t-test
Employment for the entire year
Education dichotomized into “College or advanced degree” vs. “High school or less”
Obesity defined as a BMI of 30 or greater
PCS and MCS scores range from 0–100, with a higher score indicating better functioning
DISCUSSION
We found that the national use of prescription opioid analgesics increased from 2000 through 2010. Although the number of prescriptions per person has essentially remained unchanged, the proportion of Americans receiving at least one opioid analgesic prescription has risen steeply. Based on our adjusted estimates, each year was associated with a 6% increase in the likelihood of receiving an opioid prescription from 2000 to 2010. We suspect that the disproportionate drop in opioid prescribing in 2003 may have represented a sampling variation. However, it may also be explained by other factors such as drug company litigation, drug shortages, or the nadir effect of the 2000–2003 economic recession.
We were surprised to find that, despite an increase in prescription opioid analgesic use among U.S. adults from 2000 to 2010, disability and health status metrics either declined or remained essentially unchanged among users. Leading societal guidelines, advocacy groups, and regulatory statements have argued that more aggressive pain management (mostly through opioid analgesics) should lead to improvements in both physical and psychological health.14–17 These purported health benefits of opioid analgesic use seem a logical prerequisite to justify the proven serious morbidity of prescription opioid analgesics stemming from epidemic levels of addiction, diversion, and overdose.2,7,18,19 Our findings are consistent with other recent reports that appear to challenge the public health benefit of the expansion of opioid analgesic prescribing. For instance, a comprehensive review exploring the effectiveness of long-term therapy for chronic, noncancer pain highlights the paucity of compelling data on long-term opioid analgesic therapy.20 In particular, there is limited evidence to support significant improvements in functional status and quality of life among opioid analgesic users.21 In the outpatient setting, moreover, routinely measuring pain as a “fifth vital sign” in fact has not increased the quality of pain management.22
In our study, the effect of time on opioid analgesic use likely represents a combination of unmeasured cultural factors including aggressive pharmaceutical marketing and social/regulatory policy advocating for the liberal use of opioid analgesics. Worthy of note is the well-documented marketing campaign of Purdue Pharma L.P., makers of Oxycontin, one of the most popular opioids to date.23 From a social policy perspective, the U.S. Veterans Health Administration adopted the “fifth vital sign” campaign in 2000 and enacted a national strategy to ensure that pain is routinely assessed at all patient encounters, using a 0-to-10 Numeric Rating Scale.24 Finally, The Joint Commission on Accreditation of Health Care Organizations (JCAHO) released its 2001 mandate regarding pain management standards for health care organization accreditation.17
Our logistic regression model identified independent predictors of prescription opioid analgesic use. Such information may prove helpful in targeting educational initiatives around opioid analgesic safety or in constructing opioid-related research designs. The sociodemographics of relatively young, obese, uninsured, smoking, non-Hispanic white, and female stood out as predictors even when controlling for mental and physical health status.
Limitations
There are several limitations to our study that must be acknowledged. First, MEPS data is only generalizable to non-institutionalized Americans; trends may differ among those in the military, for example. Second, MEPs data are observational and based on self-report, and, thus, are subject to potential unmeasured confounding. Our logistic model was based on a priori assumptions and univariate predictors but may have missed important unmeasured factors. Third, our data lack specificity regarding dose, quantity of drug, and duration of treatment. Therefore, it is possible that the absolute quantity of opioid analgesics has not changed within the population. Such a scenario is unlikely given prior information provided by the federal government.9
Lastly, as our study is a cross-sectional design, the lack of improvement in health and disability metrics should be interpreted with some caution. It is conceivable that disability and health metrics may have declined more in the absence of the expanded use of opioid analgesics. Future prospective observational studies are required to more rigorously evaluate the potential lack of association between expanding opioid analgesic use and disability metrics.
Conclusion
Our study suggests that the use of prescription opioid analgesics for adult Americans is on the rise. While the use of prescription opioid analgesics has increased, this increase does not appear to be associated with improvements in health status among opioid users commensurate with well-documented risks of these drugs. These data suggest that, on a public health level, there may be an opportunity to reduce opioid prescribing without worsening of clinical health metrics.
Acknowledgments
Funding:
The Department of Anesthesiology at Dartmouth Medical School provided protected research time for Drs. Brian Sites and Michael Beach to support this project. Matthew Davis was supported by award No. 5K01AT006162 by the National Institutes of Health. The views expressed herein do not represent the official view of the National Institutes of Health.
Footnotes
Conflict of Interest:
The authors declare no conflict of interest.
REFERENCES
- 1.Epidemic: Responding to America's Prescription Drug Abuse Crisis. Washington, D.C.: 2011. [Accessed January 5, 2013]. Office of National Drug Control Policy, Executive Office of the President of the United States. 2011. Available at: http://www.whitehouse.gov/sites/default/files/ondcp/policy-and-research/rx_abuse_plan.pdf. [Google Scholar]
- 2.Centers for Disease Control and Prevention. CDC Grand Rounds: prescription drug overdoses - a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61:10–13. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers, United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60:1487–1492. [PubMed] [Google Scholar]
- 4.Policy Impact: Prescription Painkiller Overdoses. [Accessed January 5, 2013];2012 Available at: http://www.cdc.gov/homeandrecreationalsafety/rxbrief.
- 5.IMS Health. [Accessed March 17, 2013]; Available at: http://www.imshealth.com/portal/site/ims. [Google Scholar]
- 6.Audit IHNP. Top Products by Rx. [Accessed January 4, 2013];2011 Available at: http://www.imshealth.com/ims/Global/Content/Corporate/Press Room/Top-Line Market Data&Trends/2011 Top-line Market Data/Top_Products_by_RX.pdf. [Google Scholar]
- 7.Manchikanti L, Helm S, 2nd, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15:ES9–ES38. [PubMed] [Google Scholar]
- 8.Drug Abuse Warning Network. National Estimates of Drug-Related Emergency Department Visits 2006. [Accessed January 5, 2013]; Available at: http://www.samhsa.gov/data/DAWN/files/ED2006/DAWN2k6ED.htm.
- 9.Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010;13:401–435. [PubMed] [Google Scholar]
- 10.Cohen SB. Design strategies and innovations in the medical expenditure panel survey. Medical Care. 2003;41:III5–III12. doi: 10.1097/01.MLR.0000076048.11549.71. [DOI] [PubMed] [Google Scholar]
- 11.Cohen JW, Monheit AC, Beauregard KM, et al. The Medical Expenditure Panel Survey: a national health information resource. Inquiry. 1996;33:373–389. [PubMed] [Google Scholar]
- 12.Medical Expenditure Panel Survey Web site. [Accessed April 3, 2013];2013 Available at: http://meps.ahrq.gov/mepsweb/.
- 13.Ware J, Kosinski M, Turner-Bowker DM, Gandek B. How to Score Version 2 of the SF-12v2 Health Survey. Lincoln, RI: QualityMetric Incorporated; 2002. [Google Scholar]
- 14.Quality improvement guidelines for the treatment of acute pain and cancer pain. American Pain Society Quality of Care Committee. JAMA. 1995;274:1874–1880. doi: 10.1001/jama.1995.03530230060032. [DOI] [PubMed] [Google Scholar]
- 15.The use of opioids for the treatment of chronic pain. A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin J Pain. 1997;13:6–8. [PubMed] [Google Scholar]
- 16.American Pain Society. Pain assessment and treatment in the managed care environment. A position statement from the American Pain Society. Case Manager. 2000;11:50–53. doi: 10.1067/mcm.2000.110313. [DOI] [PubMed] [Google Scholar]
- 17.The facts about pain management. [Accessed April 5, 2013];The Joint Commission. 2012 Available at: http://www.jointcommission.org/pain_management. [Google Scholar]
- 18.McCarthy M. Containing the opioid overdose epidemic. BMJ. 2012;345:e8340. doi: 10.1136/bmj.e8340. [DOI] [PubMed] [Google Scholar]
- 19.Thompson ME, Tommasello A, Long B. The prescription drug misuse and abuse epidemic. JAPhA. 2012;52:564–568. doi: 10.1331/JAPhA.2012.12532. [DOI] [PubMed] [Google Scholar]
- 20.Manchikanti L, Vallejo R, Manchikanti KN, Benyamin RM, Datta S, Christo PJ. Effectiveness of long-term opioid therapy for chronic non-cancer pain. Pain Physician. 2011;14:E133–E156. [PubMed] [Google Scholar]
- 21.Stein C, Reinecke H, Sorgatz H. Opioid use in chronic noncancer pain: guidelines revisited. Curr Opin Anaesthesiol. 2010;23:598–601. doi: 10.1097/ACO.0b013e32833c57a8. [DOI] [PubMed] [Google Scholar]
- 22.Mularski RA, White-Chu F, Overbay D, Miller L, Asch SM, Ganzini L. Measuring pain as the 5th vital sign does not improve quality of pain management. J Gen Intern Med. 2006;21:607–612. doi: 10.1111/j.1525-1497.2006.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Zee A. The promotion and marketing of Oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009;99:221–227. doi: 10.2105/AJPH.2007.131714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Department of Veterans Affairs, Pain as the 5th Vital Sign Toolkit. Veterans Health Administration. [Accessed April 1, 2013];2000 Available at: http://www.va.gov/painmanagement/docs/toolkit.pdf.