Abstract
By shortening the time to pathogen identification and allowing for detection of organisms missed by blood culture, new molecular methods may provide clinical benefits for the management of patients with sepsis. While a number of reviews on the diagnosis of sepsis have recently been published we here present up-to-date new developments including multiplex PCR, mass spectrometry and array techniques. We focus on those techniques that are commercially available and for which clinical studies have been performed and published.
Keywords: biomarker, blood culture, mass spectrometry, microarray, molecular diagnostics, PCR, sepsis, SeptiFast
Clinical impact of bloodstream infections and unmet medical needs
Sepsis – considered a race to the death between the pathogens and the host immune system [1] – represents a major public health problem and is among the most common reasons for admission to the intensive care unit (ICU). Mortality related to sepsis remains high, despite improving outcomes in healthcare, being the second leading cause of death in the noncoronary ICU [2, 3]. Patients who survived sepsis bear an underrecognized risk of physical and cognitive impairment and suffer a more-than-doubled risk of dying in the next 5 years compared with hospitalized controls [4].
Inappropriate antimicrobial treatment is a major concern and is associated with increased mortality [5, 6]. Reasons for inappropriate treatment include the lack of coverage of the underlying pathogen and antimicrobial resistance of the causative pathogen in nosocomial infections and in infections by emerging multiresistant Gram-negative bacteria [7]. Increased mortality may also be found following inappropriate fungal therapy [8, 9] since empiric antimycotic coverage is only recommended in high-risk patients (e.g., neutropenia, intra-abdominal infections) [10]. These findings have led to a growing interest in the development of diagnostic tests for the rapid diagnosis of pathogens causing bloodstream infections to allow early administration of adequate targeted antimicrobial therapy in critically ill patients.
Special attention should be given to patients presenting with isolated fever or leukocytosis. The systemic inflammatory response (SIRS) and a multivariable decision rule with major and minor criteria are sensitive but not specific predictors of bacteremia [11]. SIRS and the decision rule may be helpful in identifying patients who do not need blood cultures. Because these conclusions do not apply to immunocompromised patients or when endocarditis is suspected, such patients should be cautiously evaluated. Physicians have to finally decide if ordering blood cultures is necessary in patients with isolated fever or leukocytosis.
Blood culture (BC) is still considered the gold standard for diagnosis and identification of bloodstream pathogens by many [12–14]. However, this conventional laboratory method lacks sensitivity, has a low pre-test probability in certain clinical settings, and is impaired by the delay in the time to result. In order to increase the speed of diagnosis, to improve sensitivity and the clinical benefit of detection of pathogens in the blood, new methods have been developed. Molecular detection techniques for bacterial and fungal DNA have been implemented but are not in widespread clinical use [15]. By shortening the time to pathogen identification and allowing for detection of organisms missed by blood culture, molecular methods may contribute to the reduction of hospitalization and ICU stay, as well as decreases in mortality [16–18]. In the present review, we focus on the microbiological techniques available for the diagnosis of sepsis. In addition to what has been reported in a series of elegant recent reviews [4, 15, 19–21], we included up-to-date new developments including mass spectrometry and array techniques. However, based on the large number of studies published in the field, this review focuses on those techniques that are commercially available and for which clinical studies have been performed and published.
Epidemiology of sepsis
The incidence of sepsis has recently been reviewed by Angus and van der Poll [2]. In the United States, 2% of patients admitted to the hospital have severe sepsis, and 10% of all ICU admissions are patients with severe sepsis [22, 23]. Currently, more than 750,000 cases are reported in the United States per year [23]. Severe sepsis occurs as a result of both community-acquired and healthcare-associated infections. Pneumonia, intraabdominal, and urinary tract infections are the most common causes of sepsis [23–26]. Staphylococcus aureus and Streptococcus pneumonia are the most common gram-positive isolates, whereas Escherichia coli, Klebsiella spp., and Pseudomonas aeruginosa predominate among Gram-negative isolates [27]. Between 1979 and 2000, Gram-positive infections were reported more frequently than Gram-negative infections [28]. More recently, Gram-negative bacteria were isolated in 62% of patients with severe sepsis who had positive cultures, whereas Gram-positive bacteria accounted for 47% and fungi for 19% of cases [26].
Key risk factors for severe sepsis are the patient’s predisposition for infection and the likelihood of acute organ dysfunction if infection develops. Chronic diseases (e.g., acquired immunodeficiency syndrome, chronic obstructive pulmonary disease, and many cancers) and the use of immunosuppressive agents are among the most important risk factors for those infections that result in severe sepsis and septic shock [23]. Age, sex, and race or ethnic group all influence the incidence of severe sepsis, which is higher in infants and elderly persons than in other age groups, higher in males than in females, and higher in blacks than in whites [23, 29]. Host genetic factors, i.e., polymorphisms in genes encoding cytokines and other mediators involved in innate immunity, coagulation, and fibrinolysis likely contribute to the incidence and outcome of sepsis [2].
Diagnosis of sepsis using blood culture
Blood culture and biochemical pathogen identification
Conventional techniques are based on culture of a blood sample in enriched broth, followed by identification and susceptibility testing of the pathogen using standard biochemical techniques. Blood cultures are crucial for fine-tuning of antibiotic therapy since inappropriate antimicrobial therapy is a key risk factor for mortality in critically ill patients with life-threatening infections [10, 15]. Significant advances have been made in laboratory blood culture systems over the last decades, including the additions of enriched growth media, advances in automated agitation systems, and development of software that allows faster detection of bacterial growth via improved algorithms designed to track growth curves. Despite these technological advances, obtaining blood cultures (BCs) before initiating anti-infective therapy and ensuring appropriate fill volumes of 20–40 mL of blood per venipuncture within a single blood culture order remain key factors in the detection of adult bacteremia [13, 30, 31].
Blood cultures are currently performed with fully automated instruments that detect microbial growth by the analysis of CO2 release using fluorescent or colorimetric sensors; alternatively, pressure changes in the bottle headspace due to the consumption and production of gases are used to indicate microbial growth [15]. However, despite these advances, the overall time to result of blood cultures is far too long to allow physicians to make immediate treatment decisions [32]. While sensitivity of blood culture is impacted by the interval from blood draw to loading of blood culture bottles into the instrument [15, 33, 34], a delay in time to result is caused by the need to grow pathogens in broth (typically taking 24 to 72 h) [35] and the performance of Gram stains followed by additional overnight growth to yield single colonies for identification and susceptibility testing. Furthermore, negative results can only be reported after 5–7 days. In addition, inhibitory effects of the presence of antibiotic drugs and/or fastidious pathogens are limiting the sensitivity of blood cultures [36, 37]. In contrast to previous studies, a recent comparison of two automated blood culture systems revealed significant differences in time to result, overall bacterial growth, and recovery from cultures where antimicrobials had been dosed up to 48 h before culture collection; of interest, each medium failed to isolate organisms even under ideal growth conditions in the absence of antimicrobials [31].
Clancy and Nguyen [38] recently reviewed the value of blood culture in comparison with nonculture tests including PCR for the diagnosis of invasive candidiasis; β-d-glucan and PCR were found superior to blood cultures in deep-seated candidiasis. The authors conclude that while positive predictive values of nonculture tests are limited by the low prevalence of invasive candidiasis, they can be used as “biomarkers” to assess a patient’s risk of having invasive candidiasis, thereby facilitating preemptive antifungal strategies. Furthermore, excellent negative predictive values will also be useful for ruling out invasive candidiasis and discontinuing unnecessary antifungal therapy.
Current guidelines recommend the collection of at least two blood cultures before initiation of antibiotic therapy with at least one drawn percutaneously and one drawn through each vascular access device, unless the device was recently inserted [39]. Blood volume is especially important for pediatric patients, for whom it is not always possible to draw a large volume of blood. A Gram stain is performed after a positive signal was provided by the automated instrument, followed by subculture for identification and antimicrobial susceptibility testing. Preliminary susceptibility results can be obtained directly from positive blood cultures within several hours rather than awaiting fully validated susceptibility testing results following growth and isolation of single colonies [40].
Despite considerable efforts to reduce their incidence, contamination of blood cultures continues to be a significant clinical problem. The rate of contamination is estimated to be close to 3% [41] with clinical and financial cost of more than $8,000 and an additional day of hospitalization [42]. Since excess contamination rates are driven primarily by a lack of operator fastidiousness during the collection process (e.g., driven by emergency department crowding [43]), dedicated phlebotomy teams, prepackaged blood culture kits, and the use of sterile gloves are successfully used to decrease contamination rates [44]. Of interest, a recent observational study revealed that multiple interventions, including new policies, reeducation of phlebotomists and intravenous teams, preparation of special supply kit for obtaining blood samples from catheters, greatly reduced the proportion of blood cultures obtained from central lines and markedly reduced the rate of contamination with marked savings in excess hospital costs [45].
Blood culture and molecular pathogen identification
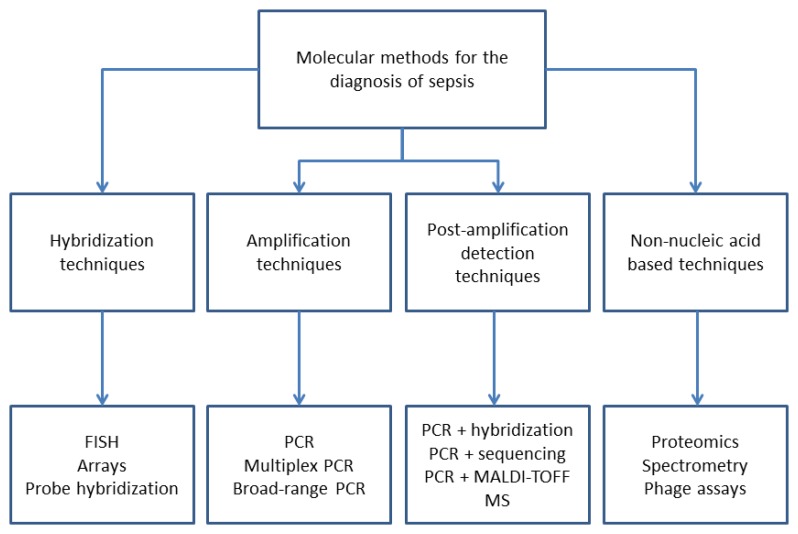
Speed is of pivotal importance for the diagnosis of sepsis. Therefore, a variety of molecular techniques has been developed for the detection of pathogens as summarized in Fig. 1. Table 1 provides an overview of commercially available molecular techniques. However, the majority of these techniques are applied to microorganisms following initial growth in blood culture bottles followed by single colony growth on solid media.
Fig. 1.
Diagnostic techniques for the diagnosis of sepsis (modified after [21])
Table 1.
Commercially available molecular assays for the diagnosis of sepsis using positive blood cultures or whole blood as sample type
| Diagnostic technique | No. of pathogens detected |
Sensitivity | Specificity | References | |
|---|---|---|---|---|---|
| A. Using positive blood cultures | |||||
| PNA-FISH, | Fluorescence-based hybridization | 10 | 94–99 | 99–100 | [15, 169] |
| ACCU-PROBE | Chemiluminescent DNA probes (rRNA) | 5 | 80.8–100 | 98.7–100 | [51 |
| HYPLEX | Multiplex PCR plus hybridization | 10 plus mec A | 96–100 | 92.5–100 | [15, 58] |
| PLEX-ID BAC | Chemiluminescent DNA probes (rRNA) | ||||
| Hyplex | Multiplex PCR plus hybridization | ||||
| PLEX-ID BAC | Broad-range PCR plus electrospray ionisation mass spectrometry |
>300 different pathogens |
95 | 98.8 | [19, 59, 60] |
| StaphPlex | Multiplex PCR plus microarray | 1 | 100 | 95.5–100 | [61] |
| Staph SR | Multiplex PCR assay | 1 plus mecA | 50–100 | 86.8–98.4 | [62, 63] |
| MALDI-TOF | Matrix-assisted laser desorption/ ionization time-of-flight mass spectrometry |
Hundreds | 76–80 | 96–100 | [20, 75] |
| Prove-it sepsis | Multiplex PCR and microarray | 50 plus mec A gene | |||
| Verigene | Nucleic-acid-based microarray | Gram-positive or -negative bacteria, resistance genes |
92–96 | n.d. | [86–88] |
| Filmarray | PCR | Gram-positive or Gram-negative bacteria, resistance genes | 91 | n.d. | [85] |
| B. Using whole blood | |||||
| Xpert MRSA/SA | Real-time PCR | 2 | 75–100 | 98.4–99.4 | [64, 170] |
| SeptiFast | Multiplex real time PCR assay for bacterial and fungal pathogens | 25, plus mecA as reflex test |
60–95 | 74–99 | See Table 2 |
| VYOO | Multiplex PCR with gel electrophoresis | 34plus mec A, vanA/B/C, SHV, CTX-M | 30–51 | n.d. | [85] |
| SepsiTest | Broad-range PCR with sequencing | >300 pathogens | 61–88.5 | 83.5–85.8 | [57, 91, 92] |
| *Concordance with blood
culture-dependent assays; n.d., not determined | |||||
The main disadvantages of analyzing specimens after they have grown in culture are the delay in time and the potential bias introduced by culture steps. Furthermore, uncultivable organisms cannot be identified by these techniques. Nevertheless, the use of molecular methods to identify pathogens following blood culture can be faster than the standard techniques involving phenotypic identification and antimicrobial susceptibility testing requiring up to 72 h after the blood culture became positive.
Hybridization
Fluorescent in situ hybridization (FISH) is among the most studied commercial techniques suitable for the detection of pathogens in positive blood cultures. In only 2.5–3 h, FISH may identify more than 95% of bacteria and yeasts commonly found in blood [46–48]. Slides of positive blood cultures are prepared, hybridized with fluorochrome-labeled oligonucleotide probes targeted to rRNA, and visualized microscopically [49]. Of note, some bacteria may be identified only at the genus level because no species-specific probes are available. A new fluorescence-based hybridization method using peptide nucleic acid probes (AdvanDx, USA) targeting uses 16S rRNA for the direct identification of S. aureus from positive blood cultures within 3 h [50–52], the test received FDA clearance. This technique was extended to allow identification of other bacterial and fungal pathogens from positive blood cultures [52]. The sensitivity and specificity for the different kits was reported to be 99% and 100%, respectively [15].
S. aureus, S. pneumoniae, enterococci, and groups A and B streptococci can be identified in about 2.5 h using a commercially available DNA probe kit (AccuProbe, Gen-Probe Inc., San Diego, CA, USA) that utilizes hybridization protection assay technology [53]. The sensitivity and specificity was reported to be high for most pathogens using bacteria grown on solid or broth media. The specificity for the detection of S. aureus was high (99.8%), while the sensitivity was low (72.4%) in one report; the sensitivity for detection of S. aureus was improved using adjusted cut-off values [54].
DNA amplification
PCR is the most commonly applied technique for the detection of pathogens from positive blood cultures. Commercially available tests use either broad-range PCR or multiplex PCR.
Broad-range amplification
Broad-range assays use primers that recognize conserved sequences of bacterial/fungal chromosomal genes encoding ribosomal DNA. The clinical usefulness of these methods is, however, limited because after the PCR amplification of a target sequence, further identification procedures are necessary. Various alternatives such as sequencing and polymorphism analysis [55, 56] or subsequent genus- or species-specific real-time PCR [57] have been developed.
Hyplex Blood Screen (BAG, Germany) is a multiplex PCR assay with subsequent identification of bacterial species (methicillin-sensitive S. aureus, methicillin-resistant S. aureus, S. epidermidis, Streptococcus pyogenes, S. pneumoniae, E. faecalis, and Enterococcus faecium, E. coli, Enterobacter aerogenes, P. aeruginosa, and Klebsiella spp.) from positive blood cultures using hybridization in an ELISA-like format. The turnaround time is 3–4 h, and the assay also allows the detection of drug resistance markers, such as van genes and several β-lactamase genes. The reported sensitivity and specificity ranged from 96.6% to 100% and 92.5% to 100%, respectively [58].
Abbott/Ibis (USA) recently combined the broad-range PCR amplification with electrospray ionization/mass spectroscopy (PCR/ESI–MS). This technique (PLEX-ID BAC Spectrum) uses primers which target genomic regions highly conserved among bacteria or fungi. Multiple pairs of primers are used to amplify selected regions of bacterial or fungal genomes after culture; the primer target sites are broadly conserved, but the amplified region carries information on the microbe’s identity in its nucleotide base composition. Following PCR amplification, a fully automated ESI–MS analysis is performed on the PCR/ESI MS instrument. The PCR/ESI–MS instrument identifies the organisms present in a clinical sample and can provide additional information including strain type, antimicrobial resistance, and virulence factors [19]. The turnaround time to obtain results is approximately 5–6 h after positive blood culture. Concordance between results obtained with PCR/ESI-MS and blood culture was 98.7% at the genus and 96.6% species levels, respectively [59, 60].
Broad spectrum amplification technologies have the potential to revolutionize diagnostics since they allow clinical laboratories to query single samples for hundreds of organisms simultaneously, freeing them from the need to restrict their focus on a few most likely etiologies. The same is true for sequencing and mass spectrometry as discussed below.
Multiplex amplification
Multiplex assays target different genes of the most frequent pathogens involved in sepsis. Amplicons may be subsequently analyzed by electrophoresis, hybridization on an enzyme-linked immunosorbent assay, or multiplex real-time PCR.
The following multiplex PCR assays were designed to detect only one pathogen and its genetic properties, such as the presence of genes encoding antibiotic resistance:
The StaphPlex system (Qiagen, USA) detects S. aureus using a unique target-enriched multiplex PCR method. This assay is designed for simultaneous detection and species-level identification of Panton–Valentine leukocidin (PVL) and several antimicrobial resistance determinants of staphylococci directly from blood culture in which Gram-positive cocci in clusters have been detected by Gram staining. The system amplifies and detects 18 Staphylococcus-specific genes simultaneously in one reaction. Drug resistance makers include mecA, aacA, ermA and ermC, tetM and tetK. The entire process, from blood culture to results, can be completed in approximately 5 h, which significantly reduces the time needed for phenotypic identification and antimicrobial susceptibility. The StaphPlex system demonstrated 100% sensitivity and specificity, ranging from 95.5% to 100.0% when used for staphylococcal cassette chromosome mec typing and PVL detection [61].
The StaphSR assay (BD GeneOhm, San Diego, CA, USA) can differentiate meticillin-susceptible S. aureus (MSSA) from meticillin-resistant S. aureus (MRSA). This assay is a multiplex real-time PCR test performed on the SmartCycler instrument with a turnaround time of 2.5 h. Initially, excellent performance characteristics were reported (sensitivity for MSSA and MRSA of 98.9% and 100%, respectively) [62] while later studies noted limitations [63].
The Xpert MRSA/SA blood culture assay (Cepheid, Sunnyvale, CA, USA) identifies S. aureus and differentiates MSSA from MRSA. This system detects sequences in the staphylococcal protein A (spa) gene, the SCCmec inserted into the S. aureus chromosomal at B insertion site, and the mecA gene. Sensitivity and specificity for S. aureus detection were 100% and 98.6%, respectively, and for MRSA detection were 98.3% and 99.4%, respectively [64]. This assay has a rapid turnaround time of approximately 60 min.
Sequencing
Several sequence-based approaches have been successfully used to identify bacteria directly from positive blood culture bottles. Qian and colleagues successfully used the MicroSeq 500 kit (Perkin-Elmer Applied Biosystems, CA), a method that sequences the first 527 bases of the amplified 16S rRNA gene, for this purpose [55]. Turenne and collaborators used single-stranded conformation polymorphism analysis of PCR amplicons to distinguish between organisms [56]. Several investigators have used pyrosequencing (Biotage, Sweden) to identify numerous bacteria, yeasts, and fungi [65, 66]. Pyrosequencing can be performed in 96-well microtitre plates in a few hours directly from bacterial colonies with a single PCR for each isolate. The main advantage of pyrosequencing is its relative rapidity and lower price compared to conventional sequencing. Pyrosequencing (Biotage, Sweden) provides rapid, short-read sequencing of 30 bases in approximately 30 min. This method has been used to classify, identify, and subtype a variety of bacterial 16S rDNA fragments [67].
Jordan et al. [68] compared the results to those obtained by culture-based identification and identified two different regions within the 23S rRNA gene that greatly improved the ability to differentiate among certain enteric Gram-negative rods associated with bloodstream infections or Streptococcus species compared to the universal 16S rRNA gene target previously described. The overall agreement between pyrosequencing and culture based identification was high (97.8%). In blood culture bottles with a single organism isolated, concordance was even higher (98.8%).
A rapid protocol for the identification of Candida species (C. albicans, C. parapsilosis, C. tropicalis, C. glabrata, C. dubliniensis, C. krusei and A. niger) from positive blood cultures was developed by combining a simple method for nucleic acid extraction and preparation using microbial storage cardboards with PCR and pyrosequencing of a small region of the 18S rRNA gene [69]. The method was completed in 4 h and tested against a collection of clinical blood cultures. Agreement of sequence identifications with standard microbiological methods was excellent. Recently, Motoshima and colleagues [70] evaluated a rapid protocol for bacterial identification based on PCR and pyrosequencing of the V1 and V3 regions of the 16S rRNA gene using DNA extracted directly from positive blood culture. The bacteria were identified by phenotyping and pyrosequencing. The results displayed 84.3% and 64.7% concordance with the results of phenotypic identification at the genus and species levels, respectively. In monomicrobial samples, the concordance between the results of pyrosequencing and phenotypic identification at the genus level was 87%. The process of pyrosequencing identification was completed within 4 h.
However, the use of multiplex PCR or broad-range amplification followed by sequence analysis of microorganisms after growth in conventional blood culture does provide the necessary clinical benefit within a few hours; the costs compared to conventional identification techniques are high. Studies using direct detection of pathogen DNA from blood have been confounded by the presence of pathogen DNA contamination introduced at the time of specimen collection and/or preparation [71].
Mass spectrometry
Matrix-assisted laser desorption/ionization (MALDI) time-of-flight mass spectrometry (TOF MS) has been used successfully for routine identification of bacterial colonies and for the direct identification of bacteria in positive blood cultures [72–74]; the current status has recently been reviewed by La Scola et al. [75]. It has also been used to detect a limited number of antimicrobial resistance genes. Among the advantages of this technique are the rapid response provided and the minimal amount of labor compared with traditional methods. Three complete systems (mass spectrometer, analysis software and spectra database) are available for clinical microbiology applications: MALDI BioTyper™ (BrukerDaltonics), AXIMA@SARAMIS™ (Shimadzu and Anagnostec) and MALDI micro MX™ (Waters Corporation). Seng et al. [72] reported 95.4% success in post-culture bacterial identification by MALDI-TOF MS; 84.1% of pathogens were identified at the species level and 11.3% were identified at the genus level. Currently, MALDI-TOFF MS still requires culture of microorganisms, and drug resistance must still be determined by conventional methods. Identifying mixed populations of bacteria seems to be difficult owing to dynamic range issues in the mass spectrometer [74].
The Bruker and Shimadzu systems were recently compared [75, 76] using 16s rRNA gene sequencing as the gold standard on a large number of clinical isolates. 94.4% of organisms were identified with the Bruker compared to 88.8% with the Shimadzu spectrometer. Both systems showed a >99% agreement with conventional and 16S identifications with anaerobes and streptococcal species remaining most frequently unidentified. Eigner et al. [77] reported correct identifications to the species level in 80% to 100% of cases. Furthermore, the usefulness of MALDI-TOF MS for the identification of yeast and yeast-like fungi was demonstrated [78, 79]. Recently, Clerc et al. [80] reported that MALDI-TOFF MS performed on pellets from positive blood cultures resulted in modification of the treatment regimen in 13.4% of adult and 2.5% of pediatric patients. Integrating rapid organism identification with matrix-assisted laser desorption/ionization time-of-flight and real-time review and intervention by an antimicrobial stewardship team was associated with decreased mortality, decreased length of ICU stay, improved time to effective and optimal antibiotic therapy, and decreased recurrent bacteremia [81]; thus, while still requiring positive blood cultures and Gram stain, organism identification by MALDI-TOF combined with antimicrobial stewardship improved the management of patients with sepsis.
Microarray
Prove-it sepsis
Prove-it Sepsis (Mobidiag, Finland) is a broad-range PCR test using positive blood cultures. This assay is a novel PCR and microarray method that is based on amplification and detection of gyrB, parE, and mecAgenes of 50 bacterial species [82]. The method allows identification of a large panel of bacterial pathogens covering around 90% of the agents commonly involved in the aetiology of sepsis. The assay is also able to determine the presence of the mecA gene. The assay had a sensitivity of 94.7% and a specificity of 98.8%, and was 18 h faster than conventional blood culture [82]. Recently, identification of clinically relevant yeasts was reported with a sensitivity of 99% and a specificity of 98% [83]. Unfortunately, microbiological results were not compared to clinical information thus far. Spiking whole blood allowed for correct identification of bacterial species with detection limits of 11–600 colony-forming unit/mL (CFU/mL) [84].
Film array blood culture identification
The Film Array Blood Culture Identification Panel detects (Biofire, US) six Gram-positive pathogens, 10 Gram-negative pathogens, and five Candida spp. associated with bloodstream infections. In addition, the test detects the antibiotic resistance genes mecA, VanA/B, and KPC. Following positive blood cultures, a result can be obtained in 1 h with 2 min of hands-on time [85]. Compared to traditional blood culture-based identification, a pathogen was identified in 91.6% of samples with monomicrobial growth; 7.8% of the undetected pathogens were not covered by the FilmArray panel. In 3.6% of samples, the FilmArray detected an additional pathogen compared to blood culture. Future studies will have to further evaluate the performance of the assay.
Verigene
The Verigene Gram-Positive Blood Culture and Gram-Negative nucleic acid assays (Nanosphere, US) are microarray-based and performed on the Verigene system for detection of a variety of Gram-positive and Gram-negative microorganisms and associated resistance markers in positive aerobic blood culture bottles. The assays take approximately 2.5 h to complete [86]. The Gram-positive assay has been evaluated using positive blood cultures drawn from adult and pediatric populations showing high sensitivity and specificity compared to routine microbiological methods [87, 88].
Diagnosis of sepsis independent of blood culture
Molecular techniques applied directly on whole blood samples are the best choice for rapid identification of a microorganism in the blood. The main advantages of PCR detection directly from the blood are the increased sensitivity and the avoidance of time-consuming culture, resulting in substantial reduction in turnaround time even compared with PCR identification from positive blood cultures. These amplification techniques include broad-range and multiplex PCR. Currently, a number of these tests have the CE label, but none of these assays is approved by the U.S. Food and Drug Administration (FDA).
Broad-range nucleic acid amplification
Broad-range PCR assays have been implemented for the detection of bacteria or fungi in blood based on the 16S or 23S rRNA gene of bacteria and the 18S rRNA gene of fungi. After amplification, the amplicons can be identified by different methods such as capillary sequencing analysis, pyrosequencing, or hybridization with specific probes [89].
Multiplex nucleic acid amplification
The multiplex real-time PCR assays allow the rapid identification of pathogens directly from blood. Multiplex PCR involves amplifying multiple targets of DNA in the same sample at the same time using a mix of primers. This technique is often based on amplification of the internal transcribed spacer region of the microorganisms. This non-coding region of the ribosomal DNA is localized among highly conserved genes, shows a high level of heterogeneity among bacterial and fungal genera and species and allows a high level of identification using a limited pool of slightly degenerated primers [15, 90]. While PCR-based techniques allow more rapid and sensitive detection of pathogens compared with conventional blood culture, the climate of opinion is that currently PCR can supplement, but not replace, blood culture. In numerous studies, combined detection rate of both methods was significantly higher compared with PCR or blood culture alone. Also, complete determination of antibiotic resistance can currently not be performed exclusively by PCR due to limited multiplexing capabilities.
SepsiTest
SepsiTest (Molzym, Germany) is a PCR-based detection and sequence identification system for organisms causing sepsis. Using 1 mL of blood, the presence of bacteremia or fungemia can be detected within 4 h via broad-range PCR for 16S and 18S rRNA genes. In positive cases, sequence analysis of the amplicon is performed for identification of more than 300 bacteria and fungi in 8–12 h. The diagnostic sensitivity and specificity of this test were 87.0% and 85.8% when compared to blood culture [91]. Grif and collaborators [92] have recently confirmed the diagnostic sensitivity and specificity (88.5% and 83.5%).
Magicplex
The Magicplex Sepsis Real-time test (Seegene, Korea) screens for more than 90 pathogens (73 Gram-positive, 12 Gram-negative, and 6 fungi) as well as three drug resistance markers (mecA, vanA and vanB) using whole blood samples. After an initial screening step for 3 h identification of pathogens takes an additional 30 min. No reports on the validation of analytical and/or clinical performance have been published thus far.
VYOO
The Multiplex PCR system VYOO (SIRSLab, Germany) combines culture-independent pathogen-derived nucleic acid concentration and multiplex PCR-based species detection. The multiplex PCR detects 34 bacterial and 7 fungal species and five most common resistance markers (mecA, vanA, vanB, blaSHV, blaCTX-M) [93]. The system allows the selective removal of human DNA and exploits the methylation differences between bacterial/fungal DNA and human DNA to enrich the clinical sample with pathogen DNA by affinity chromatography. Following amplification, products are run on an agarose gel for evaluation of the pathogen-specific electrophoretic pattern. Approximately 90% of human DNA is removed, signal loss on amplification caused by human DNA is significantly decreased, with sensitivity elevated at least 10-fold compared with samples not subjected to pathogen DNA enrichment. In an observational study performed by Bloos and colleagues, 311 concomitant blood cultures and blood for VYOO were obtained from 245 patients with suspected sepsis; 14.5% of blood cultures and 30.1% of PCRs were positive. Thus, VYOO results were available faster, were more frequently positive, and may result in earlier adjustment of antimicrobial therapy [94]. In a recent study, Fitting and colleagues determined that 70% of infected patients with positive blood cultures also gave a positive result using the VYOO technique, with improvements still needed [93]. The overall turnaround time was approximately 8 h. Results of a larger multicenter trial have not become available yet.
SeptiFast
The LightCycler® SeptiFast test (Roche Molecular Systems, Germany) is a multiplex PCR assay designed to detect 25 microorganisms that cause approximately 90% of all bloodstream infections directly from blood. SeptiFast uses real-time PCR in a non-quantitative mode to identify ten bacteria at the species level, several more at the genus level, as well as five Candida spp. and Aspergillus fumigatus. These organisms are thought to be responsible for more than 90% of all the cases of bloodstream infection [95]. 1.5 mL of whole blood was manually extracted, followed by three separate PCR reactions (one each for Gram-positives, Gram-negatives, and fungi) using fluorescent probes and melting curve analysis based on a dedicated software to identify the pathogens [95]. Internal and reagent controls are provided by the assay to control for inhibition and reaction efficiency. The overall time to result is around 5 h. Recently, a semi-automated DNA extraction protocol has been described using the MagNAPure instrument (Roche Molecular Systems), shortening the overall time to result to 3.5–4 h without a loss in accuracy [96].
There is a wealth of more than 60 publications on the clinical evaluation of SeptiFast in different patient populations (Table 2). The technical performance results were initially described by Lehmann et al. [95]. Subsequently, the test has then been evaluated mainly in adult ICU patients in multicenter studies [94, 97–99] for CE registration of SeptiFast. In these studies enrolling patients with suspected sepsis, severe sepsis, and septic shock, the positivity rate for SeptiFast was significantly higher than that of blood culture; positivity rate for SeptiFast ranged from 25% to 35%, whereas that of traditional blood culture ranged between 13% and 21%. Single-center studies in ICU patients mirrored these findings [100–102]. The key studies reported an increased sensitivity compared to blood culture (Tables 1 and 2). Patients with positive SeptiFast results also showed increased inflammatory markers including procalcitonin and IL-6 [103–105] (E. Tsalik, unpublished data), as well as increased APACHE II scores [104].
Table 2.
Study design and patient cohort/ward in published studies using the SeptiFast test
| Title of publication | Study design | Patient cohort/ward | Reference |
|---|---|---|---|
| New diagnostic tools for neonatal sepsis: The role of a real-time polymerase chain reaction for the early detection and identification of bacterial and fungal species in blood samples | Review | Oncohematological malignancies, and other febrile states |
[142] |
| Molecular diagnosis of sepsis in neutropenic patients with hematological malignancies | Prospective | Hematological malignancies | [106] |
| Multiplex polymerase chain reaction detection enhancement of bacteremia and fungemia | Retrospective | IR and ICU Sepsis | [95, 100] |
| A multiplex real-time PCR assay for rapid detection and differentiation of 25 bacterial and fungal pathogens from whole blood samples | Non clinical performance and observational data | N/A | [95] |
| Automatic detection of bacterial and fungal infections in blood | Retrospective | Sepsis patients | [171] |
| LightCycler SeptiFast assay as a tool for the rapid diagnosis of sepsis in patients during antimicrobial therapy | Prospective | ICU and febrile BMT recipients | [143] |
| Molecular diagnosis of polymicrobial sepsis | Letter to Editor | Sepsis patients | [110] |
| Blood culture systems: rapid detection – how and why? | Review | N/A | [172] |
| Laboratory diagnosis of late-onset sepsis in newborns by multiplex real-time PCR | Correspondence | Neonates | [90] |
| Invasive aspergillosis in two liver transplant recipients: diagnosis by SeptiFast | Case report | Liver Transplant recipients | [111] |
| Diagnosis of bloodstream infections in immunocompromised patients by real-time PCR | Interventional study | Cancer | [16] |
| Microbiological sepsis screening in surgical ICU patients with the “Lightcycler” Septifast Test – A pilot study | Pilot study | Surgical ICU | [173] |
| Evaluation of the LightCycler® SeptiFast test in the rapid etiologic diagnosis of infectious endocarditis | Prospective | Definite endocarditis | [122] |
| Multiplex real time PCR and blood culture for ID of blood stream pathogens in patients with suspected sepsis | Observational | ICU Sepsis | [97] |
| Utility of a commercially available multiplex real-time PCR assay to detect bacterial and fungal pathogens in febrile neutropenia | Retrospective | Febrile neutropenia after chemotherapy | [105] |
| Clinical impact of a commercially available multiplex PCR system for rapid detection of pathogens in patients with presumed sepsis | Retrospective | Presumed sepsis | [102] |
| Improved detection of blood stream pathogens by real-time PCR in severe sepsis | Observational | ICU Sepsis | [145] |
| A multicentre trial to compare blood culture with polymerase chain reaction in severe human sepsis | Observational | ICU Sepsis | [103] |
| Potential clinical utility of polymerase chain reaction in microbiological testing for sepsis | Observational | ICU Sepsis | [98] |
| Use of the LightCycler SeptiFast Test for rapid etiologic diagnosis of nosocomial infection in gynecological sepsis | Case reports | Gynecological sepsis | [174] |
| Evaluation of pathogen detection from clinical samples by real-time polymerase chain reaction using a sepsis pathogen DNA detection kit | Prospective | SIRS | [146] |
| Preliminary clinical study using a multiplex real-time PCR test for detection of bacterial 4 and fungal DNA directly in blood | Prospective; observational | ICU Sepsis | [112] |
| Molecular identification of bloodstream pathogens in patients presenting to the emergency department with suspected sepsis | Retrospective | Suspected sepsis in the ER | [175] |
| Evaluación de una PCR multiplex en tiempo real para la detección de patógenos en el tejidovalvular de pacientes con endocarditis | Prospective | Valve tissues | [126] |
| Multiplex PCR allows rapid and accurate diagnosis of bloodstream infections in newborns and children with suspected sepsis | Retrospective | Neonates and children | [119] |
| Therapeutic impact and diagnostic performance of multiplex PCR in patients with malignancies and suspected sepsis | Prospective | Cancer and sepsis | [176] |
| Cost and mortality prediction using polymerase chain reaction pathogen detection in sepsis: evidence from three observational trials | Retrospective analysis/ mathematical prediction | N/A | [155] |
| Multiplex blood PCR in combination with blood cultures for improvement of the microbiological documentation of infection in febrile neutropenia | Prospective, observational | Hematological malignancies | [107] |
| Automated extraction improves multiplex molecular detection of infection in septic patients | Analytical method comparison | N/A | [96] |
| Is detection of bacterial DNA in ascitic fluid of clinical relevance? | Prospective | Cirrhosis and ascites samples | [125] |
| Rapid qualitative urinary tract infection pathogen identification by SeptiFast real-time PCR | Retrospective | UTI | [124] |
| Molecular biological sepsis diagnostic using multiplex PCR in surgical intensive care as suitable alternative to conventional microbial culture – a representative overview | Retrospective | Surgical ICU | [177] |
| Diagnostic accuracy and potential clinical value of the LightCycler SeptiFast assay in the management of bloodstream infections occurring in neutropenic and critically ill patients | Retrospective | Neutropenia, ICU | [178] |
| Establishment of a semi-automated pathogen DNA isolation from whole blood and comparison with commercially available kits | Analytical | N/A | [131] |
| The clinical diagnostic accuracy of rapid detection of healthcare-associated bloodstream infection in intensive care using multipathogen real-time PCR technology | Prospective | HAI-associated bloodstream infection | [179] |
| Multiplex polymerase chain reaction pathogen detection in patients with suspected septicemia after trauma, emergency, and burn surgery | Prospective | ICU, ED, burn | [116, 180] |
| [The first experience of application of PCR techniques in real-time mode to diagnose bacteriemia during postoperational period in cardiosurgery patients] | Retrospective | Post-surgery | [181] |
| Performance of the LightCycler SeptiFast test Mgrade in detecting microbial pathogens in purulent fluids | Retrospective | Pyogenic infections | [182] |
| Usefulness of real-time PCR for the diagnosis of sepsis in ICU-acquired infections | Review | ICU | [101] |
| Molecular diagnosis of Aspergillus fumigatus endocarditis | Case report | CLL | [183] |
| Evaluation of a commercial multiplex PCR test (SeptiFast) in the etiological diagnosis of community-onset bloodstream infections | Retrospective | Community-onset sepsis | [5] |
| [Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry vs conventional methods in the identification of Candida non-albicans] | Analytical | Candida isolates | [184] |
| Multiplex PCR pathogen detection in two severely burned patients with suspected septicemia | Case reports | Burn | [116] |
| Comparison of conventional culture with SeptiFast real-time PCR for microbial pathogen detection in clinical specimens other than blood | Retrospective | Antimicrobial therapy | [185] |
| Procalcitonin predicts real-time PCR results in blood samples from patients with suspected sepsis | Retrospective | Suspected sepsis | [186] |
| Results and relevance of molecular detection of pathogens by SeptiFast – A retrospective analysis in 75 critically ill children | Retrospective | Pediatric ICU | [113]. |
| Accuracy of LightCycler® SeptiFast for the detection and identification of pathogens in the blood of patients with suspected sepsis: a systematic review protocol | Review | Suspected sepsis | [187] |
| LightCycler SeptiFast technology in patients with solid malignancies: clinical utility for rapid etiologic diagnosis of sepsis | Retrospective | Solid malignancies, ICU | [188] |
| PCR-based rapid sepsis diagnosis effectively guides clinical treatment in patients with new onset of SIRS | Retrospective | Abdominal sepsis | [144] |
| Rapid detection of bloodstream pathogens by real-time PCR in patients with sepsis | Retrospective | Sepsis | [92] |
| Diagnostic performance of multiple real-time polymerase chain reaction assay in patients with suspected sepsis hospitalized in an internal medicine ward | Prospective | Suspected sepsis | [189] |
| Diagnosis, management and outcome of Candida endocarditis | Prospective | Candida endocarditis | [123] |
| Bacterial lung sepsis in patients with febrile neutropenia | Review | Febrile neutropenia | [190, 191] |
| Molecular approaches in the diagnosis of sepsis in neutropenic patients with hematological malignances | Prospective | Neutropenia | [108] |
| Rapid detection of bloodstream pathogens by real-time PCR in patients with sepsis | Retrospective | Sepsis | [92] |
| Diagnostic performance of multiple real-time polymerase chain reaction assay in patients with suspected sepsis hospitalized in an internal medicine ward | Prospective | Suspected sepsis | [189] |
| Diagnosis, management and outcome of Candida endocarditis | Prospective | Candida endocarditis | [123] |
| Bacterial lung sepsis in patients with febrile neutropenia | Review | Febrile neutropenia | [190, 191] |
| Molecular approaches in the diagnosis of sepsis in neutropenic patients with hematological malignances | Prospective | Neutropenia | [108] |
| Diagnosis of infective endocarditis: comparison of the LightCycler SeptiFast real-time PCR with blood culture | Retrospective | Endocarditis | [191] |
| Multiplex PCR for rapid and improved diagnosis of bloodstream infections in liver transplant recipients | Retrospective | Liver transplant | [117] |
| Invasive candidiasis: a review of nonculture-based laboratory diagnostic methods | Review | Candidiasis | [192] |
| Diagnostic utility of LightCycler SeptiFast and procalcitonin assays in the diagnosis of bloodstream infection in immunocompromised patients | Retrospective | Immunosuppression | [193] |
| Cost analysis of real-time polymerase chain reaction microbiological diagnosis in patients with septic shock | Cost-minimization study | Septic shock | [17] |
| Evaluation of the LightCycler SeptiFast test in newborns and infants with clinical suspicion of sepsis | Retrospective | Neonatal ICU | [120] |
| Microbial diagnosis in patients with presumed severe infection in the emergency department | Retrospective | Emergency room | [104] |
| Routine use of a real-time polymerase chain reaction method for detection of bloodstream infections in neutropenic patients | Retrospective | Neutropenics | [194] |
| Comparison of two molecular assays with conventional blood culture for diagnosis of sepsis | Retrospective | Critical illness | [127] |
| Molecular detection of late-onset neonatal sepsis in
premature infants using small blood volumes: Proof-of-concept |
Experimental | Neonates | [121] |
| Comparison of three different commercial PCR assays for the detection of pathogens in critically ill sepsis patients | Prospective, observational | Sepsis | [128] |
| The value of combining blood culture and SeptiFast data for predicting complicated bloodstream infections caused by gram-positive bacteria or candida species | Prospective | Sepsis | [195] |
| Use of a multiplex polymerase chain reaction system for enhanced bloodstream pathogen detection in thoracic transplantation | Observational | Thoracic allograft recipients (heart and lung) | [118] |
| Multiplex PCR system for rapid detection of pathogens in patients with presumed sepsis – a systemic review and meta-analysis | Systemic review/meta-analysis | Patients with presumed sepsis | [99] |
| Use of SeptiFast for the detection of bacterial meningitis | Retrospective | Suspected meningitis (cerebrospinal fluid ) | Infection, in press |
To answer the question whether microbial DNA in blood correlates with bloodstream infection, Tsalik et al. [35] investigated the presence of microbial DNA in more than 300 patients admitted to the emergency department with suspected sepsis using the SeptiFast in addition to routine care including blood culture. The results convincingly showed that SeptiFast-positive results indicated the presence of infection but were not caused by circulation of bacterial DNA in patients free of infections (i.e., presence of bacteria due to contamination with skin flora).
The value of SeptiFast was also investigated in other patient populations. In patients with febrile neutropenia, increased sensitivity of SeptiFast compared to traditional blood culture was reported in several studies [105–108]. In an early study, Mancini et al. [106] found a positivity rate of 20.4% vs. 33% using blood culture and PCR, respectively. Lilienfeld-Toal et al. [105] reported a 3% positivity for blood cultures during antibiotic therapy compared to 15% using SeptiFast. In six cases, SeptiFast detected a new pathogen repetitively accompanied by a significant rise in procalcitonin levels, suggestive of a true detection of infection. All patients with probable invasive fungal infection had a positive SeptiFast result for A. fumigatus. Lamoth et al. [107] studied 141 febrile neutropenic episodes in 86 hematological patients characterized in 31% of cases as microbiologically documented compared to 35% clinically documented infections and 34% unexplained fevers. The numbers of microorganisms detected by SeptiFast vs. blood culture were similar at the onset of fever (12 microorganisms were detected by blood culture and SeptiFast, 32 by blood culture only, and 34 by SeptiFast only); however, in episodes of persistent fever, SeptiFast detected 28 new microorganisms (7 Gram-positives, 15 Gram-negatives, and 6 fungi [89% with a clinically documented site of infection]), whereas blood culture detected only four pathogens (P = 0.001). While blood culture did not detect fungi, SeptiFast identified five Candida spp. and one Aspergillus spp. in 5/7 probable or possible cases of invasive fungal infections. All studies concluded that the use of SeptiFast in combination with blood cultures improves the diagnosis of bloodstream infections in febrile neutropenia, especially when fever persists and invasive fungal infections are suspected. Idelevich et al. [109] observed that the average time between blood sampling and communication of SeptiFast result was 19 h, while the time from blood sampling to communication of Gram stain result from positive blood cultures was 32 h, and communication of preliminary identification and susceptibility from blood culture isolates took an average of 58 h. The same authors suggested that the automated cut-off for coagulase-negative staphylococci applied by the SeptiFast software (to eliminate detection of potential contaminants) should be replaced by manual readings in neutropenic patients (E. Idelevich, personal communication).
Since the markedly increased sensitivity for the detection of fungal pathogens was of particular interest, Ruhnke et al. (manuscript in preparation) monitored patients undergoing allogeneic bone marrow transplantation (BMT) twice weekly and when fever developed for the presence of bacterial and/or fungal pathogens from admission until discharge from the BMT unit. The vast majority of Gram-negative pathogens and all fungi (mostly A. fumigatus) were exclusively detected by SeptiFast. Based on these results, it is tempting to speculate that SeptiFast may be of great value as an early indicator of bloodstream infection in patients undergoing bone marrow transplantation.
A limitation for the successful diagnostic use in neutropenic patients may be the software-algorithm that increases the cut-off values for coagulase-negative staphylococci and streptococci to allow differentiation between pathogens and contaminants from the skin in ICU patients (Package insert, SeptiFast test).
The vast majority of studies using the SeptiFast test as described above used non-interventional trial designs (retrospective analysis or descriptive non-interventional). Therefore, one can only speculate about the clinical benefit of Septifast. A number of case reports showed the value of interventional use of the SeptiFast test, particularly in polymicrobial infections [90, 110] and infections in immunocompromised hosts [90, 111]. A small number of studies [102, 112] reported a potential for therapy adjustments using SeptiFast results in between 5% and 8% of ICU patients and in up to 13% of pediatric patients [113]. Furthermore, a recent randomized controlled clinical trial investigated the value of conventional blood culture vs. Septifast in patients with pulmonary or abdominal sepsis on six postoperative ICUs in Germany [114]. Block randomization was used to allocate 37 patients into the control and 41 into the intervention group in which SeptiFast results were provided. In 24.4% patients, Septifast detected at least one pathogen (mean duration from blood draw to information of ICU was 15 h). In contrast, blood culture results were communicated only after 29 h (p < 0.05). In 40% of patients with positive Septifast results, therapy was modified (two invasive mycoses, one P. aeruginosa, one S. aureus) after 18 h (26 h earlier than that in controls (p = 0.040). Thus, Septifast achieved a significant reduction in time to adaptation of therapy, especially beneficial in patients with invasive mycoses.
Most importantly, the French government funded the EVAMICA study, a prospective randomized multicenter study with two consecutive 6-month periods during which – in addition to routine blood culture – the SeptiFast test was performed or not performed at the onset of severe infections, namely, severe sepsis, first episode of febrile neutropenia, or suspicion of endocarditis have been presented [115]. Primary and secondary outcomes include the number of patients with microbial detection in blood, number of patients with adequate treatment, mortality at day 30, and the occurrence of complications. The intention-to-treat analysis for the primary outcome of microbial positivity in the blood has been presented for 1416 patients in 18 hospitals. Overall, microbes were detected in blood by blood culture and/or SeptiFast in 286 (39.1%) patients in the period when SeptiFast was used, and in 194 (28.4%) patients in the study period when SeptiFast was not used (p value <0.001). The higher microbial detection in blood during the SF period was observed in cases of severe sepsis (198/465 (42.6%) patients in the SeptiFast period vs. 125/442 (28.3%) in the nonSeptiFast period (p value < 0.001)), but not in neutropenic patients; there were more endocarditis patients with pathogens detected in the SeptiFast period (18/49, 36.7%) than in the nonSeptiFast period (1/20, 5%) (p value of 0.007). Patient characteristics were overall similar between the two periods. Multivariate analysis revealed that the SeptiFast period was associated with a significant increase in the rate of pathogen detection (OR=1.83, IC95% 1.32–2.53, p < 0.001). Additional analyses including health economic benefit, etc. are currently under investigation by the study coordinators.
Patients with burns [116], liver [111, 117], and thoracic transplantation [118] as well as children and neonates [113, 119, 120] have also been investigated with promising results. The latter population is of particular interest based on the low volume of blood available for routine blood culture [121]. SeptiFast was routinely used in more than 800 patients with suspected sepsis in a large Italian pediatric hospital [119]. Positivity rates for SeptiFast were markedly higher compared to blood culture in neonates/children with hemato-oncological diseases, children undergoing surgery or in ICUs, as well as in children in emergency departments or general pediatric wards. Among 42 blood samples from 35 neonates on ICUs, Torres-Martos [120] observed a high sensitivity and specificity of SeptiFast compared to the clinical diagnosis of sepsis. Of interest, the rate of contamination in blood culture and SeptiFast was 16.7% and 2.4%. Similarly, Tschiedel et al. [113] retrospectively analyzed a cohort of pediatric patients and observed a significantly higher pathogen detection rate using SeptiFast compared to blood culture, especially in patients pretreated with antibiotics. Furthermore, SeptiFast results were available at least 31 h before blood culture results, and antibiotic therapy was adjusted in 13% of patients based on SeptiFast results. No major advantage was observed for patients with suspected infective endocarditis since several endocarditis-related bacterial species are not included in the SeptiFast panel, and the sensitivity of the assay may not be sufficient to detect the low-grade bacteremia associated with endocarditis [15, 122]. In a small series of patients, Lefort et al. [123] observed similar performance of SeptiFast compared to blood culture for samples from patients with candida endocarditis.
In addition, a number of investigators have applied the technique to sample types other than whole blood including cerebrospinal fluid (Steinmann et al., Infection, in press), urine [124], ascites [125], and heart valves [126]. A metaanalysis of 34 studies using SeptiFast to diagnose suspected sepsis was performed by Chang et al. [99]. The overall sensitivity and specificity to detect bacterial and fungal infection was 75% and 92% with high heterogeneity in the bacterial but not the fungal subgroups.
There are only a very limited number of studies that compare the performance of molecular tests with each other. In a small study, SeptiFast showed a higher sensitivity and specificity when compared to blood culture than the SepsiTest [127]. Schreiber et al. [128] compared SeptiFast, VYOO and SepsiTest and observed some variability between the three PCR assays and the corresponding blood cultures with regards to the type of pathogen detected. Of interest, the three PCR assays appeared to be less susceptible to false-positive results than blood cultures.
Pros and cons of diagnostic techniques
Preanalytical sample processing
After collection, whole blood samples are easily transferred into blood culture bottles with culture media. Further preanalytical steps to increase the yield of traditional blood culture are currently not available. Yet, one of the critical steps in the molecular diagnosis of sepsis is the purification of microbial nucleic acids from blood to increase sensitivity [15]. An ideal DNA extraction method should be sensitive, reproducible, cost-effective, fully automated, and universal in its ability to extract bacterial and fungal DNA, thereby enabling rapid and reliable detection of pathogen DNA in septic patients. The presence of contaminating bacterial or fungal DNA in the reagents [129], the risk of carryover contamination among samples, and the interference of high level human DNA with the extraction of less abundant bacterial or fungal DNA [130] are among the main problems. Increased sensitivity without loss of specificity can be introduced by software algorithms that artificially increase the detection limits for frequent contaminants such as coagulase-negative staphylococci (CNS) and Streptococcus ssp. (as in the SeptiFast assay). Recently, a variety of new tools have been introduced to achieve selective enrichment of bacterial DNA from total DNA [129] such as the use of a protein immobilized on a column to specifically bind prokaryotic DNA for selective enrichment of bacterial DNA (e.g., SIRS-Lab’s LOOXSTER® universal) or the use of DNase specific for human DNA [131].
Overall turnaround time
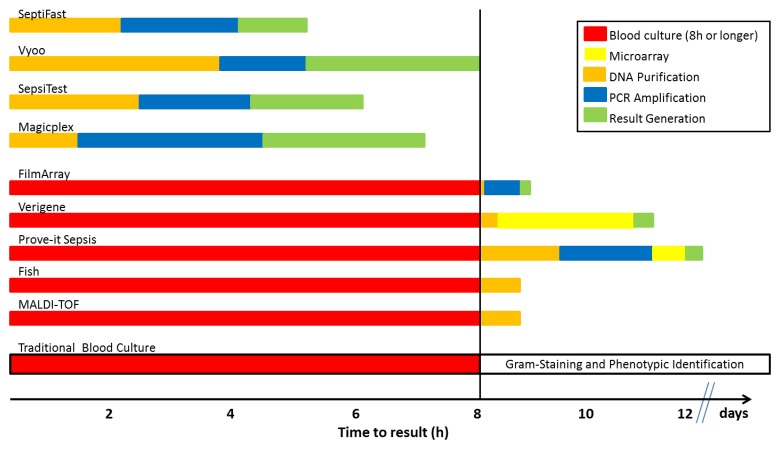
Despite some advances with rapid phenotypic tests the delay in time to result represents one of the major limitations of traditional blood culture due to the dependence on growth of organisms and biochemical identification. The turnaround time can be several days and even more prolonged when slow-growing pathogens such as fastidious bacteria and yeasts or anaerobes are present [15]. Molecular techniques performed on positive blood cultures offer faster identification of bacteria following culture but are still time-consuming because of the growth requirement in blood culture. It will therefore be difficult to address the clinician’s needs applying molecular techniques only after blood cultures have indicated the growth of organisms as the turnaround time does not meet the clinically relevant time frames. In contrast, molecular techniques performed directly on whole blood show markedly shorter turnaround time combined with increased sensitivity. First, the avoidance of a time-consuming culture step substantially reduces the turnaround time compared to routine blood culture but also compared to rapid detection of pathogens using PCR following growth in blood cultures (Fig. 2). Secondly, a large number of studies has demonstrated increased sensitivity compared to routine blood culture (Table 1) driven by the amplification using PCR and the independence from inhibitory influences of antibiotics or other factors present in the blood. On the contrary, laboratory contamination, background bacterial DNA in blood, and the potential detection of bacterial DNA from dead and living organisms are some of the main limitations of these methods [89]. Of importance, the turnaround time of the molecular technology alone does not allow to preclude its value; rapid molecular technologies have to be embedded into well-defined clinical algorithms that allow transport of the sample to the laboratory, pre-analytics, performance of the test, and result transmission to the clinician around the clock. In this regard, most studies using molecular technologies have reported reduced overall turnaround times compared to traditional blood culture but mean turnaround times remained disappointing due to the limitations of test performance after hours and/or over the weekend. Furthermore, the extent of clinical benefit (initiation, escalation, or de-escalation of antimicrobial therapy) provided by rapid turnaround time in patients who most likely are pre-treated with broad-range antibiotics as empiric therapy has to be determined.
Fig. 2.
Time to result of selected blood culture-dependent and blood culture-independent technologies for the diagnosis of sepsis; the vertical line indicates the duration of blood culture (fixed at 8 h or longer for this figure)
Detection of antimicrobial resistance
Despite significant advances in diagnostic technologies, many patients with suspected sepsis receive empiric antimicrobial therapy rather than appropriate therapy dictated by the rapid identification of the infectious agent. The result is overuse of a limited number of effective antimicrobials. A diagnostic strategy that incorporates sensitive biomarkers to indicate the presence of infection followed by pathogen-specific tests that are linked to a rapid assessment of drug resistance could revolutionize sepsis management by enabling the initiation or adjustment of definitive antimicrobial therapy. Rapid identification of antibiotic resistance is also central to infection control policies including the timely isolation of patients harboring drug-resistant organisms. A limited number of bacterial resistance genes can be routinely detected using commercially molecular assays such as mecA, vanA and vanB [132], in most cases combined with the detection of Gram-positive pathogens. PCR tests for the presence of genes that encode resistance in Gram-negative bacteria including ESBL and carbapenemases are also commercially available (e.g., Hyplex systems, Amplex, Germany; Check-Points, the Netherlands). A DNA microarray with high coverage of known variants allows rapid molecular testing for ESBLs following growth and isolation of single colonies [133]. However, it remains to be shown whether the molecular detection of antimicrobial resistance genes can replace phenotypic microbiological characterization of antimicrobial susceptibility since the presence of resistance genes does not always translate in clinically relevant resistance.
Sample transport, lab workflow, and result reporting
Implementation of the new molecular techniques in the clinical microbiology laboratory is needed especially in university and emergency hospitals which are able to meet the daily increasing demand for such specific tests. The laboratory workflow incorporate optimized handling pre- and post-analytics including immediate sample transfer to the laboratory after collection, processing of samples at night and on weekends, and immediate reporting of validated results to highly specialized experts [134]. As trained personnel are needed to perform molecular assays, difficulties might appear in applying the test in the routine of some microbiology laboratories [135]. Furthermore, the current trend of centralization of clinical microbiology laboratories may on one hand add value due to more organized and efficient transport of samples to these laboratories and computer-based communication of results; however, the distance between sample collection at the bedside and the laboratory may also increase the transport time. Thus, laboratories introducing molecular technologies for the rapid diagnosis of sepsis need to take a holistic look at the patient management from sample collection, laboratory workflow, to patient management and infection control measures.
Clinical aspects
The value of blood cultures for confirming the clinical diagnosis of sepsis, severe sepsis, and septic shock is suboptimal. Although most untreated patients with bacterial meningitis have positive blood cultures, only 30% of patients with bacterial pneumonia and intra-abdominal infections have positive cultures. Only 5% to 15% of all cultures drawn for any reason, and only 50% of patients with septic shock, are showing positive results [136]. It has been demonstrated that PCR adds clinically valuable information to blood culture, and it delivers it much earlier [35], offering a great potential in clinical practice. For acute care physicians, rapid molecular tests targeting various microorganisms involved in specific syndromes, such as meningitis, endocarditis, pneumonia, or sepsis, would allow for rapid diagnosis and early aggressive targeted therapy. As a result, this will diminish the development of antibiotic resistance and decrease costs [137]. The clinical benefit of PCR in monitoring patients after allogenic stem cell transplantation has elegantly been shown by Hebart et al. [138]. Using results generated by a laboratory-developed PCR test from whole blood to initiate amphotericin B therapy, a significant decrease in mortality was observed. The notion of a universal detection is directly linked to the future potential effect of PCR-based assays for clinical practice in the emergency units or other acute critical-care settings. To determine the appropriateness of empiric antimicrobial therapy and the extent to which therapy would be altered based on the result of a rapid PCR test, Stoneking et al. [139] retrospectively investigated consecutive patients with positive blood cultures in an emergency department. If PCR had revealed the causative organism at the time of admission, antimicrobial therapy would have been changed to narrower-spectrum antibiotics in 55% of cases. Therapy would have been changed because the organism was not covered in 21.3% of cases, and therapy would remain the same in 23.0% of cases. Rapid diagnosis using PCR showed a statistically significant advantage (p < 0.0001) over Infectious Disease Society of America protocols in facilitating accurate antimicrobial therapies. In contrast, Pletz et al. [140, 141] reported that the use of current rapid PCR-based diagnostics cannot address some of the most common causes for inappropriate antimicrobial use, i.e., antimicrobial resistance, poor penetration of antibiotics into tissues, and underdosing. They propose to focus on pathogens and resistance genes that are currently not covered by guideline-recommended treatment regimens rather than introducing complexity by increasing the panel of multiplex PCRs. However, none of these studies critically evaluate the feasibility of de-escalation or tailored antimicrobial therapy in critically ill patients under broad-spectrum antimicrobial therapy. Using a chart review in a large medico-surgical ICU department (antibiotic strategies were reviewed by ID specialists three times per week), Heenen et al. [141] observed that even close collaboration among intensivists and ID specialists allowed de-escalation in less than 50% of cases.
Implementation of new molecular techniques has focused on clinical settings, such as ICU, hematology, cardiology, neonatology, or pediatric units, where rapid identification of systemic bacterial infection is critical due to high mortality rates. The clinical use of Septifast has been demonstrated in numerous scientific reports (Table 2). Septifast has shown promising performances as an adjunct to blood culture for neutropenic [105–107, 110, 111], neonatal and pediatric [90, 113, 119, 142, 143], intensive care [100, 101, 103, 128, 144, 145], and general medicine [97, 100, 146] patients. No major advantage was observed for patients with suspected infective endocarditis using blood [122, 126], while the performance on heart valves was superior to blood culture [126]. The lower performance in some studies can be explained in part by the absence of several endocarditis-related bacterial species from the SeptiFast menu.
Lodes et al. [144] for the first time demonstrated the successful use of SeptiFast to guide treatment of ICU patients at risk for abdominal sepsis; improved detection of specific pathogens had a positive impact on therapeutic decision-making when adding SeptiFast in patients with new onset of SIRS. However, the implementation of a rapid PCR assay alone is not enough to improve antibiotic use. Rapid molecular technologies with timely reporting of results to the clinician must be embedded into an antibiotic stewardship program to fully impact patient management at the bedside [147]. A recent report by Perez and colleagues [137] assessed the value of an intervention bundle consisting of MALDI-TOF mass spectrometry, rapid antimicrobial susceptibility testing, and near real-time antimicrobial stewardship practices on length of hospital stay and associated hospital costs. While only including patients diagnosed with aerobic bacterial infections (thereby excluding those with infections caused by yeasts or anaerobic bacteria), the average turnaround time for final culture identification and susceptibility after intervention was reduced by approximately one day. The mean hospital length of stay in the preintervention vs. intervention group survivors was 12 vs. 9 days. After multivariate analysis, factors independently associated with decreased length of hospitalization included MALDI-TOF-based antimicrobial stewardship intervention and active therapy at 48 h. Of interest, the use of an infectious disease or clinical pharmacist significantly enhanced the utility of the rapid indentification and antimicrobial susceptibility by tailoring antimicrobial therapy. These findings confirm previous reports showing that decreased turnaround time are unlikely to affect time to appropriate antimicrobial therapy or length of hospital stay [148–151].
Economical aspects
Molecular technologies are more expensive than conventional approaches. The direct costs of PCR reagents, equipment, dedicated space, personnel training, and labor have been reported to be as high as €300 per reaction. The labor intensity needed for most assays as well as technical limitations of most thermocyclers to do multiple runs of PCR simultaneously have prevented routine around-the-clock testing in the clinical setting. However, in assessing the overall benefit of PCR, direct monetary costs should not be the only consideration since the assay has several significant advantages over traditional methods. Moreover, it has been shown that the benefits of a PCR outweighed its cost [152]. In 2004, Burchardi and Schneider estimated at €1200 ($1560) the average total cost per ICU day in seven countries with highly developed healthcare systems [153] which is approximately 12 times higher than the estimated direct cost of a PCR test. Authors noted that staffing costs represented from 40% to >60% of the total ICU budget and variable costs, including drugs, other consumables and laboratory and diagnostic services, amounted to only 30% of total costs [153]. They stated that because of the high proportion of fixed costs in ICU treatment, the total cost of ICU care is mainly dependent on the length of ICU stay. By reducing the turnaround time, molecular technologies offer a more rapid diagnosis and may reduce the ICU and hospital length of stay [16].
Few studies have determined the health-economic aspects of commercial tests for the detection of sepsis [73, 148, 154]. During an evidence-based intervention that integrated mass spectrometry, rapid antimicrobial susceptibility testing and near real-time antimicrobial stewardship practices savings of approximately $20.000 savings/patient were reported compared to the pre-intervention period; the authors projected an annual savings of approximately $18 million with the implementation of this strategy [137]. While clinical outcomes and savings appear marked, the bundle investigated used a rapid molecular test that was applied to positive blood cultures but not directly to the blood.
Economic analyses have also been done for molecular tests performed directly from blood. The impact of the use of the SeptiFast test on healthcare costs and medical outcomes has been determined by Lehmann and colleagues [155] using a mathematical prediction model. In 221 sepsis episodes of 189 post-surgical and ICU sepsis patients from two studies which involved 1,147 (thereof 316 inadequately treated) medical or surgical ICU patients, a total of 13.1% of PCR tests enabled earlier adequate treatment. The authors predicted that the cost for PCR testing (approximately €300/test) can be fully recovered for patients above €717 (605 to €1,710) daily treatment cost. A 2.6% (2.0 to 3.2%) absolute reduction of mortality is expected. Cost per incremental survivor calculates to €11,477 (9,321 to €14,977) and incremental cost-effectiveness ratio to €3,107 (2,523 to €4,055) per quality-adjusted life-year. Generally, for ICU patients with >25% incidence of inadequate empiric antimicrobial treatment, and at least 15% with a positive blood culture, the SeptiFast test represents a cost-neutral adjunct method. More recently, a cost-minimization study was carried out in patients admitted with a diagnosis of severe sepsis or septic shock to the ICU of a university hospital in Spain [17]. During an initial 6-month period, the reliability of the SeptiFast test was assessed, and patients were treated using routine management, the physicians supervising treatment did not receive the SeptiFast result; in the subsequent 6 months, the Septifast result was made available to the supervising physician, and patients were managed accordingly. Clinical outcomes (28-day and 6-month mortalities, length of ICU stay, length of hospital stay, and antibiotic use), as well as costs of antibiotics, ICU stay, non-ICU hospital stay, were compared between the two periods (Table 3). The mortality rate was similar in both groups, but the additional use of SeptiFast results shortened the ICU stay and lowered the use (and costs) of antibiotics. The mean total costs were €42,198 in the control vs. €32,228 in the group in whom the SeptiFast test was used (p < 0.05). The Xpert MRSA PCR test [148] and FISH [156] were also shown to allow timely, effective therapy of S. aureus bacteremia associated with a decrease in the length of stay and healthcare costs.
Table 3.
Patient outcome and hospital costs for patients with bloodstream infection treated using routine medical management with or without the SeptiFast test*
| Routine management (Mean ± SD) |
Routine management plus SeptiFast (Mean ± SD) |
p value | |
|---|---|---|---|
| 28-day mortality | 13 (27%) | 14 (26%) | n.s. |
| 6-month mortality | 20 (37%) | 20 (41.6%) | n.s. |
| Stay in ICU | 31.0 ± 19.4 | 22.9 ± 29.9 | <0.05 |
| Stay in hospital | 21.3 ± 23.4 | 18.3 ± 21.4 | <0.05 |
| Stay in ICU survivors | 24.1 ± 21.9 | 18.3 ± 11.4 | <0.05 |
| Number of antibiotics used per patient | 5.1 ± 3.1 | 4.2 ± 2.2 | <0.05 |
| Antibiotic treatment cost per patient | 3576 € | 2812 € | <0.05 |
| Cost of ICU stay | 32798 € | 24246 € | <0.05 |
| Cost of ward stay | 5824 € | 4988 € | <0.05 |
| Total cost | 42198 € | 32228 € | <0.05 |
| n.s., not significant *Modified after ref. [17] | |||
Future directions
Despite major technical and clinical advances in the field, there are still a large number of unanswered questions before new approaches may change the management of patients with sepsis. The Infectious Disease Society of America has recently published recommendations for the development and clinical implementation of improved diagnostic tests [18]. Achieving these goals will not only require the engagement and coordination of clinical stakeholders, including not only healthcare systems, professional societies, and individual clinicians but also of congress, funding and regulatory bodies, public health agencies, and the diagnostics industry. In addition, the use of biomarkers, “companion” diagnostics for clinical trials, technological advances and new technologies stand out as future directions. We believe that a number of advances will have to act jointly to pave the way for innovative approaches to the diagnosis of sepsis. To be successful, new diagnostic approaches have on one hand to be rapid and actionable, but also need to be embedded in well-planned and implemented algorithms for the management of patients with suspected sepsis.
Rapid molecular test for the detection of pathogens may prove useful for the enrichment of subjects with a “proven” pathogen or patients with specific clinical syndromes for targeted microorganisms prior to or concomitant with enrollment in clinical trials; other applications of rapid molecular tests are the use as “companion” diagnostics where the prescription of antimicrobial drugs is dependent on the result of a specific diagnostic test, or the enrichment of subjects in clinical trials for the treatment of rare pathogens [18].
The past focus on pathogen detection has not provided the expected results but has rather stagnated in the phase of method comparison studies. Therefore, the measurement of host responses to infection was identified as an alternative to pathogen-based diagnostics (reviewed in ref. [4]). Biomarkers or the combination of pathogen plus biomarker detection may result in improved management of patients with suspected sepsis; it may also be used as a “companion” diagnostic approach to enrich for patients in the setting of clinical trials using new drugs against sepsis targeting host responses or pathogens (http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM359184.pdf). With increasing interest in biomarker-guided immunotherapy [1] for sepsis, concepts for innovative diagnostic tests will have to be redefined.
Unfortunately, even the best validated biomarkers are currently only used to aid in the diagnosis of sepsis. The detection of biomarkers can be based on peptides and proteins, or the detection of nucleic acids. In regards to the former, procalcitonin, often used in combination with interleukin-6 [157] is regarded as the champion so far when identifying or ruling out bacterial infections. Other markers under investigation are the soluble triggering receptor expressed on myeloid cells (sTREM-1) [158, 159] soluble urokinase-type plasminogen activator receptor (suPAR) [160], ST2, a receptor involved in helper T cell responses, and the natriuretic peptides adrenomedullina and pro-adrenomedullin [161]. Recently, Langsley et al. [162] analyzed the metabolomes and proteomes of patients with sepsis at hospital admittance to allow prediction of outcome. Profiles of proteins and metabolites in the fatty acid transport and b-oxidation, gluconeogenesis, and the citric acid cycle differed consistently among several sets of patients, and diverged more as death approached.
Nucleic acid-based detection techniques have also been described; mitochondrial DNA released into the circulation following cellular damage elicits inflammatory signals (damage-associated molecular patterns (DAMPs)) [163]. Given that the immune response to sepsis is complex and difficult to evaluate with single analytes, high throughput technologies, such as multiplex PCR or arrays, have substantive utility as diagnostic tests determining the status of the patient’s immune system. Sutherland et al. [164] validated the relative expression of 42 genes that represent innate and adaptive immune function, cell cycling, white blood cells, differentiation, extracellular remodeling, and immune modulation pathways for the diagnosis of sepsis. More recently, a reverse transcription PCR low-density array using host genes exclusively showed promise in the detection of subjects with experimentally induced influenza infection and had sensitivities and specificities of 89% and 94%, respectively, in a cohort of patients with influenza in an emergency department. Thus, an algorithm combining clinical features together with measurements of five metabolites could predict patient outcome and may be useful to guide the treatment of individual patients with sepsis [162].
Biological advances have been paralleled by technical advances focusing on improvements to traditional blood culture and new technologies using whole blood. To improve and speed up the time to positive blood culture, colorimetric sensor arrays were developed based on the detection of headspace volatile organic compounds from bacterial species with a preliminary accuracy of 95% (http://specifictechnologies.net/?page_id=8). This technology could allow a more rapid detection of bloodstream infections, and the pathogens involved before molecular or mass spectrometry assays are used on traditional positive blood cultures. T2 magnetic resonance in a portable device was applied directly to whole blood and rapidly detected fungal pathogens using PCR followed by hybridization on probe-decorated nanoparticles microclusters that induce changes in magnetic resonance signals [165].
A number of manufacturers (GenePOC, IQuum, Quidel among others) are developing point of care molecular solutions for a wide variety of bacterial and viral pathogens. The unmet medical need for a rapid tool to diagnose bloodstream infection is particularly apparent in the emergency room as well as in the setting of disasters. First-line responders must be equipped with easy-to-use devices to perform critical tests at the point of need, even in austere low resource environments. The importance and process of clinical needs assessments of a suitable point-of-care solution has been discussed [166]; critical components may include sampling device design, range of targets detected, performance characteristics, disposal of samples, and the ability to operate on battery power [167].
However, even the most promising new technological approach will not be resulting in marked improvements in patient management if not implemented as part of larger algorithms that must be based on the close interaction between the clinician and the laboratory including antibiotic stewardship [149]. In this regard, standardized algorithms for patient management, rigorous adherence to infection control and antibiotic stewardship programs [168], as well as other factors including lab IT, training for staff in laboratories and wards/ERs are key components of successful programs. Thus, future research should focus on a holistic “ideal” approach to the diagnosis of sepsis.
Acknowledgments
We wish to thank Kathy Schneider and Rares Olariu for expert support. E.L.L., K.-P.H. and G.K. do not declare any conflict of interest. O.L. is Chief Medical Officer at Roche Molecular Diagnostics.
Contributor Information
O. Liesenfeld, 1Medical and Scientific Affairs, Roche Molecular Diagnostics, Pleasanton, CA 94566, USA.
L. Lehman, 2Department of Anaesthesiology and Pain Medicine, Inselspital, University of Bern, Freiburgstrasse, CH-3010 Bern, Switzerland.
K.-P. Hunfeld, 3Institute for Laboratory Medicine, Microbiology and Infection Control, Northwest Medical Centre, Steinbacher Hohl 2-26, D-60488 Frankfurt am Main, Germany.
G. Kost, 4Department of Pathology and Laboratory Medicine, School of Medicine, University of California, Davis, CA 95616, USA.
References
- 1.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013 Dec;13(12):862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013 Aug 29;369(9):840–581. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 3.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013 Feb;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 4.Reinhart K, Bauer M, Riedemann NC, Hartog CS. New approaches to sepsis: molecular diagnostics and biomarkers. Clin Microbiol Rev. 2012 Oct;25(4):609–634. doi: 10.1128/CMR.00016-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Josefson P, Strålin K, Ohlin A, Ennefors T, Dragsten B, Andersson L, Fredlund H, Mölling P, Olcén P. Evaluation of a commercial multiplex PCR test (SeptiFast) in the etiological diagnosis of community-onset bloodstream infections. Eur J Clin Microbiol Infect Dis. 2011 Sep;30(9):1127–1134. doi: 10.1007/s10096-011-1201-6. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A, Haery C, Paladugu B, Kumar A, Symeoneides S, Taiberg L, Osman J, Trenholme G, Opal SM, Goldfarb R, Parrillo JE. The duration of hypotension before the initiation of antibiotic treatment is a critical determinant of survival in a murine model of Escherichia coli septic shock: association with serum lactate and inflammatory cytokine levels. J Infect Dis. 2006 Jan 15;193(2):251–258. doi: 10.1086/498909. [DOI] [PubMed] [Google Scholar]
- 7.Nordmann P, Gniadkowski M, Giske CG, Poirel L, Woodford N, Miriagou V. Identification and screening of carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect. 2012 May;18(5):432–438. doi: 10.1111/j.1469-0691.2012.03815.x. [DOI] [PubMed] [Google Scholar]
- 8.Labelle AJ, Micek ST, Roubinian N, Kollef MH. Treatment-related risk factors for hospital mortality in Candida bloodstream infections. Crit Care Med. 2008 Nov;36(11):2967–2672. doi: 10.1097/CCM.0b013e31818b3477. [DOI] [PubMed] [Google Scholar]
- 9.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006 Jul 1;43(1):25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 10.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008 Jan;34(1):17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coburn B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood cultures? JAMA. 2012 Aug 1;308(5):502–511. doi: 10.1001/jama.2012.8262. [DOI] [PubMed] [Google Scholar]
- 12.Barenfanger J, Graham DR, Kolluri L, Sangwan G, Lawhorn J, Drake CA, Verhulst SJ, Peterson R, Moja LB, Ertmoed MM, Moja AB, Shevlin DW, Vautrain R, Callahan CD. Decreased mortality associated with prompt Gram staining of blood cultures. Am J Clin Pathol. 2008 Dec;130(6):870–876. doi: 10.1309/AJCPVMDQU2ZJDPBL. [DOI] [PubMed] [Google Scholar]
- 13.Patel R, Vetter EA, Harmsen WS, Schleck CD, Fadel HJ, Cockerill FR., 3rd Optimized pathogen detection with 30- compared to 20-milliliter blood culture draws. J Clin Microbiol. 2011 Dec;49(12):4047–4051. doi: 10.1128/JCM.01314-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirn TJ, Weinstein MP. Update on blood cultures: how to obtain, process, report, and interpret. Clin Microbiol Infect. 2013 Jun;19(6):513–520. doi: 10.1111/1469-0691.12180. [DOI] [PubMed] [Google Scholar]
- 15.Mancini N, Carletti S, Ghidoli N, Cichero P, Burioni R, Clementi M. The era of molecular and other non-culture-based methods in diagnosis of sepsis. Clin Microbiol Rev. 2010 Jan;23(1):235–251. doi: 10.1128/CMR.00043-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varani S, Stanzani M, Paolucci M, Melchionda F, Castellani G, Nardi L, Landini MP, Baccarani M, Pession A, Sambri V. Diagnosis of bloodstream infections in immunocompromised patients by real-time PCR. J Infect. 2009 May;58(5):346–351. doi: 10.1016/j.jinf.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez J, Mar J, Varela-Ledo E, Garea M, Matinez-Lamas L, Rodriguez J, Regueiro B. Cost analysis of real-time polymerase chain reaction microbiological diagnosis in patients with septic shock. Anaesth Intensive Care. 2012 Nov;40(6):958–963. doi: 10.1177/0310057X1204000606. [DOI] [PubMed] [Google Scholar]
- 18.Caliendo AM, Gilbert DN, Ginocchio CC, Hanson KE, May L, Quinn TC, Tenover FC, Alland D, Blaschke AJ, Bonomo RA, Carroll KC, Ferraro MJ, Hirschhorn LR, Joseph WP, Karchmer T, MacIntyre AT, Reller LB, Jackson AF. Better tests, better care: improved diagnostics for infectious diseases. Clin Infect Dis. 2013 Dec;57(Suppl 3):S139–S170. doi: 10.1093/cid/cit578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ecker DJ, Sampath R, Li H, Massire C, Matthews HE, Toleno D, Hall TA, Blyn LB, Eshoo MW, Ranken R, Hofstadler SA, Tang YW. New technology for rapid molecular diagnosis of bloodstream infections. Expert Rev Mol Diagn. 2010 May;10(4):399–415. doi: 10.1586/erm.10.24. [DOI] [PubMed] [Google Scholar]
- 20.Leggieri N, Rida A, François P, Schrenzel J. Molecular diagnosis of bloodstream infections: planning to (physically) reach the bedside. Curr Opin Infect Dis. 2010 Aug;23(4):311–319. doi: 10.1097/QCO.0b013e32833bfc44. [DOI] [PubMed] [Google Scholar]
- 21.Venkatesh M, Flores A, Luna RA, Versalovic J. Molecular microbiological methods in the diagnosis of neonatal sepsis. Expert Rev Anti Infect Ther. 2010 Sep;8(9):1037–1048. doi: 10.1586/eri.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995 Jan 11;273(2):117–123. [PubMed] [Google Scholar]
- 23.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001 Jul;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012 Mar;40(3):754–761. doi: 10.1097/CCM.0b013e318232db65. [DOI] [PubMed] [Google Scholar]
- 25.Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, Gårdlund B, Marshall JC, Rhodes A, Artigas A, Payen D, Tenhunen J, Al-Khalidi HR, Thompson V, Janes J, Macias WL, Vangerow B, Williams MD. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012 May 31;366(22):2055–2064. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 26.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009 Dec 2;302(21):2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 27.Opal SM, Garber GE, LaRosa SP, Maki DG, Freebairn RC, Kinasewitz GT, Dhainaut JF, Yan SB, Williams MD, Graham DE, Nelson DR, Levy H, Bernard GR. Systemic host responses in severe sepsis analyzed by causative microorganism and treatment effects of drotrecogin alfa (activated) Clin Infect Dis. 2003 Jul 1;37(1):50–58. doi: 10.1086/375593. [DOI] [PubMed] [Google Scholar]
- 28.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003 Apr 17;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 29.Mayr FB, Yende S, Linde-Zwirble WT, Peck-Palmer OM, Barnato AE, Weissfeld LA, Angus DC. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. JAMA. 2010 Jun 23;303(24):2495–2503. doi: 10.1001/jama.2010.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Grady NP, Barie PS, Bartlett JG, Bleck T, Carroll K, Kalil AC, Linden P, Maki DG, Nierman D, Pasculle W, Masur H. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008 Apr;36(4):1330–1349. doi: 10.1097/CCM.0b013e318169eda9. [DOI] [PubMed] [Google Scholar]
- 31.Zadroga R, Williams DN, Gottschall R, Hanson K, Nordberg V, Deike M, Kuskowski M, Carlson L, Nicolau DP, Sutherland C, Hansen GT. Comparison of 2 blood culture media shows significant differences in bacterial recovery for patients on antimicrobial therapy. Clin Infect Dis. 2013 Mar;56(6):790–797. doi: 10.1093/cid/cis1021. [DOI] [PubMed] [Google Scholar]
- 32.Vallés J, Rello J, Ochagavía A, Garnacho J, Alcalá MA. Community-acquired bloodstream infection in critically ill adult patients: impact of shock and inappropriate antibiotic therapy on survival. Chest. 2003 May;123(5):1615–1624. doi: 10.1378/chest.123.5.1615. [DOI] [PubMed] [Google Scholar]
- 33.Sautter RL, Bills AR, Lang DL, Ruschell G, Heiter BJ, Bourbeau PP. Effects of delayed-entry conditions on the recovery and detection of microorganisms from BacT/ALERT and BACTEC blood culture bottles. J Clin Microbiol. 2006 Apr;44(4):1245–1249. doi: 10.1128/JCM.44.4.1245-1249.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwetz I, Hinrichs G, Reisinger EC, Krejs GJ, Olschewski H, Krause R. Delayed processing of blood samples influences time to positivity of blood cultures and results of Gram stain-acridine orange leukocyte Cytospin test. J Clin Microbiol. 2007 Aug;45(8):2691–2694. doi: 10.1128/JCM.00085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsalik EL, Jones D, Nicholson B, Waring L, Liesenfeld O, Park LP, Glickman SW, Caram LB, Langley RJ, van Velkinburgh JC, Cairns CB, Rivers EP, Otero RM, Kingsmore SF, Lalani T, Fowler VG, Woods CW. Multiplex PCR to diagnose bloodstream infections in patients admitted from the emergency department with sepsis. J Clin Microbiol. 2010 Jan;48(1):26–33. doi: 10.1128/JCM.01447-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreillon P, Que YA. Infective endocarditis. Lancet. 2004 Jan 10;363(9403):139–149. doi: 10.1016/S0140-6736(03)15266-X. [DOI] [PubMed] [Google Scholar]
- 37.Watkin RW, Lang S, Lambert PA, Littler WA, Elliott TS. The microbial diagnosis of infective endocarditis. J Infect. 2003 Jul;47(1):1–11. doi: 10.1016/s0163-4453(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 38.Clancy CJ, Nguyen MH. Finding the "missing 50%" of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013 May;56(9):1284–1292. doi: 10.1093/cid/cit006. [DOI] [PubMed] [Google Scholar]
- 39.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008 Jan;36(1):296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 40.Chapin KC, Musgnug MC. Direct susceptibility testing of positive blood cultures by using Sensititre broth microdilution plates. J Clin Microbiol. 2003 Oct;41(10):4751–4754. doi: 10.1128/JCM.41.10.4751-4754.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bekeris LG, Tworek JA, Walsh MK, Valenstein PN. Trends in blood culture contamination: a College of American Pathologists Q-Tracks study of 356 institutions. Arch Pathol Lab Med. 2005 Oct;129(10):1222–1225. doi: 10.5858/2005-129-1222-TIBCCA. [DOI] [PubMed] [Google Scholar]
- 42.Gander RM, Byrd L, DeCrescenzo M, Hirany S, Bowen M, Baughman J. Impact of blood cultures drawn by phlebotomy on contamination rates and health care costs in a hospital emergency department. J Clin Microbiol. 2009 Apr;47(4):1021–1024. doi: 10.1128/JCM.02162-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halverson S, Malani PN, Newton DW, Habicht A, Vander Have K, Younger JG. Impact of hourly emergency department patient volume on blood culture contamination and diagnostic yield. J Clin Microbiol. 2013 Jun;51(6):1721–1726. doi: 10.1128/JCM.03422-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim NH, Kim M, Lee S, Yun NR, Kim KH, Park SW, Kim HB, Kim NJ, Kim EC, Park WB, Oh MD. Effect of routine sterile gloving on contamination rates in blood culture: a cluster randomized trial. Ann Intern Med. 2011 Feb 1;154(3):145–151. doi: 10.7326/0003-4819-154-3-201102010-00003. [DOI] [PubMed] [Google Scholar]
- 45.Boyce JM, Nadeau J, Dumigan D, Miller D, Dubowsky C, Reilly L, Hannon CV. Obtaining blood cultures by venipuncture versus from central lines: impact on blood culture contamination rates and potential effect on central line-associated bloodstream infection reporting. Infect Control Hosp Epidemiol. 2013 Oct;34(10):1042–1047. doi: 10.1086/673142. [DOI] [PubMed] [Google Scholar]
- 46.Rüssmann H, Kempf VA, Koletzko S, Heesemann J, Autenrieth IB. Comparison of fluorescent in situ hybridization and conventional culturing for detection of Helicobacter pylori in gastric biopsy specimens. J Clin Microbiol. 2001 Jan;39(1):304–308. doi: 10.1128/JCM.39.1.304-308.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kempf VA, Mändle T, Schumacher U, Schäfer A, Autenrieth IB. Rapid detection and identification of pathogens in blood cultures by fluorescence in situ hybridization and flow cytometry. Int J Med Microbiol. 2005 Apr;295(1):47–55. doi: 10.1016/j.ijmm.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 48.Oliveira K, Haase G, Kurtzman C, Hyldig-Nielsen JJ, Stender H. Differentiation of Candida albicans and Candida dubliniensis by fluorescent in situ hybridization with peptide nucleic acid probes. J Clin Microbiol. 2001 Nov;39(11):4138–4141. doi: 10.1128/JCM.39.11.4138-4141.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paolucci M, Landini MP, Sambri V. Conventional and molecular techniques for the early diagnosis of bacteraemia. Int J Antimicrob Agents. 2010 Dec;36(Suppl 2):S6–S16. doi: 10.1016/j.ijantimicag.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 50.Chapin K, Musgnug M. Evaluation of three rapid methods for the direct identification of Staphylococcus aureus from positive blood cultures. J Clin Microbiol. 2003 Sep;41(9):4324–4327. doi: 10.1128/JCM.41.9.4324-4327.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oliveira K, Brecher SM, Durbin A, Shapiro DS, Schwartz DR, De Girolami PC, Dakos J, Procop GW, Wilson D, Hanna CS, Haase G, Peltroche-Llacsahuanga H, Chapin KC, Musgnug MC, Levi MH, Shoemaker C, Stender H. Direct identification of Staphylococcus aureus from positive blood culture bottles. J Clin Microbiol. 2003 Feb;41(2):889–891. doi: 10.1128/JCM.41.2.889-891.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shepard JR, Addison RM, Alexander BD, Della-Latta P, Gherna M, Haase G, Hall G, Johnson JK, Merz WG, Peltroche-Llacsahuanga H, Stender H, Venezia RA, Wilson D, Procop GW, Wu F, Fiandaca MJ. Multicenter evaluation of the Candida albicans/Candida glabrata peptide nucleic acid fluorescent in situ hybridization method for simultaneous dual-color identification of C. albicans and C. glabrata directly from blood culture bottles. J Clin Microbiol. 2008 Jan;46(1):50–55. doi: 10.1128/JCM.01385-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davis TE, Fuller DD. Direct identification of bacterial isolates in blood cultures by using a DNA probe. J Clin Microbiol. 1991 Oct;29(10):2193–2196. doi: 10.1128/jcm.29.10.2193-2196.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindholm L, Sarkkinen H. Direct identification of gram-positive cocci from routine blood cultures by using AccuProbe tests. J Clin Microbiol. 2004 Dec;42(12):5609–5613. doi: 10.1128/JCM.42.12.5609-5613.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qian Q, Tang YW, Kolbert CP, Torgerson CA, Hughes JG, Vetter EA, Harmsen WS, Montgomery SO, Cockerill FR, 3rd, Persing DH. Direct identification of bacteria from positive blood cultures by amplification and sequencing of the 16S rRNA gene: evaluation of BACTEC 9240 instrument true-positive and false-positive results. J Clin Microbiol. 2001 Oct;39(10):3578–3582. doi: 10.1128/JCM.39.10.3578-3582.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turenne CY, Witwicki E, Hoban DJ, Karlowsky JA, Kabani AM. Rapid identification of bacteria from positive blood cultures by fluorescence-based PCR-single-strand conformation polymorphism analysis of the 16S rRNA gene. J Clin Microbiol. 2000 Feb;38(2):513–520. doi: 10.1128/jcm.38.2.513-520.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gebert S, Siegel D, Wellinghausen N. Rapid detection of pathogens in blood culture bottles by real-time PCR in conjunction with the pre-analytic tool MolYsis. J Infect. 2008 Oct;57(4):307–316. doi: 10.1016/j.jinf.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 58.Wellinghausen N, Wirths B, Essig A, Wassill L. Evaluation of the Hyplex BloodScreen Multiplex PCR-Enzyme-linked immunosorbent assay system for direct identification of gram-positive cocci and gram-negative bacilli from positive blood cultures. J Clin Microbiol. 2004 Jul;42(7):3147–3152. doi: 10.1128/JCM.42.7.3147-3152.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaleta EJ, Clark AE, Johnson DR, Gamage DC, Wysocki VH, Cherkaoui A, Schrenzel J, Wolk DM. Use of PCR coupled with electrospray ionization mass spectrometry for rapid identification of bacterial and yeast bloodstream pathogens from blood culture bottles. J Clin Microbiol. 2011 Jan;49(1):345–353. doi: 10.1128/JCM.00936-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacob D, Sauer U, Housley R, Washington C, Sannes-Lowery K, Ecker DJ, Sampath R, Grunow R. Rapid and high-throughput detection of highly pathogenic bacteria by Ibis PLEX-ID technology. PLoS One. 2012;7(6):e39928. doi: 10.1371/journal.pone.0039928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang YW, Kilic A, Yang Q, McAllister SK, Li H, Miller RS, McCormac M, Tracy KD, Stratton CW, Han J, Limbago B. StaphPlex system for rapid and simultaneous identification of antibiotic resistance determinants and Panton-Valentine leukocidin detection of staphylococci from positive blood cultures. J Clin Microbiol. 2007 Jun;45(6):1867–1873. doi: 10.1128/JCM.02100-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stamper PD, Cai M, Howard T, Speser S, Carroll KC. Clinical validation of the molecular BD GeneOhm StaphSR assay for direct detection of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus in positive blood cultures. J Clin Microbiol. 2007 Jul;45(7):2191–2196. doi: 10.1128/JCM.00552-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Snyder JW, Munier GK, Heckman SA, Camp P, Overman TL. Failure of the BD GeneOhm StaphSR assay for direct detection of methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolates in positive blood cultures collected in the United States. J Clin Microbiol. 2009 Nov;47(11):3747–3748. doi: 10.1128/JCM.01391-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolk DM, Struelens MJ, Pancholi P, Davis T, Della-Latta P, Fuller D, Picton E, Dickenson R, Denis O, Johnson D, Chapin K. Rapid detection of Staphylococcus aureus and methicillin-resistant S. aureus (MRSA) in wound specimens and blood cultures: multicenter preclinical evaluation of the Cepheid Xpert MRSA/SA skin and soft tissue and blood culture assays. J Clin Microbiol. 2009 Mar;47(3):823–826. doi: 10.1128/JCM.01884-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Innings A, Krabbe M, Ullberg M, Herrmann B. Identification of 43 Streptococcus species by pyrosequencing analysis of the rnpB gene. J Clin Microbiol. 2005 Dec;43(12):5983–5991. doi: 10.1128/JCM.43.12.5983-5991.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haanperä M, Jalava J, Huovinen P, Meurman O, Rantakokko-Jalava K. Identification of alpha-hemolytic streptococci by pyrosequencing the 16S rRNA gene and by use of VITEK 2. J Clin Microbiol. 2007 Mar;45(3):762–770. doi: 10.1128/JCM.01342-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tärnberg M, Jakobsson T, Jonasson J, Forsum U. Identification of randomly selected colonies of lactobacilli from normal vaginal fluid by pyrosequencing of the 16S rDNA variable V1 and V3 regions. APMIS. 2002 Nov;110(11):802–810. doi: 10.1034/j.1600-0463.2002.1101106.x. [DOI] [PubMed] [Google Scholar]
- 68.Jordan JA, Butchko AR, Durso MB. Use of pyrosequencing of 16S rRNA fragments to differentiate between bacteria responsible for neonatal sepsis. J Mol Diagn. 2005 Feb;7(1):105–110. doi: 10.1016/s1525-1578(10)60015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quiles-Melero I, García-Rodriguez J, Romero-Gómez MP, Gómez-Sánchez P, Mingorance J. Rapid identification of yeasts from positive blood culture bottles by pyrosequencing. Eur J Clin Microbiol Infect Dis. 2011 Jan;30(1):21–24. doi: 10.1007/s10096-010-1045-5. [DOI] [PubMed] [Google Scholar]
- 70.Motoshima M, Yanagihara K, Morinaga Y, Matsuda J, Hasegawa H, Kohno S, Kamihira S. Identification of bacteria directly from positive blood culture samples by DNA pyrosequencing of the 16S rRNA gene. J Med Microbiol. 2012 Nov;61(Pt 11):1556–1562. doi: 10.1099/jmm.0.049163-0. [DOI] [PubMed] [Google Scholar]
- 71.Peters RP, Mohammadi T, Vandenbroucke-Grauls CM, Danner SA, van Agtmael MA, Savelkoul PH. Detection of bacterial DNA in blood samples from febrile patients: underestimated infection or emerging contamination? FEMS Immunol Med Microbiol. 2004 Oct 1;42(2):249–253. doi: 10.1016/j.femsim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 72.Seng P, Drancourt M, Gouriet F, La Scola B, Fournier PE, Rolain JM, Raoult D. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis. 2009 Aug 15;49(4):543–551. doi: 10.1086/600885. [DOI] [PubMed] [Google Scholar]
- 73.Schubert S, Weinert K, Wagner C, Gunzl B, Wieser A, Maier T, Kostrzewa M. Novel, improved sample preparation for rapid, direct identification from positive blood cultures using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry. J Mol Diagn. 2011 Nov;13(6):701–706. doi: 10.1016/j.jmoldx.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.La Scola B, Raoult D. Direct identification of bacteria in positive blood culture bottles by matrix-assisted laser desorption ionisation time-of-flight mass spectrometry. PLoS One. 2009 Nov 25;4(11):e8041. doi: 10.1371/journal.pone.0008041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.La Scola B. Intact cell MALDI-TOF mass spectrometry-based approaches for the diagnosis of bloodstream infections. Expert Rev Mol Diagn. 2011 Apr;11(3):287–298. doi: 10.1586/erm.11.12. [DOI] [PubMed] [Google Scholar]
- 76.Cherkaoui A, Hibbs J, Emonet S, Tangomo M, Girard M, Francois P, Schrenzel J. Comparison of two matrix-assisted laser desorption ionization-time of flight mass spectrometry methods with conventional phenotypic identification for routine identification of bacteria to the species level. J Clin Microbiol. 2010 Apr;48(4):1169–1175. doi: 10.1128/JCM.01881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eigner U, Holfelder M, Oberdorfer K, Betz-Wild U, Bertsch D, Fahr AM. Performance of a matrix-assisted laser desorption ionization-time-of-flight mass spectrometry system for the identification of bacterial isolates in the clinical routine laboratory. Clin Lab. 2009;55(7-8):289–296. [PubMed] [Google Scholar]
- 78.Marklein G, Josten M, Klanke U, Müller E, Horré R, Maier T, Wenzel T, Kostrzewa M, Bierbaum G, Hoerauf A, Sahl HG. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for fast and reliable identification of clinical yeast isolates. J Clin Microbiol. 2009 Sep;47(9):2912–2917. doi: 10.1128/JCM.00389-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bader O, Weig M, Taverne-Ghadwal L, Lugert R, Gross U, Kuhns M. Improved clinical laboratory identification of human pathogenic yeasts by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Microbiol Infect. 2011 Sep;17(9):1359–1365. doi: 10.1111/j.1469-0691.2010.03398.x. [DOI] [PubMed] [Google Scholar]
- 80.Clerc O, Prod'hom G, Vogne C, Bizzini A, Calandra T, Greub G. Impact of matrix-assisted laser desorption ionization time-of-flight mass spectrometry on the clinical management of patients with Gram-negative bacteremia: a prospective observational study. Clin Infect Dis. 2013 Apr;56(8):1101–1117. doi: 10.1093/cid/cis1204. [DOI] [PubMed] [Google Scholar]
- 81.Huang AM, Newton D, Kunapuli A, Gandhi TN, Washer LL, Isip J, Collins CD, Nagel JL. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis. 2013 Nov;57(9):1237–1245. doi: 10.1093/cid/cit498. [DOI] [PubMed] [Google Scholar]
- 82.Tissari P, Zumla A, Tarkka E, Mero S, Savolainen L, Vaara M, Aittakorpi A, Laakso S, Lindfors M, Piiparinen H, Mäki M, Carder C, Huggett J, Gant V. Accurate and rapid identification of bacterial species from positive blood cultures with a DNA-based microarray platform: an observational study. Lancet. 2010 Jan 16;375(9710):224–230. doi: 10.1016/S0140-6736(09)61569-5. [DOI] [PubMed] [Google Scholar]
- 83.Aittakorpi A, Kuusela P, Koukila-Kähkölä P, Vaara M, Petrou M, Gant V, Mäki M. Accurate and rapid identification of Candida spp. frequently associated with fungemia by using PCR and the microarray-based Prove-it Sepsis assay. J Clin Microbiol. 2012 Nov;50(11):3635–3640. doi: 10.1128/JCM.01461-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Laakso S, Mäki M. Assessment of a semi-automated protocol for multiplex analysis of sepsis-causing bacteria with spiked whole blood samples. Microbiologyopen. 2013 Apr;2(2):284–292. doi: 10.1002/mbo3.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Altun O, Almuhayawi M, Ullberg M, Ozenci V. Clinical evaluation of the FilmArray blood culture identification panel in identification of bacteria and yeasts from positive blood culture bottles. J Clin Microbiol. 2013 Dec;51(12):4130–4136. doi: 10.1128/JCM.01835-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scott LJ. Verigene® gram-positive blood culture nucleic acid test. Mol Diagn Ther. 2013 Apr;17(2):117–122. doi: 10.1007/s40291-013-0021-z. [DOI] [PubMed] [Google Scholar]
- 87.Samuel LP, Tibbetts RJ, Agotesku A, Fey M, Hensley R, Meier FA. Evaluation of a microarray-based assay for rapid identification of Gram-positive organisms and resistance markers in positive blood cultures. J Clin Microbiol. 2013 Apr;51(4):1188–1192. doi: 10.1128/JCM.02982-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sullivan KV, Turner NN, Roundtree SS, Young S, Brock-Haag CA, Lacey D, Abuzaid S, Blecker-Shelly DL, Doern CD. Rapid detection of Gram-positive organisms by use of the Verigene Gram-positive blood culture nucleic acid test and the BacT/Alert Pediatric FAN system in a multicenter pediatric evaluation. J Clin Microbiol. 2013 Nov;51(11):3579–3584. doi: 10.1128/JCM.01224-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peters RP, van Agtmael MA, Danner SA, Savelkoul PH, Vandenbroucke-Grauls CM. New developments in the diagnosis of bloodstream infections. Lancet Infect Dis. 2004 Dec;4(12):751–760. doi: 10.1016/S1473-3099(04)01205-8. [DOI] [PubMed] [Google Scholar]
- 90.Paolucci M, Capretti MG, Dal Monte P, Corvaglia L, Landini MP, Varani S, Pession A, Faldella G, Sambri V. Laboratory diagnosis of late-onset sepsis in newborns by multiplex real-time PCR. J Med Microbiol. 2009 Apr;58(Pt 4):533–534. doi: 10.1099/jmm.0.003848-0. [DOI] [PubMed] [Google Scholar]
- 91.Wellinghausen N, Kochem AJ, Disqué C, Mühl H, Gebert S, Winter J, Matten J, Sakka SG. Diagnosis of bacteremia in whole-blood samples by use of a commercial universal 16S rRNA gene-based PCR and sequence analysis. J Clin Microbiol. 2009 Sep;47(9):2759–2765. doi: 10.1128/JCM.00567-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grif K, Heller I, Prodinger WM, Lechleitner K, Lass-Flörl C, Orth D. Improvement of detection of bacterial pathogens in normally sterile body sites with a focus on orthopedic samples by use of a commercial 16S rRNA broad-range PCR and sequence analysis. J Clin Microbiol. 2012 Jul;50(7):2250–2254. doi: 10.1128/JCM.00362-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fitting C, Parlato M, Adib-Conquy M, Memain N, Philippart F, Misset B, Monchi M, Cavaillon JM, Adrie C. DNAemia detection by multiplex PCR and biomarkers for infection in systemic inflammatory response syndrome patients. PLoS One. 2012;7(6):e38916. doi: 10.1371/journal.pone.0038916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bloos F, Sachse S, Kortgen A, Pletz MW, Lehmann M, Straube E, Riedemann NC, Reinhart K, Bauer M. Evaluation of a polymerase chain reaction assay for pathogen detection in septic patients under routine condition: an observational study. PLoS One. 2012;7(9):e46003. doi: 10.1371/journal.pone.0046003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lehmann LE, Hunfeld KP, Emrich T, Haberhausen G, Wissing H, Hoeft A, Stüber F. A multiplex real-time PCR assay for rapid detection and differentiation of 25 bacterial and fungal pathogens from whole blood samples. Med Microbiol Immunol. 2008 Sep;197(3):313–324. doi: 10.1007/s00430-007-0063-0. [DOI] [PubMed] [Google Scholar]
- 96.Regueiro BJ, Varela-Ledo E, Martinez-Lamas L, Rodriguez-Calviño J, Aguilera A, Santos A, Gomez-Tato A, Alvarez-Escudero J. Automated extraction improves multiplex molecular detection of infection in septic patients. PLoS One. 2010 Oct 13;5(10):e13387. doi: 10.1371/journal.pone.0013387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Westh H, Lisby G, Breysse F, Böddinghaus B, Chomarat M, Gant V, Goglio A, Raglio A, Schuster H, Stuber F, Wissing H, Hoeft A. Multiplex real-time PCR and blood culture for identification of bloodstream pathogens in patients with suspected sepsis. Clin Microbiol Infect. 2009 Jun;15(6):544–551. doi: 10.1111/j.1469-0691.2009.02736.x. [DOI] [PubMed] [Google Scholar]
- 98.Lehmann LE, Alvarez J, Hunfeld KP, Goglio A, Kost GJ, Louie RF, Raglio A, Regueiro BJ, Wissing H, Stüber F. Potential clinical utility of polymerase chain reaction in microbiological testing for sepsis. Crit Care Med. 2009 Dec;37(12):3085–3090. doi: 10.1097/CCM.0b013e3181b033d7. [DOI] [PubMed] [Google Scholar]
- 99.Chang SS, Hsieh WH, Liu TS, Lee SH, Wang CH, Chou HC, Yeo YH, Tseng CP, Lee CC. Multiplex PCR system for rapid detection of pathogens in patients with presumed sepsis - a systemic review and meta-analysis. PLoS One. 2013;8(5):e62323. doi: 10.1371/journal.pone.0062323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Louie RF, Tang Z, Albertson TE, Cohen S, Tran NK, Kost GJ. Multiplex polymerase chain reaction detection enhancement of bacteremia and fungemia. Crit Care Med. 2008 May;36(5):1487–1492. doi: 10.1097/CCM.0b013e31816f487c. [DOI] [PubMed] [Google Scholar]
- 101.Wallet F, Loïez C, Herwegh S, Courcol RJ. Usefulness of real-time PCR for the diagnosis of sepsis in ICU-acquired infections. Infect Disord Drug Targets. 2011 Aug;11(4):348–353. doi: 10.2174/187152611796504845. [DOI] [PubMed] [Google Scholar]
- 102.Dierkes C, Ehrenstein B, Siebig S, Linde HJ, Reischl U, Salzberger B. Clinical impact of a commercially available multiplex PCR system for rapid detection of pathogens in patients with presumed sepsis. BMC Infect Dis. 2009 Aug 11;9:126. doi: 10.1186/1471-2334-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bloos F, Hinder F, Becker K, Sachse S, Mekontso Dessap A, Straube E, Cattoir V, Brun-Buisson C, Reinhart K, Peters G, Bauer M. A multicenter trial to compare blood culture with polymerase chain reaction in severe human sepsis. Intensive Care Med. 2010 Feb;36(2):241–247. doi: 10.1007/s00134-009-1705-z. [DOI] [PubMed] [Google Scholar]
- 104.Hettwer S, Wilhelm J, Schürmann M, Ebelt H, Hammer D, Amoury M, Hofmann F, Oehme A, Wilhelms D, Kekulé AS, Klöss T, Werdan K. Microbial diagnostics in patients with presumed severe infection in the emergency department. Med Klin Intensivmed Notfmed. 2012 Feb;107(1):53–62. doi: 10.1007/s00063-011-0051-4. [DOI] [PubMed] [Google Scholar]
- 105.von Lilienfeld-Toal M, Lehmann LE, Raadts AD, Hahn-Ast C, Orlopp KS, Marklein G, Purr I, Cook G, Hoeft A, Glasmacher A, Stüber F. Utility of a commercially available multiplex real-time PCR assay to detect bacterial and fungal pathogens in febrile neutropenia. J Clin Microbiol. 2009 Aug;47(8):2405–2410. doi: 10.1128/JCM.00491-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mancini N, Clerici D, Diotti R, Perotti M, Ghidoli N, De Marco D, Pizzorno B, Emrich T, Burioni R, Ciceri F, Clementi M. Molecular diagnosis of sepsis in neutropenic patients with haematological malignancies. J Med Microbiol. 2008 May;57(Pt 5):601–604. doi: 10.1099/jmm.0.47732-0. [DOI] [PubMed] [Google Scholar]
- 107.Lamoth F, Jaton K, Prod'hom G, Senn L, Bille J, Calandra T, Marchetti O. Multiplex blood PCR in combination with blood cultures for improvement of microbiological documentation of infection in febrile neutropenia. J Clin Microbiol. 2010 Oct;48(10):3510–3516. doi: 10.1128/JCM.00147-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guido M, Quattrocchi M, Zizza A, Pasanisi G, Pavone V, Lobreglio G, Gabutti G, De Donno A. Molecular approaches in the diagnosis of sepsis in neutropenic patients with haematological malignances. J Prev Med Hyg. 2012 Jun;53(2):104–108. [PubMed] [Google Scholar]
- 109.Idelevich E, Niederbracht Y, Penner H, Tafelski S, Nachtigall I, Berdel WE, Peters G, Silling G, Becker K. Utility of the LightCycler® SeptiFast test in haematologic patients with neutropenic fever or sepsis: a randomized controlled trial. Berlin, Germany: European Conference of Clinical Microbiology and Infectious Diseases (ECCMID); 2013. [Google Scholar]
- 110.Mancini N, Carletti S, Ghidoli N, Cichero P, Ossi CM, Ieri R, Poli E, Burioni R, Clementi M. Molecular diagnosis of polymicrobial sepsis. J Clin Microbiol. 2009 Apr;47(4):1274–1275. doi: 10.1128/JCM.00011-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Steinmann J, Buer J, Rath PM, Paul A, Saner F. Invasive aspergillosis in two liver transplant recipients: diagnosis by SeptiFast. Transpl Infect Dis. 2009 Apr;11(2):175–178. doi: 10.1111/j.1399-3062.2009.00367.x. [DOI] [PubMed] [Google Scholar]
- 112.Wallet F, Nseir S, Baumann L, Herwegh S, Sendid B, Boulo M, Roussel-Delvallez M, Durocher AV, Courcol RJ. Preliminary clinical study using a multiplex real-time PCR test for the detection of bacterial and fungal DNA directly in blood. Clin Microbiol Infect. 2010 Jun;16(6):774–779. doi: 10.1111/j.1469-0691.2009.02940.x. [DOI] [PubMed] [Google Scholar]
- 113.Tschiedel E, Steinmann J, Buer J, Onnebrink JG, Felderhoff-Müser U, Rath PM, Dohna-Schwake C. Results and relevance of molecular detection of pathogens by SeptiFast--a retrospective analysis in 75 critically ill children. Klin Padiatr. 2012 Jan;224(1):12–16. doi: 10.1055/s-0031-1285878. [DOI] [PubMed] [Google Scholar]
- 114.Tafelski S, Nachtigall I, Idelevich EA, Silling G, Becker K, Faust J, Trefzer T, Tamarkin A, Adam T, Bereswill S, Deja M, Spies C. Impact of SeptiFast for pathogen detection in critical-care patients with sepsis: results from a randomized controlled clinical trial. Berlin, Germany: European Conference of Clinical Microbiology and Infectious Diseases (ECCMID); 2013. [Google Scholar]
- 115.Cambau E, Courcol R, Veerabudun V, Bretagne S, Durand-Zaleski I, Bastuji-Garin S, EVAMICA group . Does performing DNA detection in blood improve the microbial diagnosis of severe infections? First results of the EVAMICA study. Berlin, Germany: European Conference of Clinical Microbiology and Infectious Diseases (ECCMID); 2013. [Google Scholar]
- 116.Tran NK, Greenhalgh DG, Palmieri TL, Kost GJ. Multiplex PCR pathogen detection in two severely burned patients with suspected septicemia. J Burn Care Res. 2011 Nov-Dec;32(6):e172–e177. doi: 10.1097/BCR.0b013e318231c140. [DOI] [PubMed] [Google Scholar]
- 117.Rath PM, Saner F, Paul A, Lehmann N, Steinmann E, Buer J, Steinmann J. Multiplex PCR for rapid and improved diagnosis of bloodstream infections in liver transplant recipients. J Clin Microbiol. 2012 Jun;50(6):2069–2071. doi: 10.1128/JCM.00745-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chaidaroglou A, Manoli E, Marathias E, Gkouziouta A, Saroglou G, Alivizatos P, Degiannis D. Use of a multiplex polymerase chain reaction system for enhanced bloodstream pathogen detection in thoracic transplantation. J Heart Lung Transplant. 2013 Jul;32(7):707–713. doi: 10.1016/j.healun.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 119.Lucignano B, Ranno S, Liesenfeld O, Pizzorno B, Putignani L, Bernaschi P, Menichella D. Multiplex PCR allows rapid and accurate diagnosis of bloodstream infections in newborns and children with suspected sepsis. J Clin Microbiol. 2011 Jun;49(6):2252–2258. doi: 10.1128/JCM.02460-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Torres-Martos E, Pérez-Ruiz M, Pedrosa-Corral I, Peña-Caballero M, Jiménez-Valera MM, Pérez-Ramírez MD, Navarro-Marí JM. [Evaluation of the LightCycler® SeptiFast test in newborns and infants with clinical suspicion of sepsis] Enferm Infecc Microbiol Clin. 2013 Jun-Jul;31(6):375–379. doi: 10.1016/j.eimc.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 121.Kasper DC, Altiok I, Mechtler TP, Böhm J, Straub J, Langgartner M, Pollak A, Herkner KR, Berger A. Molecular detection of late-onset neonatal sepsis in premature infants using small blood volumes: proof-of-concept. Neonatology. 2013;103(4):268–273. doi: 10.1159/000346365. [DOI] [PubMed] [Google Scholar]
- 122.Casalta JP, Gouriet F, Roux V, Thuny F, Habib G, Raoult D. Evaluation of the LightCycler SeptiFast test in the rapid etiologic diagnostic of infectious endocarditis. Eur J Clin Microbiol Infect Dis. 2009 Jun;28(6):569–573. doi: 10.1007/s10096-008-0672-6. [DOI] [PubMed] [Google Scholar]
- 123.Lefort A, Chartier L, Sendid B, Wolff M, Mainardi JL, Podglajen I, Desnos-Ollivier M, Fontanet A, Bretagne S, Lortholary O. Diagnosis, management and outcome of Candida endocarditis. Clin Microbiol Infect. 2012 Apr;18(4):E99–E109. doi: 10.1111/j.1469-0691.2012.03764.x. [DOI] [PubMed] [Google Scholar]
- 124.Lehmann LE, Hauser S, Malinka T, Klaschik S, Weber SU, Schewe JC, Stüber F, Book M. Rapid qualitative urinary tract infection pathogen identification by SeptiFast real-time PCR. PLoS One. 2011 Feb 16;6(2):e17146. doi: 10.1371/journal.pone.0017146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Appenrodt B, Lehmann LE, Thyssen L, Gentemann M, Rabe C, Molitor E, Trebicka J, Stüber F, Sauerbruch T. Is detection of bacterial DNA in ascitic fluid of clinical relevance? Eur J Gastroenterol Hepatol. 2010 Dec;22(12):1487–1494. doi: 10.1097/MEG.0b013e328340c43a. [DOI] [PubMed] [Google Scholar]
- 126.Fernández AL, Varela E, Martínez L, Martínez A, Sierra J, González-Juanatey JR, Regueiro B. Evaluation of a multiplex real-time PCR assay for detecting pathogens in cardiac valve tissue in patients with endocarditis. Rev Esp Cardiol. 2010 Oct;63(10):1205–1208. doi: 10.1016/s1885-5857(10)70236-x. [DOI] [PubMed] [Google Scholar]
- 127.Leitner E, Kessler HH, Spindelboeck W, Hoenigl M, Putz-Bankuti C, Stadlbauer-Köllner V, Krause R, Grisold AJ, Feierl G, Stauber RE. Comparison of two molecular assays with conventional blood culture for diagnosis of sepsis. J Microbiol Methods. 2013 Mar;92(3):253–255. doi: 10.1016/j.mimet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 128.Schreiber J, Nierhaus A, Braune SA, de Heer G, Kluge S. Comparison of three different commercial PCR assays for the detection of pathogens in critically ill sepsis patients. Med Klin Intensivmed Notfmed. 2013 May;108(4):311–318. doi: 10.1007/s00063-013-0227-1. [DOI] [PubMed] [Google Scholar]
- 129.Hansen T, Skånseng B, Hoorfar J, Löfström C. Evaluation of direct 16S rDNA sequencing as a metagenomics-based approach to screening bacteria in bottled water. Biosecur Bioterror. 2013 Sep;11(Suppl 1):S158–S165. doi: 10.1089/bsp.2012.0073. [DOI] [PubMed] [Google Scholar]
- 130.Handschur M, Karlic H, Hertel C, Pfeilstöcker M, Haslberger AG. Preanalytic removal of human DNA eliminates false signals in general 16S rDNA PCR monitoring of bacterial pathogens in blood. Comp Immunol Microbiol Infect Dis. 2009 May;32(3):207–219. doi: 10.1016/j.cimid.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 131.Wiesinger-Mayr H, Jordana-Lluch E, Martró E, Schoenthaler S, Noehammer C. Establishment of a semi-automated pathogen DNA isolation from whole blood and comparison with commercially available kits. J Microbiol Methods. 2011 Jun;85(3):206–213. doi: 10.1016/j.mimet.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 132.Torres MJ, Criado A, Ruiz M, Llanos AC, Palomares JC, Aznar J. Improved real-time PCR for rapid detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis clinical isolates. Diagn Microbiol Infect Dis. 2003 Mar;45(3):207–212. doi: 10.1016/s0732-8893(02)00521-7. [DOI] [PubMed] [Google Scholar]
- 133.Leinberger DM, Grimm V, Rubtsova M, Weile J, Schröppel K, Wichelhaus TA, Knabbe C, Schmid RD, Bachmann TT. Integrated detection of extended-spectrum-beta-lactam resistance by DNA microarray-based genotyping of TEM, SHV, and CTX-M genes. J Clin Microbiol. 2010 Feb;48(2):460–471. doi: 10.1128/JCM.00765-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Raoult D, Fournier PE, Drancourt M. What does the future hold for clinical microbiology? Nat Rev Microbiol. 2004 Feb;2(2):151–159. doi: 10.1038/nrmicro820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Karumaa S, Kärpänoja P, Sarkkinen H. PCR identification of bacteria in blood culture does not fit the daily workflow of a routine microbiology laboratory. J Clin Microbiol. 2012 Mar;50(3):1031–1033. doi: 10.1128/JCM.01271-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Murray PR, Masur H. Current approaches to the diagnosis of bacterial and fungal bloodstream infections in the intensive care unit. Crit Care Med. 2012 Dec;40(12):3277–3282. doi: 10.1097/CCM.0b013e318270e771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Perez KK, Olsen RJ, Musick WL, Cernoch PL, Davis JR, Land GA, Peterson LE, Musser JM. Integrating rapid pathogen identification and antimicrobial stewardship significantly decreases hospital costs. Arch Pathol Lab Med. 2013 Sep;137(9):1247–1254. doi: 10.5858/arpa.2012-0651-OA. [DOI] [PubMed] [Google Scholar]
- 138.Hebart H, Klingspor L, Klingebiel T, Loeffler J, Tollemar J, Ljungman P, Wandt H, Schaefer-Eckart K, Dornbusch HJ, Meisner C, Engel C, Stenger N, Mayer T, Ringden O, Einsele H. A prospective randomized controlled trial comparing PCR-based and empirical treatment with liposomal amphotericin B in patients after allo-SCT. Bone Marrow Transplant. 2009 Apr;43(7):553–561. doi: 10.1038/bmt.2008.355. [DOI] [PubMed] [Google Scholar]
- 139.Stoneking LR, Patanwala AE, Winkler JP, Fiorello AB, Lee ES, Olson DP, Wolk DM. Would earlier microbe identification alter antibiotic therapy in bacteremic emergency department patients? J Emerg Med. 2013 Jan;44(1):1–8. doi: 10.1016/j.jemermed.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 140.Pletz MW, Wellinghausen N, Welte T. Will polymerase chain reaction (PCR)-based diagnostics improve outcome in septic patients? A clinical view. Intensive Care Med. 2011 Jul;37(7):1069–1076. doi: 10.1007/s00134-011-2245-x. [DOI] [PubMed] [Google Scholar]
- 141.Heenen S, Jacobs F, Vincent JL. Antibiotic strategies in severe nosocomial sepsis: why do we not de-escalate more often? Crit Care Med. 2012 May;40(5):1404–1409. doi: 10.1097/CCM.0b013e3182416ecf. [DOI] [PubMed] [Google Scholar]
- 142.Mussap M, Molinari MP, Senno E, Gritti P, Soro B, Mannelli S, Fabris C. New diagnostic tools for neonatal sepsis: the role of a real-time polymerase chain reaction for the early detection and identification of bacterial and fungal species in blood samples. J Chemother. 2007 Oct;19(Suppl 2):31–34. doi: 10.1080/1120009x.2007.11782441. [DOI] [PubMed] [Google Scholar]
- 143.Vince A, Lepej SZ, Barsić B, Dusek D, Mitrović Z, Serventi-Seiwerth R, Labar B. LightCycler SeptiFast assay as a tool for the rapid diagnosis of sepsis in patients during antimicrobial therapy. J Med Microbiol. 2008 Oct;57(Pt 10):1306–1307. doi: 10.1099/jmm.0.47797-0. [DOI] [PubMed] [Google Scholar]
- 144.Lodes U, Bohmeier B, Lippert H, König B, Meyer F. PCR-based rapid sepsis diagnosis effectively guides clinical treatment in patients with new onset of SIRS. Langenbecks Arch Surg. 2012 Mar;397(3):447–455. doi: 10.1007/s00423-011-0870-z. [DOI] [PubMed] [Google Scholar]
- 145.Lehmann LE, Hunfeld KP, Steinbrucker M, Brade V, Book M, Seifert H, Bingold T, Hoeft A, Wissing H, Stüber F. Improved detection of blood stream pathogens by real-time PCR in severe sepsis. Intensive Care Med. 2010 Jan;36(1):49–56. doi: 10.1007/s00134-009-1608-z. [DOI] [PubMed] [Google Scholar]
- 146.Yanagihara K, Kitagawa Y, Tomonaga M, Tsukasaki K, Kohno S, Seki M, Sugimoto H, Shimazu T, Tasaki O, Matsushima A, Ikeda Y, Okamoto S, Aikawa N, Hori S, Obara H, Ishizaka A, Hasegawa N, Takeda J, Kamihira S, Sugahara K, Asari S, Murata M, Kobayashi Y, Ginba H, Sumiyama Y, Kitajima M. Evaluation of pathogen detection from clinical samples by real-time polymerase chain reaction using a sepsis pathogen DNA detection kit. Crit Care. 2010;14(4):R159. doi: 10.1186/cc9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Frye AM, Baker CA, Rustvold DL, Heath KA, Hunt J, Leggett JE, Oethinger M. Clinical impact of a real-time PCR assay for rapid identification of staphylococcal bacteremia. J Clin Microbiol. 2012 Jan;50(1):127–133. doi: 10.1128/JCM.06169-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bauer KA, West JE, Balada-Llasat JM, Pancholi P, Stevenson KB, Goff DA. An antimicrobial stewardship program's impact with rapid polymerase chain reaction methicillin-resistant Staphylococcus aureus/S. aureus blood culture test in patients with S. aureus bacteremia. Clin Infect Dis. 2010 Nov 1;51(9):1074–1080. doi: 10.1086/656623. [DOI] [PubMed] [Google Scholar]
- 149.Holtzman C, Whitney D, Barlam T, Miller NS. Assessment of impact of peptide nucleic acid fluorescence in situ hybridization for rapid identification of coagulase-negative staphylococci in the absence of antimicrobial stewardship intervention. J Clin Microbiol. 2011 Apr;49(4):1581–1582. doi: 10.1128/JCM.02461-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Charani E, Edwards R, Sevdalis N, Alexandrou B, Sibley E, Mullett D, Franklin BD, Holmes A. Behavior change strategies to influence antimicrobial prescribing in acute care: a systematic review. Clin Infect Dis. 2011 Oct;53(7):651–662. doi: 10.1093/cid/cir445. [DOI] [PubMed] [Google Scholar]
- 151.Roberts RR, Hota B, Ahmad I, Scott RD, 2nd, Foster SD, Abbasi F, Schabowski S, Kampe LM, Ciavarella GG, Supino M, Naples J, Cordell R, Levy SB, Weinstein RA. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis. 2009 Oct 15;49(8):1175–1184. doi: 10.1086/605630. [DOI] [PubMed] [Google Scholar]
- 152.Haberland CA, Benitz WE, Sanders GD, Pietzsch JB, Yamada S, Nguyen L, Garber AM. Perinatal screening for group B streptococci: cost-benefit analysis of rapid polymerase chain reaction. Pediatrics. 2002 Sep;110(3):471–480. doi: 10.1542/peds.110.3.471. [DOI] [PubMed] [Google Scholar]
- 153.Burchardi H, Schneider H. Economic aspects of severe sepsis: a review of intensive care unit costs, cost of illness and cost effectiveness of therapy. Pharmacoeconomics. 2004;22(12):793–813. doi: 10.2165/00019053-200422120-00003. [DOI] [PubMed] [Google Scholar]
- 154.Forrest GN, Mehta S, Weekes E, Lincalis DP, Johnson JK, Venezia RA. Impact of rapid in situ hybridization testing on coagulase-negative staphylococci positive blood cultures. J Antimicrob Chemother. 2006 Jul;58(1):154–158. doi: 10.1093/jac/dkl146. [DOI] [PubMed] [Google Scholar]
- 155.Lehmann LE, Herpichboehm B, Kost GJ, Kollef MH, Stüber F. Cost and mortality prediction using polymerase chain reaction pathogen detection in sepsis: evidence from three observational trials. Crit Care. 2010;14(5):R186. doi: 10.1186/cc9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Forrest G. Role of antifungal susceptibility testing in patient management. Curr Opin Infect Dis. 2006 Dec;19(6):538–543. doi: 10.1097/QCO.0b013e328010682c. [DOI] [PubMed] [Google Scholar]
- 157.Tsalik EL, Jaggers LB, Glickman SW, Langley RJ, van Velkinburgh JC, Park LP, Fowler VG, Cairns CB, Kingsmore SF, Woods CW. Discriminative value of inflammatory biomarkers for suspected sepsis. J Emerg Med. 2012 Jul;43(1):97–106. doi: 10.1016/j.jemermed.2011.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Moyer MW. New biomarkers sought for improving sepsis management and care. Nat Med. 2012 Jul 6;18(7):999. doi: 10.1038/nm0712-999. [DOI] [PubMed] [Google Scholar]
- 159.Su L, Han B, Liu C, Liang L, Jiang Z, Deng J, Yan P, Jia Y, Feng D, Xie L. Value of soluble TREM-1, procalcitonin, and C-reactive protein serum levels as biomarkers for detecting bacteremia among sepsis patients with new fever in intensive care units: a prospective cohort study. BMC Infect Dis. 2012 Jul 18;12:157. doi: 10.1186/1471-2334-12-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Backes Y, van der Sluijs KF, Mackie DP, Tacke F, Koch A, Tenhunen JJ, Schultz MJ. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: a systematic review. Intensive Care Med. 2012 Sep;38(9):1418–1428. doi: 10.1007/s00134-012-2613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Al Shuaibi M, Bahu RR, Chaftari AM, Al Wohoush I, Shomali W, Jiang Y, Debiane L, Raad S, Jabbour J, Al Akhrass F, Hachem RY, Raad I. Pro-adrenomedullin as a novel biomarker for predicting infections and response to antimicrobials in febrile patients with hematologic malignancies. Clin Infect Dis. 2013 Apr;56(7):943–950. doi: 10.1093/cid/cis1029. [DOI] [PubMed] [Google Scholar]
- 162.Langley RJ, Tsalik EL, van Velkinburgh JC, Glickman SW, Rice BJ, Wang C, Chen B, Carin L, Suarez A, Mohney RP, Freeman DH, Wang M, You J, Wulff J, Thompson JW, Moseley MA, Reisinger S, Edmonds BT, Grinnell B, Nelson DR, Dinwiddie DL, Miller NA, Saunders CJ, Soden SS, Rogers AJ, Gazourian L, Fredenburgh LE, Massaro AF, Baron RM, Choi AM, Corey GR, Ginsburg GS, Cairns CB, Otero RM, Fowler VG, Jr., Rivers EP, Woods CW, Kingsmore SF. An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci Transl Med. 2013 Jul 24;5(195):195ra95. doi: 10.1126/scitranslmed.3005893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010 Mar 4;464(7285):104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sutherland A, Thomas M, Brandon RA, Brandon RB, Lipman J, Tang B, McLean A, Pascoe R, Price G, Nguyen T, Stone G, Venter D. Development and validation of a novel molecular biomarker diagnostic test for the early detection of sepsis. Crit Care. 2011 Jun 20;15(3):R149. doi: 10.1186/cc10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Neely LA, Audeh M, Phung NA, Min M, Suchocki A, Plourde D, Blanco M, Demas V, Skewis LR, Anagnostou T, Coleman JJ, Wellman P, Mylonakis E, Lowery TJ. T2 magnetic resonance enables nanoparticle-mediated rapid detection of candidemia in whole blood. Sci Transl Med. 2013 Apr 24;5(182):182ra54. doi: 10.1126/scitranslmed.3005377. [DOI] [PubMed] [Google Scholar]
- 166.Weigl BH, Gaydos CA, Kost G, Beyette FR, Jr., Sabourin S, Rompalo A, de Los Santos T, McMullan JT, Haller J. The Value of Clinical Needs Assessments for Point-of-Care Diagnostics. Point Care. 2012 Jun;11(2):108–113. doi: 10.1097/POC.0b013e31825a241e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kost GJ, Mecozzi DM, Brock TK, Curtis CM. Assessing Point-of-Care Device Specifications and Needs for Pathogen Detection in Emergencies and Disasters. Point Care. 2012 Jun 1;11(2):119–125. doi: 10.1097/POC.0b013e31825a25cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Leuthner KD, Doern GV. Antimicrobial stewardship programs. J Clin Microbiol. 2013 Dec;51(12):3916–3920. doi: 10.1128/JCM.01751-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hall L, Le Febre, KM, Deml SM, Wohlfiel SL, Wengenack NL. Evaluation of the Yeast Traffic Light PNA FISH probes for identification of Candida species from positive blood cultures. J Clin Microbiol. 2012 Apr;50(4):1446–1448. doi: 10.1128/JCM.06148-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kelley PG, Grabsch EA, Farrell J, Xie S, Montgomery J, Mayall B, Howden BP. Evaluation of the Xpert™ MRSA/SA Blood Culture assay for the detection of Staphylococcus aureus including strains with reduced vancomycin susceptibility from blood culture specimens. Diagn Microbiol Infect Dis. 2011 Jul;70(3):404–407. doi: 10.1016/j.diagmicrobio.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 171.Molina JM, Córdoba J, Ramírez P, Gobernado M. [Automatic detection of bacterial and fungal infections in blood] Enferm Infecc Microbiol Clin. 2008 Jul;26(Suppl 9):75–80. doi: 10.1016/S0213-005X(08)76544-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gaibani P, Rossini G, Ambretti S, Gelsomino F, Pierro AM, Varani S, Paolucci M, Landini MP, Sambri V. Blood culture systems: rapid detection–how and why? Int J Antimicrob Agents. 2009;34(Suppl 4):S13–S15. doi: 10.1016/S0924-8579(09)70559-X. [DOI] [PubMed] [Google Scholar]
- 173.Lodes U, Meyer F, König B, Lippert H. [Microbiological sepsis screening in surgical ICU patients with the "lightCycler" Septifast test–a pilot study] Zentralbl Chir. 2009 Jun;134(3):249–253. doi: 10.1055/s-0028-1098776. [DOI] [PubMed] [Google Scholar]
- 174.Figueroa JR, Ortiz J, Morales I. Use of the LightCycler SeptiFast test for rapid etiologic diagnosis of nosocomial infection in gynecological sepsis. Gynecol Obstet Invest. 2010;70(3):215–216. doi: 10.1159/000318868. [DOI] [PubMed] [Google Scholar]
- 175.Avolio M, Diamante P, Zamparo S, Modolo ML, Grosso S, Zigante P, Tosoni N, De Rosa R, Stano P, Camporese A. Molecular identification of bloodstream pathogens in patients presenting to the emergency department with suspected sepsis. Shock. 2010 Jul;34(1):27–30. doi: 10.1097/SHK.0b013e3181d49299. [DOI] [PubMed] [Google Scholar]
- 176.Maubon D, Hamidfar-Roy R, Courby S, Vesin A, Maurin M, Pavese P, Ravanel N, Bulabois CE, Brion JP, Pelloux H, Timsit JF. Therapeutic impact and diagnostic performance of multiplex PCR in patients with malignancies and suspected sepsis. J Infect. 2010 Oct;61(4):335–342. doi: 10.1016/j.jinf.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 177.Lodes U, Lippert H, Meyer F. [Molecular biological sepsis diagnostic using multiplex PCR in surgical intensive care as suitable alternative to conventional microbial culture – a representative overview] Zentralbl Chir. 2011 Apr;136(2):135–142. doi: 10.1055/s-0031-1271407. [DOI] [PubMed] [Google Scholar]
- 178.Bravo D, Blanquer J, Tormo M, Aguilar G, Borrás R, Solano C, Clari MA, Costa E, Muñoz-Cobo B, Argüeso M, Pineda JR, Navarro D. Diagnostic accuracy and potential clinical value of the LightCycler SeptiFast assay in the management of bloodstream infections occurring in neutropenic and critically ill patients. Int J Infect Dis. 2011 May;15(5):e326–e331. doi: 10.1016/j.ijid.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 179.Dark P, Dunn G, Chadwick P, Young D, Bentley A, Carlson G, Warhurst G. The clinical diagnostic accuracy of rapid detection of healthcare-associated bloodstream infection in intensive care using multipathogen real-time PCR technology. BMJ Open. 2011 Jun 30;1(1):e000181. doi: 10.1136/bmjopen-2011-000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tran NK, Wisner DH, Albertson TE, Cohen S, Greenhalgh D, Palmieri TL, Polage C, Kost GJ. Multiplex polymerase chain reaction pathogen detection in patients with suspected septicemia after trauma, emergency, and burn surgery. Surgery. 2012 Mar;151(3):456–463. doi: 10.1016/j.surg.2011.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Popov DA, Vostrikova T. [The first experience of application of PCR techniques in real-time mode to diagnose bacteriemia during postoperational period in cardiosurgery patients] Klinicheskaia Laboratornaia Diagnostika. 2011:49–52. [PubMed] [Google Scholar]
- 182.Sancho-Tello S, Bravo D, Borrás R, Costa E, Muñoz-Cobo B, Navarro D. Performance of the LightCycler SeptiFast test Mgrade in detecting microbial pathogens in purulent fluids. J Clin Microbiol. 2011 Aug;49(8):2988–2991. doi: 10.1128/JCM.00359-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Palomares JC, Bernal S, Marín M, Holgado VP, Castro C, Morales WP, Martin E. Molecular diagnosis of Aspergillus fumigatus endocarditis. Diagn Microbiol Infect Dis. 2011 Aug;70(4):534–537. doi: 10.1016/j.diagmicrobio.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 184.Martínez-Lamas L, Pérez del Molino ML, Pardo F, Varela E, Regueiro BJ. [Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry vs conventional methods in the identification of Candida non-albicans] Enferm Infecc Microbiol Clin. 2011 Oct;29(8):568–572. doi: 10.1016/j.eimc.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 185.Mencacci A, Leli C, Cardaccia A, Montagna P, Moretti A, Bietolini C, Meucci M, Perito S, Cenci E, Bistoni F. Comparison of conventional culture with SeptiFast real-time PCR for microbial pathogen detection in clinical specimens other than blood. J Med Microbiol. 2011 Dec;60(Pt 12):1774–1778. doi: 10.1099/jmm.0.034280-0. [DOI] [PubMed] [Google Scholar]
- 186.Mencacci A, Leli C, Cardaccia A, Meucci M, Moretti A, D'Alò F, Farinelli S, Pagliochini R, Barcaccia M, Bistoni F. Procalcitonin predicts real-time PCR results in blood samples from patients with suspected sepsis. PLoS One. 2012;7(12):e53279. doi: 10.1371/journal.pone.0053279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dark P, Wilson C, Blackwood B, McAuley DF, Perkins GD, McMullan R, Gates S, Warhurst G. Accuracy of LightCycler(R) SeptiFast for the detection and identification of pathogens in the blood of patients with suspected sepsis: a systematic review protocol. BMJ Open. 2012;2(1):e000392. doi: 10.1136/bmjopen-2011-000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dubská L, Vyskočilová M, Minaříková D, Jelínek P, Tejkalová R, Valík D. LightCycler SeptiFast technology in patients with solid malignancies: clinical utility for rapid etiologic diagnosis of sepsis. Crit Care. 2012 Jan 17;16(1):404. doi: 10.1186/cc10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pasqualini L, Mencacci A, Leli C, Montagna P, Cardaccia A, Cenci E, Montecarlo I, Pirro M, di Filippo F, Cistaro E, Schillaci G, Bistoni F, Mannarino E. Diagnostic performance of a multiple real-time PCR assay in patients with suspected sepsis hospitalized in an internal medicine ward. J Clin Microbiol. 2012 Apr;50(4):1285–1288. doi: 10.1128/JCM.06793-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lanoix JP, Schmit JL, Douadi Y. Bacterial lung sepsis in patients with febrile neutropenia. Curr Opin Pulm Med. 2012 May;18(3):175–180. doi: 10.1097/MCP.0b013e328351f8e8. [DOI] [PubMed] [Google Scholar]
- 191.Mencacci A, Leli C, Montagna P, Cardaccia A, Meucci M, Bietolini C, Cenci E, Pasticci MB, Bistoni F. Diagnosis of infective endocarditis: comparison of the LightCycler SeptiFast real-time PCR with blood culture. J Med Microbiol. 2012 Jun;61(Pt 6):881–883. doi: 10.1099/jmm.0.040113-0. [DOI] [PubMed] [Google Scholar]
- 192.Ahmad S, Khan Z. Invasive candidiasis: a review of nonculture-based laboratory diagnostic methods. Indian J Med Microbiol. 2012 Jul-Sep;30(3):264–269. doi: 10.4103/0255-0857.99482. [DOI] [PubMed] [Google Scholar]
- 193.Mauro MV, Cavalcanti P, Perugini D, Noto A, Sperlì D, Giraldi C. Diagnostic utility of LightCycler SeptiFast and procalcitonin assays in the diagnosis of bloodstream infection in immunocompromised patients. Diagn Microbiol Infect Dis. 2012 Aug;73(4):308–311. doi: 10.1016/j.diagmicrobio.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 194.Paolucci M, Stanzani M, Melchionda F, Tolomelli G, Castellani G, Landini MP, Varani S, Lewis RE, Sambri V. Routine use of a real-time polymerase chain reaction method for detection of bloodstream infections in neutropaenic patients. Diagn Microbiol Infect Dis. 2013 Feb;75(2):130–134. doi: 10.1016/j.diagmicrobio.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 195.Fernández-Cruz A, Marín M, Kestler M, Alcalá L, Rodriguez-Créixems M, Bouza E. The value of combining blood culture and SeptiFast data for predicting complicated bloodstream infections caused by Gram-positive bacteria or Candida species. J Clin Microbiol. 2013 Apr;51(4):1130–1136. doi: 10.1128/JCM.02882-12. [DOI] [PMC free article] [PubMed] [Google Scholar]