Abstract
Human papillomavirus (HPV) is the well-known second most cause of cervical cancer in women worldwide. According to the WHO survey, 70% of the total cervical cancers are associated with types HPV 16 and 18. Presently used prophylactic vaccine for HPV contains mainly capsid protein of L1 virus like particles (VLPs). Correct folding of VLPs and display of neutralizing epitopes are the major constraint for VLP-based vaccines. Further, monoclonal antibodies (mAbs) play a vital role in developing therapeutics and diagnostics. mAbs are also useful for the demonstration of VLP conformation, virus typing and product process assessment as well. In the present study, we have explored the usefulness of mAbs generated against sf-9 expressed HPV 16 VLPs demonstrated as type-specific and conformational dependent against HPV 16 VLPs by ELISA. High affinity and high pseudovirion neutralization titer of mAbs indicated their potential for the development of prophylactic vaccines for HPV. Also, the type-specific and conformational reactivity of the mAbs to HPV 16 VLPs in sf-9 cells by immunofluorescence assay proved their diagnostic potential.
Keywords: HPV 16 VLP, monoclonal antibody, pseudovirus neutralization assay
Introduction
Human papillomavirus type 16 (HPV 16) has been reported as a major cause of a wide range of oncogenic malignancies which includes cervical, anogenital, head, and neck cancers [1–3]. The high-risk HPV subtypes were identified in almost all cervical cancers, and it was estimated that globally about 70% of cervical cancers are due to types 16 and 18 HPV [4]. Its wide spread to various continents has become a major focus for the past two decades. The structure and biology of HPV has become a major challenge in understanding the disease. The sequence similarity between the genome of different serotypes of HPV is liable in understanding the infection and pathogenesis of the disease. Conformational and type-specific characterized mAbs have been developed previously to various serotypes of HPV to study the infection [5, 6]. Monoclonal antibodies (mAbs) have been widely applied to study the viral characteristics relating to their structure or host–cell interactions and in designing potential vaccines [7, 8].
Human papillomavirions are nonenveloped, icosahedral, and smaller in size about 55 nm in diameter [9]. The capsid is made up of two viral proteins, a major L1 capsid protein (53 to 57 kDa) and a minor L2 capsid protein (76 to 86 kDa). It has been proved that neutralizing epitopes are presented on the surface of L1 major capsid protein. Both conformational and linear epitopes have been identified on the surface of HPV 16 VLPs. The major capsid proteins of HPV L1 are being capable of forming virus capsid in vitro by self-assembly into virus-like particles (VLPs). These VLPs are immunogenic in humans and are capable of inducing neutralizing antibodies [10, 11]. Presently available prophylactic vaccines for HPV are composed of VLPs formed by self-assembly of the recombinant L1 capsid proteins of HPV 6, HPV 11, HPV 16, and HPV 18 [10, 12].
Monoclonal antibodies have been emerging as an important therapeutic modality for the treatment of cancer due to their high specificity, low toxicity, and the ability to activate components of the immune system. Several reports have demonstrated that monoclonal antibodies generated against HPV showed binding to the HPV VLPs of both conformational and linear epitopes. It is provided as a valuable tool for the immunological analysis of capsids and capsomeres which are produced by recombinant methods [13] and serological studies [14–16]. Neutralizing mAbs to HPV are mostly type-specific and conformation-dependent due to the hypervariable nature of their respective epitopes, which typically reside in the surface-exposed loop regions of the L1 protein [6, 11, 17, 18]. Most of the cross-reactive mAbs were generally found to be non-neutralizing as described previously [17, 19].
The present study describes the detailed characterization of mAbs developed against HPV 16 VLPs. The mAbs were characterized using a panel of tests including antigen-specific enzyme-linked immunosorbant assay (ELISA), neutralization and immunoassay. Selected mAbs were found to be conformational-specific and has shown potent neutralization toward HPV 16.
Materials and methods
Production of HPV 16 VLPs using baculo viral system
HPV 16 VLPs were produced using sf-9 insect cells, according to Shang-Zhong et al. and Zheng et al. [20, 21].
Production of monoclonal antibodies
Four- to six-week-old female Balb/c mice were immunized (2 μg/dosage/animal) with recombinant HPV type-16 (VLPs) expressed in sf-9 (Spodoptera frugiperda) cells according to Le Cann et al. [22]. Fused hybrids were generated by the conventional hybridoma production techniques [23]. Two rounds of limiting dilution were performed to establish the monoclonality of the progeny hybridomas. Different ELISAs were performed for the selection of best reacting mAbs against HPV 16 VLPs.
Specificity of mAbs to conformational VLPs by ELISA
The type-specific and conformational reactivity of the mAbs was performed by HPV VLP ELISA [24]. Briefly, HPV VLPs 16, 18, 31, and 52 (100 ng/well) were coated onto Maxisorp™ 96-well microtiter plates (Nunc, Denmark) in PBS (pH 7.2) and incubated at 4 °C for overnight. The free sites were blocked using 2% (w/v) skimmed milk solution in PBS containing 0.05% (v/v) Tween-20 (PBST), at 37 °C for 2 h. ELISA plates were washed thrice with PBST. 100 µl of culture supernatants of mAbs were added and incubated at 37 °C for 2 h. After incubation, plates were washed and incubated at 37 °C for 1 h with the secondary conjugate, sheep anti-mouse IgG peroxidase (1:25,000) (Sigma-Aldrich). The reaction was developed with 3,3´,5,5´-tetramethylbenzidine (TMB, Sigma-Aldrich, USA). Absorbance was read at 450 nm using ELISA plate reader (Molecular Devices, USA). Immunized mice sera and conformational type-specific antibodies were used as positive controls (H16V5, H18J4, H31A6, H52B4) (kindly provided by Professor Neil D. Christensen, Gittlen Cancer Research Foundation, Pennsylvania State University, Hershey, PA, USA).
Binding activity of mAbs to linear epitopes by ELISA
All mAbs were tested for their reactivity toward linear epitopes of HPV 16 VLPs by ELISA according to Rao et al. [24]. Briefly, HPV VLPs 16, 18, 31, and 52 (100 ng/well) were coated onto Maxisorp™ 96-well microtiter plates in carbonate buffer (pH 9) and incubated at 4 °C overnight. The unbound free sites were blocked using 2% (w/v) skimmed milk solution in PBS and incubated, at 37 °C for 2 h. ELISA plates were washed thrice with PBST. 100 µl of selected mAbs culture supernatants were added and incubated at 37 °C for 2 h. Conformational and type-specific antibodies H16V5, H18J4, H31A6, and H52B4 were used as a positive control for this test. After incubation, plates were washed with PBST and incubated at 37 °C for 1 h with sheep anti-mouse IgG peroxidase (1:25,000) (Sigma-Aldrich). The reaction was developed with the TMB substrate and absorbance was read at 450 nm using ELISA plate reader.
Binding activity of mAbs to capsomeres by L1-GST capture ELISA
The L1-GST ELISA was performed to determine the binding activity of mAbs to capsomeres according to Chen et al. [18]. Briefly, glutathione-coupled casein was coated using carbonate-bicarbonate buffer at 200 ng/well in a Maxisorp™ 96-well Microtiter. The plates were incubated overnight at 4 °C. The plates were washed thrice with PBS containing 0.05% (v/v) Tween-20 (PBST), and the unbound active surface in the wells were blocked using 2% (w/v) casein in PBST for 2 h at 37 °C. The ELISA plates were washed thrice with PBST, and HPV 16 L1-GST proteins (400 ng/well) were captured by incubating with the casein glutathione at 37 °C for 1 h. The plates were washed as mentioned above and 100 µl of mAbs culture supernatants along with positive control immunized mice sera (IMS) was added and incubated at 37 °C for 1 h. The plates were washed, and sheep anti-mouse IgG HRPO conjugate (1:25,000; Sigma-Aldrich) was added and incubated for 1 h at 37 °C. After incubation, the reaction was developed using H2O2 (0.03%, v/v) as a substrate and TMB as the chromogen. The reaction was stopped after 10 minutes with 1.25M sulphuric acid. Absorbance was read at 450 nm using ELISA plate reader.
Immunoblotting
Baculo-expressed L1 capsid protein of HPV16 VLPs was fractioned on 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis under denaturing conditions. The proteins were electrophoretically transferred onto polyvinylidene fluoride (PVDF) membrane (Immobilon-P, Millipore) using standard techniques. The unbound active surfaces of the PVDF membrane were blocked with 5% (w/v) skimmed milk powder in PBST at 37 °C for 2 h. The membrane was washed and incubated with the mAbs culture supernatant at 37 °C for 2 h. After incubation, membrane was washed and incubated with anti-mouse IgG HRPO conjugate (1:10,000) (Sigma-Aldrich) at 37 °C for 1 h. The reaction was visualized with diamino benzidine tetrahydrochloride (DAB) (Sigma-Aldrich) chromogen.
Pseudovirion neutralization assay
The neutralizing abilities of the antibodies were performed by in vitro pseudovirion neutralization method according to the procedures described previously [25, 26]. Briefly, HPV pseudovirions (PsV) were pre-treated with the culture supernatants of the respective mAbs at 4 °C for 1 h and incubated with the human embryonic kidney HEK-293 TT (Invitrogen) cells at 37 °C for 72 h. The mAb H16V5 was used as a positive control for this test. The reduction in secreted alkaline phosphatase (SEAP) (Clonetech, USA) activity was measured using an MLX microplate luminometer (Beckman, USA).
Isotyping
Isotyping analysis of the mAbs was performed to identify the monoclonality of the clones using Isostrips method (Roche) as per the manufacturer’s instructions. In detail, 150 μl of mAb culture supernatants were added into individual tubes which had colored latex beads and agitated so that the beads are completely resuspended. The isostrips were positioned in each tube and were observed for 5 min. The strips were then observed for the blue bands at the places indicated for light chain type and different subclasses of heavy chain.
Indirect immunofluorescence assay
HPV VLP type 16 expressing sf-9 cells were cultured as monolayers in Labtek culture slides (Nunc) with Grace’s insect cell culture media (Invitrogen) at 37 °C and maintained at 5% CO2 for 48h. The cells were fixed using 10% methanol in PBSA and washed with PBS. The mAbs culture supernatants 100 µl were added to the cells and incubated at 37 °C for 1 h. Later, the cell monolayer was washed with PBS and incubated with sheep anti-mouse IgG FITC (Fluorescein Isothiocyanate) at a dilution of 1:50 (Sigma-Aldrich) with Evan’s blue (1:2000) (Sigma-Aldrich) and incubated at 37 °C for 1 h. Culture slides were washed and observed under a fluorescent microscope.
Results
The hybrid clones were produced by fusion of hyper-immunized mice splenocytes and the mouse myeloma partner SP2/0. The monoclonality of the hybrids was established by two rounds of conventional limiting dilution. Seven monoclones were selected based on the binding activity as indicated by ELISA for further study.
Reactivity of mAbs to conformational VLPs
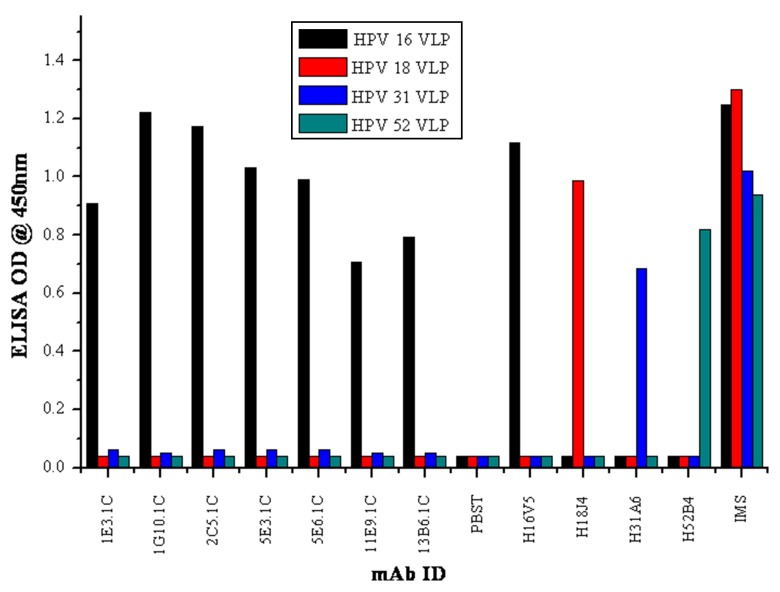
The clones selected in the study have demonstrated high reactivity toward HPV 16 VLPs. All the mAbs (1E3.1C, 1G10.1C, 2C5.1C, 5E3.1C, 5E6.1C, 11E9.1C, and 13B6.1C) were found to be highly type-specific and recognized conformational-dependent epitopes of HPV 16 VLPs. The selected mAbs did not show cross-reactivity with other VLPs of HPV 18, 31, and 52. Among all the mAbs, only one mAb 1G10.1C had shown high reactivity to HPV 16 VLPs. The positive control, immunized mice sera (IMS), showed a broad reactivity with all VLPs, and conformational reactive control mAbs (H16V5, H18J4, H31A6, H52B4) showed specific reactivity with their respective VLPs (Fig. 1).
Fig. 1.
Specificity of mAbs to conformational VLPs of HPV 16, 18, 31, and 52 by indirect ELISA
Reactivity of mAbs to Linear VLPs
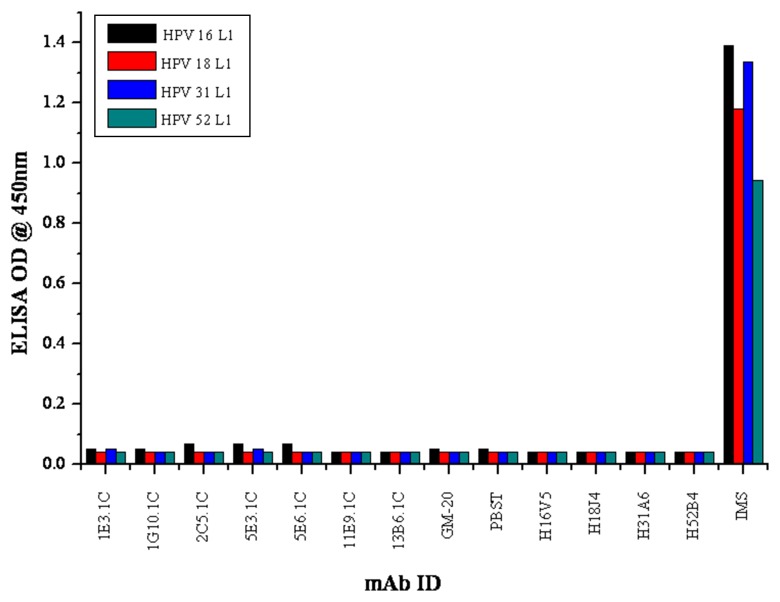
All the mAbs were tested for their ability to detect the linear or the denatured forms of different HPV VLPs by indirect ELISA. All the seven mAbs (2C5.1C, 1G10.1C, 2C5.1C, 5E3.1C, 5E6.1C, 11E9.1C, and 13B6.1C) did not show any cross-reactivity to L1 proteins of HPV 16, 18, 31, and 52. The positive control, IMS, showed a broad reactivity with all linear VLPs. The positive conformational reactive mAbs (H16V5, H18J4, H31A6, and H52B4) did not show specific reactivity with their respective linear VLPs (Fig. 2).
Fig. 2.
Specificity of mAbs to linear proteins (L1) of HPV 16, 18, 31, and 52 by indirect ELISA
Reactivity of mAbs to native L1 epitopes by L1-GST ELISA
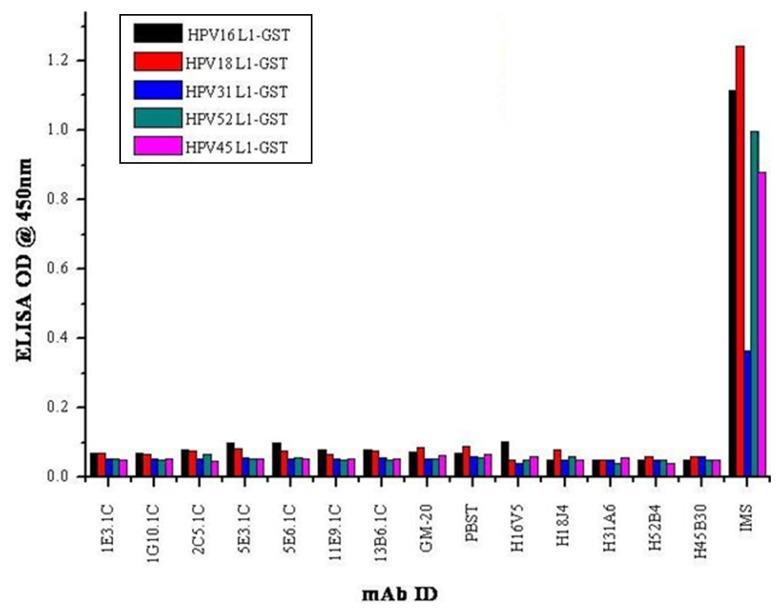
The HPV16 L1-GST fusion protein was produced in E. coli (data not shown) and further used in ELISA as capsomeres. The identified type-specific and conformational-dependent mAbs did not react with L1-GST proteins of HPV 16, 18, 31, 45, and 52 as indicated by ELISA. Positive control IMS showed a broad cross-reactivity with all HPV 16, 18, 31, 45 and 52 L1-GST proteins. Based on the above results, all the mAbs were identified as conformational-dependent (Fig. 3).
Fig. 3.
Capsomere reactivity of mAbs demonstrated by HPV L1-GST ELISA
Western blot analysis
The linear reactivity of the mAbs was tested with the denatured form of HPV 16 L1 proteins by immunoblot analysis. The type-specific and conformational reactive mAbs (1E3.1C, 1G10.1C, 2C5.1C, 5E3.1C, 5E6.1C, 11E9.1C and 13B6.1C) did not show reactivity toward L1 linear proteins of HPV 16. IMS was used as a positive control which showed good reactivity with the denatured form of HPV 16 L1 proteins in the western blot (Fig. 4).
Fig. 4.
HPV 16 mAbs reactivity toward baculo expressed L1 proteins. Lanes 1–8 are mAbs (1E3.1C, 1G10.1C, 2C5.1C, 5E3.1C, 5E6.1C, 11E9.1C, 13B6.1C, and H16V5), lane 9 is immunized mouse sera and lane 10 is PBST
Neutralization efficiency of mAbs to HPV 16 pseudovirions
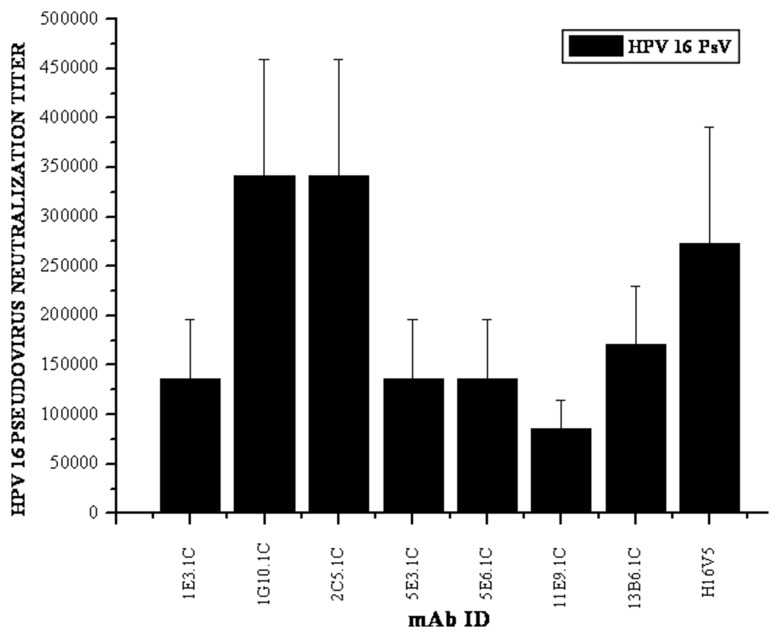
The mAb 1G10.1C was selected for further study based on the binding activity to determine the neutralizing activity against HPV 16 Pseudovirion neutralization (HPV 16 PsVs). It is the most widely used method to analyze the neutralization abilities of antibodies to HPV capsids. The neutralizing ability of the mAbs toward HPV VLPs is very important in qualitative and quantitative analyses of the antigen in VLP-based vaccines. The prerequisite of these assays in estimating the neutralization abilities of the antibodies has been previously described by Roden et al., Christensen et al., and Yeger et al. [11, 27, 28]. Mature culture supernatants of the mAbs (1:1000 dilution) were tested with HPV 16 pseudovirions (PsVs). The mAbs 1G10.1C and 2C5.1C have shown a higher titer of 2 × 105, and other mAbs 1E3.1C, 5E3.1C, 5E6.1C and 13B6.1C were also neutralized when compared with the positive control mAb H16V5 showed a titer of 1 × 105. The mAb 11E9.1C showed lower titer of 5 × 104 (Fig. 5).
Fig. 5.
The neutralizing activity of the mAbs as demonstrated by the pseudovirion neutralization assay against HPV 16
Isotype analysis of mAbs
The isotyping of the monoclones was analyzed to find the sub-class of immunoglobulin of the antibodies. All the tested monoclones were identified as sub-class of IgG (Table 1).
Table 1.
Isotyping of HPV 16 mAbs as heavy chain and light chain
| S. No | MAb ID | Heavy chain | Light chain |
|---|---|---|---|
| 1 | 1E3.1C | IgGl | kappa |
| 2 | 1G10.1C | IgGl | kappa |
| 4 | 2C5.1C | IgGl | kappa |
| 5 | 5E3.1C | IgG2a | kappa |
| 6 | 5E6.1C | IgG2a | kappa |
| 7 | 11E9.1C | IgGl | kappa |
| 8 | 13B6.1C | IgGl | kappa |
Indirect detection of intact HPV VLPs
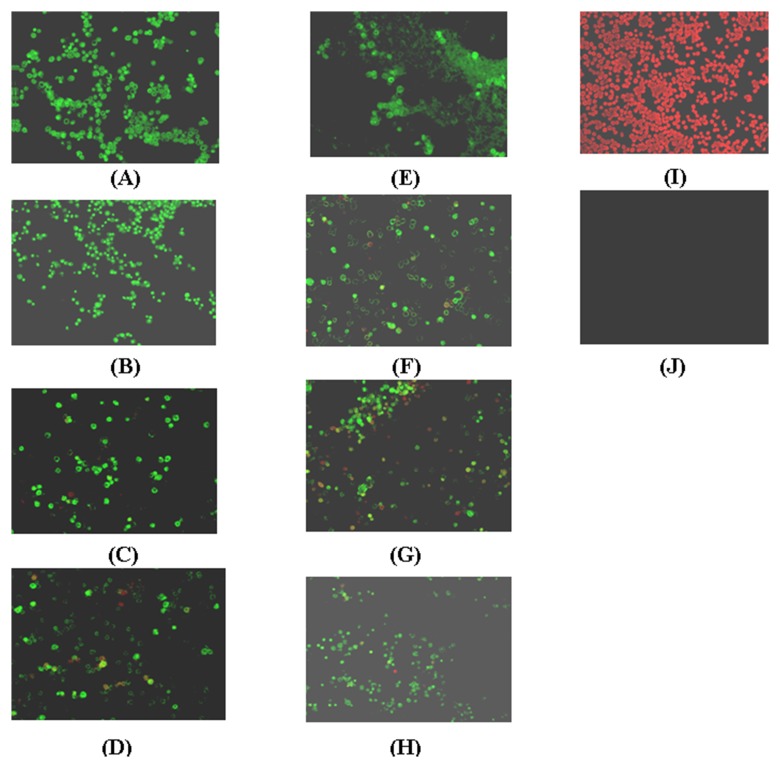
The mAbs were analyzed for the ability to detect the intact HPV VLPs (capsids) in native state by an indirect immunoflourescence assay. All the mAbs were able to detect the HPV 16 VLPs inside the insect cells (sf-9) upon infection with recombinant baculovirus. The mAb 1G10.1C and 2C5.1C have shown good fluorescence reactivity compared with the other five mAbs 1E3.1C, 5E3.1C, 5E6.1C and 13B6.1C toward the HPV 16 VLPs in their conformational state (Fig. 6).
Fig. 6.
Demonstration of immunofluorescence shows the ability of the conformational type-specific reactivity of mAb toward intact HPV VLPs expressed in sf-9 cells. (A) 1G10.1C (B) 2C5.1C (C) 1E3.1C (D) 5E3.1C (E) 5E6.1C (F) 11E9.1C (G) 13B6.1C (H) H16V5 (I) only sf-9 cells with evan’s blue without antibody (J) only sf-9 cells without evan’s blue and antibody
Discussion
HPV infection is the most common sexually transmitted disease and several high-risk types have been strongly associated with different genital malignancies. The biology and structure of HPV have often made it difficult to characterize the etiological agent. Recombinantly expressed VLPs closely resemble as infectious particles by ultrastructural studies [29]. The advantage of papilloma virus (PV) VLPs is the correctly assembled viral surface conformational epitopes structure that is involved in triggering virus neutralizing antibodies [10, 29–31]. Type-specific and conformational reactive mAbs are helpful in understanding the structure of HPV and pathogenesis of the disease [7, 8]. In vaccine industries, mAbs are useful for making potential recombinant vaccines by modifying with antibody-reacting neutralizing epitopes. The production of type specific mAbs would require rigorous screening strategies owing to the high percentage similarity shared between serotypes of HPV [2]. The VLP ELISAs have been identified as the core for HPV antibody screening systems [10, 6]. In the present study, we developed a panel monoclonal antibodies against baculo-expressed HPV 16 VLPs [10, 29, 32].
Monoclonal antibodies for HPV 16 VLPs have been primarily screened for their reactivity toward HPV 16 VLPs by using VLP ELISAs and L1-GST ELISA. Based on the reactivity, two mAbs, 1G10.1C and 2C5.1C, were identified as the most type-specific and conformational-dependent and highly reactive in a VLP-based ELISA similar to that of the mAb H16V5. Our results showed that selected mAbs reacted well with intact VLPs but not with the denatured VLPs, and the results are in good agreement with that of Rizk et al. [8]. Consistent with previous reports of other type-restricted and conformation-dependent VLP mAbs. Further, all the mAbs were tested for the neutralizing ability by PsVs. Among all the mAbs tested, only 1G10.1C and 2C5.1C mAbs have shown high neutralization titer and equally prevented virus infection in HPV 16 pseudovirion neutralization assay. Both the mAbs exhibited very high neutralizing titers of 2 × 105. The high neutralizing mAbs would be helpful in producing efficacious prophylactic vaccines [27, 33] and as possible treatment options for HPV-related malignancies. The type-specific reactivity of mAbs to HPV 16 VLPs in permeabilized sf-9 cells was visualized by immunofluorescence staining. It has indicated that the formation of intact VLPs by perfect assembling of HPV 16 L1 in sf-9 cells suggests that they may also be useful in virus–host interaction studies and diagnosis of HPV16-related infections [7, 34, 35].
Conclusion
HPV 16 is the most common etiology for cervical cancer in the world. This study provides an insight into the monoclonal antibodies reacting to conformational-dependent and neutralizing epitopes of HPV 16. The high pseudovirion neutralization of the mAbs 1G10.1C and 2C5.1C against HPV 16 pseudovirions (PsVs) may provide further application in the production of efficacious vaccines. Also these two mAbs can be explored in various fields, viz., diagnostics, therapeutics, apart from viral kinetics and epidemiological studies.
Contributor Information
P. Vidyasagar, Indian Immunologicals Limited, Gachibowli, Hyderabad, Andhra Pradesh 500032, India
V. N. Sridevi, Indian Immunologicals Limited, Gachibowli, Hyderabad, Andhra Pradesh 500032, India.
S. Rajan, Indian Immunologicals Limited, Gachibowli, Hyderabad, Andhra Pradesh 500032, India
A. Praveen, Indian Immunologicals Limited, Gachibowli, Hyderabad, Andhra Pradesh 500032, India
A. Srikanth, Indian Immunologicals Limited, Gachibowli, Hyderabad, Andhra Pradesh 500032, India
G. Abhinay, Indian Immunologicals Limited, Gachibowli, Hyderabad, Andhra Pradesh 500032, India
V. Siva Kumar, Indian Immunologicals Limited, Gachibowli, Hyderabad, Andhra Pradesh 500032, India
R. R. Verma, Indian Immunologicals Limited, Gachibowli, Hyderabad, Andhra Pradesh 500032, India
L. Rajendra, Indian Immunologicals Limited, Gachibowli, Hyderabad, Andhra Pradesh 500032, India
References
- 1.Goon PK, Stanley MA, Ebmeyer J, Steinsträsser L, Upile T, Jerjes W, Bernal-Sprekelsen M, Görner M, Sudhoff HH. HPV & head and neck cancer: a descriptive update. Head Neck Oncol. 2009 Oct 14;1:36. doi: 10.1186/1758-3284-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJ. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003 Feb 6;348(6):518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 3.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, Clifford GM. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007 Aug 1;121(3):621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 4.Hariri S, Dunne E, Saraiya M, Unger E, Markowitz L. Human Papillomavirus, Chapter 5-1. 2011. [Google Scholar]
- 5.Christensen ND, Dillner J, Eklund C, Carter JJ, Wipf GC, Reed CA, Cladel NM, Galloway DA. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology. 1996 Sep 1;223(1):174–184. doi: 10.1006/viro.1996.0466. [DOI] [PubMed] [Google Scholar]
- 6.Combita AL, Touzé A, Bousarghin L, Christensen ND, Coursaget P. Identification of two cross-neutralizing linear epitopes within the L1 major capsid protein of human papillomaviruses. J Virol. 2002 Jul;76(13):6480–6486. doi: 10.1128/JVI.76.13.6480-6486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Culp TD, Spatz CM, Reed CA, Christensen ND. Binding and neutralization efficiencies of monoclonal antibodies, Fab fragments, and scFv specific for L1 epitopes on the capsid of infectious HPV particles. Virology. 2007 May 10;361(2):435–446. doi: 10.1016/j.virol.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizk RZ, Christensen ND, Michael KM, Müller M, Sehr P, Waterboer T, Pawlita M. Reactivity pattern of 92 monoclonal antibodies with 15 human papillomavirus types. J Gen Virol. 2008 Jan;89(Pt 1):117–129. doi: 10.1099/vir.0.83145-0. [DOI] [PubMed] [Google Scholar]
- 9.Williams MG, Howatson AF, Almeida JD. Morphological characterization of the virus of the human common wart (verruca vulgaris) Nature. 1961 Mar 18;189:895–897. doi: 10.1038/189895a0. [DOI] [PubMed] [Google Scholar]
- 10.Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen ND, Reed CA, Cladel NM, Hall K, Leiserowitz GS. Monoclonal antibodies to HPV-6 L1 virus-like particles identify conformational and linear neutralizing epitopes on HPV-11 in addition to type-specific epitopes on HPV-6. Virology. 1996 Oct 15;224(2):477–486. doi: 10.1006/viro.1996.0554. [DOI] [PubMed] [Google Scholar]
- 12.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, Jenkins D, Schuind A, Costa Clemens SA, Dubin G. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006 Apr 15;367(9518):1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 13.Thönes N, Herreiner A, Schädlich L, Piuko K, Müller M. A direct comparison of human papillomavirus type 16 L1 particles reveals a lower immunogenicity of capsomeres than viruslike particles with respect to the induced antibody response. J Virol. 2008 Jun;82(11):5472–5485. doi: 10.1128/JVI.02482-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heim K, Christensen ND, Hoepfl R, Wartusch B, Pinzger G, Zeimet A, Baumgartner P, Kreider JW, Dapunt O. Serum IgG, IgM, and IgA reactivity to human papillomavirus types 11 and 6 virus-like particles in different gynecologic patient groups. J Infect Dis. 1995 Aug;172(2):395–402. doi: 10.1093/infdis/172.2.395. [DOI] [PubMed] [Google Scholar]
- 15.Strickler HD, Hildesheim A, Viscidi RP, Shah KV, Goebel B, Drummond J, Waters D, Sun Y, Hubbert NL, Wacholder S, Brinton LA, Han CL, Nasca PC, McClimens R, Turk K, Devairakkam V, Leitman S, Martin C, Schiller JT. Interlaboratory agreement among results of human papillomavirus type 16 enzyme-linked immunosorbent assays. J Clin Microbiol. 1997 Jul;35(7):1751–1756. doi: 10.1128/jcm.35.7.1751-1756.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wikström A, van Doornum GJ, Quint WG, Schiller JT, Dillner J. Identification of human papillomavirus seroconversions. J Gen Virol. 1995 Mar;76(Pt 3):529–539. doi: 10.1099/0022-1317-76-3-529. [DOI] [PubMed] [Google Scholar]
- 17.Giroglou T, Sapp M, Lane C, Fligge C, Christensen ND, Streeck RE, Rose RC. Immunological analyses of human papillomavirus capsids. Vaccine. 2001 Feb 8;19(13-14):1783–93. doi: 10.1016/s0264-410x(00)00370-4. [DOI] [PubMed] [Google Scholar]
- 18.Chen XS, Garcea RL, Goldberg I, Casini G, Harrison SC. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol Cell. 2000 Mar;5(3):557–567. doi: 10.1016/s1097-2765(00)80449-9. [DOI] [PubMed] [Google Scholar]
- 19.Margaret S, Douglas R, Lowyb I. Chapter 12: Prophylactic HPV vaccines: Underlying mechanisms. 2006. [DOI] [PubMed] [Google Scholar]
- 20.Xi SZ, Banks LM. Baculovirus expression of the human papillomavirus type 16 capsid proteins: detection of L1-L2 protein complexes. J Gen Virol. 1991 Dec;72(Pt 12):2981–2988. doi: 10.1099/0022-1317-72-12-2981. [DOI] [PubMed] [Google Scholar]
- 21.Zheng B, Wang J, Jiang H. [Production of HPV 16 major capsid protein L1 with baculovirus expression system] Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2001 Dec;15(4):314–316. [PubMed] [Google Scholar]
- 22.Le Cann P, Coursaget P, Iochmann S, Touze A. Self-assembly of human papillomavirus type 16 capsids by expression of the L1 protein in insect cells. FEMS Microbiol Lett. 1994 Apr 15;117(3):269–274. doi: 10.1111/j.1574-6968.1994.tb06778.x. [DOI] [PubMed] [Google Scholar]
- 23.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 24.Hanumantha Rao N, Baji Babu P, Rajendra L, Sriraman R, Pang YY, Schiller JT, Srinivasan VA. Expression of codon optimized major capsid protein (L1) of human papillomavirus type 16 and 18 in Pichia pastoris; purification and characterization of the virus-like particles. Vaccine. 2011 Oct 6;29(43):7326–7334. doi: 10.1016/j.vaccine.2011.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buck CB, Pastrana DV, Lowy DR, Schiller JT. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol Med. 2005;119:445–462. doi: 10.1385/1-59259-982-6:445. [DOI] [PubMed] [Google Scholar]
- 26.Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, Krüger Kjaer S, Lowy DR, Schiller JT. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004 Apr 10;321(2):205–216. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 27.Yeager MD, Aste-Amezaga M, Brown DR, Martin MM, Shah MJ, Cook JC, Christensen ND, Ackerson C, Lowe RS, Smith JF, Keller P, Jansen KU. Neutralization of human papillomavirus (HPV) pseudovirions: a novel and efficient approach to detect and characterize HPV neutralizing antibodies. Virology. 2000 Dec 20;278(2):570–577. doi: 10.1006/viro.2000.0674. [DOI] [PubMed] [Google Scholar]
- 28.Roden RB, Greenstone HL, Kirnbauer R, Booy FP, Jessie J, Lowy DR, Schiller JT. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. J Virol. 1996 Sep;70(9):5875–5883. doi: 10.1128/jvi.70.9.5875-5883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagensee ME, Olson NH, Baker TS, Galloway DA. Three-dimensional structure of vaccinia virus-produced human papillomavirus type 1 capsids. J Virol. 1994 Jul;68(7):4503–4505. doi: 10.1128/jvi.68.7.4503-4505.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose RC, Bonnez W, Da Rin C, McCance DJ, Reichman RC. Serological differentiation of human papillomavirus types 11, 16 and 18 using recombinant virus-like particles. J Gen Virol. 1994 Sep;75(Pt 9):2445–2449. doi: 10.1099/0022-1317-75-9-2445. [DOI] [PubMed] [Google Scholar]
- 31.Christensen ND, Cladel NM, Reed CA, Budgeon LR, Embers ME, Skulsky DM, McClements WL, Ludmerer SW, Jansen KU. Hybrid papillomavirus L1 molecules assemble into virus-like particles that reconstitute conformational epitopes and induce neutralizing antibodies to distinct HPV types. Virology. 2001 Dec 20;291(2):324–334. doi: 10.1006/viro.2001.1220. [DOI] [PubMed] [Google Scholar]
- 32.Christensen ND, Höpfl R, DiAngelo SL, Cladel NM, Patrick SD, Welsh PA, Budgeon LR, Reed CA, Kreider JW. Assembled baculovirus-expressed human papillomavirus type 11 L1 capsid protein virus-like particles are recognized by neutralizing monoclonal antibodies and induce high titres of neutralizing antibodies. J Gen Virol. 1994 Sep;75(Pt 9):2271–2276. doi: 10.1099/0022-1317-75-9-2271. [DOI] [PubMed] [Google Scholar]
- 33.Culp TD, Spatz CM, Reed CA, Christensen ND. Binding and neutralization efficiencies of monoclonal antibodies, Fab fragments, and scFv specific for L1 epitopes on the capsid of infectious HPV particles. Virology. 2007 May 10;361(2):435–446. doi: 10.1016/j.virol.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olcese VA, Chen Y, Schlegel R, Yuan H. Characterization of HPV16 L1 loop domains in the formation of a type-specific, conformational epitope. BMC Microbiol. 2004 Jul 19;4:29. doi: 10.1186/1471-2180-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ressler S, Scheiden R, Dreier K, Laich A, Müller-Holzner E, Pircher H, Morandell D, Stein I, Viertler HP, Santer FR, Widschwendter A, Even J, Jansen-Dürr P, Capesius C, Zwerschke W. High-risk human papillomavirus E7 oncoprotein detection in cervical squamous cell carcinoma. Clin Cancer Res. 2007 Dec 1;13(23):7067–7072. doi: 10.1158/1078-0432.CCR-07-1222. [DOI] [PubMed] [Google Scholar]