Abstract
Interplay between a natural killer (NK)–cell receptor, NKp30, and other cells in the salivary glands profoundly affects pathogenesis of the autoimmune disease Sjögren’s syndrome (Rusakiewicz et al., this issue).
Sjögren’s syndrome (SS) is a chronic autoimmune disorder that affects mainly the exocrine glands. By current estimates, ~0.5% of the population is afflicted with some form of SS, making it one of the most prevalent autoimmune diseases. SS is defined clinically either as primary Sjögren’s syndrome (pSS) or as secondary SS when accompanied by other autoimmune manifestations, such as rheumatoid arthritis or lupus. Although the major symptoms of SS are dry eyes and mouth, systemic effects often manifest as peripheral neuropathies with severe fatigue and pain. SS patients develop circulating autoantibodies that react with multiple cellular proteins and immune (T and B cell) infiltrates within the exocrine glands, thus leading to its classification as an autoimmune disorder (1).
For the past few decades, the focus of SS research has been on investigating the role of adaptive immunity in the disease process. With multiple reports demonstrating an up-regulated type I interferon (IFN) signature in SS patients (2), the role of innate immunity activation and its influence on SS is now actively being investigated (3). Natural killer (NK) cells are crucial components of the innate immune system, but they also influence adaptive immune responses. Through combinations of activating and inhibitory receptor-engagement, NK cells interact with multiple cell types and influence their fate (4). In this issue of Science Translational Medicine, Rusakiewicz et al. address the role of the NK cell and its NKp30 receptor in the pathogenesis of SS (5).
NK cells have been shown to both promote and regulate autoimmune diseases (6). The role of NK cells in SS pathogenesis is unclear because of contradictory data in the literature. For example, both increased and decreased numbers of peripheral NK cells have been reported in patients (7, 8). NK cells have been shown to exhibit impaired activity that can or cannot be augmented in vitro. The reasons for these disparate findings can be attributed to patient demographics, stage of disease, methodologies used, and, most importantly, the subset of NK cells investigated. Moreover, the sheer focus of many studies on the killing ability of NK cells as a functional readout has caused the helper aspect of NK-cell interactions to be overlooked. The new work (5) highlights the helper role of NK cells.
Rusakiewicz et al. propose that NK cells are critical early regulators of the autoimmune process in SS. Although this hypothesis can be readily tested in animal models, investigation is challenging in SS patients who present with well-established chronic autoimmune disease. The known heterogeneity of NK cell phenotypes and function further complicates the investigation. In the present study, the authors used a multipronged approach to establish the role of NK cells, in particular those that express the NKp30 receptor, as important contributors to SS pathogenesis.
Like other autoimmune disorders, SS shows a strong association with multiple genetic factors. Rusakiewicz et al. showed that the single-nucleotide polymorphism (SNP) rs11575837 (G>A), which is located in the promoter region of the NKp30 gene locus, is significantly associated with susceptibility to pSS. The major allele (G) occurred more frequently in SS patients and was associated with higher levels of NKp30 expression on peripheral-blood NK cells, relative to controls. In control subjects, the minor variant (A) occurred more frequently and was associated with reduced NKp30 expression.
NKp30 is an activation receptor on NK cells and is critical in NK cell–dendritic cell (DC) crosstalk during an innate immune response (9). Signaling through NKp30 leads to up-regulation of activation marker CD69 and production of the cytokines IFN-γ and tumor necrosis factor–α (TNF-α). In turn, interaction of NKp30 with its ligand B7-H6 on DCs induces proliferation and activation of immature and mature DCs. The genetic finding from this study implies that the reduced expression of NKp30 protects from SS by modulating the NK-DC interaction, and thereby limiting the subsequent activation of adaptive autoimmune responses.
To further test their hypothesis, the authors focused on studying NK cells in peripheral blood and minor salivary gland biopsies from pSS patients. Lymphopenia and fewer circulating NK cells is a common characteristic seen in SS patients relative to controls (8). However, in this study, relative to controls, pSS patients displayed an increased frequency of circulating CD56bright NK cells (which have been implicated in chemokine and cytokine production) compared to the CD56dim CD16+ cytotoxic NK cell subset. Further, NK cells from patients displayed higher amounts of NKp30 expression than did those from controls, and SS NK cells produced more IFN-γ after NKp30 cross-linking in culture. However, one must exercise caution when interpreting these data, because a subset of pSS patients in the study were shown to harbor the rs11575837 minor variant as well as lower in vitro IFN-γ production relative to controls and other pSS patients. These confounding observations indicate that other factors also influence SS pathogenesis.
So how do NK cells participate in SS pathogenesis? In the NZB/W F1 mouse model of SS, after innate-immune activation, NK cells and DCs infiltrate the salivary glands first, followed by B cells and CD4+ helper T cells (10). This process occurs in response to the up-regulated expression of multiple chemokines in the salivary glands, which suggests that NK cells and DCs lay the ground work for the formation of classical inflammatory foci (composed mainly of T cells, B cells, and macrophages) in the salivary glands. Indeed, Rusakiewicz et al. (5) found that in pSS patients with low focus scores, NK cells existed primarily within these inflammatory foci. In contrast, in pSS patients with high focus scores, NK cells existed mostly outside of and along the edges of the inflammatory foci.
These observations raise a critical question: What is the role of NK cells in later stages of the disease? A possible explanation comes from the final experiment in this study. The NKp30 ligand, B7-H6, is constitutively expressed in malignant hematopoietic cells but can be induced on multiple cell types after exposure to stress or cytokines. The authors used immortalized human salivary gland cell lines and primary salivary gland epithelial cell (SGEC) lines from pSS patients and showed that incubation with TNF-α resulted in the expression of B7-H6. Further, SGEC B7-H6 interacted with NKp30-expressing transfected human Jurkat cells and drove efficient signal transduction in culture. Together, these results suggest the intriguing possibility that NKp30-positive NK cells may directly interact with B7-H6 induced on SGECs present along the periphery of the inflammatory foci and set up a vicious cycle that contributes to the chronic nature of the disease. In this model, the innate cells play a role, not only in the initiation of SS, but also in disease progression and maintenance of chronicity.
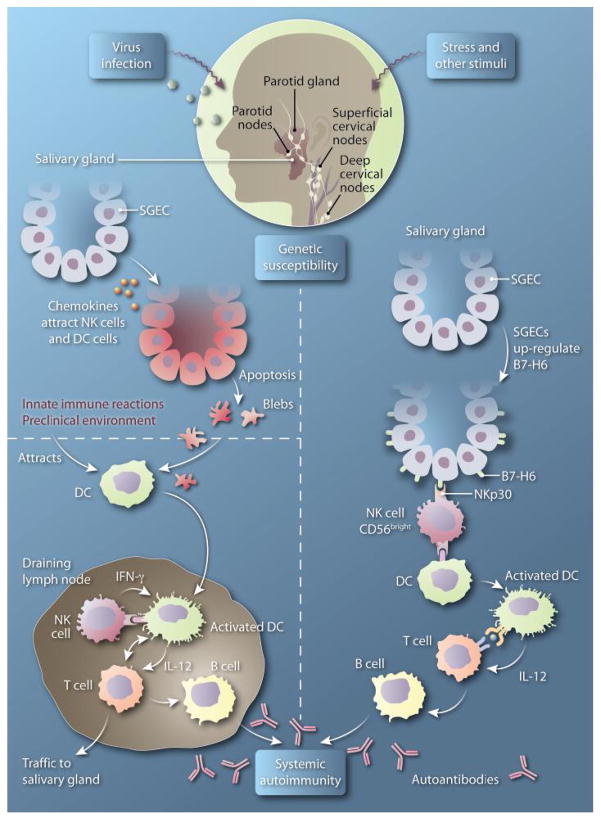
On the basis of data presented by Rusakiewicz et al. (5), one can envision a new model for NK cell involvement in the pathogenesis of SS (Fig. 1, left). This model relies on the helper role of NK cells rather than the traditional killer role. In this model, in the preclinical stage of the disease, activation of innate immunity by events such as viral infection leads to the up-regulation of chemokines within the salivary glands and to SGEC apoptosis. These events cause an initial wave of NK cells and DCs to infiltrate the tissue. The DCs pick up the apoptotic debris and traffic to the local draining lymph nodes, wherein they interact with NK cells that express NKp30 receptors. This coupling triggers maturation of DCs followed by activation of autoreactive T cells. The ensuing T cell–B cell interaction results in the generation of autoantibodies by the activated B cells and, thus, a systemic autoimmune response.
Fig. 1. Other little helper.
(Left) In lymph nodes that drain the salivary glands, NK cells interact with DCs carrying apoptotic debris from SGECs, resulting in DC maturation. Mature DCs interact with and activate T cells, which then couple with and activate B cells, thus initiating the systemic autoimmune response. (Right) Within the salivary glands, NKp30-expressing NK cells interact with SGECs that express B7-H6. This interaction leads to the production of multiple chemokines, which spurs inflammatory cell infiltration within the salivary gland. NK-activated DCs interact with infiltrating T cells. The resulting activated T cells couple with and activate B cells, which produce autoantibodies and thus initiate and sustain a localized autoimmune response. IL-12, interleukin-12.
We suggest an additional disease-driving pathway (Fig. 1, right). In response to external stimuli or stress, SGECs up-regulate expression of B7-H6, which spurs their interaction with NKp30-expressing NK cells in the salivary glands. The resulting localized production of IFN-γ and other cytokines results in the release of several chemokines that can now attract, to the salivary glands, other inflammatory cells as well as T cells activated in the periphery by self-antigens. This branch of the model applies to both early and later stages of the disease, can contribute to chronicity, and might explain the intriguing observation of the authors that, in patients with higher-grade inflammation, NK cells were present mostly outlining the inflammatory foci.
This new model for SS pathogenesis raises the following translational possibilities. The rs11575837 SNP can become an important member of the expanding panel of biomarkers for SS. Moreover, blockade of the NK-DC interaction can be explored as a therapeutic option for the treatment of SS.
Acknowledgments
The authors are supported by NIH grants DE022977 and AI079621.
Footnotes
Competing interests: The authors declare that they have no competing interests.
References
- 1.Nikolov NP, Illei GG. Pathogenesis of Sjögren’s syndrome. Curr Opin Rheumatol. 2009:465–470. doi: 10.1097/BOR.0b013e32832eba21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao Y, Liu Z, Jallal B, Shen N, Rönnblom L. Type I interferons in Sjögren’s syndrome. Autoimmun Rev. 2013:558–566. doi: 10.1016/j.autrev.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Szczerba BM, Rybakowska PD, Dey P, Payerhin KM, Peck AB, Bagavant H, Deshmukh US. Type I interferon receptor deficiency prevents murine Sjogren’s syndrome. J Dent Res. 2013:444–449. doi: 10.1177/0022034513483315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long EO, Sik Kim H, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: Integration of signals for activation and inhibition. Annu Rev Immunol. 2013:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rusakiewicz S, Nocturne G, Lazure T, Semeraro M, Flament C, Caillat-Zucman S, Sène D, Delahaye N, Vivier E, Chaba K, Poirier-Colame V, Nordmark G, Eloranta M-L, Eriksson P, Theander E, Forsblad-d’Elia H, Omdal R, Wahren-Herlenius M, Jonsson R, Rönnblom L, Nititham J, Taylor KE, Lessard CJ, Moser Sivils KL, Gottenberg J-E, Criswell LA, Miceli-Richard C, Zitvogel L, Mariette X. NCR3/NKp30 contributes to pathogenesis in primary Sjögren’s syndrome. Sci Trans Med. 2013:195ra96. doi: 10.1126/scitranslmed.3005727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian Z, Gershwin ME, Zhang C. Regulatory NK cells in autoimmune disease. J Autoimmun. 2012:206–215. doi: 10.1016/j.jaut.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Szodoray P, Papp G, Horvath IF, Barath S, Sipka S, Nakken B, Zeher M. Cells with regulatory function of the innate and adaptive immune system in primary Sjögren’s syndrome. Clin Exp Immunol. 2009:343–349. doi: 10.1111/j.1365-2249.2009.03966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izumi Y, Ida H, Huang M, Iwanaga N, Tanaka F, Aratake K, Arima K, Tamai M, Kamachi M, Nakamura H, Origuchi T, Kawakami A, Anderson P, Eguchi K. Characterization of peripheral natural killer cells in primary Sjögren’s syndrome: Impaired NK cell activity and low NK cell number. J Lab Clin Med. 2006:242–249. doi: 10.1016/j.lab.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Kaifu T, Escalière B, Gastinel LN, Vivier E, Baratin M. B7-H6/NKp30 interaction: A mechanism of alerting NK cells against tumors. Cell Mol Life Sci. 2011:3531–3539. doi: 10.1007/s00018-011-0802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nandula SR, Scindia YM, Dey P, Bagavant H, Deshmukh US. Activation of innate immunity accelerates sialoadenitis in a mouse model for Sjögren’s syndrome-like disease. Oral Dis. 2011:801–807. doi: 10.1111/j.1601-0825.2011.01839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]