Abstract
Climatic changes forecasted in the coming years are likely to result in substantial alterations to the distributions and populations of vectors of arthropod-borne pathogens. Characterization of the effect of temperature shifts on the life history traits of specific vectors is needed to more accurately define how such changes could impact the epidemiological patterns of vector-borne disease. Here, we determined the effect of temperatures including 16, 20, 24, 28, and 32°C on development time, immature survival, adult survival, mosquito size, blood feeding, and fecundity of both field and colonized populations of the Culex mosquitoes Culex pipiens L., Culex quinquefasciatus Say, and Culex restuans Theobald. Our results demonstrate that temperature significantly affects all of these traits, yet also that the extent of this effect is at times incongruent among temperatures, as well as being population and species-specific. Comparisons of colonized mosquitoes with field populations generally demonstrate decreased adult and immature survival, increased blood feeding and egg production, and significant variation in the effects of temperature, indicating that such colonies are not fully representative of natural populations. Results with field populations in general indicate that increases in temperature are likely to accelerate mosquito development, and that this effect is greater at temperatures below 24°C, but also that temperature significantly increases mortality. Among field populations, Cx. restuans were most affected by temperature increases, with decreased longevity relative to other species and significant increases in adult and immature mortality measured with each incremental temperature increase. Despite the unique climates characteristic of the geographic ranges of Cx. quinquefasciatus and Cx. pipiens, evidence of significant species-specific adaptation to temperature ranges was not seen. Taken together, these results indicate that geographic region, as well as species and population differences, must be considered when measuring the effect of temperature on vector populations.
Keywords: temperature, Culex, life history traits
The Intergovernmental Panel on Climate Change has predicted continued and accelerating global warming, resulting in a 2–4°C rise in mean global temperature over the next century (Intergovernmental Panel on Climate Change [IPCC 2007]). These changes in climate are likely to result in significant changes to ecological landscapes and patterns of infectious disease. Vector-borne pathogens, which are transmitted by ectothermic arthropods, are likely to be particularly sensitive to increases in global temperatures (Kilpatrick and Randolph 2012). Although it is generally well-established that increased temperatures have the potential to significantly increase proliferation of pathogens in arthropods (Dohm et al. 2002, Reisen et al. 2006, Kilpatrick et al. 2008), the relationship between temperature and life history traits of arthropod vectors is less clear. Defining this relationship is therefore necessary if we are to predict how a warming climate could impact the distribution, abundance, and vectorial capacity of disease vectors.
Mosquitoes in the Culex pipiens species complex, Cx. pipiens L. and Culex quinquefasciatus Say, as well as Culex restuans Theobald, have worldwide distributions, close association with humans, and are frequently vectors of pathogens of both human and animal diseases (Farajollahi et al. 2011). In N. America, Culex mosquitoes are primary vectors of West Nile virus and other arboviral pathogens (Bernard and Kramer 2001, Turell et al. 2002, Kilpatrick et al. 2005, Diaz–Badillo et al. 2011), yet vector competence has been shown to differ among and within-species, and to be temperature-dependent (Kilpatrick et al. 2008, Kilpatrick et al. 2010). In addition, there are a wide variety of behavioral, physiological, and morphological variations among local populations of Culex mosquitoes that could potentially impact vectorial capacities (Gunay et al. 2011).
Environmental conditions have been associated with significant variation in both adult and immature stage characteristics of insects, including larval growth rates, development times, body size, fecundity, and longevity (Shelton 1973, Loetti et al. 2011). Temperature is a particularly important abiotic factor for mosquitoes and other arthropods, as it directly affects mortality, life span, and development rates that can cause changes in morphology (Su and Mulla 2001, Debat et al. 2003, Gunay et al. 2011). The majority of insects function optimally within a narrow range of temperatures, and deviations from this range can cause stress during development, which may result in developmental inconsistencies (Mpho et al. 2002). In addition, there are often trade-offs between development time and both adult size and fitness, which can have significant downstream effects on survival, feeding behavior, and fecundity (Shelton 1973, Mohammed and Chadee 2011). Alterations to these life history traits can lead to substantial variations in vectorial capacity of mosquitoes that harbor and transmit pathogens (Dye 1992, Delatte et al. 2009).
It is now well-documented that colonized and field populations can differ significantly in genetic and phenotypic diversity, resulting in variability in larval and adult life-history traits (Mpho et al. 2002, Aguilar et al. 2005). While field conditions are highly dynamic and diverse, the homogeneous conditions common to laboratory colonies may result in the loss of allelic diversity and overall population fitness (Aguilar et al. 2005). Defining these differences is essential for interpreting studies of vector populations, which often rely on highly colonized populations to assess the effects of environmental conditions on vectorial capacity and fitness.
Here, we determined the effect of temperature on life history traits of Cx. pipiens, Cx. quinquefasciatus, and Cx. restuans mosquitoes. Specifically, we measured temperature-dependent variation in development time, immature survival, adult survival, mosquito size, blood-feeding, and fecundity both among species and between colonized and field-derived populations. Our results demonstrate the significant effects changing temperature has on these traits and identify critical population and species-specific differences, which have broad importance in contributing to our understanding of how changing climates will impact pathogen transmission.
Materials and Methods
Mosquitoes
All colonized Culex mosquitoes were maintained in 30.5-cm3 cages in an environmental chamber at 27 ± 2°C with a relative humidity of 45–65% and a photoperiod of 16:8 (L:D) h before the collection of experimental egg rafts. Cx. pipiens colony mosquitoes were originally collected in Pennsylvania in 2004 (courtesy of M. Hutchinson) and have been highly colonized at the Wadsworth Center Arbovirus laboratory. Cx. quinquefasciatus colony mosquitoes were derived from a laboratory colony provided by D. Fonseca (Rutgers University, New Brunswick, NJ) and initially derived from egg rafts from a highly colonized line from Benzon Research Inc. (Carlisle, PA). Cx. pipiens and Cx. restuans field egg rafts were collected in August, 2010 from Takoma Park, MD, near Washington, DC, and reared in an environmental chamber maintained at 25–27°C, with a photoperiod of 16:8 (L:D) h for a single generation. Field Cx. quinquefasciatus egg rafts were collected in Orange County, CA (courtesy of R. Cummings). We confirmed species identifications and the absence of hybridization using morphological identification of larvae, and molecular identification using species-specific primers (Smith and Fonseca 2004, Bahnck and Fonseca 2006).
Mosquito Development
After hatching of individual groups, 400 larvae per population were counted and equally distributed into two transparent holding pans with 1 liter of filtered water each. Larvae were then placed in environmental chambers (Therma, San Jose, CA) and subjected to a photoperiod of 16:8 (L:D) h under five constant temperatures (16, 20, 24, 28, and 32 ± 1°C). Water dishes were placed inside the chambers to maintain humidity, and temperatures were monitored and recorded daily. Larvae were fed a 1:1:1 ratio of ground koi food, ground rabbit pellets, and bovine liver powder (MP Biomedicals, Solon, OH) in increasing quantities as follows: 60 mg—first instar, 100 mg—second instar, 120 mg—third instar, and 160 mg—fourth instar. Developing larvae were monitored daily for mortality, and dead larvae were counted, recorded, and discarded. Daily pupation also was recorded, and pupae were placed in distilled water in emergence jars (BioQuip Products Inc., Rancho Dominguez, CA) in preparation for eclosion.
Mosquito Survival, Feeding, and Fecundity
Newly emergent mosquitoes were sexed, counted, and housed at corresponding temperatures in 3.8-L mesh top paper cups provided with cotton pads soaked in 10% sucrose ad libitum. Survival of all groups was monitored and recorded daily. Dead adults were removed and frozen at −20°C. Wings from ≈20 adults per group per temperature were removed and placed on slides with double-sided tape and were subsequently measured from the alular notch to the distal margin, excluding the fringe using a Zeiss microscope, Axiocam camera, and Axiovision software (Carl Zeiss Microscopy, Gottingen, Germany) to estimate mosquito size (Dodson et al. 2011). Adults were grouped and housed as they emerged in 3-d intervals and held for 5–7 d to allow for mating. Mosquitoes were starved overnight for 12–24 h and offered defibrinated goose blood (Hema Resources, Aurora, OR) with 2.5% sucrose for 1-h. Mosquitoes were then anesthetized using CO2, and blood-fed females were sorted, counted, and separated for housing into individual holding cups. Unfed females were kept in the original cup with males, and offered a second bloodmeal 5–7 d later. Oviposition dishes were placed in holding cups containing blood-fed females and were checked daily for the presence of egg rafts. Statistical analyses were performed using GraphPad Prism 4.0 and Statsplus 9.0.
Results
Immature Development and Survival
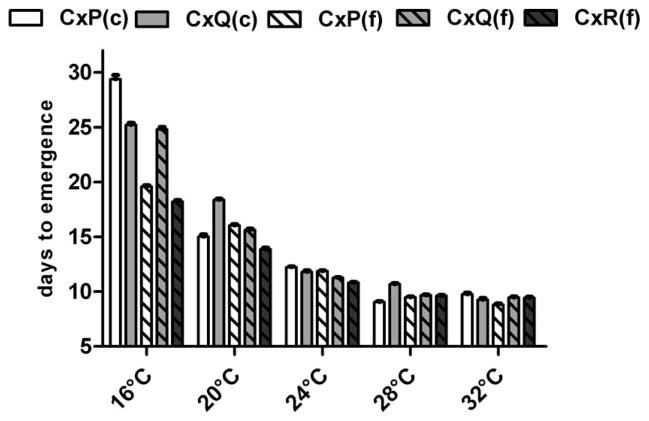
We found significant differences in development time among species, population (field vs. colony), and temperature (Fig. 1). In general, increased temperatures accelerated development for all groups, yet this effect diminished with temperatures >24°C (Fig. 1; Kruskall–Wallis test; P < 0.05). Specifically, the rate of development increased by an average of 2.9-fold with temperature increases from 16 to 24°C (P < 0.001) and an average of 2.3-fold with increases from 24 to 32°C (P < 0.001). When considered separately, development times of individual groups were significantly different when comparing rearing temperatures from 16 to 28°C (Dunn’s multiple comparison test; P < 0.05). Increases from 28 to 32°C were also associated with decreased development times of colony-derived Cx. quinquefascisatus (Mann–Whitney test; P < 0.0001), yet did not significantly alter development time of other groups. The largest effect of temperature on development time was measured for Cx. pipiens colony mosquitoes, for which mean time to emergence ranged from 29 d at 16°C to 9 d at 28°C, the temperature closest to colony rearing temperatures (26–28°C). Development times of field-derived Cx. pipiens were significantly shorter than colonized Cx. pipiens at 16°C (Mann–Whitney test; P < 0.0001). Although differences between field and colony populations of Cx. quinquefasciatus were not as large as those measured at 16°C in Cx. pipiens, significantly shorter development times were also measured in field-derived populations relative to colonized Cx. quinquefasciatus at 16, 20, and 28°C (Mann–Whitney test; P < 0.05).
Fig. 1.
Development time in mean days to emergence ± SEM of field (f)and colony (c) Cx. pipiens (CxP), Cx. quinquefasciatus (CxQ), and Cx. restuans (CxR) at various temperatures.
Species-specific differences in development time of field populations were also observed at all temperatures other than 28°C (Fig. 1). Specifically, Cx. quinquefasciatus and Cx. restuans emerged an average of 0.7 d later than Cx. pipiens at 32°C and 0.9 d earlier at 24°C (Kruskal–Wallis test; P < 0.0001). Differences measured at lower temperatures were greater, with shorter development times measured in Cx. quinquefasciatus relative to Cx. pipiens at 16°C (5 d) and with Cx. restuans relative to both other species at 16 and 20°C. Although still significant (Kruskal–Wallis test; P < 0.0001), the smallest range in development time was measured in Cx. restuans, for which development time spanned from 18 d (16°C) to 8 d (32°C; Fig. 1).
Because proportions of larvae and pupae surviving were similar within groups, data were combined and depicted as proportions surviving to adult (Table 1; Fig. 2A). Temperature, population, and species were all associated with significant differences in immature survival, yet this trait overall varied less than both development time and adult survival. Immature survival was highest at 16°C for all groups, yet the degree to which increasing temperatures altered survival varied among species and populations (Table 1; Fig. 2A). Field populations of Cx. pipiens and Cx. quinquefasciatus were more robust than colonized mosquitoes, showing only modest fluctuations in immature survival resulting from temperature variation. Despite this, the proportion of field-derived Cx. quinquefasciatus surviving at 28°C (0.95) was significantly higher than field-derived Cx. pipiens (0.85; χ2 test, P = 0.009). In contrast to the other groups, there was a significant negative correlation between temperature and immature survival for field-derived Cx. restuans, as well as colony Cx. quinquefasciatus, with <40% of immatures surviving at 32°C for both groups (linear regression analyses, r2 = 0.85; P = 0.025; Fig. 2A). Colony Cx. pipiens again demonstrated adaptation to the temperature closest to that which they are reared (28°C), for which proportion surviving was significantly higher than both the 24 and 32°C groups (χ2 test; P < 0.01; Table 1). The low proportion of colony Cx. pipiens surviving at 24°C (0.61) can be attributed primarily to male death, as male/female ratio of emerged adults was significantly skewed toward females relative to other groups (χ2 test; P<0.0001). No other significant variations in sex ratios were observed among groups.
Table 1.
Proportion of Culex (Cx) pipiens (P), quinquefasciatus (Q), and restuans (R) surviving immature stages of development at various temperatures
| Group | CxP | P (pop)a | CxQ | P (pop)a | CxR | P (species)b |
|---|---|---|---|---|---|---|
| 16°C | ||||||
| Field | 0.92 | 0.0592 | 0.96 | <0.0001 | 0.83 | <0.0001 |
| Colony | 0.87 | 0.81 | — | 0.012 | ||
| 20°C | ||||||
| Field | 0.87 | 0.0007 | 0.84 | 0.0358 | 0.82 | 0.2460 |
| Colony | 0.77 | 0.89 | — | <0.0001 | ||
| 24°C | ||||||
| Field | 0.87 | <0.0001 | 0.86 | <0.0001 | 0.81 | 0.0434 |
| Colony | 0.61 | 0.70 | — | 0.0090 | ||
| 28°C | ||||||
| Field | 0.82 | 0.7730 | 0.95 | <0.0001 | 0.61 | <0.0001 |
| Colony | 0.83 | 0.53 | — | <0.0001 | ||
| 32°C | ||||||
| Field | 0.83 | <0.0001 | 0.77 | <0.0001 | 0.34 | <0.0001 |
| Colony | 0.46 | 0.38 | — | 0.0181 | ||
| P (temp)c | <0.0001 | <0.0001 | <0.0001 | |||
χ2 test tests measuring the effect of temperature on immature survival among field or colony populations of the same species.
χ2 test tests measuring the effect of species on immature survival for individual populations and temperatures.
χ2 test tests comparing immature survival of field and colony populations of individual species and temperatures.
Fig. 2.
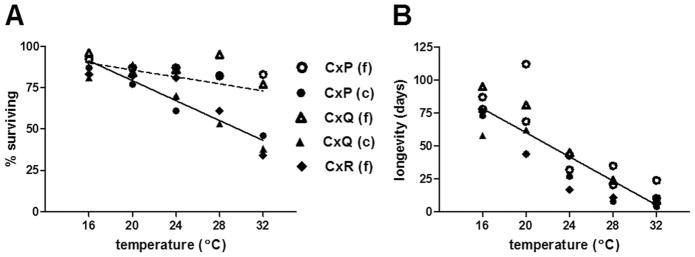
Relationship between temperature and survival of field-derived (f) and colonized (c) Culex (Cx) mosquitoes, Cx. pipiens (P), Cx. quinquefasciatus (Q), and Cx. restuans (R). (A) Relationship between temperature and proportion of larvae surviving to adult. Statistically similar relationships are depicted by individual lines. A significant correlation was identified for Cx. restuans and colonized Cx. quinquefasciatus (solid line; linear regression analysis, r2 = 0.85; P = 0.025; y = 139.5–3.01×), but not for Cx. pipiens or field-derived Cx. quinquefasciatus (dotted line; r2 =0.57; P=0.139). (B) Relationship between temperature and adult longevity. Slopes were statistically similar for all groups (linear regression analysis of mean, y = 151.6–4.57×; r2 = 0.94; P = 0.006), but intercepts were significantly different among groups (F = 4.9; P = 0.003).
Adult Size and Survival
Average wing length was used to assess the relationship between temperature and body size of Culex populations (Fig. 3). With the exception of the colonized Cx. pipiens, a negative correlation between body size and temperature was measured, correlating with the increased development time in each group (Fig. 3; linear regression analyses; P<0.001). Although the size of colony Cx. pipiens was generally less affected by temperature than other groups, mean wing length of mosquitoes reared at 28°C was significantly larger than at all other temperatures (t-test; P < 0.001). Field-derived Cx. pipiens wing lengths were significantly larger than Cx. quinquefasciatus and Cx. restuans at all temperatures and colony-derived Cx. pipiens at 16, 20, and 24°C (t-tests; P < 0.01). No differences in wing length were identified between field and colony populations of Cx. quinquefasciatus.
Fig. 3.
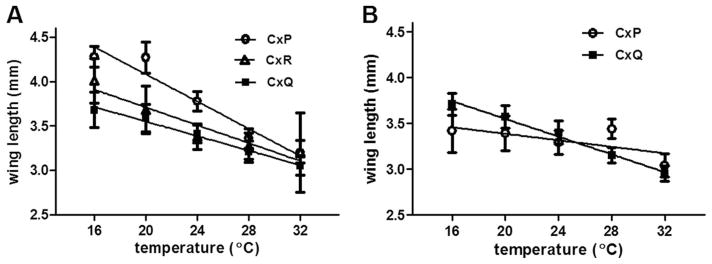
Mean wing length (millimeters) of (A) field-derived and (B) colonized Culex (Cx) mosquitoes at various rearing temperatures. A negative correlation between temperature and winglength was measured in all groups with the exception of colony Cx. pipiens (linear regression analysis, P=0.205). Relationships were as follows: Cx. pipens (P; field) y =5.62–0.08×; r2 = 0.94; P = 0.0064; Cx. quinquefasciatus (Q; field) y = 4.37–0.04×; r2 = 0.99; P = 0.0006; Cx. restuans (R; field) y = 4.72–0.05×; r2 = 0.91; P = 0.016; Cx. quinqs (colony) y = 4.52–0.05×; r2 = 0.99; P = 0.0005.
Median female longevity decreased with temperature and differed between species and populations, with modest two- and three-way interactions among variables contributing significantly to the measured variation in longevity (three-factor analysis of variance [ANOVA]; P < 0.001). Temperature alone accounted for 45.1% of the variation in longevity (F = 7328.1; P < 0.0001), and the combined effect of all three factors accounted for 61.5% of the variation (F= 291.9; P < 0.0001), indicating a significant effect of species and population but also variation in longevity among individuals independent of these factors. For all groups, there was a significant negative correlation between temperature and longevity (Fig. 2B; linear regression analysis; r2 = 0.94; P = 0.006) for which slopes (i.e., the effect of temperature) were not significantly different among groups (P = 0.65) despite differences in longevity (Fig. 4). The largest decreases in median survival resulted from temperature increases from 28 to 32°C for both colonized populations and field-derived Cx. quinquefasciatus, and for temperature increases from 20 to 24°C for field populations of Cx. pipiens and Cx. restuans. Field populations of both Cx. pipiens and Cx. quinquefasciatus survived significantly longer than Cx. restuans at all temperatures, and survival of field-derived Cx. pipiens was greater than both other species at all temperatures but 16 and 24°C (Mantel–Cox test; P < 0.001). With the exception of Cx. quinquefasciatus at 28 and 32°C, field populations generally outlived colony populations by highly significant margins (Gehan–Breslow–Wilcoxon test; P < 0.001). The most significant of these differences was observed with Cx. pipiens at 20°C, for which median survival for the field-derived population was 112 d, as compared with just 44 d for the colony-derived population (Fig. 4).
Fig. 4.
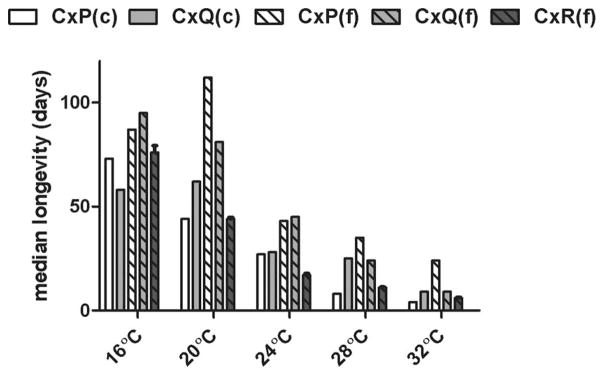
Median longevity ± SEM of field (f) and colonized (c) Cx. pipens (CxP), Cx. quinquefasciatus (CxQ), and Cx. restuans (CxR) at various temperatures.
Blood Feeding and Fecundity
Temperatures, population, and species significantly altered Culex blood feeding (Fig. 5). The colony populations had significantly higher proportion blood fed relative to field-derived populations at all temperatures for Cx. quinquefasciatus and all but 28 and 32°C for Cx. pipiens, for which field population feeding was significantly higher (χ2 test; P < 0.001). In fact, individuals from field populations of Cx. quinquefasciatus fed only at 24 and 28°C (Fig. 5A). Species comparisons of colony-derived mosquitoes demonstrates significantly higher proportions of Cx. pipiens fed at the lowest temperature (16°C; Fig. 5A; χ2 test, P = 0.0021), yet significantly higher proportions of Cx. quinquefasciatus fed at the two highest temperatures (28 and 32°C; Fig. 4A; χ2 test, P<0.001). Only a single Cx. restuans female fed during the study, emphasizing the difficulty of colonizing this species and the general reluctance of field-derived populations to feed from artificial systems.
Fig. 5.
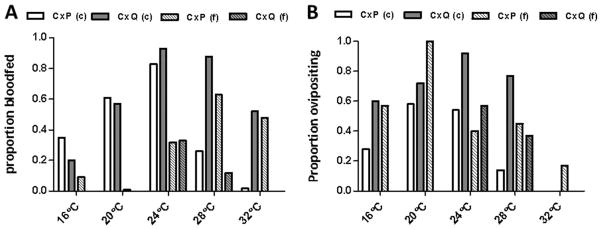
Blood feeding and fecundity of Culex mosquitoes of field (f) and colonized (c) Cx. pipens (CxP), Cx. quinquefasciatus (CxQ), and Cx. restuans (CxR) at various temperatures. (A) Proportion of blood-fed Culex mosquitoes at various temperatures. (B) Proportion of blood-fed Culex mosquitoes laying egg rafts at various temperatures.
Although the relatively small number of fed females in many groups made assessing fecundity differences difficult, some significant variation among groups was identified (Fig. 5B). Overall, egg raft production among blood-fed females was highest for both populations of Cx. pipiens at 20°C and both populations of Cx. quinquefasciatus at 24°C. In addition, with the exception of 24°C, for which proportions were statistically similar, a higher proportion of blood-fed field Cx. pipiens laid egg rafts than did colony Cx. pipiens at all temperatures. In contrast, blood-fed colony Cx. quinquefasciatus mosquitoes produced proportionally more egg rafts than field Cx. quinquefasciatus at all temperatures, and combined egg raft output was highest for this population (Fig. 5B; χ2 test, P < 0.001).
Discussion
Although many studies have demonstrated a role for temperature in mosquito development and population dynamics of individual species (Lachmajer and Hien 1975, Rueda et al. 1990, Reisen 1995), quantifying the specific relationships between temperature and life history traits as well as direct comparison of the relative importance of temperature among medically important mosquito vectors is critical to our understanding of how climate shifts could ultimately alter vectorial capacities and patterns of disease transmission (Dye 1992; Rogers and Randolph 2000, 2006). Here, we provide a unique comparison of the effect of temperature on both field-derived and colonized Cx. pipiens and Cx. quinquefasciatus, as well as a field population of Cx. restuans. Our results demonstrate that temperature significantly affects rates of immature development, survival of immature stages, adult size, adult longevity, blood feeding, and fecundity of Culex mosquitoes, and that both species and colonization are additional factors significantly altering these life history traits and their susceptibility to temperature shifts.
In concordance with previous studies with other mosquito species, results here demonstrate a positive correlation between temperature and development rate (Lachmajer and Hien 1975, Rueda et al. 1990, Bayoh and Lindsay 2003), indicating that increasing temperatures could generally lead to a more rapid proliferation of Culex populations. Despite this, temperatures >24°C had more modest effects on development time than increases below this temperature, demonstrating that rising mean temperatures in milder regions may have lesser effects on the rate of Culex proliferation than would increases in regions where mean summer temperatures range from 16 to 24°C. In addition, the increased mortality observed at higher temperatures in all groups could result in an overall decline in the reproductive output of Culex mosquitoes despite modestly accelerated development. Indeed, only field Cx. pipiens produced egg rafts at 32°C. Similar effects of high temperature on adult longevity have been observed in other studies (Hawley 1985), including in Cx. quinquefasciatus (Rueda et al. 1990) and Culex tarsalis (Reisen 1995).
Although temperature was responsible for the majority of the variation in life history traits measured here and all groups were significantly affected by rearing temperatures, important species and population differences were also identified. Others have acknowledged differences in fitness of field and colonized mosquito populations (Tabachnick 2003), yet results here indicate that species and population differences likely require defining such differences for individual studies. Among the most distinct differences was the sensitivity of colonized larvae and pupae to variable temperatures, as opposed to the robustness of field Cx. pipiens and Cx. quinquefasciatus, for which little effect of temperature on immature survival was measured. The largest effect of temperature was observed with colonized Cx. pipiens mosquitoes, for which mean development time at 16°C was significantly higher than all other groups. Furthermore, colonized Cx. pipiens was the only group for which the shortest development time was measured at 28°C rather than 32°C. A lack of phenotypic variability was also observed with winglength of colony Cx. pipiens, where only modest variation in size was measured with variable temperatures, with 28°C producing the largest mosquitoes. For all other groups, a significant negative correlation was measured between size and temperature (i.e., development time) as has been shown in previous studies (Rueda et al. 1990, Chadee and Beier 1997, Gunay et al. 2011, Mohammed and Chadee 2011). In addition, survival of both immature stages and adults was significantly lower for colonized populations, and overall blood feeding and egg production were generally higher relative to field mosquitoes. These differences, which are consistent with previous studies investigating the effects of colonization (Aguilar et al. 2005, Vitek and Livdahl 2006), are likely a reflection of selection events resulting from long-term colonization and rearing at generally constant environmental conditions, indicating that the use of colonies to measure the effects of temperature or other environmental variables on life history traits may at times provide imprecise representations of natural populations.
Measurement of species-specific variation among field populations provided a direct assessment of the potential implications of natural temperature fluctuations among Culex mosquitoes. Cx. restuans were less sensitive to alterations in development time than Cx. pipiens or Cx. quinquefasciatus, yet had significantly shorter longevity than both other species at every temperature and were the only field population for which immature survival was significantly decreased at temperatures >24°C. These results suggest that Cx. restuans, which are important vectors of West Nile virus (Kilpatrick et al. 2005, Degroote and Sugumaran 2012) and more prevalent than Cx. pipiens in some rural areas of the eastern United States at certain times during the transmission season (Ebel et al. 2005, Diuk–Wasser et al. 2006), are likely to be the most susceptible of the studied species to population fluctuations as a result of increasing temperatures. Consistent with these findings, previous studies have indicated that Cx. restuans populations tend to peak in late spring and early summer, followed by a decline in the hotter summer months (Reiter 1988, Andreadis et al. 2001).
Cx. quinquefasciatus and Cx. pipiens are sibling species separated by a hybridization zone stretching from ≈ 30°N and 40°N latitude in N. America (Kothera et al. 2009, Farajollahi et al. 2011, Huang et al. 2011), with pure Cx. quinquefasciatus to the south and Cx. pipiens to the north. This distribution would suggest that Cx. quinquefasciatus are more likely to be adapted to higher temperatures, yet only modest differences in life history traits were measured between the two species. Immature survival was significantly higher for Cx. quinquefasciatus at 28°C (but not 24 or 32°C), and development was slightly accelerated at 24°C, yet median longevity for Cx. pipiens was similar to Cx. quinquefasciatus at 24°C and in fact significantly higher than Cx. quinquefasciatus at both 28 and 32°C. This suggests that Cx. quinquefasciatus, as they currently reside in areas with mean summer temperatures generally between 24 and 28°C (www.noaa.gov), are likely to experience decreased survival as a result of rising temperatures. Because development rate is only modestly increased at temperatures >24°C, this increased mortality would decrease population size and, potentially, vectorial capacity of Cx. quinquefasciatus. Cx. pipiens, as they reside in areas with mean summer temperatures between 16 and 24°C, could also experience decreases in mean longevity with rising temperatures, but this negative effect on the population size could be compensated by the fact that greater increases in development rate occur with temperature increases within this range. Consistent with mortality results, field-derived Cx. pipiens blood feeding was also significantly greater than Cx. quinquefasciatus at temperatures >24°C, which could serve to further enhance differences in vectorial capacities. However, when considering the effect of temperature on vectorial capacity, the likelihood of increased pathogen proliferation at higher temperature, and therefore increased vector competence, must also be considered (Dohm et al. 2002, Reisen et al. 2006, Kilpatrick et al. 2008). The fact that the field populations of Cx. pipiens were derived from the southern end of this species’ range (Washington DC area) may partially explain why decreased fitness at higher temperatures was not observed, and is consistent with the presence of significant population level variation. In support of this, colonized Cx. pipiens, originally derived from Pennsylvania, were indeed more susceptible to adult mortality and displayed decreased blood feeding at temperatures >24°C when compared with colonized Cx. quinquefasciatus, yet the potential effects of colonization make these differences difficult to interpret. Genetic diversity identified among populations and individuals of these species, particularly in the case of Cx. pipiens (Fonseca et al. 2004, Kilpatrick et al. 2007, Kothera et al. 2009, Huang et al. 2011), together with identification of substantial variation in vector competence for West Nile virus among Culex populations (Kilpatrick et al. 2010), support the idea of significant population-level phenotypic variation in life history traits and in susceptibility to environmental fluctuations, yet regardless of population variation, these results suggest that differences in mosquito fitness or in the capacity for adaptation to warmer temperatures cannot by itself explain the differences in geographic distributions of these sibling species. Because Cx. quinquefasciatus do not have the capacity to enter true diapause as Cx. pipiens do (Hayes 1975), the inability to successfully overwinter may explain why Cx. quinquefasciatus cannot establish themselves in the northern United States, yet the explanation for the lack of Cx. pipiens moving south requires further study.
A potentially important variable not considered in these studies is the fluctuating daily temperature experienced by natural Culex populations. Although constant temperatures may provide an accurate representation of the effects of daily mean temperature on mosquito development (Milby and Meyer 1986), some species and populations may be more likely to experience changes in life history traits as a result of diurnal fluctuations (Paaijmans et al. 2010, Lambrechts et al. 2011, Mohammed and Chadee 2011). Laboratory rearing containers are also poor representations of natural adult habitats, and differences in mating success, blood-feeding behavior, and overall reproduction output could differ significantly in natural settings. In addition, the largest effect of climate change on Culex populations could result from rising temperatures during breaks in transmission. Milder fall or winter months could serve to significantly alter the size and success of overwintering populations, and milder spring months may advance or accelerate the commencement of pathogen transmission (Ciota et al. 2011). Despite these caveats, our results suggest life history traits of Culex mosquitoes are governed, to a large degree, by temperature and to a lesser extent by species and population differences and that these differences could have profound effects on the influence of climate change on the epidemiological patterns of vector-borne disease.
Acknowledgments
We thank R. Cummings, Lana Hall, and Ben Vincent for collecting the egg rafts in the field and members of the Arbovirus laboratory insectary staff for assisting with these studies. We also thank the Wadsworth Center Tissue culture and Media facility for providing media for these studies. Funding was provided by National Institutes of Health grant 1R01AI090159, and National Science Foundation grant EF-0914866.
References Cited
- Aguilar R, Dong Y, Warr E, Dimopoulos G. Anopheles infection responses; laboratory models versus field malaria transmission systems. Acta Trop. 2005;95:285–291. doi: 10.1016/j.actatropica.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Andreadis TG, Anderson JF, Vossbrinck CR. Mosquito surveillance for West Nile virus in Connecticut, 2000: isolation from Culex pipiens, Cx. restuans, Cx. salinarius, and Culiseta melanura. Emerg Infect Dis. 2001;7:670–674. doi: 10.3201/eid0704.010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahnck CM, Fonseca DM. Rapid assay to identify the two genetic forms of Culex (Culex) pipiens L. (Diptera: Culicidae) and hybrid populations. Am J Trop Med Hyg. 2006;75:251–255. [PubMed] [Google Scholar]
- Bayoh MN, Lindsay SW. Effect of temperature on the development of the aquatic stages of Anopheles gambiae sensu stricto (Diptera: Culicidae) Bull Entomol Res. 2003;93:375–381. doi: 10.1079/ber2003259. [DOI] [PubMed] [Google Scholar]
- Bernard KA, Kramer LD. West Nile virus activity in the United States, 2001. Viral Immunol. 2001;14:319–338. doi: 10.1089/08828240152716574. [DOI] [PubMed] [Google Scholar]
- Chadee DD, Beier JC. Factors influencing the duration of blood-feeding by laboratory-reared and wild Aedes aegypti (Diptera: Culicidae) from Trinidad, West Indies. Ann Trop Med Parasitol. 1997;91:199–207. doi: 10.1080/00034983.1997.11813130. [DOI] [PubMed] [Google Scholar]
- Ciota AT, Drummond CL, Drobnack J, Ruby MA, Kramer LD, Ebel GD. Emergence of Culex pipiens from overwintering hibernacula. J Am Mosq Control Assoc. 2011;27:21–9. doi: 10.2987/8756-971X-27.1.21. [DOI] [PubMed] [Google Scholar]
- Debat V, Begin M, Legout H, David JR. Allometric and nonallometric components of Drosophila wing shape respond differently to developmental temperature. Evolution. 2003;57:2773–2784. doi: 10.1111/j.0014-3820.2003.tb01519.x. [DOI] [PubMed] [Google Scholar]
- Degroote JP, Sugumaran R. National and regional associations between human West Nile virus incidence and demographic, landscape, and land use conditions in the coterminous United States. Vector Borne Zoonotic Dis. 2012;12:657–665. doi: 10.1089/vbz.2011.0786. [DOI] [PubMed] [Google Scholar]
- Delatte H, Gimonneau G, Triboire A, Fontenille D. Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. J Med Entomol. 2009;46:33–41. doi: 10.1603/033.046.0105. [DOI] [PubMed] [Google Scholar]
- Diaz–Badillo A, Bolling BG, Perez–Ramirez G, Moore CG, Martinez–Munoz JP, Padilla–Viveros AA, Camacho–Nuez M, Diaz–Perez A, Beaty BJ, Munoz ML. The distribution of potential West Nile virus vectors, Culex pipiens pipiens and Culex pipiens quinquefasciatus (Diptera: Culicidae), in Mexico City. Parasit. Vectors 4. 2011:70. doi: 10.1186/1756-3305-4-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diuk–Wasser MA, Brown HE, Andreadis TG, Fish D. Modeling the spatial distribution of mosquito vectors for West Nile virus in Connecticut, USA. Vector Borne Zoonotic Dis. 2006;6:283–295. doi: 10.1089/vbz.2006.6.283. [DOI] [PubMed] [Google Scholar]
- Dodson BL, Kramer LD, Rasgon JL. Larval nutritional stress does not affect vector competence for West Nile virus (WNV) in Culex tarsalis. Vector Borne Zoonotic Dis. 2011;11:1493–1497. doi: 10.1089/vbz.2011.0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohm DJ, O’Guinn ML, Turell MJ. Effect of environmental temperature on the ability of Culex pipiens (Diptera : Culicidae) to transmit West Nile virus. J Med Entomol. 2002;39:221–225. doi: 10.1603/0022-2585-39.1.221. [DOI] [PubMed] [Google Scholar]
- Dye C. The analysis of parasite transmission by bloodsucking insects. Annu Rev Entomol. 1992;37:1–19. doi: 10.1146/annurev.en.37.010192.000245. [DOI] [PubMed] [Google Scholar]
- Ebel GD, Rochlin I, Longacker J, Kramer LD. Culex restuans (Diptera: culicidae) relative abundance and vector competence for West Nile virus. J Med Entomol. 2005;42:838–843. doi: 10.1603/0022-2585(2005)042[0838:CRDCRA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Farajollahi A, Fonseca DM, Kramer LD, Marm KA. “Bird biting” mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect Genet Evol. 2011;11:1577–1585. doi: 10.1016/j.meegid.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca DM, Keyghobadi N, Malcolm CA, Mehmet C, Schaffner F, Mogi M, Fleischer RC, Wilkerson RC. Emerging vectors in the Culex pipiens complex. Science. 2004;303:1535–1538. doi: 10.1126/science.1094247. [DOI] [PubMed] [Google Scholar]
- Gunay F, Alten B, Ozsoy ED. Narrow-sense heritability of body size and its response to different developmental temperatures in Culex quinquefasciatus (Say 1923) J Vector Ecol. 2011;36:348–354. doi: 10.1111/j.1948-7134.2011.00175.x. [DOI] [PubMed] [Google Scholar]
- Hawley WA. The effect of larval density on adult longevity of a mosquito, Aedes sierrensis: epidemiological consequences. J Anim Ecol. 1985;54:955–964. [Google Scholar]
- Hayes J. Seasonal changes in population structure of Culex pipiens quinquefasciatus Say (Diptera: Culicidae): study of an isolated population. J Med Entomol. 1975;12:167–178. doi: 10.1093/jmedent/12.2.167. [DOI] [PubMed] [Google Scholar]
- Huang S, Molaei G, Andreadis TG. Reexamination of Culex pipiens hybridization zone in the Eastern United States by ribosomal DNA-based single nucleotide polymorphism markers. Am J Trop Med Hyg. 2011;85:434–441. doi: 10.4269/ajtmh.2011.10-0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (IPCC) Intergovernmental Panel on Climate Change. Third Assessment Report. Cambridge University Press; Cambridge, United Kingdom: 2007. [Google Scholar]
- Kilpatrick AM, Kramer LD, Campbell S, Alleyne EO, Dobson AP, Daszak P. West Nile virus risk assessment and the bridge vector paradigm. Emerg Infect Dis. 2005;11:425–429. doi: 10.3201/eid1103.040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P, Fonseca DM. Genetic influences on mosquito feeding behavior and the emergence of zoonotic pathogens. Am J Trop Med Hyg. 2007;77:667–671. [PubMed] [Google Scholar]
- Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 2008;4:e1000092. doi: 10.1371/journal.ppat.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Fonseca DM, Ebel GD, Reddy MR, Kramer LD. Spatial and temporal variation in vector competence of Culex pipiens and Cx. restuans mosquitoes for West Nile virus. Am J Trop Med Hyg. 2010;83:607–613. doi: 10.4269/ajtmh.2010.10-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Randolph SE. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet. 2012;380:1946–1955. doi: 10.1016/S0140-6736(12)61151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothera L, Zimmerman EM, Richards CM, Savage HM. Microsatellite characterization of subspecies and their hybrids in Culex pipiens complex (Diptera: Culicidae) mosquitoes along a north-south transect in the central United States. J Med Entomol. 2009;46:236–248. doi: 10.1603/033.046.0208. [DOI] [PubMed] [Google Scholar]
- Lachmajer J, Hien DS. Effect of the environmental conditions on eggs and water living stages of Aedes aegypti (Linn.) and Aedes albopictus (Skuse), vectors of dengue haemorrhagic fever in Viet-Nam. Bull Inst Marit Trop Med Gdynia. 1975;26:353–367. [PubMed] [Google Scholar]
- Lambrechts L, Paaijmans KP, Fansiri T, Carrington LB, Kramer LD, Thomas MB, Scott TW. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc Natl Acad Sci USA. 2011;108:7460–7465. doi: 10.1073/pnas.1101377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetti V, Schweigmann NJ, Burroni NE. Temperature effects on the immature development time of Culex eduardoi Casal & Garcia (Diptera: Culicidae) Neotrop Entomol. 2011;40:138–142. doi: 10.1590/s1519-566x2011000100021. [DOI] [PubMed] [Google Scholar]
- Milby MM, Meyer RP. The influence of constant versus fluctuating water temperatures on the pre-imaginal development of Culex tarsalis. J Am Mosq Control Assoc. 1986;2:7–10. [PubMed] [Google Scholar]
- Mohammed A, Chadee DD. Effects of different temperature regimens on the development of Aedes aegypti (L.) (Diptera: Culicidae) mosquitoes. Acta Trop. 2011;119:38–43. doi: 10.1016/j.actatropica.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Mpho M, Callaghan A, Holloway GJ. Temperature and genotypic effects on life history and fluctuating asymmetry in a field strain of Culex pipiens. Heredity (Edinb) 2002;88:307–312. doi: 10.1038/sj.hdy.6800045. [DOI] [PubMed] [Google Scholar]
- Paaijmans KP, Blanford S, Bell AS, Blanford JI, Read AF, Thomas MB. Influence of climate on malaria transmission depends on daily temperature variation. Proc Natl Acad Sci USA. 2010;107:15135–15139. doi: 10.1073/pnas.1006422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen WK. Effect of temperature on Culex tarsalis (Diptera: Culicidae) from the Coachella and San Joaquin Valleys of California. J Med Entomol. 1995;32:636–645. doi: 10.1093/jmedent/32.5.636. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Fang Y, Martinez VM. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera : Culicidae) J Med Entomol. 2006;43:309–317. doi: 10.1603/0022-2585(2006)043[0309:EOTOTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Reiter P. Weather, vector biology, and arboviral recrudescence. In: Monath TP, editor. The Arboviruses: Epidemiology and Ecology. CRC; Boca Raton, FL: 1988. pp. 245–255. [Google Scholar]
- Rogers DJ, Randolph SE. The global spread of malaria in a future, warmer world. Science. 2000;289:1763–1766. doi: 10.1126/science.289.5485.1763. [DOI] [PubMed] [Google Scholar]
- Rogers DJ, Randolph SE. Climate change and vector-borne diseases. Adv Parasitol. 2006;62:345–381. doi: 10.1016/S0065-308X(05)62010-6. [DOI] [PubMed] [Google Scholar]
- Rueda LM, Patel KJ, Axtell RC, Stinner RE. Temperature-dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae) J Med Entomol. 1990;27:892–898. doi: 10.1093/jmedent/27.5.892. [DOI] [PubMed] [Google Scholar]
- Shelton RM. The effect of temperatures on development of eight mosquito species. Mosq News. 1973;33:1–12. [Google Scholar]
- Smith JL, Fonseca DM. Rapid assays for identification of members of the Culex (Culex) Pipiens complex, their hybrids, and other sibling species (Dipteria: Culicidae) Am J Trop Med Hyg. 2004;70:339–345. [PubMed] [Google Scholar]
- Su T, Mulla MS. Effects of temperature on development, mortality, mating and blood feeding behavior of Culiseta incidens (Diptera: Culicidae) J Vector Ecol. 2001;26:83–92. [PubMed] [Google Scholar]
- Tabachnick WJ. Reflections on the Anopheles gambiae genome sequence, transgenic mosquitoes and the prospect for controlling malaria and other vector borne diseases. J Med Entomol. 2003;40:597–606. doi: 10.1603/0022-2585-40.5.597. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Sardelis MR, O’Guinn ML, Dohm DJ. Potential vectors of West Nile virus in North America. J Curr Top Microbiol Immunol. 2002;267:241–52. doi: 10.1007/978-3-642-59403-8_12. [DOI] [PubMed] [Google Scholar]
- Vitek CJ, Livdahl TP. Field and laboratory comparison of hatch rates in Aedes albopictus (Skuse) J Am Mosq Control Assoc. 2006;22:609–614. doi: 10.2987/8756-971X(2006)22[609:FALCOH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]