Abstract
The post-menopausal loss of estrogen is key in the increased incidence of Alzheimer’s disease (AD) in women. However, estrogen therapy (ET) clinical trials have produced conflicting results. The APOE gene of apolipoprotein E (apoE) likely modulates the effects of ET in AD. APOE4 is the greatest genetic risk factor for AD, increasing risk up to 15-fold compared with APOE3, and the negative effect of APOE4 on AD risk and neuropathology is greater in women than men. The interactive effects of APOE and ET may converge on modulation of amyloid-beta (Aβ) levels, as independently both the loss of estrogen and APOE4 increases Aβ accumulation. Thus, in this study, 3-month old female EFAD mice (5xFAD mice crossed with apoE-targeted replacement mice), which express increased levels of Aβ42 and human APOE were ovariectomized and treated for 3 months with either 17-β estradiol (OVXET+, 0.25mg total) or vehicle control (OVXET−) and the effects on Aβ accumulation were determined. Compared to the OVXET− cohort, in the OVXET+ cohort, extracellular amyloid and Aβ deposition in the hippocampus and cortex were decreased with APOE2 and APOE3, but were increased with APOE4 by IHC. Biochemical analysis demonstrated increased total and insoluble Aβ levels with APOE4, and decreased soluble Aβ42 levels with both APOE3 and APOE4, after ET. These data suggest that ET administered at menopause may benefit APOE4 negative women by decreasing extracellular and soluble Aβ42. However, for APOE4 carriers, the efficacy of ET will be dependent on the relative impact of extracellular and soluble Aβ on AD-induced neurodegeneration.
Keywords: Alzheimer’s disease, Estrogen, Amyloid-β, APOE, soluble Aβ, AD transgenic mouse model
INTRODUCTION
Alzheimer's Disease (AD) is the most common form of dementia and over the age of 65, 2/3 of the cases afflict women [1]. The increased prevalence of AD in women remains even after adjusting for age and education levels [1]. Epidemiological analysis [18, 24] and controlled clinical trials [11] indicate that the post-menopausal loss of estrogen is a major mechanism for the increased AD risk in women. However, data on the effect of hormone replacement therapy (HRT) or estrogen therapy (ET) on AD risk and cognitive decline are conflicting [5, 13, 19]. Thus far, identified-confounding factors include the timing and duration of ET and whether ET was used alone or in combination with progesterone.
An additional factor that may modulate the effects of ET in AD is the APOE gene. APOE4 is the greatest genetic risk factor for AD, increasing the risk up to 15-fold, whereas APOE2 decreases risk 4-fold, compared to APOE3 17]. Importantly, the negative effect of APOE4 on AD progression may be more pronounced in women, as APOE3/4 is reported to increase AD risk ~4-fold compared to APOE3/3 in women, but not men [2, 12]. Further, in patients with Mild Cognitive Impairment (MCI), APOE4-dependent cognitive impairment and hippocampal volume loss is greater in women than men [6]. Thus, APOE4 exerts an adverse effect directly on AD risk and on markers associated with AD in post-menopausal women. Although these data indicate that ET treatment may be particularly efficacious at improving cognition in APOE4 carriers however published data are inconsistent [7, 8, 14]. In a placebo-controlled trial/study [16], ET was associated with less cognitive decline in APOE4 negative but not in APOE4 positive women [20]. In partial contrast, the Nurses’ Health Study demonstrated that ET provided no effects in older women, regardless of APOE genotype [9], and in follow-up analysis, HRT was associated with a worse rate of cognitive decline, an effect more pronounced with APOE4 8]. However, HRT has also been demonstrated to decrease the risk of dementia associated with APOE4 14], and to improve markers of accelerated aging in APOE4 carriers [7]. Thus, preclinical studies are required to determine interactive effects of APOE and ET on AD relevant pathology.
One potential interactive effect of APOE and ET is modulation of amyloid beta-42 (Aβ) levels. Aβ42, is considered the proximal neurotoxin in AD. Two major forms include extracellular Aβ42 present in amyloid plaques, and soluble Aβ42, considered a major cause of cognitive impairment in AD. Independently, both APOE4 and the loss of estrogen increase Aβ levels. Compared to APOE3, APOE4 increases the extracellular and soluble Aβ levels in transgenic mouse models expressing familial-AD mutations (FAD-Tg) [22] and in AD patients [17]. Importantly, amyloid deposition is increased in middle age females compared to men, an effect increased by APOE4 4]. In APP23 mice, the loss of estrogen accelerates plaque formation [23] and in 3xTG mice, a FAD-Tg mouse that also expresses human Tau, ovariectomy (OVX) exacerbates extracellular Aβ accumulation, an effect prevented with ET [3]. Although these data indicate that ET may lower Aβ levels, an effect particularly beneficial with APOE4, there is no direct in vivo data. Therefore in this study EFAD mice, which express 5xFAD mutations and human APOE 22], were utilized to determine whether APOE modulates the effect of ET on Aβ accumulation after OVX.
MATERIALS AND METHODS
EFAD mice and treatment protocols
All protocols follow the UIC Institutional Animal Care and Use Committee protocols (IUCAC number 11–121). EFAD mice are the result of crossing 5XFAD mice (APP K670N/M671L + I716V + V717I and PS1 M146L + L286V) and apoE-targeted replacement mice [22]. There are three lines of EFAD mice; E2FAD (APOE2), E3FAD (APOE3) and E4FAD (APOE4). All breeding and colony maintenance was conducted at Taconic labs as described in [22]. At Taconic laboratories, 3-month old EFAD mice received a bilateral ovariectomy (OVX) performed under anesthesia, and a pellet containing either 0.25mg of a 90-day continual release of 17β-estradiol (OVXET+, Innovative Research of America #NC-111) or placebo pellet (OVXET−) was implanted subcutaneously in the posterior aspect of the neck. At 5.75 months, animals were shipped to the University of Illinois, Chicago for tissue harvest at 6 months.
Tissue harvest
EFAD mice were anesthetized with sodium pentobarbital (50 mg/kg) and transcardially perfused with ice-cold PBS containing protease inhibitors (Calbiochem, set 3). Brains were dissected at the midline, with the left hemi- fixed in 4% paraformaldehyde (PFA) for 48 h, followed by storage at 4°C in PBS + 0.05% sodium azide (NaN3) until use. Right hemi-brains were dissected on ice into cortex, hippocampus and cerebellum, snap frozen in liquid nitrogen, and stored at −80°C until use [22].
Biochemical analysis of Aβ42 levels
Dissected brains were sequentially homogenized in PBS, followed by PBS containing 1% Triton X-100 (PBSX), and then 5 M guanidine (Gu) as described [21]. Aβ42 levels were measured (Wako) by ELISA and all data are expressed per mg of brain tissue.
IHC analysis
Brains were incubated using two sequential 30% sucrose solutions (in TBS) for 24 h each. For immunohistochemistry, tissues were sectioned on a freezing microtome at 40μm intervals and stored in cryoprotectant (30% glycerol, 30% ethylene glycol in PBS). Every 8th section for 6 consecutive sections was stained for either amyloid plaques (Thioflavin-S, Thio-S) or for Aβ.
Thio-S staining for plaques
Thio-S plaque staining and quantification was conducted by an investigator blinded to APOE genotype and treatment beginning with the lateral-most section in the region of interest (ROI) [22]. Sections were washed in TBS (6×5 min), mounted on glass coverslips, allowed to dry, rehydrated in Milli-Q water for 2 min and stained in 0.1% Thio-S (dissolved in 50% EtOH + 50% 1×PBS) for 5 min in the dark. Tissue was destained in 80% EtOH (2×5 min) in the dark and mounted with VectaShield fluorescence mounting media.
Immunohistochemistry for Aβ
Free-floating sections were processed for immunohistochemical staining as described in [22], using the anti-Aβ antibody MOAΒ-2 (mouse IgG2b, 1:1000 dilution of 0.5mg/ml stock) an anti-NeuN antibody (mouse IgG1, 1:1000 dilution, Chemicon), Alexa fluorophore-conjugated isotype specific secondary antibodies (diluted 1:200) and ProLong Gold antifade mounting media containing DAPI (Invitrogen).
Image analysis
Slides were mounted into a Nanozoomer whole slide scanner at the University of Chicago. The scanner was programmed to measure FITC, DAPI and Texas Red. Tissue sections were outlined with a marking tool and slides were scanned. Images were then imported into Image J and converted to 8-bit gray images. A threshold for signal was established and optimized. Image J was programmed to measure number, size and % area covered.
Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc analysis using GraphPad Prism version 4 for Macintosh. p < 0.05 was considered significant.
RESULTS
Extracellular amyloid plaque and Aβ deposition is decreased with APOE2 and APOE3 but increased with APOE4, in ovariectomized EFAD mice treated with estradiol
To determine the interactions between APOE and ET on Aβ accumulation, female EFAD mice, which express increased levels of Aβ42 and human APOE were utilized [22]. EFAD mice develop extracellular Aβ deposition that initiates in the subiculum and the deep layers of the frontal cortex. 3-month old female EFAD mice were ovariectomized (OVX), and treated for 3 months with an implant containing either 17-β estradiol pellet (OVXET+, 0.25mg total) or vehicle control (OVXET−). This method models ET given at the beginning of the menopause, which represents a significantly indication of ET use in women. Comparing the ET dose and treatment duration between humans and the EFAD mice used for this study is complicated by a number of factors. For dose, the overall confounding factor is the pharmacokinetics of estradiol in mice vs humans, which is dependent on the drug formulation, route/method of administration, as well as absorbance and clearance parameters. However, 0.25 mg dose of 17-estradiol in mice corresponds to levels in pro-estrus phase [10].This stage in mice is equivalent to the early follicular phase of the menstrual cycle in humans. Typically, most hormone therapies replace to follicular levels in humans using 100 mg patch of ET biweekly (for example [15]). That aging is not a linear process in either mice or humans makes it particularly problematic to compare duration equivalency of treatment, as it is necessarily based on age. Simplistically, female mice live 2.3y and humans 86y, a ratio of ~1:39. Thus, a 3-month treatment in mice is ~10 human years. Based on these calculations, 0.25mg treatment over 3 months in EFAD mice is the equivalent to a 100mg patch of ET bi-weekly for 10 years
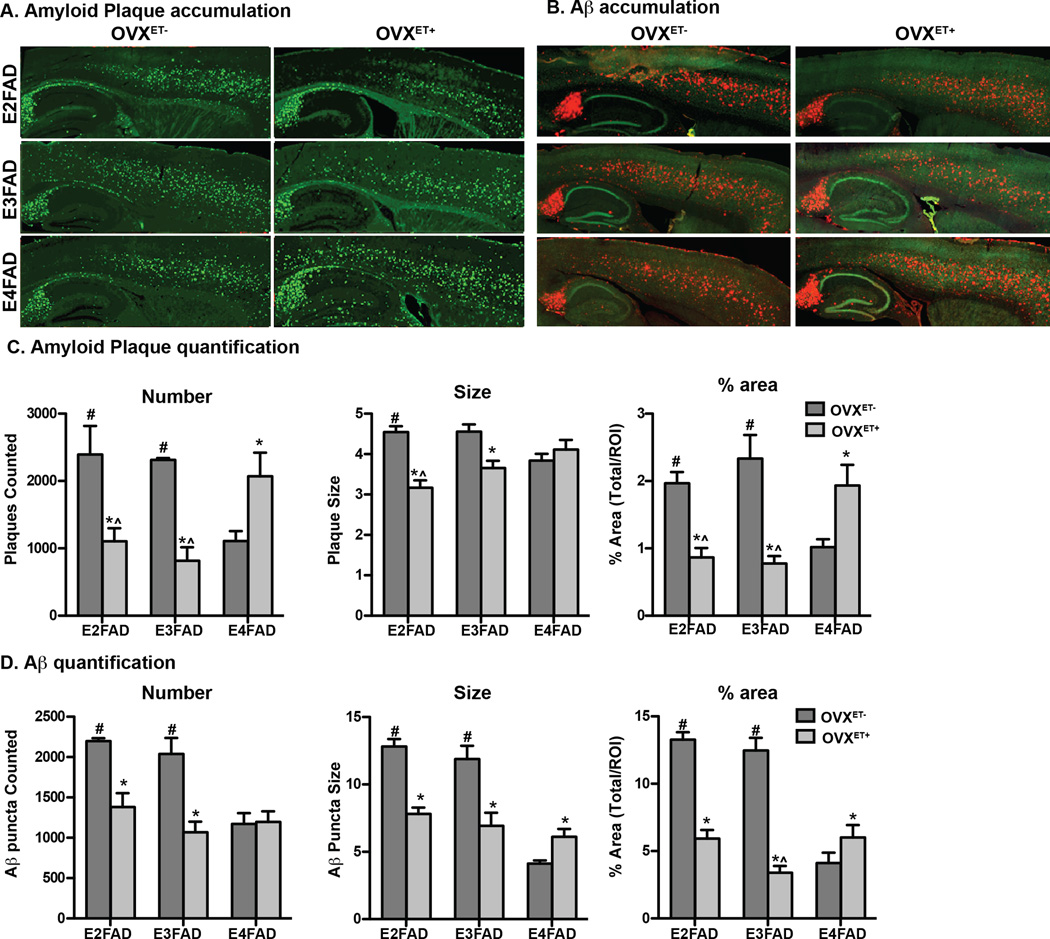
Although there is a debate as to the exact role of amyloid deposits in causing memory dysfunction in AD, extracellular amyloid deposits are one of the pathological hallmarks used for diagnosis of AD post-mortem, and amyloid brain imaging is increasingly utilized pre-mortem to aid in the diagnosis of AD. Amyloid plaques consist primarily of the Aβ42 peptide, therefore amyloid plaque accumulation using Thio-S staining (Figure 1A and C), and extracellular Aβ deposition, using the Aβ-specific antibody MOAB-2 (Figure 1B and D) was assessed.
Figure 1. Extracellular amyloid plaque and Aβ deposition is decreased with APOE2 and APOE3 but increased with APOE4, in ovariectomized EFAD mice treated with estradiol.
Representative image of sagittal sections from ovariectomized EFAD mice treated with vehicle (OVXET−) or estradiol (OVXET+), and A. stained with Thio-S or B. immunostained for Aβ (red) and NeuN (green) (x10 magnification). Quantification of number, size and % area covered in the frontal cortex of C. Thio-S stained plaque plaques or D. extracellular Aβ. Data are expressed as the mean ± S.E.M, and were analyzed by one-way ANOVA followed by Tukey’s multiple comparison post hoc analysis. n = 5–6. *p < 0.05 OVXET− versus OVXET+. #p < 0.05 vs E4FAD for OVXET−, ^p < 0.05 vs E4FAD for OVXET+
Compared to the OVXET− cohort, in the OVXET+ cohort, extracellular Thio-S (Figure 1A for representative image and 1C for quantification) and Aβ (Figure 1B for representative image and 1D for quantification) accumulation was decreased with APOE2 and APOE3 (plaque count, size and % area covered) but increased with APOE4 (% area covered) in the cortex and hippocampus (data not shown). Indeed, the % area covered by Thio-S was decreased ~60 % for E2FAD and E3FAD and increased by ~90% in E4FAD mice. Thus, after ovariectomy, ET with APOE4 increases extracellular Aβ and amyloid plaques in brain regions that are susceptible to relatively high (hippocampus) and intermediate (cortex) extracellular Aβ accumulation.
Total Aβ levels are increased with APOE4 in ovariectomized EFAD mice treated with estradiol
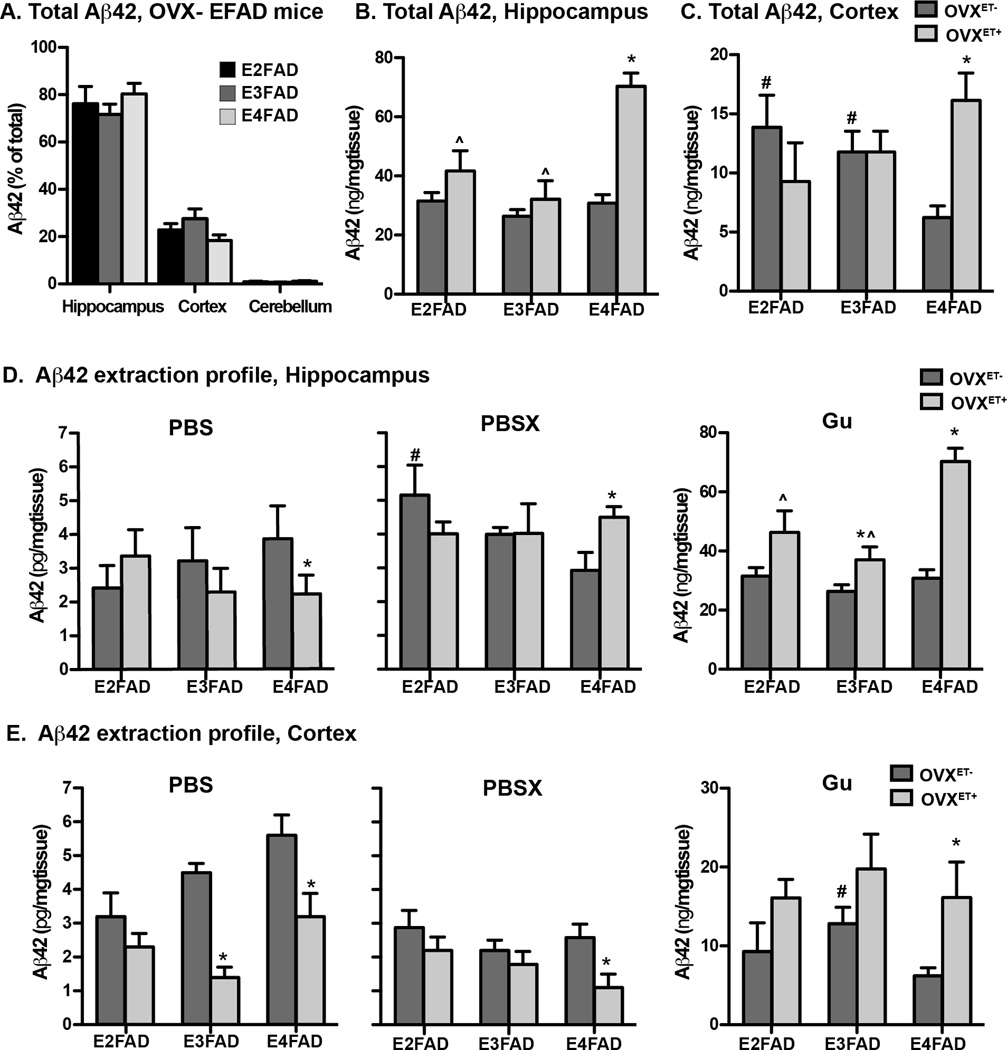
In order for a more in depth analysis of Aβ42 levels in OVXET−/+ EFAD mice, biochemical (BC) analysis was conducted. With all three APOE genotypes the levels of Aβ42 were highest in the hippocampus, followed by the cortex, while the cerebellum has virtually no Aβ42 (Figure 2A) in the OVX− group. Compared to OVXET−, in the OVXET+ group, total Aβ42 levels were increased in the hippocampus (Figure 2B) and cortex (Figure 2C) for APOE4, consistent with the increased extracellular amyloid plaques and Aβ42 deposition (Figure 1), but were unchanged with APOE2 and APOE3.
Figure 2. Total and insoluble Aβ42 levels are increased with APOE4, whereas soluble Aβ42 levels are decreased with APOE3 and APOE4 in ovariectomized EFAD mice treated with estradiol.
A. Total Aβ42 levels in the CX, HC and CB of ovariectomized EFAD mice as measured by ELISA. Total Aβ42 levels in the B. hippocampus and C. cortex of ovariectomized EFAD mice treated with vehicle (OVXET−) or estradiol (OVXET+). Aβ42 extraction profile using a three-step sequential protein extraction (PBS, PBSX, Gu) in the D hippocampus and E. cortex. Data are expressed as the mean ± S.E.M, and were analyzed by one-way ANOVA followed by Tukey’s multiple comparison post hoc analysis. n = 5–6. *p < 0.05 OVXET− versus OVXET+. #p < 0.05 vs E4FAD for OVXET−, ^p < 0.05 vs E4FAD for OVXET+
Soluble Aβ42 levels are decreased with APOE3 and APOE4 in ovariectomized EFAD mice treated with estradiol
Accumulating evidence over the last decade suggests that soluble Aβ in brain is the proximate cause in the initiation of AD development. Therefore, a 3-step biochemical extraction protocol was used to sequentially extract soluble (PBS), detergent (PBSX), and insoluble (Gu) Aβ42. In the hippocampus (Figure 2D), compared to OVXET−, in the OVXET+ group soluble Aβ42 levels were decreased and insoluble levels increased for APOE4, whereas Aβ42 levels were unchanged with APOE2 and APOE3.
In the cortex (Figure 2E), compared to OVXET−, in the OVXET+ group, soluble Aβ42 levels were decreased for both APOE3 and APOE4, whereas for APOE2, soluble Aβ42 levels were low in both the OVXET− and OVXET+ groups. Insoluble Aβ42 levels were increased for APOE4 in the OVXET+ group compared to OVXET−.
In both susceptible regions, the data are consistent with ET decreasing soluble Aβ42 levels, but increasing insoluble Aβ with APOE4. In addition, with APOE3, ET decreased soluble Aβ in the cortex, a region susceptible to intermediate extracellular Aβ accumulation, but not the hippocampus.
DISCUSSION
The increased risk of developing AD in women, and evidence that the post menopausal loss of estrogen may underlie these effects, has led to the hypothesis that ET is a potential treatment to prevent or treat AD. However, prospective analysis and clinical trials have produced conflicting results [5, 13, 19]. Upon further analysis, these studies appear consistant with the notion that ET should be initiated at the time of menopause to elicit beneficial effects. Future clinical trials (ELITE/KEEPS) may shed more light on this issue. An important, but understudied, factor that may modulate the therapeutic effects of ET is human APOE. However, it is unclear whether carriers of all human APOE genotypes will equally profit from ET, or whether ET should only be administered to certain APOE genotypes i.e. either APOE4 carriers or non-carriers [7–9, 14, 20]. This issue necessitates the need for preclinical ET studies in AD-relevant mouse models that express human APOE, using markers pertinent to human AD as read-outs. In this study, for the first time, the interactive effects of APOE and ET administered at the time of ovariectomy (OVX) on Aβ accumulation were determined in EFAD mice.
Previous data using FAD-Tg mice have demonstrated that the loss of estrogen increases, and ET treatment after OVX decreases, extracellular Aβ accumulation [3]. However, as these studies used FAD-Tg mice that express mouse APOE, which is structurally and functionally distinct from any of the human APOE genotypes, the relevance of these data are unclear. Thus, the first important finding from this study is that ET initiated at the same time as OVX decreased extracellular amyloid plaque and Aβ deposition with APOE2 and APOE3, and decreased soluble Aβ with APOE3 in the cortex a region susceptible to intermediate Aβ accumulation. Although comparing preclinical with clinical data is not clear-cut, these data are seemingly in agreement with the Cardiovascular Health study, in which current estrogen use was found to diminish the risk of cognitive impairment compared with never users by 41% in APOE4 negative women [20]. One potentially cautionary point is that in the hippocampus, which is a brain region susceptible to high extracellular Aβ deposition in EFAD mice, ET did not lower soluble Aβ42 levels with APOE3. Currently, there is debate in the AD field on the relative contribution of extracellular Aβ and soluble Aβ42 to AD progression. If soluble Aβ is a significant contributor to neurodegeneration, our data may indicate that the efficacy of ET on cognition may require treatment in the preclinical stage of AD, where extracellular Aβ levels are lower. However, a 12-month placebo controlled study directly demonstrated a beneficial ET effect on cognition in APOE4 non-carrier AD patients [16]. Tentatively, ET may prove beneficial for APOE4 non-carriers for lowering extracellular Aβ and in prevention and treatment paradigms.
As APOE4 22] and the loss of estrogen, both increase amyloid deposition in FAD-Tg mice, a reasonable hypothesis is that ET should prove particularly effective at lowering Aβ in APOE4 carriers. However, the second important finding of this study is that ET increased extracellular amyloid plaque and Aβ deposition, but lowered soluble Aβ42 levels in E4FAD mice. As mentioned previously, these data indicate that the efficacy of ET in human trials may depend on the relative contribution of soluble and extracellular Aβ to AD progression. However, the increased extracellular Aβ may be relevant for the findings that ET had no effect on cognitive impairment in APOE4 carriers in the Cardiovascular Health study, and in a trial of ET in AD patients [16]. Furthermore, a recent prospective analysis has indicated that ET actually worsens rates of cognitive decline in APOE4 carriers, although whether this effect is related to increased Aβ accumulation is unclear [8]. The results of this current study are particularly timely given the debate on the appropriate use of amyloid imaging. For APOE4 positive post-menopausal women using ET, amyloid imaging and CSF Aβ42 measurements may provide important information for interpreting the effects of extracellular plaques on cognition, and identifying potential high-risk groups for developing cognitive impairment and AD. Essentially, if an APOE4 carrier is enrolled in an ET clinical trial, then careful monitoring of amyloid levels could be incorporated in order to prevent future negative cognitive effects induced by Aβ.
This study focused on the effect of ET on Aβ accumulation for each APOE genotype. However, comparing APOE genotypes reveals an interesting region-specific response to ET. It would be expected that in OVXET− EFAD mice, extracellular amyloid and Aβ would be highest with APOE4. However, in the cortex of OVXET− EFAD mice, extracellular amyloid, Aβ staining and Aβ42 concentrations were lower with APOE4 compared to APOE3 and APOE2, while levels in the hippocampus were equivalent among APOE genotypes. Furthermore, in the cortex, estradiol treatment raised extracellular plaque and insoluble Aβ42 levels in E4FAD mice to the levels in E3FAD OVXET−. Importantly, this was not observed in the hippocampus; insoluble Aβ42 levels in OVXET+ E4FAD mice were higher than OVXET− E3FAD and E2FAD mice. Thus, interactions between OVX and APOE on Aβ accumulation are likely dependent on the brain region, i.e. susceptibility to Aβ accumulation.
The interactive effects between ET and APOE are likely multifactorial, including effects, the cardiovascular system [20], cellular aging [7] and Aβ accumulation. Our ongoing studies are aimed at addressing these issues, including the potential mechanisms underlying these different effects, the therapeutic treatment window of ET and the effect of combined progesterone and estrogen HRT.
Conclusions
The present study provides the first in vivo evidence that human APOE differentially regulate the effects of ET on Aβ accumulation. Overall data indicate that ET may be beneficial with APOE2 and APOE3 for decreasing extracellular and soluble Aβ42 however, the efficacy of ET treatment with APOE4 will be dependent of the relative contribution extracellular and soluble Aβ to neurodegeneration during AD progression.
Highlights.
The interactive effects of APOE and ERT after OVX on Aβ accumulation were determined in EFAD mice.
ERT decreased extracellular amyloid plaque and Aβ deposition with APOE2 and APOE3.
ERT increased extracellular amyloid plaque and Aβ deposition, but lowered soluble Aβ42 with APOE4.
First in vivo evidence that hAPOE differentially regulates the effects of ERT on Aβ deposition.
Aβ42 levels may be a critical read-out for APOE4 carriers enrolled in ERT trials.
Acknowledgements
FUNDING SOURCES
This work was supported in part, by National Institutes of Health Grants P01AG030128 (through the NIA) (M.J.L) Alzheimer’s Association Grant ZEN-08-899000 (M.J.L.); University of Illinois at Chicago Center for Clinical and Translational Science Grant UL1RR029879 (M.J.L.); and Baxter Healthcare.
Abbreviations
- Aβ
amyloid-β
- apoE
apolipoprotein E
- AD
Alzheimer’s disease
- ET
estrogen therapy
- FAD
familial AD
- Thio-S
thioflavin S
- HRT
hormone replacement therapy
- OVX
ovariectomy
- OVXET−
ovariectomy without ET
- OVXET+
ovariectomy with ET
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JR, Dartigues JF, Kragh-Sorensen P, Baldereschi M, Brayne C, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. EURODEM Incidence Research Group. Neurology. 1999;53:1992–1997. doi: 10.1212/wnl.53.9.1992. [DOI] [PubMed] [Google Scholar]
- 2.Bretsky PM, Buckwalter JG, Seeman TE, Miller CA, Poirier J, Schellenberg GD, Finch CE, Henderson VW. Evidence for an interaction between apolipoprotein E genotype, gender, and Alzheimer disease. Alzheimer disease and associated disorders. 1999;13:216–221. doi: 10.1097/00002093-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corder EH, Ghebremedhin E, Taylor MG, Thal DR, Ohm TG, Braak H. The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: modification by age, sex, and APOE polymorphism. Annals of the New York Academy of Sciences. 2004;1019:24–28. doi: 10.1196/annals.1297.005. [DOI] [PubMed] [Google Scholar]
- 5.Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA : the journal of the American Medical Association. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 6.Fleisher A, Grundman M, Jack CR, Jr, Petersen RC, Taylor C, Kim HT, Schiller DH, Bagwell V, Sencakova D, Weiner MF, DeCarli C, DeKosky ST, van Dyck CH, Thal LJ. Sex, apolipoprotein E epsilon 4 status, and hippocampal volume in mild cognitive impairment. Archives of neurology. 2005;62:953–957. doi: 10.1001/archneur.62.6.953. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs EG, Kroenke C, Lin J, Epel ES, Kenna HA, Blackburn EH, Rasgon NL. Accelerated cell aging in female APOE-epsilon4 carriers: implications for hormone therapy use. PloS one. 2013;8:e54713. doi: 10.1371/journal.pone.0054713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang JH, Grodstein F. Postmenopausal hormone therapy, timing of initiation, APOE and cognitive decline. Neurobiology of aging. 2012;33:1129–1137. doi: 10.1016/j.neurobiolaging.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang JH, Weuve J, Grodstein F. Postmenopausal hormone therapy and risk of cognitive decline in community-dwelling aging women. Neurology. 2004;63:101–107. doi: 10.1212/01.wnl.0000132522.13574.67. [DOI] [PubMed] [Google Scholar]
- 10.Nequin LG, Alvarez J, Schwartz NB. Measurement of serum steroid and gonadotropin levels and uterine and ovarian variables throughout 4 day and 5 day estrous cycles in the rat. Biology of reproduction. 1979;20:659–670. doi: 10.1095/biolreprod20.3.659. [DOI] [PubMed] [Google Scholar]
- 11.Paganini-Hill A, Henderson VW. Estrogen replacement therapy and risk of Alzheimer disease. Archives of internal medicine. 1996;156:2213–2217. [PubMed] [Google Scholar]
- 12.Payami H, Zareparsi S, Montee KR, Sexton GJ, Kaye JA, Bird TD, Yu CE, Wijsman EM, Heston LL, Litt M, Schellenberg GD. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. American journal of human genetics. 1996;58:803–811. [PMC free article] [PubMed] [Google Scholar]
- 13.Resnick SM, Coker LH, Maki PM, Rapp SR, Espeland MA, Shumaker SA. The Women's Health Initiative Study of Cognitive Aging (WHISCA): a randomized clinical trial of the effects of hormone therapy on age-associated cognitive decline. Clin Trials. 2004;1:440–450. doi: 10.1191/1740774504cn040oa. [DOI] [PubMed] [Google Scholar]
- 14.Ryan J, Carriere I, Scali J, Dartigues JF, Tzourio C, Poncet M, Ritchie K, Ancelin ML. Characteristics of hormone therapy, cognitive function, and dementia: the prospective 3C Study. Neurology. 2009;73:1729–1737. doi: 10.1212/WNL.0b013e3181c34b0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slowinska-Srzednicka J, Zgliczynski S, Jeske W, Stopinska-Gluszak U, Srzednicki M, Brzezinska A, Zgliczynski W, Sadowski Z. Transdermal 17 beta-estradiol combined with oral progestogen increases plasma levels of insulin-like growth factor-I in postmenopausal women. Journal of endocrinological investigation. 1992;15:533–538. doi: 10.1007/BF03348801. [DOI] [PubMed] [Google Scholar]
- 16.Valen-Sendstad A, Engedal K, Stray-Pedersen B, Group AS, Strobel C, Barnett L, Meyer N, Nurminemi M. Effects of hormone therapy on depressive symptoms and cognitive functions in women with Alzheimer disease: a 12 month randomized, double-blind, placebo-controlled study of low-dose estradiol and norethisterone. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2010;18:11–20. doi: 10.1097/JGP.0b013e3181beaaf4. [DOI] [PubMed] [Google Scholar]
- 17.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waring SC, Rocca WA, Petersen RC, O'Brien PC, Tangalos EG, Kokmen E. Postmenopausal estrogen replacement therapy and risk of AD: a population-based study. Neurology. 1999;52:965–970. doi: 10.1212/wnl.52.5.965. [DOI] [PubMed] [Google Scholar]
- 19.Wharton W, Baker LD, Gleason CE, Dowling M, Barnet JH, Johnson S, Carlsson C, Craft S, Asthana S. Short-term hormone therapy with transdermal estradiol improves cognition for postmenopausal women with Alzheimer's disease: results of a randomized controlled trial. Journal of Alzheimer's disease : JAD. 2011;26:495–505. doi: 10.3233/JAD-2011-110341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yaffe K, Haan M, Byers A, Tangen C, Kuller L. Estrogen use, APOE, and cognitive decline: evidence of gene-environment interaction. Neurology. 2000;54:1949–1954. doi: 10.1212/wnl.54.10.1949. [DOI] [PubMed] [Google Scholar]
- 21.Youmans KL, Leung S, Zhang J, Maus E, Baysac K, Bu G, Vassar R, Yu C, Ladu MJ. Amyloid-beta42 alters apolipoprotein E solubility in brains of mice with five familial AD mutations. Journal of neuroscience methods. 2011;196:51–59. doi: 10.1016/j.jneumeth.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Youmans KL, Tai LM, Nwabuisi-Heath E, Jungbauer L, Kanekiyo T, Gan M, Kim J, Eimer WA, Estus S, Rebeck GW, Weeber EJ, Bu G, Yu C, Ladu MJ. APOE4-specific Changes in Abeta Accumulation in a New Transgenic Mouse Model of Alzheimer Disease. The Journal of biological chemistry. 2012;287:41774–41786. doi: 10.1074/jbc.M112.407957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer's disease animal model. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:19198–19203. doi: 10.1073/pnas.0505203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JC. Hormone replacement therapy and incidence of Alzheimer disease in older women: the cache county study. JAMA : the journal of the American Medical Association. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]