Abstract
The effects of mechanical ventilation on the surface electrocardiogram (ECG) have not been systematically investigated and the anticipated changes such as rightward P and QRS axes shifts, reduced QRS voltage, and slow R-wave progression are not supported by definitive data. We sought to determine the effects of mechanical ventilation on the surface ECG in hemodynamically stable adults without active cardiopulmonary disease. Seventeen patients in good overall health who were undergoing elective outpatient surgery had serial ECGs done preoperatively, intraoperatively, and postoperatively. No clinically significant changes in QRS or P-wave axis were detected. R-wave progression was not altered and there were no significant differences in the QRS amplitudes pre-, intra-, or postoperatively in either the precordial or limb leads. The present study shows that hemodynamically stable patients without active cardiopulmonary disease undergoing elective surgery demonstrate relatively minor ECG changes from baseline despite the addition of positive pressure ventilation. Clinicians should not assume that substantial changes in P wave or QRS axis or amplitude in patients undergoing mechanical ventilation are due to the effects of positive pressure ventilation alone.
Keywords: Electrocardiography, Positive pressure ventilation, Lung, Airway, Heart
Introduction
Mechanical ventilation is required in both the relatively healthy individual undergoing elective surgery and the critically ill patient in the emergency room or intensive care unit. Surprisingly, the electrocardiographic changes associated with mechanical ventilation in humans have not been thoroughly investigated in the literature and the expected changes, if any, are often hypothesized without definitive data.
Pulmonary diseases such asthma, emphysema, pulmonary embolism, and pulmonary hypertension are associated with well-described electrocardiographic changes [1–3]. In chronic obstructive pulmonary disease, the more characteristic, although insensitive, electrocardiogram(ECG) changes are an increase in P wave amplitude, tendency for right axis deviation, low-voltage QRS, and slow R-wave progression [3]. Hyperinflation, as seen in obstructive pulmonary diseases such as asthma and emphysema, has been associated with progressive P wave verticalization (i.e., a P-wave axis ≥60°) [4]. “Verticalization” of the frontal P wave axis is thought to be the most consistent finding in chronic obstructive lung disease with emphysema [5, 6]. This effect on the frontal P-wave axis is attributed to the anatomical relationship of the right atrium and the diaphragm [7, 8]. The mean frontal vector of the P wave has been used as an electrocardiographic indicator of airway obstruction [4]. Moreover, one study has shown that with increasing vital capacity, the atrial depolarization axis deviates to the right [8]. However, the impact of hyperinflation per se on the electrocardiogram, in the absence of pulmonary pathology, has not been assessed systematically in humans to our knowledge.
We sought to determine the isolated impact of mechanical ventilation on the 12-lead ECG in the absence of hemodynamic instability, known active cardiopulmonary disease, and without other metabolic derangements or fluid shifts. Our hypothesis was that lung volume increases induced by mechanical ventilation would lead to expansion of the chest wall and flattening of the diaphragms, ultimately leading to a consistent vertical shift of the P and QRS axes [4, 5, 7, 8] and reduced amplitude of the QRS complex in the precordial leads.
Patients and Methods
Approval was obtained from the institutional review board who waived the requirement for informed consent. Consecutive patients in generally good health who were to undergo surgery under general anesthesia were enrolled in the study. Patients with known or a history of active cardiopulmonary disease were excluded. All patients underwent general anesthesia with endotracheal intubation. Patients were premedicated in the holding area with 2 mg of midazolam. A 1-mg defasciculating dose of vecuronium was administered and intubation was facilitated by succinylcholine 100 mg. Anesthetic induction was achieved using propofol 2 mg/kg and fentanyl 100 mcg. Patients were endotracheally intubated with a number 7.0 ETT for females and 7.5 for males. Anesthesia was maintained using desflurane and further fentanyl as required. All subjects were ventilated with volume-controlled ventilation at a set volume of 10 ml/kg of ideal body weight and a positive end expiratory pressure of 5 cmH2O. The respiratory rate was set to maintain an end-tidal CO2 between 35 and 40 mmHg. All patients were monitored with a pulse oximeter and an end-tidal CO2 detector. No arterial blood gases were required clinically either intraoperatively or postoperatively. After completion of the surgical procedure, all patients were extubated and transferred to the postanesthesia care unit (PACU). All patients remained hemodynamically stable throughout the perioperative course. A 12-lead ECG was performed preoperatively while the patient was in the supine position. The electrodes remained in place for subsequent ECGs. Ten minutes after induction of anesthesia and before the start of surgery (before any incisions), a second ECG was obtained while the patient was in the supine position. Following extubation and clinical reassessment for adequacy of ventilation, a final ECG was obtained in the PACU while the patient was in the supine position.
PR and QT/QTc intervals, QRS duration, heart rate (HR), and QRS and P-wave axes were computed by automated ECG measurement algorithms (Spacelabs Burdick, Eclipse Plus, Deerfield, WI). Two cardiologists blinded to the timing of the ECG recordings independently reviewed each ECG to confirm that the computed values were correct. Two patients had incorrect computed P wave axes that were corrected after visual inspection. Hodges’ formula was used to calculate QTc [9]. In a blinded fashion, a single physician manually measured the peak-to-peak QRS amplitudes. The peak-to-peak QRS amplitude was defined as the R-wave voltage plus the S-wave voltage, unless the absolute value of the Q wave was greater than that of the S wave. In the latter case, the sum of the R-wave and Q-wave voltages represented the peak-to-peak QRS amplitude. Measurements were made with reference to the TP segment as the baseline. Using a standard ruler, lines were drawn to highlight the baseline for measurement of the peak height or depth from baseline. Measurements were made with calipers and estimated to the nearest 0.05 mV. Intraobserver variation for the measurements of QRS amplitude in nine random ECGs was 0.016 ± 2.8%. The peak-to-peak QRS amplitude was calculated for each QRS complex in each of the 12 leads for all 17 patients pre-, intra-, and postoperatively. In instances when the baseline could not be reliably set or the peak or trough of a complex was distorted so that measurement would be imprecise, these complexes were excluded. Only sinus beats were included. In most cases, three complexes per lead were averaged and ultimately this mean value from each patient was used to calculate a mean peak-to-peak QRS amplitude for each lead pre-, intra-, and postoperatively. Low voltage was defined as a QRS amplitude less than 0.5 mV in each of the six limb leads and less than 1.0 mV in each of the six precordial leads [10]. Slow R-wave progression was defined as either an R wave in lead V3 less than 0.2 mV and/or less than 0.4 mV in V4, or R < S in V4 [11, 12]. The following were considered normal ECG parameters: sinus rhythm, rate of 60–99 bpm; P-wave axis of between −10° and +100°; QRS axis of between −30° and +100°; PR interval between 120 and 200 ms; QRS duration less than 110 ms; and QTc interval less than 450 ms [10]. All means were expressed as ± standard deviation (SD). The ECG parameters studied were normally distributed and therefore a parametric statistical analysis was appropriate using SigmaStat (Systat Software, Chicago, IL). The preoperative results were compared to intraoperative values and postoperative values using Student’s paired t tests. A p ≤ 0.0016 was considered statistically significant after a Bonferroni correction for multiple comparisons was done [13].
Results
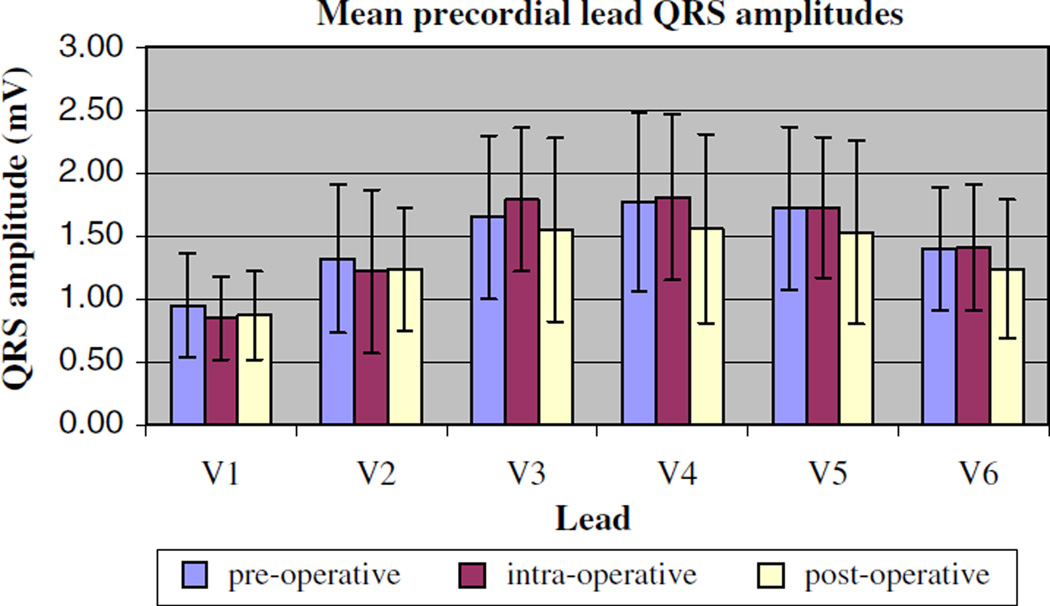
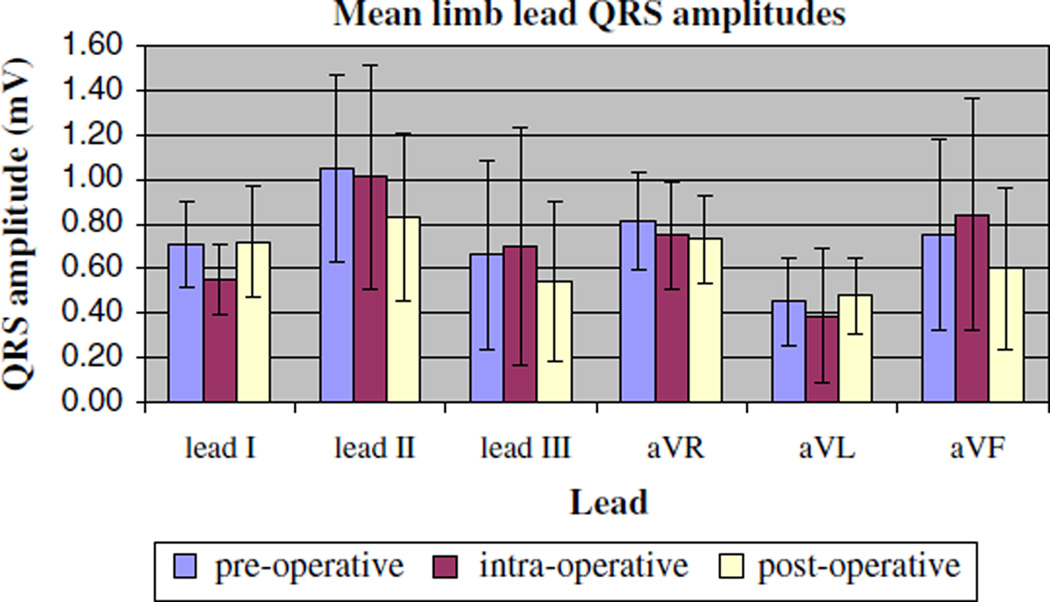
For the group of 17 subjects (Table 1), there was no clinically significant change in mean heart rate, QRS duration, or PR interval with mechanical ventilation. The QTc was slightly but significantly more prolonged postoperatively (427 ± 30 ms) compared with the preoperative value (406 ± 23 ms, p < 0.001) (Table 2). No differences were seen in the QRS amplitudes pre-, intra-, and postoperatively in either the precordial or limb leads (Figs. 1 and 2). Moreover, criteria for low-voltage QRS and slow R-wave progression were not met intraoperatively in any of the patients. There was no clinically significant change in the QRS axis with intraoperative mechanical ventilation. The maximum change of a QRS axis in any single patient was 27° (from 29° to 56°), and no patient met criteria for right axis deviation intraoperatively. The mean P-wave axis change with mechanical ventilation also did not meet statistical significance (61° ± 15° intraoperatively) compared with the preoperative value (51° ± 19°, p = 0.032). Notably, 3 of the 17 patients, all of whom had a P-wave axis less than 60° preoperatively (range = +37° to +40°), showed a verticalization of their axis intraoperatively (range = +66° to +67°). This verticalization did not persist in two of three patients once mechanical ventilation was discontinued in the postoperative period.
Table 1.
Patient characteristics (n = 17)
| Mean age (years) (± SD) | 54.4 ± 19.6 |
| Gender | 10 males, 7 females |
| Mean height (m) (± SD) | 1.7 ± 0.1 |
| Mean weight (kg) (± SD) | 76.1 ± 15.1 |
| Surgery | |
| Peripherala | 3/17 |
| Abdominalb | 6/17 |
| Orthopedicc | 8/17 |
| Comorbid illness | |
| Hypertension | 4/17 |
| Diabetes | 2/17 |
| Coronary artery disease | 1/17d |
| Medications | |
| Beta blockers | 2/17 |
| Calcium channel blockers | 2/17 |
Varicose vein stripping (3)
Cholecystectomy (3), colectomy (3)
Arthroscopic medial meniscus repair (2), total knee revision (1), laminectomy (1), open reduction and internal fixation of the right acetabulum (1), revision and fixation of left intertrochanteric hip fracture (1), removal of hardware following internal fixation of right ankle (1), resection of heterotrophic ossification of the hip (1) d The patient had undergone coronary artery bypass surgery and a postoperative exercise stress test done 1 year prior to current surgery showed no evidence of ischemia
The patient had undergone coronary artery bypass surgery and a postoperative exercise stress test done 1 year prior to current surgery showed no evidence of ischemia
Table 2.
Mean heart rate, intervals, and axes pre-, intra-, and postoperatively (± SD)
| Preoperative | Intraoperative | Postoperative | p valuesa | |
|---|---|---|---|---|
| HR (bpm) | 71 ± 12 | 77 ± 15 | 72 ± 11 | 0.066 and 0.80 |
| PR interval (ms) | 155 ± 29 | 155 ± 54 | 159 ± 26 | 0.98 and 0.27 |
| QRS duration (ms) | 84 ± 8.2 | 84 ± 8.8 | 86 ± 6.6 | 0.75 and 0.10 |
| QT interval (ms) | 387 ± 34 | 393 ± 39 | 408 ± 32 | 0.43 and 0.0056 |
| QTcinterval (ms) | 406 ± 23 | 421 ± 22 | 427 ± 30 | 0.0021 and <0.001 |
| QRS axis (degrees) | 39 ± 31 | 50 ± 32 | 32 ± 29 | <0.001 and 0.090 |
| P wave axis (degrees) | 51 ± 19 | 61 ± 15 | 53 ± 15 | 0.032 and 0.68 |
Preoperative compared with intraoperative and postoperative
Fig. 1.
Mean precordial lead amplitudes measured preoperatively, intraoperatively, and postoperatively. Data are presented as mean values ± SD for all 17 patients
Fig. 2.
Mean limb lead amplitudes measured preoperatively, intraoperatively, and postoperatively. Data are presented as mean values ± SD for all 17 patients
Discussion
Current clinical teaching supports the notion that positive-pressure ventilation alone may cause clinically significant ECG changes such as a rightward shift of the P-wave and QRS axes, low voltages, or slow R-wave progression. The key finding of the present study is that hemodynamically stable patients without active cardiopulmonary disease who are undergoing elective surgery demonstrate relatively minor ECG changes from baseline with the addition of positive-pressure ventilation. A recent study in rabbits [14] on the ECG changes with positive end-expiratory pressure (PEEP) detected reductions in P-wave and T-wave amplitudes but no change in HR and only minor decreases in R-wave amplitude. Our study did not specifically analyze P- or T-wave amplitudes, and the amount of PEEP applied in the study by Joulia et al. [14] was relatively large compared to the amount used in the present study and what is typically used clinically. One interesting finding of the present study is the shift of the mean frontal P-wave axis intraoperatively and resolution postoperatively. One could speculate that some of these subjects may have anatomical differences with respect to the relationship of their right atrium and diaphragm such that the tidal volumes administered intraoperatively were enough to displace their atrial axis. However, we did not obtain further anatomical information from either a chest radiograph or transthoracic echocardiogram to investigate these findings. Alternatively, P-wave axis shifts may occur secondary to subtle shifts in sinus pacemaker activity related to autonomic effects [15]. The increase in QTc interval seen in this study has been reported as an effect of some inhaled anesthetics [16, 17]. However, there is no report to our knowledge that associates any of the medications used in the present study with a prolonged QTc. No electrolyte or other metabolic derangements were suspected in any of the patients included in the study. However, clinically unrecognized electrolyte abnormalities or other metabolic perturbations affecting ventricular repolarization cannot be ruled out. The degree of QTc prolongation seen in this study, although minor, would be worth exploring in a subsequent study.
Patients with obstructive pulmonary diseases such as emphysema and asthma often demonstrate predictable ECG manifestations associated with abnormal lung mechanics [1, 2, 18]. Relatively acute pulmonary disease such as a pulmonary embolism can also impact the ECG [19]. Similarly, positive pressure ventilation is typically thought of as an acute change in normal cardiopulmonary physiology and as such is assumed to have predictable and consistent ECG changes.
There are a few possible reasons that we did not detect major electrocardiographic changes due to mechanical ventilation in our study. The first possibility is that positive-pressure ventilation in hemodynamically stable patients without active cardiopulmonary disease has no consistent impact on the ECG. Another possibility is that changes are intermittent and therefore only detectable with continuous monitoring or that any changes induced are not of sufficient magnitude to be detected by surface ECG alone. In addition, the difference between the patient’s baseline tidal volumes preoperatively and the 10 cc/kg intraoperative tidal volumes used in our study may not be relatively large enough to induce the ECG changes previously described with hyperinflation [4]. Moreover, the electrical impedance of diseased lung tissue and/or chest wall in patients with emphysema and asthma may impart many of the characteristic ECG changes rather than increased lung volumes in isolation. In critically ill patients undergoing mechanical ventilation, occasionally unrecognized variables associated with critical illness may cause surface ECG changes that are not directly related to the introduction of positive pressure alone. For example, Madias et al. [20] demonstrated that QRS amplitude fluctuation was independently associated with fluid balance in patients with anasarca. Moreover, in their control and study populations, mechanical ventilation alone was not associated with any changes in QRS amplitude [20]. Association of other variables with mechanical ventilation has likely perpetuated clinical assumptions about putative ventilator-induced ECG changes.
The small number of patients included in this study limits the power to detect the rare instance in which the intraoperative ECG may in fact demonstrate perturbations caused by mechanical ventilation. However, we doubt that we have missed a clinically important effect of mechanical ventilation on the ECG because we observed no instances of clinically important ECG changes induced by mechanical ventilation (e.g., low-voltage ECG in the precordial leads). Thus, we are unable to perform rigorous power calculations as to how many subjects we would need to study to show a statistically significant increase in important ECG changes. Moreover, because of the study design our patient population had no known active cardiopulmonary disease and was both metabolically and hemodynamically more stable than many of the patients encountered in other clinical settings. Other factors may also modulate our results such as the ages of the participants and their underlying lung and chest wall compliances. Therefore, our results may not to be generalized to the intensive care unit, the emergency room, and patients undergoing emergent surgery. However, limiting our study to patients without active cardiopulmonary disease strengthens the study because we were able to minimize confounding variables in an attempt to isolate the effects of mechanical ventilation. Another limitation of the study was the fact that we used only one set of ventilator parameters. A range of tidal volumes or different levels of positive end-expiratory pressures may have induced subtle ECG changes that we did not detect. However, given that our chosen ventilator settings are typically used in this clinical context, we believe we have excluded a major impact of these but not other ventilator settings. Because higher PEEP levels are sometimes used in the management of acute respiratory distress syndrome (and in some cases to prevent its development), we would support further research into these areas.
In summary, mechanical ventilation in people without active cardiopulmonary disease who are undergoing elective surgical procedures is not consistently associated with major intra- or postoperative ECG changes. This study supports a cautious view of the previously unproven notion that positive-pressure ventilation alone can induce clinically important ECG changes. Therefore, clinicians should not assume that new ECG changes in patients undergoing mechanical ventilation are due to the effects of the ventilator alone. Despite the plausibility of this hypothesis in mechanically ventilated patients, other explanations should be explored for any observed changes.
Acknowledgments
Dr. Malhotra was the principal investigator of the NIA Beeson Award (AG024837-01), RO1-HL73146-01, SCOR Neurobiology of Sleep and Sleep Apnea Project 1 (HL060292), and American Heart Association Established Investigator Award.
Abbreviations
- ECG
Electrocardiogram
- HR
Heart rate
- PEEP
Positive end-expiratory pressure
- PACU
Postanesthesia care unit
Footnotes
The patients included in this study and all of the electrocardiographic data were obtained at the Beth Israel Deaconess Medical Center.
Contributor Information
Jason Elinoff, Critical Care Medicine Department, National Institutes of Health, Bethesda, MD 20892, USA, elinoffj@cc.nih.gov.
Daniel Talmor, Department of Anesthesia, Critical Care and Pain Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA 02215, USA, dtalmor@bidmc.harvard.edu.
Balachundhar Subramaniam, Department of Anesthesia, Critical Care and Pain Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA 02215, USA, bsubrama@bidmc.harvard.edu.
Dimitrios Karmpaliotis, Cardiology of Georgia, Fuqua Heart Center, Piedmont Hospital, Atlanta, GA 30309, USA, dkarmpaliotis@cardioga.com.
Ary L. Goldberger, Department of Internal Medicine, Divisions of Cardiology and Interdisciplinary Medicine/Biotechnology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA 02215, USA, agoldber@caregroup.harvard.edu
Atul Malhotra, Department of Internal Medicine, Divisions of Pulmonary/Critical Care and Sleep Divisions, Brigham and Women’s Hospital and Harvard Medical School, 75 Francis St, Boston, MA 02115, USA, amalhotra1@partners.org.
References
- 1.Spodick DH. Electrocardiographic studies in pulmonary diseaseIElectrocardiographic abnormalities in diffuse lung disease. Circulation. 1959;20:1067–1072. doi: 10.1161/01.cir.20.6.1067. [DOI] [PubMed] [Google Scholar]
- 2.Armen RN, Kantor M, Weiser N. Pulmonary heart disease with emphasis on electrocardiographic diagnosis. Circulation. 1958;17:164–175. doi: 10.1161/01.cir.17.2.164. [DOI] [PubMed] [Google Scholar]
- 3.Rubin LJ. Pulmonary heart disease. In: Crapo JD, Glassroth J, Karlinsky JB, King TE, editors. Baum’s textbook of pulmonary disease. 7th edn. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 778–779. [Google Scholar]
- 4.Krishnan SS, Stewart J, Amin N, Griffing RT, Dozer AJ. Electrocardiographic prediction of hyperinflation in children. Am J Resp Crit Care Med. 1997;156:2011–2014. doi: 10.1164/ajrccm.156.6.9611024. [DOI] [PubMed] [Google Scholar]
- 5.Shah N, Koller S, Janower M, Spodick D. Diaphragm levels as determinants of P axis in restrictive versus obstructive pulmonary disease. Chest. 1995;107:697–700. doi: 10.1378/chest.107.3.697. [DOI] [PubMed] [Google Scholar]
- 6.Baljepally R, Spodick DH. Electrocardiographic screening for emphysema: the frontal plane P axis. Clin Cardiol. 1999;22:226–228. doi: 10.1002/clc.4960220313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saha NC. Study of the P wave in normal and obstructive lung disease in Delhi. Am Heart J. 1970;80:154–161. doi: 10.1016/0002-8703(70)90162-6. [DOI] [PubMed] [Google Scholar]
- 8.Gross D. The correlations between vital capacity and the P wave of the electrocardiogram. Acta Med Scand. 1956;156:97–107. doi: 10.1111/j.0954-6820.1956.tb00521.x. [DOI] [PubMed] [Google Scholar]
- 9.Luo S, Michler K, Johnston P, Macfarlane PW. A comparison of commonly used QT correction formulae: the effect of heart rate on the QTc of normal ECGs. J Electrocardiol. 2004;37(Suppl):81–90. doi: 10.1016/j.jelectrocard.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Goldberger A. Clinical electrocardiography: a simplified approach. 7th edn. St. Louis: Mosby/Elsevier; 2006. [Google Scholar]
- 11.Zema MJ, Kligfield P. Electrocardiographic poor R wave progression. I: correlation with the frank vectorcardiogram. J Electrocardiol. 1979;12:3–10. doi: 10.1016/s0022-0736(79)80038-2. [DOI] [PubMed] [Google Scholar]
- 12.Goldberger AL. A specific ECG triad associated with congestive heart failure. Pacing Clin Electrophysiol. 1982;5:593–599. doi: 10.1111/j.1540-8159.1982.tb02285.x. [DOI] [PubMed] [Google Scholar]
- 13.Weisstein EW. “Bonferroni correction. ” From Math- World—a wolfram web resource. [Accessed 2 June 2007];2004 Available at http://mathworld.wolfram.com/BonferroniCorrection.html. [Google Scholar]
- 14.Joulia F, Barthelemy P, Lafay V, Zattara-Hartmann MC, Jammes Y. Electrocardiogram changes during positive pressure breathing in rabbits. Eur J Appl Physiol Occup Physiol. 1996;73:56–60. doi: 10.1007/BF00262809. [DOI] [PubMed] [Google Scholar]
- 15.Mitro P, Spegar J. Dynamic changes of P wave duration and P wave axis during head-up tilt test in patients with vasovagal syncope. Pacing Clin Electrophysiol. 2006;29:742–746. doi: 10.1111/j.1540-8159.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- 16.Schmeling WT, Warltier DC, McDonald DJ, Madsen KE, Atlee JL, Kampine JP. Prolongation of the QT interval by enflurane, isoflurane, and halothane in humans. Anesth Analg. 1991;72:137–144. doi: 10.1213/00000539-199102000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Guler N, Bilge M, Eryonucu B, Kati I, Demirel CB. The effects of halothane and sevoflurane on QT dispersion. Acta Cardiol. 1999;54:311–315. [PubMed] [Google Scholar]
- 18.Fessler HE. Heart-lung interactions: applications in the critically ill. Eur Respir J. 1997;10:226–237. doi: 10.1183/09031936.97.10010226. [DOI] [PubMed] [Google Scholar]
- 19.Stein PD, Terrin ML, Hales CA, Palevsky HI, Saltzman HA, Thompson BT, Weg JG. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest. 1991;100:598–603. doi: 10.1378/chest.100.3.598. [DOI] [PubMed] [Google Scholar]
- 20.Madias JE, Bazaz R, Agarwal H, Win M, Medepalli L. Anasarca-mediated attenuation of the amplitude of electrocardiogram complexes: a description of a heretofore unrecognized phenomenon. J Am Coll Cardiol. 2001;38:756–764. doi: 10.1016/s0735-1097(01)01429-2. [DOI] [PubMed] [Google Scholar]