Abstract
Lymphoma is rising in incidence and there is a continued need for new and novel therapeutic options. Lymphomas are extremely radiosensitive, but the majority of patients are not candidates for involved field radiation therapy. An intact immune system has a critical role in suppressing lymphomagenesis. Here we discuss the contribution of various components of the immune system in suppressing the development of lymphoma, as elucidated from mouse models. We review the nature of the immune response to lymphoma in non-immunocompromised patients. Finally, we discuss the potential role of immunomodulation, in concert with radiation therapy, as a component of future therapeutic strategies for lymphoma.
Keywords: Lymphoma, Hodgkin lymphoma, Radiotherapy, Immunotherapy, Macrophage, Calreticulin
Introduction
The incidence of lymphoma rose in the late twentieth century in the US and other developed countries (1, 2). While the cause of this is uncertain, studies have suggested that it may be related to changing patterns of childhood infectious disease. The incidence of Hodgkin Lymphoma (HL) is higher in those without early exposure to measles, rubella, mumps and pertussis (3, 4). There is epidemiologic evidence to support the hypothesis that the rise in non-Hodgkin lymphoma (NHL) is also secondary to decreases in childhood infectious diseases (5). In this model, early infection activates Type 1 immunity that provides protection from lymphoma cell proliferation later in life. Type 1 immunity is associated with tumor rejection and would correlate well with reduced cancer risk, while, Type 2 immunity supports tumor progression and therefore would be associated with increased cancer risk in children. Although lymphoma is often curable, it remains the sixth leading cause of US cancer deaths and improved therapeutic options are needed (6). Here we describe the role of radiation therapy in lymphoma treatment, and we explore potential means of optimizing immunomodulatory therapy in combination with radiotherapy in the treatment of lymphoma.
Lymphoma: clinical background
There are 94 lymphoma subtypes defined in the 2008 World Health Organization classification (7, 8). Broadly, these can be categorized as Hodgkin lymphoma, Aggressive B-cell Non Hodgkin lymphoma, Indolent B-cell Non Hodgkin lymphoma, and mature T-cell and NK-cell neoplasms. Within each category, disease entities have a similar prognosis and are treated with the same therapy. Increasingly, however, we are appreciating molecular heterogeneity of tumors within each category and even within each subtype, and learning that responsiveness of molecular subtypes to different therapies is variable (9). This is a busy and exciting time in lymphoma research.
The median age at diagnosis of Hodgkin lymphoma (HL) is 30 years old, making curative and safe treatment a paramount concern. The Reed-Sternberg cell is the pathognomonic finding, easily identified under the microscope, which allowed the early appreciation of HL as a distinct pathologic entity (10). It was not until recently that modern tools including laser microdissection and cDNA expression library sequencing established the identity of the Reed-Sternberg cell as a B cell (11). Despite a lack of clarity of the cellular origin of HL, curative treatment has been available since the 1960’s. Current treatment in the United States usually begins with Adriamycin, Bleomycin, Vinblastine and Dacarbazine (ABVD) chemotherapy. Some patients also receive radiation therapy. The disease is very sensitive to radiation, with 20 Gy Involved Field Radiation Therapy (IFRT), in combination with 2 cycles ABVD, affording long–term disease control in early favorable disease (12, 13). This is a significantly lower dose of radiation than is required to treat nonhematologic neoplasms. More than 70% of the highest risk advanced stage HL patients are cured with frontline therapy 14). Nonetheless, late toxicities including secondary malignancies, occurring ten years and more after treatment as a result of radiation exposure remain a concern. Ongoing clinical trials aim to further reduce the amount of radiation delivered to young HL patients (15).
Diffuse Large B-cell lymphoma (DLBCL) is the most common of the aggressive NHLs (16). It occurs at higher rates in immunocompromised or older individuals. Like HL, DLBCL is treated with curative intent. Standard chemotherapy consists of Rituximab, cyclophosphamide, adriamycin, vincrisitine and prednisone (R-CHOP). Radiation therapy is also effective in DLBCL, and involved field radiation is offered to some patients after chemotherapy (17). Approximately 65% of newly diagnosed advanced stage DLBCL patients are cured with one course of treatment (18).When involved field radiation therapy is included in the treatment of DLBCL it is typically given to approximately 35 Gy (17).
Indolent B-cell NHLs are so-named because they are slow growing diseases. The most common is follicular lymphoma (FL). Indolent lymphoma appears to be particularly radiosensitive. Radiation therapy to early stage disease is the only known curative regimen for FL, but less than 25% of newly diagnosed FL patients are early stage (19–21). There is no known curative therapy for advanced stage indolent lymphomas. Patients are not treated immediately upon diagnosis unless they require palliation or have a high burden of disease. Because treatment is not curative, there is no single standard therapy for FL (22). Median survival is approximately 12 years from diagnosis (23). Most patients are treated several times, achieving remissions of shorter and shorter duration, before succumbing to the disease. Very low dose total body irradiation, in total doses of 1 – 4 Gy has palliative utility (24–26).
T cell lymphomas represent approximately 15% of all NHL (27). Historically T cell lymphomas have been treated like B cell lymphomas and therefore CHOP chemotherapy is the accepted frontline treatment. While a majority of patients respond to CHOP remissions are very short in duration (28). Improving on T-NHL therapy is an important goal in clinical oncology. The difficulty in meeting this challenge is magnified by the rarity of T-NHL, the existence of many subtypes of T-NHL that are often hard to diagnose pathologically, and the dynamic classification of T-NHL that makes it difficult to interpret historical data. T cell lymphoma involves extranodal organs more commonly than do B-NHLs, with skin being a major site of disease for several of the T-NHLs. Cutaneous T-NHL involving only the skin is treated with skin-directed therapy including UV or electron beam radiation therapy (29).
Radiation therapy in Lymphoma
Radiation therapy has an important role in the treatment of lymphomas. Early in the twentieth century irradiation was discovered and explored in medical settings. When directed to lymphoma treatment, tumor regression was seen and thus radiation therapy became the first effective means of treating lymphoma. By the 1950’s chemotherapy was introduced and was also found to cause regression of lymphoma. Currently both modalities are used in lymphoma patient care. Current clinical trial efforts aim to optimize doses of each, with a goal of maintaining excellent long term disease control while limiting late toxicities (16, 30).
Lymphocytes respond to γ–irradiation during interphase; resting lymphocytes are more sensitive to γ–irradiation than are activated lymphocytes (31).Radiation of lymphocytes causes an early interphase, premitotic, apoptotic death (32). The response of lymphocytes to γ-irradiation is distinct from the mitotic, senescent, or postmitotic necrosis or apoptosis that account for radiation toxicity in most non-lymphoid malignancies (33, 34). It is not known whether lipid peroxidation on the cell membrane, leading to modulation of signal transduction, or radiation-induced cross-linking of the nuclear DNA, is the initiating event in activating lymphocyte apoptosis (32). Ultimately, radiation of lymphocytes results in DNA fragmentation in an apoptotic process that requires RNA and protein synthesis (13). Lymphocytes are more sensitive to radiation in vivo than in vitro, a phenomenon that may be mediated by nitric oxide in vivo (35).
The role of IFRT radiation therapy in lymphoma is controversial. The major US intergroup clinical trial consortiums differ, with the currently open Cancer and Leukemia Group B trials for treatment of early stage HL including radiation therapy only for individuals with a positive interim PET scan after 2 cycles of ABVD (15). Similarly, early stage DLBCL is often treated with chemotherapy alone, though some advocate for combination chemotherapy with radiation therapy (36). Low dose total body irradiation, in which patients are treated with a total of 1.5–4 Gy of γ–irradiation, is associated with 50–80% response rates in FL patients (24, 25), but is not widely used.
Standard radiation treatment delivers a lethal dose of therapy to sites of disease. Lymphoma is particularly radiosensitive; curative regimens incorporate 20–35 Gy, in contradistinction to the approximately 65 Gy of radiation required for definitive treatment of squamous cell and adenocarcinomas. The clinical use of radiation in lymphoma is limited because approximately 75% of lymphoma patients have widespread disease on diagnosis. For these patients, definitive treatment doses of radiation are prohibitively toxic. Radiation therapy is thus underutilized in lymphoma therapy. Radiation therapy is currently offered only to those with limited involvement on diagnosis, with a site of bulky disease requiring consolidative radiation after chemotherapy, or for palliation.
Cells of the Immune System
The immune system is classically divided into an early, innate response and an antigen-specific adaptive response. The innate response is typified by an array of myeloid and lymphoid cells, namely macrophages, dendritic cells, natural killer (NK) cells, and NKT cells that rapidly exert their effector functions through the expression of a limited repertoire of germ-line encoded receptors. Conversely, the adaptive response is primarily composed of two types of lymphocytes, B cells and T cells, which clonally express a large repertoire of antigenic receptors that are produced by site-specific somatic recombination. During an immune response, naïve B and T lymphocytes encounter antigens in specialized lymphoid organs and undergo a process of cell division and maturation, including somatic hypermutation in B-cells, before exerting their effector functions (37).
Tumor Immune Surveillance
The immune system plays a key role in both the elimination of primary tumors and preventing tumor recurrence. The immune system can identify and eliminate tumor cells on the basis of their expression of tumor-specific antigens, tumor associated antigens or molecules induced by cellular stress. This process is referred to as tumor immune surveillance, whereby the immune system identifies neoplastic or malignant cells and eliminates them before they can cause harm.
A straight-forward approach to clearly demonstrate the role of the immune system in controlling tumor development is to remove specific components of the mouse immune system and examine tumor onset and progression (38). These studies have been conducted primarily through the use of gene-targeted knockout mice, and have revealed that a number of immune effector cells and pathways are important for suppression of tumor development. For example, mice lacking T cell and NK cell cytotoxic effector pathways have been reported to spontaneously develop tumors (39, 40). Specifically, mice that lack perforin, a cytotoxic molecule used by cytotoxic cells CD8+ T cells and NK cells develop lymphomas with age. These spontaneous lymphomas are of B cell origin, develop in older mice (>1 year of age) and when transplanted into wild type (WT) mice, these tumors are rejected by CD8+ T cells. In mice lacking both perforin and β2-microglobulin (β2M), B cell lymphomas occur earlier and with increased incidence compared with mice lacking only perforin (41). In addition, B cell lymphomas derived from mice lacking both perforin and β2M are rejected by either Natural killer (NK) cells or γδ T cells following transplantation to WT mice, rather than by CD8+ T cells (as in tumors derived from mice lacking only perforin), demonstrating that cell surface expression of MHC class I molecules by tumor cells can be an important factor in determining which effector cells mediate immune protective effects. Intriguingly, mutations in the gene encoding perforin have been identified in a subset of lymphoma patients (42).
Additionally, mice lacking the apoptosis-related protein TNF related apoptosis-inducing ligand (TRAIL) or expressing a defective mutant form of the death-inducing molecule FASL are susceptible to spontaneous lymphomas that develop with age (43, 44). These aging studies have clearly demonstrated a critical role for cytotoxic pathways in immunoregulation and/or immunosuppression of spontaneous tumor development in mice. Several cytokine-deficient mice also develop spontaneous malignancies (40, 45, 46). In a study by Street et al, approximately 50% of IFN-γ–deficient C57BL/6 mice developed T cell lymphomas that were predominantly disseminated lymphomas, although some cases of thymic lymphoma were also noted (40). Interestingly, the susceptibility of IFN-γ−/− mice to T cell lymphomas is strain dependent. In addition, C57BL/6 mice lacking both IFN-γ and perforin display accelerated B cell lymphoma onset compared with perforin-deficient mice, which led the authors to conclude that IFN-γ has an important role in modifying the progression to B cell lymphoma in perforin-deficient mice.
Many clinical observations support the concept of tumor immune surveillance in humans and it has been reviewed extensively (38, 47). According to Finn (47), immunosuppression to prevent transplant rejection is associated with an increased risk (3- to 100-fold increase) of developing lymphoma. One study examined 905 recipients of transplanted hearts, lungs, or both between 1989 and 2004 for the effect that immunosuppression, used for preventing graft rejection, had on the incidence of cancer (48). Overall, 102 newly diagnosed cancers were detected in these patients, 7.1 times as many as in the general population. The predominant types were leukemias and lymphomas, which is 26.2 times as many as in the general population. In addition to patients receiving immunosuppressive drugs, tumors also occur commonly in patients with primary and acquired immunodeficiencies; however, these are generally thought to have a viral etiology.
An off-target immunologic response to lymphoma can precede the clinical detection of the lymphoma that drives it. Paraneoplastic phenomena are particularly common in HL patients but are seen in NHL and in other malignancies (49–52). A variety of paraneoplastic neurologic syndromes result from cross-reactivity between the antitumor immune response and neurologic antigens. When a paraneoplastic syndrome occurs, neurologic symptoms often precede the diagnosis of a previously undetected tumor. Paraneoplastic cerebellar degeneration (PCD) is a neurologic syndrome that arises in some patients with Hodgkin disease. These patients typically exhibit high titers of thioredoxin reductase 1–specific autoantibodies. Both autoantibodies and CD8+ T cells specific for antigens shared by tumors and Purkinje cells have been detected in the blood of PCD patients. Tumors from patients with PCD often show prominent infiltration with lymphocytes and plasma cells, which is indicative of a local immune response at the tumor site. In most cases, PCD is terminal; the only patients that generally survive this condition are those that achieve complete tumor remission in response to therapy. These data suggest that the tumor is the probable driver and the initiator of the immune response that is both self and tumor reactive. Interestingly, PCD symptoms can precede tumor diagnosis by a number of years, indicating that antitumor responses might be primed even by undetectable, microscopic tumors early in their development.
Clinical and epidemiologic studies have suggested a strong association between chronic infection, inflammation, and the development of lymphoma. For example, infection with Hepatitis C, Helicobacter pylori, Borrelia burgdorferi or Chlamidophila psittaci is associated with development of marginal zone lymphoma (53–56). EBV is associated with Burkitt lymphoma, HL, and some DLBCL (57, 58). In a recent review, Lin and Karin summarize the role of several cytokines, produced by immune cells in response to inflammatory triggers, in tumor development and progression (59). The prolonged production of TNF-α can induce NF-κB–dependent expression of antiapoptotic and proliferative genes, enhance angiogenesis, and adversely affect immune surveillance by conferring resistance to TRAIL (60). Cytokines such as IL-6 can exacerbate the effects of TNF-α and promote cell cycle progression. IL-6 has been implicated in the development of multiple myeloma (MM) and an elevated risk of Hodgkin lymphoma (61). In MM, IL-6 is produced by stromal cells in the bone marrow, and its synthesis by these cells can be further enhanced by their interaction with malignant plasma cells (62). Furthermore, in response to infection-activated TLR signaling, MM cells also produce IL-6, which promotes their growth in an autocrine manner (63). Thus, novel IL-6 antagonists are being evaluated for treatment of MM (64).
Immune responses to lymphoma
B cells and T lymphocytes, namely CD4+ T helper cells and CD8+ cytotoxic T cells, are able to recognize with extreme specificity the subtle differences that occur in normal cells upon infection or transformation (65). According to studies in mice, both the adaptive and innate compartments of the immune system function in the cancer immune-surveillance network. Established tumors have circumvented either one or both arms of immunity. Several ‘tumor immune-escape’ mechanisms have been unveiled that have an inhibitory effect either directly or indirectly on the effector cells of the immune system. Restoring the function of the effector cells, thus allowing the immune system to eliminate the tumor target cells, is one of the major goals of immunotherapy (66). Most tumor antigens that are targets for the immune system are defined as tumor-associated antigens (TAA). These protein antigens are derived from differential expression of normal coding sequences by neoplastic cells as compared to normal cells. TAA are present in different amounts, location, or cellular context which can lead to preferential recognition of the tumor by the immune system. There are few tumor-specific antigens (TSA). TSAs can arise from point mutations or other genetic alterations specific to a given tumor or group of tumors, such as fusion proteins generated by translocations, or from alterations in posttranslational modification. TSA can be derived from oncogenic viruses associated with some types of cancer, such as Epstein-Barr virus–derived antigens in lymphomas.
Tumors can induce the differentiation of circulating myeloid cells into suppressor cells, collectively referred to as myeloid derived suppressor cells (MDSCs), which impact on immune surveillance mechanisms (67, 68). MDSCs are a complex mix of CD11b+ and Gr-1+ mononuclear cells that mediate the suppression of T cells (69). It appears that MDSCs initially require activation by T cells, subsequently they inhibit CD4+ and CD8+ T cells in an MHC-independent manner (70). MDSCs express the inducible forms of nitric oxide synthase (NOS) 2 and arginase (ARG1), enzymes involved in the metabolism of arginine. Recently, Bronte’s group showed that both NOS2 and ARG1 are expressed by MDSCs in an IL-13–and IFN-γ–dependent manner and function synergistically to induce T cell dysfunction (70). MDSCs can also induce the development of regulatory T cells (Tregs) (71).
CD4+Tregs are characterized by their expression of CD25 (the α chain of the IL-2 receptor), cytotoxic T lymphocyte–associated antigen 4 (CTLA4), glucocorticoid-induced TNF receptor–related protein (GITR), and the transcription factor forkhead box P3 (FOXP3) (72). Tregs are generated both in the thymus and the periphery (73–76). They exert their suppressive activity on multiple immune cells, such as T cells, NK cells, NKT cells, and B cells, through both contact-dependent and contact-independent mechanisms. In the majority of cancer a high number of Tregs in the tumor is associated with a poor clinical outcome (77–79), although the opposite observation has been made in hematologic malignancies (80, 81). Some B cell lymphomas recruit functional Tregs into the tumor through CC chemokine ligand 22 (CCL22) produced by the tumor cells (82), as has also been shown in ovarian carcinoma (77). The neoplastic B cells of FL induce FOXP3 expression in CD4+ T cells in the tumor microenvironment (83). In FL, tumor infiltration by Tregs expressing FOXP3 may be associated with improved overall survival, opposite to what is seen in other cancers (80, 84), though these observations were made on patients who were treated with various regimens, and there has been some discrepancy in findings between groups. The histologic distribution of FOXP3 cells in the FL biopsy tissue was shown by Farhina et al to be critical in predicting outcomes (85). In this study, all patients were treated with the same multiagent chemotherapy, allowing outcome comparisons on the basis of histology. Here, outcomes were not correlated with numbers of CD4, CD25 and FOXP3 Tregs in the neoplastic lymph nodes. Progression free survival and rate of transformation was, however, related to the distribution of the Tregs within the lymph node: when Tregs were diffusely distributed patients had a longer survival than when Tregs were arranged in a follicular pattern (85).
Another type of myeloid suppressor cell that are recruited to tumors and promote tumor growth by enhancing inflammation and angiogenesis are the tumor-associated macrophages (TAMs) (86, 87). The production of IL-10 by tumor cells and TAMs is thought to promote the development of Burkitt lymphoma through the production of the TNF family member BAFF, which promotes B cell and lymphoma survival (88). An elevated amount of IL-10 in the plasma has been correlated with poor prognosis in diffuse large B cell lymphoma patients (89). Notably, B cell tumors were reported to progress much slower in IL-10 deficient mice (90).In a clinical study, Lenz and colleagues profiled gene expression in pretreatment biopsy specimens from 181 patients with diffuse large-B-cell lymphoma who received CHOP and 233 patients who received R-CHOP. The authors found that three gene-expression signatures--termed “germinal-center B-cell,” “stromal-1,” and “stromal-2”--predicted survival both in patients who received CHOP and patients who received R-CHOP, and concluded that survival after treatment of diffuse large-B-cell lymphoma is influenced by differences in immune cells, fibrosis, and angiogenesis in the tumor microenvironment (16). Similarly, in a series of diagnostic tissue biopsies from FL patients, one subset of tumor samples had an expression profile characteristic of T cells while a second group expressed a macrophage profile (44). FL tumors enriched in macrophages were also correlated with shorter survival, a finding that has been corroborated by several studies.
The TLRs are a family of specialized immune receptors that induce protective immune responses when they detect oligodeoxynucleotides containing one or more unmethylated CpGdinucleotides (CpG ODN) (91, 92). These highly conserved pathogen-expressed molecules are prevalent in bacterial and viral DNA but not in vertebrate genomes. TLR agonists are an exciting new class of vaccine adjuvants, with CpG ODN, which targets TLR9, the furthest along in clinical testing (93). The TLR agonist adjuvant activity is based on its ability to activate human B cells and plasmacytoid dendritic cells, the two main cell types that express TLR9 in humans. Relapsed NHL patients treated with the B-class CpG ODN PF-3512676 (formerly known as CPG 7909 and ODN 2006) led to clinical responses in 2 of 23 patients, indicating potential efficacy of this route of administration in humans (94). Monotherapy with the TLR9 agonist CPG 7909 or another B-class CpG ODN, 1018 ISS, activates NK cells and induces a Th1 cytokine response in humans with B cell lymphomas (94, 95). Of 23 NHL patients in the i.v. dose-escalation study of PF-3512676 there were two late clinical responses, which suggests a potential role for this approach as one component of a combination treatment regimen (94). Although CpG ODN are strong mitogens for normal B cells, there was no apparent exacerbation of the lymphoma in these patients, perhaps due to the increased immunogenicity of the tumor cells or the preferential induction of apoptosis in tumor cells stimulated through TLR9 (93). Twenty-eight patients with advanced cutaneous T cell lymphoma who had failed an average of six prior therapies were enrolled in a dose-escalation study of PF-3512676 in which they were treated with weekly s.c. administration of the agent in sequential cohorts starting at 0.08 mg/kg and escalating to 0.36 mg/kg. There were three complete and six partial responses and little toxicity beyond injection site reactions and flu-like symptoms. Responses were observed as early as two weeks, occurred in all of the dose groups except for the lowest, and persisted for the duration of the study (96). Collectively, these clinical trials of TLR9 therapy as a single agent are encouraging for a good safety profile, but the frequency of objective responses has been relatively low, and the focus of ongoing clinical trials therefore has shifted to combination therapies in an attempt to increase the clinical effectiveness of administering TLR9 agonists.
Combined radiation and immunotherapy in lymphoma – an emerging therapeutic strategy
Low dose total body irradiation, in which patients are treated with a total of 1.5–4 Gy of γ–irradiation, is associated with 50–80% response rates in FL patients (24, 25). These responses are attributed in part to the exquisite sensitivity of this lymphoma to radiation, and perhaps also to activation of a reactive proliferative T cell response. In one series, patients had peripheral blood drawn before and after low-dose total body irradiation (97). Increases in the percentage of CD4+ T cells were seen after therapy and were associated with favorable outcomes. Another study of low dose total body irradiation in FL patients utilized core biopsies taken before and 24 hours after treatment with two doses of 2 Gy (98). Three gene expression profiles were induced by treatment. One represented p53 and its pathway, a second was a cell cycle signature and a third represented an immune response. One immune signature was an activated macrophage signal, though no increase in CD68 positive cells in the biopsies was detected histologically. The authors propose that low dose radiation causes FL cells to undergo apoptosis, while sparing macrophages, which are then activated by phosphatidylserine exposure to clear the FL.
Immature dendritic cells (DC) efficiently capture antigens. Following maturation, DC become potent simulators of T cells. This process is accompanied by cytoskeletal reorganization, loss of adhesiveness, aquisition of cellular motility, migration to lymphoid tissues, reduced phagocytic uptake, and enhanced T cell activation potential. Mature DC can secrete chemokines and cytokines that attract other immune cells and activate resting T cells.
Preclinical studies of radio/chemotherapy-elicited immunogenic cell death in a variety of tumor mouse models have shown that the release of danger signals by tumor cells is the main molecular mechanism initiating DC engulfment of dead cell particles; DC activation results in the induction of a specific adaptive immune response (Figure 1). When the normally endoplasmic reticulum-resident chaperone calreticulin is relocated to the plasma membrane, concomitant with the surface expression of heat shock proteins in dying cells, these chaperones act as immunogenic signals for DC activation. Thus, T cell-mediated clearance of irradiated cells is facilitated by therapy that exposes calreticulin on malignant cell surfaces. γ–radiation therapy induces this, leading to T cell-dependent tumor regression in a colon cancer model (99). Recent studies with lymphoma cell lines and follicular lymphoma patient samples indicate that γ–irradiation also induces calreticulin translocation to the cell membrane in lymphoid malignancies. Patient follicular lymphoma cells treated ex vivo with γ–irradiation and loaded into dendritic cells were most effective as a cancer vaccine when high levels of calreticulin and HSP90 were expressed on the surface (100, 101).In addition, when follicular lymphoma cell death is induced by Rituximab, new lymphoma-specific T cell responses were detected in 80% of treated patients (102), though these were of unknown therapeutic importance.
Figure 1. Schematic of radiation-induced immunogenic cell death in lymphoma cells.
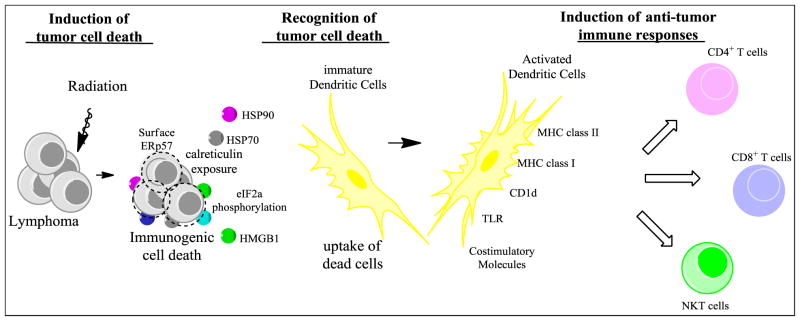
In addition to directly killing tumor cells, exposure to radiation can cause tumors cells to release “eat-me” and danger signals resulting in a type of cell death that induces an immune response. During the process of immunogenic cell death, the endoplasmic reticulum (ER) becomes stressed, which leads to the phosphorylation of eIF2γ. Then calreticulin and disulphide isomerase ERp57, both of which are usually contained in the lumen of the ER, translocate to the cell surface, followed by surface expression of heat shock proteins (HSP) 70 and HSP 90. Dying tumor cells also release the nuclear factor high-mobility group box 1 (HMGB1). This results in engulfment of dead cell particles by immature dendritic cells (DCs) and their subsequent maturation and activation. Activated DCs express toll like receptors (TLR), upregulate antigen presenting molecules (MHC class I & II, CD1d), and express high levels of costimulatory molecules. Expression of these proteins on DCs promotes the cross-priming of tumor antigens and can lead to the induction of a potent anti-tumor immune response.
Radioimmunoconjugates offer the potential to deliver low dose radiation therapy to sites of disease not detected on imaging. Two are available, Yttrium-90 labeled ibritumomabtiuxetan (Zevalin), which has a beta-emitter conjugated to an anti-CD20 antibody, and Iodine-131 labeled tositumomab (Bexxar), where an alpha-emitter is conjugated to an anti-CD20 antibody. Both have activity in FL (103, 104). Here the radiation is delivered to the sites of disease based upon the distribution of CD20, which is expressed on most NHL but not on hematologic stem cells, plasma cells, or non-hematologic cells. The radiation has a short penetration, but the radiation dose is delivered to nearby cells rather than to the CD20+ cell to which the isotope is bound. While Zevalin and Bexxar are active agents, neither has found widespread clinical use, in part because Rituximab itself is quite effective through antibody-dependent cell-mediated cytotoxicity and complement-mediated cytotoxicity.
Low dose radiation is a component of an emerging immunotherapeutic strategy for treatment of slow growing lymphomas, based on preclinical mouse studies (96, 105). Here, radiation to lymphoma is used to create a vaccine, in situ, thus obviating the need for ex vivo generation of patient-specific vaccine material. Two recently published papers from Stanford describe a Phase I/II clinical trial experience in humans. In the first study a TLR9 oligonucleotide agonist was injected into a single disease site in 15 patients with indolent B cell NHL. Patients then received 4 Gy total of radiotherapy to the injected site, followed by an additional 9 injections of TLR agonist. Abscopal clinical responses (regression of tumors distant from the treated site) were assessed, with a 27% objective response rate found. The greatest responses were seen at a median of >24 weeks, consistent with a treatment-generated immunologic response (105). Re-treatment with TLR9 agonist in a responding then relapsed patient resulted in a second, rapid, response. While not as efficacious as one would like, this therapy has an excellent side effect profile and represents an exciting alternative to current treatment choices including standard radiation or chemotherapy. The second study used the same approach to treat patients with mycosis fungoides, a T-cell lymphoma involving the skin (106). Here only one of the first cohort responded and a booster treatment was added in treatment of subsequent cohorts. While a 38% response rate was achieved the durations of responses were quite short at a median of 7 weeks. Pre- and post-treatment blood and tissue samples were analyzed without a humoral antibody to tumor detected.
Conclusions
It is clear that optimizing an immunologic response to lymphoma could become a valuable component of care, ideally leading to complete and durable responses. Immunotherapy may be most easily utilized in the slower growing lymphomas, including follicular B cell and mycosis fungoides in which human trials of immunotherapy have been reported. Radiation induces immunogenic changes in lymphoma cells, and contributes a vaccine effect in a few recent studies, and may remain a critical component of therapeutic approaches in the future. While the current reasoning for the use of radiation is to induce cytotoxicity directly in tumor cells, considerable evidence demonstrates that radiation effects extend beyond the elimination of the radiosensitive fraction of tumor cells. Given the recent advances in our understanding of mechanisms that regulate the development of effective anti-tumor immune responses, as well as improved knowledge of the effects of radiation on cells, the potential for combination radiotherapy and immunotherapy to achieve both local and definitive systemic lymphoma control is an exciting possibility.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health: NCI K01 CA131487, R21 CA162273, and R21 CA162277.
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Ariad S, Lipshitz I, Benharroch D, Gopas J, Barchana M. A sharp rise in the incidence of Hodgkin’s lymphoma in young adults in Israel. Isr Med Assoc J. 2009;11:453–5. [PubMed] [Google Scholar]
- 2.Clarke CA, Glaser SL. Changing incidence of non-Hodgkin lymphomas in the United States. Cancer. 2002;94:2015–23. doi: 10.1002/cncr.10403. [DOI] [PubMed] [Google Scholar]
- 3.Alexander FE, Jarrett RF, Lawrence D, Armstrong AA, Freeland J, Gokhale DA, et al. Risk factors for Hodgkin’s disease by Epstein-Barr virus (EBV) status: prior infection by EBV and other agents. Br J Cancer. 2000;82:1117–21. doi: 10.1054/bjoc.1999.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glaser SL, Keegan TH, Clarke CA, Trinh M, Dorfman RF, Mann RB, et al. Exposure to childhood infections and risk of Epstein-Barr virus--defined Hodgkin’s lymphoma in women. Int J Cancer. 2005;115:599–605. doi: 10.1002/ijc.20787. [DOI] [PubMed] [Google Scholar]
- 5.Cramer DW, Finn OJ. Epidemiologic perspective on immune-surveillance in cancer. Curr Opin Immunol. 2011;23:265–71. doi: 10.1016/j.coi.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LA, Eheman C, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:714–36. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program. 2009:523–31. doi: 10.1182/asheducation-2009.1.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer TIAfRo. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: World Health Organization; 2008. [Google Scholar]
- 9.Thieblemont C, Briere J, Mounier N, Voelker HU, Cuccuini W, Hirchaud E, et al. The germinal center/activated B-cell subclassification has a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B-cell lymphoma: a bio-CORAL study. J Clin Oncol. 2011;29:4079–87. doi: 10.1200/JCO.2011.35.4423. [DOI] [PubMed] [Google Scholar]
- 10.Hodgkin T. On some Morbid Appearances of the Absorbent Glands and Spleen. London: Royal Medical and Chirurgical Society; 1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cossman J, Annunziata CM, Barash S, Staudt L, Dillon P, He WW, et al. Reed-Sternberg cell genome expression supports a B-cell lineage. Blood. 1999;94:411–6. [PubMed] [Google Scholar]
- 12.Engert A, Plutschow A, Eich HT, Lohri A, Dorken B, Borchmann P, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363:640–52. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]
- 13.Engert A, Josting A, Haverkamp H, Villalobos M, Lohri A, Sokler M, et al. Epoetin alfa in patients with advanced-stage Hodgkin’s lymphoma: results of the randomized placebo-controlled GHSG HD15EPO trial. J Clin Oncol. 2010;28:2239–45. doi: 10.1200/JCO.2009.25.1835. [DOI] [PubMed] [Google Scholar]
- 14.Engert A, Eichenauer DA, Dreyling M. Hodgkin’s lymphoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20 (Suppl 4):108–9. doi: 10.1093/annonc/mdp144. [DOI] [PubMed] [Google Scholar]
- 15.Straus DJ. Chemotherapy alone for early-stage Hodgkin’s lymphoma. N Engl J Med. 2012;366:470–1. doi: 10.1056/NEJMe1113291. [DOI] [PubMed] [Google Scholar]
- 16.Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359:2313–23. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phan J, Mazloom A, Medeiros LJ, Zreik TG, Wogan C, Shihadeh F, et al. Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. J Clin Oncol. 2010;28:4170–6. doi: 10.1200/JCO.2009.27.3441. [DOI] [PubMed] [Google Scholar]
- 18.Berger F, Traverse-Glehen A, Felman P, Callet-Bauchu E, Baseggio L, Gazzo S, et al. Clinicopathologic features of Waldenstrom’s macroglobulinemia and marginal zone lymphoma: are they distinct or the same entity? Clin Lymphoma. 2005;5:220–4. doi: 10.3816/clm.2005.n.003. [DOI] [PubMed] [Google Scholar]
- 19.Pugh TJ, Ballonoff A, Newman F, Rabinovitch R. Improved survival in patients with early stage low-grade follicular lymphoma treated with radiation: a Surveillance, Epidemiology, and End Results database analysis. Cancer. 2010;116:3843–51. doi: 10.1002/cncr.25149. [DOI] [PubMed] [Google Scholar]
- 20.Guadagnolo BA, Li S, Neuberg D, Ng A, Hua L, Silver B, et al. Long-term outcome and mortality trends in early-stage, Grade 1–2 follicular lymphoma treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2006;64:928–34. doi: 10.1016/j.ijrobp.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Wilder RB, Jones D, Tucker SL, Fuller LM, Ha CS, McLaughlin P, et al. Long-term results with radiotherapy for Stage I-II follicular lymphomas. Int J Radiat Oncol Biol Phys. 2001;51:1219–27. doi: 10.1016/s0360-3016(01)01747-3. [DOI] [PubMed] [Google Scholar]
- 22.Friedberg JW, Taylor MD, Cerhan JR, Flowers CR, Dillon H, Farber CM, et al. Follicular lymphoma in the United States: first report of the national LymphoCare study. J Clin Oncol. 2009;27:1202–8. doi: 10.1200/JCO.2008.18.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wahlin BE, Yri OE, Kimby E, Holte H, Delabie J, Smeland EB, et al. Clinical significance of the WHO grades of follicular lymphoma in a population-based cohort of 505 patients with long follow-up times. Br J Haematol. 2012;156:225–33. doi: 10.1111/j.1365-2141.2011.08942.x. [DOI] [PubMed] [Google Scholar]
- 24.Mendenhall NP, Noyes WD, Million RR. Total body irradiation for stage II-IV non-Hodgkin’s lymphoma: ten-year follow-up. J Clin Oncol. 1989;7:67–74. doi: 10.1200/JCO.1989.7.1.67. [DOI] [PubMed] [Google Scholar]
- 25.Safwat A. The role of low-dose total body irradiation in treatment of non-Hodgkin’s lymphoma: a new look at an old method. Radiother Oncol. 2000;56:1–8. doi: 10.1016/s0167-8140(00)00167-5. [DOI] [PubMed] [Google Scholar]
- 26.Haas RL, Poortmans P, de Jong D, Aleman BM, Dewit LG, Verheij M, et al. High response rates and lasting remissions after low-dose involved field radiotherapy in indolent lymphomas. J Clin Oncol. 2003;21:2474–80. doi: 10.1200/JCO.2003.09.542. [DOI] [PubMed] [Google Scholar]
- 27.Rizvi MA, Evens AM, Tallman MS, Nelson BP, Rosen ST. T-cell non-Hodgkin lymphoma. Blood. 2006;107:1255–64. doi: 10.1182/blood-2005-03-1306. [DOI] [PubMed] [Google Scholar]
- 28.Dearden CE, Johnson R, Pettengell R, Devereux S, Cwynarski K, Whittaker S, et al. Guidelines for the management of mature T-cell and NK-cell neoplasms (excluding cutaneous T-cell lymphoma) Br J Haematol. 2011;153:451–85. doi: 10.1111/j.1365-2141.2011.08651.x. [DOI] [PubMed] [Google Scholar]
- 29.Wilcox RA. Cutaneous T-cell lymphoma: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86:928–48. doi: 10.1002/ajh.22139. [DOI] [PubMed] [Google Scholar]
- 30.Campbell BA, Connors JM, Gascoyne RD, Morris WJ, Pickles T, Sehn LH. Limited-stage diffuse large B-cell lymphoma treated with abbreviated systemic therapy and consolidation radiotherapy: involved-field versus involved-node radiotherapy. Cancer. 2012;118:4156–65. doi: 10.1002/cncr.26687. [DOI] [PubMed] [Google Scholar]
- 31.Sellins KS, Cohen JJ. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol. 1987;139:3199–206. [PubMed] [Google Scholar]
- 32.Leibel SAaP, TL . Textbook of Radiation Oncology. 2. Philadelphia: W.B. Saunders; 2004. [Google Scholar]
- 33.Dewey WC, Ling CC, Meyn RE. Radiation-induced apoptosis: relevance to radiotherapy. Int J Radiat Oncol Biol Phys. 1995;33:781–96. doi: 10.1016/0360-3016(95)00214-8. [DOI] [PubMed] [Google Scholar]
- 34.Forrester HB, Albright N, Ling CC, Dewey WC. Computerized video time-lapse analysis of apoptosis of REC:Myc cells X-irradiated in different phases of the cell cycle. Radiat Res. 2000;154:625–39. doi: 10.1667/0033-7587(2000)154[0625:cvtlao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 35.Sharma D, Sandur SK, Rashmi R, Maurya DK, Suryavanshi S, Checker R, et al. Differential activation of NF-kappaB and nitric oxide in lymphocytes regulates in vitro and in vivo radiosensitivity. Mutat Res. 2010;703:149–57. doi: 10.1016/j.mrgentox.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yahalom J. Radiation therapy after R-CHOP for diffuse large B-cell lymphoma: the gain remains. J Clin Oncol. 2010;28:4105–7. doi: 10.1200/JCO.2010.29.5089. [DOI] [PubMed] [Google Scholar]
- 37.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–9. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–46. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med. 2000;192:755–60. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Street SE, Trapani JA, MacGregor D, Smyth MJ. Suppression of lymphoma and epithelial malignancies effected by interferon gamma. J Exp Med. 2002;196:129–34. doi: 10.1084/jem.20020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Street SE, Hayakawa Y, Zhan Y, Lew AM, MacGregor D, Jamieson AM, et al. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J Exp Med. 2004;199:879–84. doi: 10.1084/jem.20031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clementi R, Locatelli F, Dupre L, Garaventa A, Emmi L, Bregni M, et al. A proportion of patients with lymphoma may harbor mutations of the perforin gene. Blood. 2005;105:4424–8. doi: 10.1182/blood-2004-04-1477. [DOI] [PubMed] [Google Scholar]
- 43.Zerafa N, Westwood JA, Cretney E, Mitchell S, Waring P, Iezzi M, et al. Cutting edge: TRAIL deficiency accelerates hematological malignancies. J Immunol. 2005;175:5586–90. doi: 10.4049/jimmunol.175.9.5586. [DOI] [PubMed] [Google Scholar]
- 44.Davidson WF, Giese T, Fredrickson TN. Spontaneous development of plasmacytoid tumors in mice with defective Fas-Fas ligand interactions. J Exp Med. 1998;187:1825–38. doi: 10.1084/jem.187.11.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Airoldi I, Di Carlo E, Cocco C, Sorrentino C, Fais F, Cilli M, et al. Lack of Il12rb2 signaling predisposes to spontaneous autoimmunity and malignancy. Blood. 2005;106:3846–53. doi: 10.1182/blood-2005-05-2034. [DOI] [PubMed] [Google Scholar]
- 46.Enzler T, Gillessen S, Manis JP, Ferguson D, Fleming J, Alt FW, et al. Deficiencies of GM-CSF and interferon gamma link inflammation and cancer. J Exp Med. 2003;197:1213–9. doi: 10.1084/jem.20021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–15. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 48.Roithmaier S, Haydon AM, Loi S, Esmore D, Griffiths A, Bergin P, et al. Incidence of malignancies in heart and/or lung transplant recipients: a single-institution experience. J Heart Lung Transplant. 2007;26:845–9. doi: 10.1016/j.healun.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 49.Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, Posner JB, Dalmau J. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000;123:1481–94. doi: 10.1093/brain/123.7.1481. [DOI] [PubMed] [Google Scholar]
- 50.Graus F, Dalmou J, Rene R, Tora M, Malats N, Verschuuren JJ, et al. Anti-Hu antibodies in patients with small-cell lung cancer: association with complete response to therapy and improved survival. J Clin Oncol. 1997;15:2866–72. doi: 10.1200/JCO.1997.15.8.2866. [DOI] [PubMed] [Google Scholar]
- 51.Voltz RD, Posner JB, Dalmau J, Graus F. Paraneoplastic encephalomyelitis: an update of the effects of the anti-Hu immune response on the nervous system and tumour. J Neurol Neurosurg Psychiatry. 1997;63:133–6. doi: 10.1136/jnnp.63.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Darnell RB, DeAngelis LM. Regression of small-cell lung carcinoma in patients with paraneoplastic neuronal antibodies. Lancet. 1993;341:21–2. doi: 10.1016/0140-6736(93)92485-c. [DOI] [PubMed] [Google Scholar]
- 53.Bertoni F, Coiffier B, Salles G, Stathis A, Traverse-Glehen A, Thieblemont C, et al. MALT lymphomas: pathogenesis can drive treatment. Oncology (Williston Park) 2011;25(12):1134–42. 1147. [PubMed] [Google Scholar]
- 54.Arcaini L, Bruno R. Hepatitis C virus infection and antiviral treatment in marginal zone lymphomas. Curr Clin Pharmacol. 2010;5:74–81. doi: 10.2174/157488410791110751. [DOI] [PubMed] [Google Scholar]
- 55.Stefanovic A, Lossos IS. Extranodal marginal zone lymphoma of the ocular adnexa. Blood. 2009;114:501–10. doi: 10.1182/blood-2008-12-195453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferreri AJ, Ernberg I, Copie-Bergman C. Infectious agents and lymphoma development: molecular and clinical aspects. J Intern Med. 2009;265:421–38. doi: 10.1111/j.1365-2796.2009.02083.x. [DOI] [PubMed] [Google Scholar]
- 57.Moormann AM, Snider CJ, Chelimo K. The company malaria keeps: how co-infection with Epstein-Barr virus leads to endemic Burkitt lymphoma. Curr Opin Infect Dis. 2011;24:435–41. doi: 10.1097/QCO.0b013e328349ac4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piccaluga PP, Gazzola A, Agostinelli C, Bacci F, Sabattini E, Pileri SA. Pathobiology of Epstein-Barr virus-driven peripheral T-cell lymphomas. Semin Diagn Pathol. 2011;28:234–44. doi: 10.1053/j.semdp.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–83. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 61.Bommert K, Bargou RC, Stuhmer T. Signalling and survival pathways in multiple myeloma. Eur J Cancer. 2006;42:1574–80. doi: 10.1016/j.ejca.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 62.Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann TA, et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87:1104–12. [PubMed] [Google Scholar]
- 63.Jego G, Bataille R, Geffroy-Luseau A, Descamps G, Pellat-Deceunynck C. Pathogen-associated molecular patterns are growth and survival factors for human myeloma cells through Toll-like receptors. Leukemia. 2006;20:1130–7. doi: 10.1038/sj.leu.2404226. [DOI] [PubMed] [Google Scholar]
- 64.Feng J, Yang Z, Li Y, Hu M, Yu M, Qin W, et al. The rational designed antagonist derived from the complex structure of interleukin-6 and its receptor affectively blocking interleukin-6 might be a promising treatment in multiple myeloma. Biochimie. 2006;88:1265–73. doi: 10.1016/j.biochi.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 65.Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys. 2005;63:655–66. doi: 10.1016/j.ijrobp.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rescigno M, Avogadri F, Curigliano G. Challenges and prospects of immunotherapy as cancer treatment. Biochim Biophys Acta. 2007;1776:108–23. doi: 10.1016/j.bbcan.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 67.Bhardwaj N. Harnessing the immune system to treat cancer. J Clin Invest. 2007;117:1130–6. doi: 10.1172/JCI32136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–66. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–90. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 72.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117(5):1167–74. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liston A, Rudensky AY. Thymic development and peripheral homeostasis of regulatory T cells. Curr Opin Immunol. 2007;19:176–85. doi: 10.1016/j.coi.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 74.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fu S, Zhang N, Yopp AC, Chen D, Mao M, Chen D, et al. TGF-beta induces Foxp3 + T-regulatory cells from CD4 + CD25 − precursors. Am J Transplant. 2004;4:1614–27. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 76.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–53. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 77.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 78.Wang ZK, Yang B, Liu H, Hu Y, Yang JL, Wu LL, et al. Regulatory T cells increase in breast cancer and in stage IV breast cancer. Cancer Immunol Immunother. 2012;61:911–6. doi: 10.1007/s00262-011-1158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shah W, Yan X, Jing L, Zhou Y, Chen H, Wang Y. A reversed CD4/CD8 ratio of tumor-infiltrating lymphocytes and a high percentage of CD4(+)FOXP3(+) regulatory T cells are significantly associated with clinical outcome in squamous cell carcinoma of the cervix. Cell Mol Immunol. 2011;8:59–66. doi: 10.1038/cmi.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carreras J, Lopez-Guillermo A, Fox BC, Colomo L, Martinez A, Roncador G, et al. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood. 2006;108:2957–64. doi: 10.1182/blood-2006-04-018218. [DOI] [PubMed] [Google Scholar]
- 81.Alvaro T, Lejeune M, Salvado MT, Bosch R, Garcia JF, Jaen J, et al. Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005;11:1467–73. doi: 10.1158/1078-0432.CCR-04-1869. [DOI] [PubMed] [Google Scholar]
- 82.Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107:3639–46. doi: 10.1182/blood-2005-08-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. CD70+ non-Hodgkin lymphoma B cells induce Foxp3 expression and regulatory function in intratumoral CD4+CD25 T cells. Blood. 2007;110:2537–44. doi: 10.1182/blood-2007-03-082578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, Dirnhofer S. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin’s lymphoma. Haematologica. 2008;93:193–200. doi: 10.3324/haematol.11702. [DOI] [PubMed] [Google Scholar]
- 85.Farinha P, Al-Tourah A, Gill K, Klasa R, Connors JM, Gascoyne RD. The architectural pattern of FOXP3-positive T cells in follicular lymphoma is an independent predictor of survival and histologic transformation. Blood. 2010;115(2):289–95. doi: 10.1182/blood-2009-07-235598. [DOI] [PubMed] [Google Scholar]
- 86.Colombo MP, Mantovani A. Targeting myelomonocytic cells to revert inflammation-dependent cancer promotion. Cancer Res. 2005;65:9113–6. doi: 10.1158/0008-5472.CAN-05-2714. [DOI] [PubMed] [Google Scholar]
- 87.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 88.Ogden CA, Pound JD, Batth BK, Owens S, Johannessen I, Wood K, et al. Enhanced apoptotic cell clearance capacity and B cell survival factor production by IL-10-activated macrophages: implications for Burkitt’s lymphoma. J Immunol. 2005;174:3015–23. doi: 10.4049/jimmunol.174.5.3015. [DOI] [PubMed] [Google Scholar]
- 89.Lech-Maranda E, Bienvenu J, Michallet AS, Houot R, Robak T, Coiffier B, et al. Elevated IL-10 plasma levels correlate with poor prognosis in diffuse large B-cell lymphoma. Eur Cytokine Netw. 2006;17:60–6. [PubMed] [Google Scholar]
- 90.Czarneski J, Lin YC, Chong S, McCarthy B, Fernandes H, Parker G, et al. Studies in NZB IL-10 knockout mice of the requirement of IL-10 for progression of B-cell lymphoma. Leukemia. 2004;18:597–606. doi: 10.1038/sj.leu.2403244. [DOI] [PubMed] [Google Scholar]
- 91.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 92.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 93.Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest. 2007;117:1184–94. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Link BK, Ballas ZK, Weisdorf D, Wooldridge JE, Bossler AD, Shannon M, et al. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J Immunother. 2006;29:558–68. doi: 10.1097/01.cji.0000211304.60126.8f. [DOI] [PubMed] [Google Scholar]
- 95.Friedberg JW, Kim H, McCauley M, Hessel EM, Sims P, Fisher DC, et al. Combination immunotherapy with a CpG oligonucleotide (1018 ISS) and rituximab in patients with non-Hodgkin lymphoma: increased interferon-alpha/beta-inducible gene expression, without significant toxicity. Blood. 2005;105:489–95. doi: 10.1182/blood-2004-06-2156. [DOI] [PubMed] [Google Scholar]
- 96.Kim YH, Gratzinger D, Harrison C, Brody JD, Czerwinski DK, Ai WZ, et al. In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood. 2012;119:355–63. doi: 10.1182/blood-2011-05-355222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Safwat A, Bayoumy Y, El-Sharkawy N, Shaaban K, Mansour O, Kamel A. The potential palliative role and possible immune modulatory effects of low-dose total body irradiation in relapsed or chemo-resistant non-Hodgkin’s lymphoma. Radiother Oncol. 2003;69:33–6. doi: 10.1016/s0167-8140(03)00247-0. [DOI] [PubMed] [Google Scholar]
- 98.Knoops L, Haas R, de Kemp S, Majoor D, Broeks A, Eldering E, et al. In vivo p53 response and immune reaction underlie highly effective low-dose radiotherapy in follicular lymphoma. Blood. 2007;110:1116–22. doi: 10.1182/blood-2007-01-067579. [DOI] [PubMed] [Google Scholar]
- 99.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 100.Di Nicola M, Zappasodi R, Carlo-Stella C, Mortarini R, Pupa SM, Magni M, et al. Vaccination with autologous tumor-loaded dendritic cells induces clinical and immunologic responses in indolent B-cell lymphoma patients with relapsed and measurable disease: a pilot study. Blood. 2009;113:18–27. doi: 10.1182/blood-2008-06-165654. [DOI] [PubMed] [Google Scholar]
- 101.Zappasodi R, Pupa SM, Ghedini GC, Bongarzone I, Magni M, Cabras AD, et al. Improved clinical outcome in indolent B-cell lymphoma patients vaccinated with autologous tumor cells experiencing immunogenic death. Cancer Res. 70:9062–72. doi: 10.1158/0008-5472.CAN-10-1825. [DOI] [PubMed] [Google Scholar]
- 102.Hilchey SP, Hyrien O, Mosmann TR, Livingstone AM, Friedberg JW, Young F, et al. Rituximab immunotherapy results in the induction of a lymphoma idiotype-specific T-cell response in patients with follicular lymphoma: support for a “vaccinal effect” of rituximab. Blood. 2009;113:3809–12. doi: 10.1182/blood-2008-10-185280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Witzig TE, Flinn IW, Gordon LI, Emmanouilides C, Czuczman MS, Saleh MN, et al. Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20:3262–9. doi: 10.1200/JCO.2002.11.017. [DOI] [PubMed] [Google Scholar]
- 104.Press OW, Unger JM, Braziel RM, Maloney DG, Miller TP, Leblanc M, et al. Phase II trial of CHOP chemotherapy followed by tositumomab/iodine I-131 tositumomab for previously untreated follicular non-Hodgkin’s lymphoma: five-year follow-up of Southwest Oncology Group Protocol S9911. J Clin Oncol. 2006;24:4143–9. doi: 10.1200/JCO.2006.05.8198. [DOI] [PubMed] [Google Scholar]
- 105.Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol. 2010;28:4324–32. doi: 10.1200/JCO.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim YH, Gratzinger D, Harrison C, et al. In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood. 2012;119:355–363. doi: 10.1182/blood-2011-05-355222. [DOI] [PMC free article] [PubMed] [Google Scholar]


