Abstract
Background and aims
Current creatine-based criteria for defining acute kidney injury (AKI) are validated in general hospitalised patients but their application to cirrhotics (who are younger and have reduced muscle mass) is less certain. We aimed to evaluate current definitions of AKI (acute kidney injury network (AKIN) criteria) in a population of cirrhotic patients and correlate this with outcomes.
Methods
We prospectively identified patients with AKI and clinical, radiological or histological evidence of cirrhosis. We compared them with a control group with evidence of cirrhosis and no AKI.
Results
162 cirrhotic patients were studied with a mean age of 56.8±14 years. They were predominantly male (65.4%) with alcoholic liver disease (78.4%). 110 patients had AKI: 44 stage 1, 32 stage 2 and 34 stage 3. They were well matched in age, sex and liver disease severity with 52 cirrhotics without AKI. AKI was associated with increased mortality (31.8% vs 3.8%, p<0.001). Mortality increased with each AKI stage; 3.8% in cirrhotics without AKI, 13.5% stage 1, 37.8% stage 2 and 43.2% stage 3 (p<0.001 for trend). Worsening liver disease (Child–Pugh class) correlated with increased mortality: 3.1% class A, 23.6% class B and 32.8% class C (p=0.006 for trend). AKI was associated with increased length of stay: median 6.0 days (IQR 4.0–8.75) versus 16.0 days (IQR 6.0–27.5), p<0.001. Multivariate analysis identified AKI and Child–Pugh classes B and C as independent factors associated with mortality.
Conclusions
The utility of AKIN criteria is maintained in cirrhotic patients. Decompensated liver disease and AKI appear to be independent variables predicting death in cirrhotics.
Keywords: Chronic Liver Disease, Cirrhosis, Portal Hypertension, Hepatorenal Syndrome, Hepatic Circulation
Introduction
Mortality from liver disease is increasing.1 Key drivers of this increase are the rising prevalence of alcoholic liver disease, viral hepatitis and fatty liver disease.2 Liver disease is now the fifth highest cause of death in the UK, with a 25% increase in mortality between 2001 and 2009.3 Renal dysfunction is a common complication of cirrhosis and confers a poor prognosis.4 Renal dysfunction occurs in 20% of patients with cirrhosis admitted to hospital, often linked with other complications of cirrhosis such as variceal bleeding and spontaneous bacterial peritonitis.5 In a systematic review of 118 studies, the presence of renal dysfunction was a powerful predictor of death in decompensated cirrhosis.6 Hepatorenal syndrome (HRS) has an extremely poor prognosis with a median survival time of 3 months,7 falling to just 1 month in those with untreated Type 1 HRS.8 Serum creatine is one of three variables comprising the model of end-stage liver disease score which is widely used in predicting short term mortality in allocating priority for orthotopic liver transplantation.9
Many definitions of renal dysfunction in liver disease are based on creatine thresholds, such as those used in HRS classification.10 This approach does not account for individual variation in baseline renal function or that a significant decline in renal function can occur with much smaller changes in creatine. There has also been a lack of standardisation in defining these thresholds.10–13 A more evolved approach to defining acute renal dysfunction has come with the widespread acceptance of consensus criteria for acute kidney injury (AKI) that provide a method of diagnosing and describing the severity of renal dysfunction based on individualised changes in serum creatine and urine output. The Kidney Disease: Improving Global Outcomes (KDIGO) diagnostic criteria are the most current,14 combining the earlier acute kidney injury network (AKIN)15 and Risk of renal dysfunction; Injury to the kidney; Failure of kidney function; Loss of kidney function; and End-stage kidney disease (RIFLE) classifications.16 The use of these criteria has been validated in a variety of settings including the critically ill and general hospitalised patients.17–21 Importantly, these criteria recognise that even a small decline in renal function is associated with poor outcomes.22 AKI therefore encompasses a wide spectrum of illness in a large number of patients from an abrupt rise in serum creatine of only 26.4 µmol/l (0.3 mg/dl) to critically unwell patients requiring renal replacement therapy.
However, there are several reasons why creatine-based definitions of AKI may perform differently in patients with liver disease. Cirrhotics are younger and have reduced muscle mass compared with other hospitalised patients.23 Severe hyperbilirubinaemia gives a falsely low value of serum creatine with chemical rather than the enzymatic measurement techniques.24 These factors may result in lower than expected baseline creatine values in this patient group relative to glomerular filtration rate.25 A small number of studies have begun to evaluate the current AKI criteria in cirrhotics. Some have focused on specific groups, such as those in intensive care or decompensated patients with ascites, which may limit their generalisability.26–28 The only prospective trial using robust AKIN definitions in a cirrhotic population was performed in four tertiary centres in the USA, but did not include a control group.29 These data may differ from European experience with a low proportion of compensated cirrhosis (only 2% had Child–Pugh class A disease), high incidence of hepatitis C (44%) and high rates of inpatient dialysis (24%). We therefore sought to add to these studies by further evaluating the utility of current diagnostic criteria for AKI in patients with cirrhosis and examine their association with patient outcomes.
Methods
Setting
The Royal Derby Hospital is a 1139-bed teaching hospital that provides all major medical and surgical specialities (excepting neuro- and cardiothoracic surgery). The liver unit is a tertiary referral centre, serving a population of 700 000. There is a central chemical pathology laboratory for all inpatient and outpatient samples. A compensated kinetic Jaffe method with an inter-assay coefficient of variance of 2.3% at 96 µmol/l (Roche P-analyser, Roche Diagnostics, West Sussex, UK) was used to measure all serum creatine values throughout the study period (normal creatine range 70–120 µmol/l).
Detection of AKI
We have devised and implemented a real-time electronic reporting system for AKI that identifies all cases of AKI across our hospital. This is based on the AKIN diagnostic criteria, which are summarised in table 1.15 This system has been in clinical use since 2010 and its diagnostic accuracy has been established. A full description of the system is available elsewhere.30
Table 1.
Classification/staging system for AKI according to AKIN15
| AKI stage | Serum creatine criteria | Urine output criteria |
|---|---|---|
| AKI stage 1 | Increase in serum creatine ≥0.3 mg/dl or increase to ≥150%–200% from baseline | Urine output<0.5 ml/kg/h for >6 h |
| AKI stage 2 | Increase of serum creatine to >200%–300% from baseline | Urine output<0.5 ml/kg/h for >12 h |
| AKI stage 3 | Increase of serum creatine to >300% from baseline OR serum creatine ≥4.0 mg/dl after a rise of at least 0.5 mg/dl OR treatment with renal replacement therapy |
Urine output<0.3 ml/kg/h for 24 h OR anuria for 12 h |
AKI, acute kidney injury; AKIN, acute kidney injury network.
This system also allows prospective data collection for all cases of AKI at our centre. A daily electronic report of all AKI episodes is automatically generated that details patient location (community vs hospital-acquired), patient age and baseline creatine. These data are supplemented by the highest AKI stage, last serum creatine in stay (to assess renal recovery), length of hospital stay and whether the patient survived to hospital discharge. Comorbidity data (based on ICD-10) coding and Charlson score are also included. Approval to use anonymised data in this way was obtained from the National Information Governance Board and a Local Research Ethics Committee waived requirement of individual patient consent.
Identification of patients with cirrhosis
All patients under the care of the Gastroenterology Department were selected from the prospectively identified hospital-wide population of those with AKI in the period 1 June 2010—31 October 2011. These patients were then manually screened to identify those with cirrhosis. Cirrhosis was diagnosed on the basis of any clinical (eg, decompensated chronic liver disease), radiological (eg, coarse liver, splenomegaly, presence of varices), or laboratory parameters (eg, prolonged international normalised ratio (INR) low platelets) and/or histological evidence from liver biopsy when available.29 31
A control group of patients with cirrhosis without AKI were retrospectively identified from the admission register to the liver unit over the same time period. Sequential patients were manually screened for evidence of cirrhosis in the same way as previously detailed. Patients were included only once in the control group regardless of the number of admissions during the 18-month period. Data were taken exclusively from the initial admission.
Child–Pugh score and class were retrospectively established for all cirrhotic patients included in the study. Where possible, this was calculated from electronic records within 3 months of the acute admission. If not possible, it was established from the results nearest to normal during the admission.
Statistical analysis
Parametric data are presented as mean±SD and non-parametric data as median (inter-quartile range). χ2 Test was used to compare categorical data and t test or Mann–Whitney test to compare continuous data depending on whether data were parametric or non-parametric. A Kaplan–Meier survival curve was calculated based on mortality data censored for admission. Binary logistic regression was used to test significant univariate associations with inhospital mortality. p Values of<0.05 were considered significant. All analyses were performed using SPSS V.19.
Results
Cirrhotic population
A total of 162 patients with cirrhosis were included, 110 with AKI and 52 controls. Of the 110 patients with cirrhosis who sustained AKI, 44 patients (40%) had AKI stage 1, 32 patients stage 2 (29.1%) and 34 (30.9%) stage 3. The mean age of the cirrhotic population was 56.8±13.6 years. Patients were predominantly male (65.4%) and 78.4% had liver disease caused by alcohol. The remainder of patients had fatty liver disease (6.2%), cryptogenic cirrhosis (5.5%), autoimmune hepatitis (3.7%), Hepatitis C (HCV) (1.9%), primary biliary cirrhosis (PBC) (1.9%) and other causes (2.5%). A total of 50 patients (30.9%) had biopsy-proven cirrhosis; 6.2% were recorded as having pre-existing chronic kidney disease. The mean baseline creatine was 78.4±29.4 µmol/l.
AKI versus non-AKI in patients with cirrhosis
Patient characteristics were similar when comparing those with and without AKI, as summarised in table 2. There was no significant difference between ages of the groups (59.6±13.4 vs 50.9±12.2, p=0.842), sex (62.7% vs 71.2% male, p=0.292) or aetiology of liver disease (79.1% vs 76.9% alcohol, p=0.754). There was also comparable severity of liver disease (p=0.135 for trend) and similar comorbidities between the groups. The only differences were the prevalence of diabetes (29.1% in the AKI group vs 11.5% in the control group, p=0.014) and mean baseline creatine value (85.5±28.8 µmol/l in the AKI group vs 64.7±25.6 µmol/l in the control group, p=0.03).
Table 2 .
Comparisons between cirrhotic groups: cirrhotics with acute kidney injury (AKI) and cirrhotic controls without AKI
| Factor | Cirrhotics with AKI | Cirrhotic controls without AKI | Significance |
|---|---|---|---|
| AKI stage 0 AKI stage 1 AKI stage 2 AKI stage 3 |
– 44 (40%) 32 (29.1%) 34 (30.9%) |
52 (100%) – – – |
|
| Severity of liver disease | |||
| Mean CPS | 8.87 | 8.29 | p=0.364 |
| CP A | 17 (15.5%) | 15 (28.8%) | p=0.135 |
| CP B | 52 (47.2%) | 21 (40.4%) | |
| CP C | 41 (37.1%) | 16 (30.8%) | |
| Comorbidities | |||
| Diabetes | 32 (29.1%) | 6 (11.5%) | p=0.014 |
| Lung disease | 9 (8.2%) | 7 (13.5%) | p=0.293 |
| IHD | 5 (4.5%) | 0 (0%) | p=0.118 |
| Acute MI | 5 (4.5%) | 0 (0%) | p=0.118 |
| CCF | 5 (4.5%) | 1 (1.9%) | p=0.409 |
| CKD | 8 (7.3%) | 2 (3.8%) | p=0.398 |
| Stroke | 4 (3.6%) | 0 (0%) | p=0.164 |
| Connective tissue disease | 2 (1.8%) | 0 (0%) | p=0.328 |
| Dementia | 0 (0%) | 1 (1.9%) | p=0.145 |
| Peptic ulcer disease | 3 (2.7%) | 1 (1.9%) | p=0.758 |
| Peripheral vascular disease | 3 (2.7%) | 1 (1.9%) | p=0.758 |
| Cancer | 3 (2.7%) | 0 (0%) | p=0.229 |
| Diabetic complications | 0 (0%) | 1 (1.9%) | p=0.145 |
AKI, acute kidney injury; CPS, Child-Pugh score; CP A, Child-Pugh A, B and C etc; IHD, ischaemic heart disease; MI, myocardial infarction; CCF, congested cardiac failure; CKD, chronic kidney disease.
Factors associated with outcomes
The presence of AKI in the cirrhotic population was associated with significantly increased mortality, 31.8% versus 3.8% in the control group (p<0.001), summarised in figure 1. We also observed that mortality increased as AKI became more severe, as shown in figure 2. Inhospital mortality rates were 13.5% in AKI stage 1, 37.8% in AKI stage 2 and 43.2% in AKI stage 3 (χ2 for trend, p<0.001). A Kaplan–Meier survival curve shows inhospital mortality associated with each stage of AKI (figure 3). This difference was significant between AKI stages 1 and 2 (p=0.001), but not between AKI stages 2 and 3 (p=0.793). AKI was also associated with an increased length of stay: median 6.0 days (IQR 4.0–8.75) versus 16.0 days (IQR 6.0–27.5), p<0.001.
Figure 1.
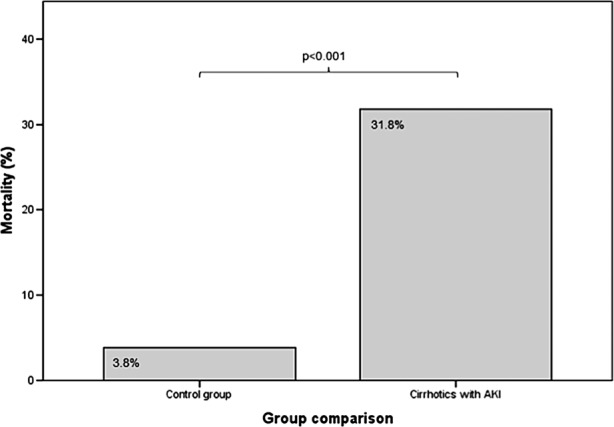
Overall mortality of cirrhotics with and without (control group) acute kidney injury (AKI).
Figure 2.
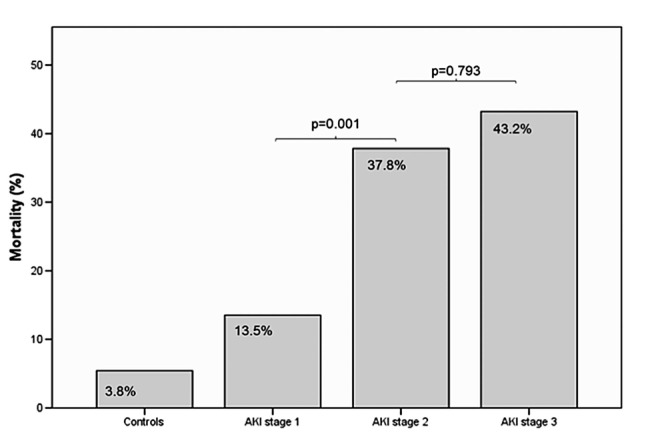
Mortality across acute kidney injury (AKI) stages.
Figure 3.
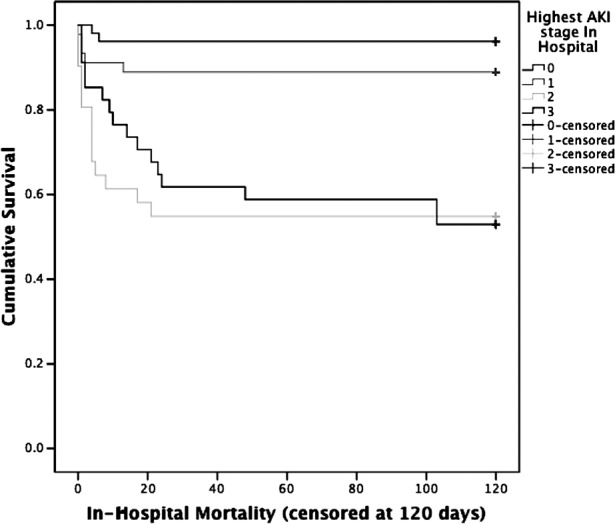
Kaplan–Meier survival curve showing inhospital mortality in cirrhotics with and without acute kidney injury (AKI) (censored at 120 days).
There was also an association between mortality and increasing severity of liver disease as measured by Child–Pugh class. Mortality rates were 3.1% in class A, 23.6% in class B and 32.8% in class C (χ2 for trend=0.006) (figure 4).
Figure 4.
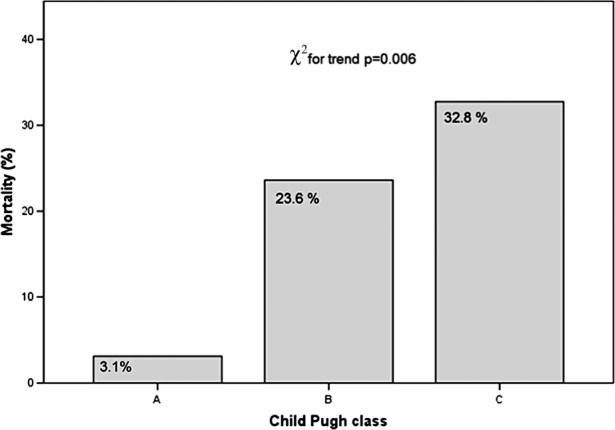
Mortality with increasing severity of liver disease by Child–Pugh class.
We compared survivors and non-survivors to identify additional factors associated with inhospital mortality. Previous myocardial infarction (1.6% vs 8.1%, p=0.044), peripheral vascular disease (0.8% vs 8.1%, p=0.012), connective tissue disease (0% vs 5.4%, p=0.009) and the presence of pre-existing chronic kidney disease (CKD) (4% vs 13.5%, p=0.035) were associated with increased mortality. Other comorbid conditions were not associated with death, including diabetes (21.6% vs 29.7%, p=0.305) and baseline creatine (median 77.8±31.4 vs 80.3±22.0 µmol/l, p=0.181). In our population, there was no difference in age between survivors and non-survivors (median 55.9±13.9 vs 59.8±12.3 years, p=0.193).
Binary logistic regression including all significant univariate associations revealed that the only factors independently associated with increased mortality were AKI, Child–Pugh class B and Child–Pugh class C (Nagelkerke r2=0.256, Hosmer and Lemeshow test for goodness of fit p=0.567). Table 3 shows this model in full.
Table 3 .
Multivariate analysis of factors independently associated with mortality
| Factor | Significance | HR | 95% CIs |
|---|---|---|---|
| Acute kidney injury | 0.02 | 10.6 | 2.4 to 47.0 |
| Child–Pugh B | 0.04 | 8.1 | 1.0 to 65.4 |
| Child–Pugh C | 0.016 | 13.1 | 1.6 to 106.0 |
Discussion
This study demonstrates that current creatine-based definitions of AKI retain their utility in cirrhotic patients, with the presence and increasing severity of AKI strongly associated with inhospital mortality. Our results add to the relatively small number of studies examining the use of such criteria in cirrhotic patients and provide an important comparison between those with and without AKI.
Previous criteria for identifying renal dysfunction, such as the HRS definitions,10 use arbitrary creatine thresholds which fail to incorporate an individual's baseline renal function and exclude smaller changes in creatine values despite their impact on patient outcome. Newer AKI criteria, such as the AKIN or KDIGO classifications, have several advantages. Small but significant changes in an individual's baseline creatine are included to improve sensitivity; these criteria also describe the severity of AKI depending on the magnitude of creatine rise (or degree of oliguria). These criteria are now widely accepted and have been validated in a variety of clinical scenarios,14 but the differences in creatine profile seen in liver patients mandate that these criteria are examined specifically in this patient group. To date, there have been a small number of studies that have attempted this. Carvalho et al32 retrospectively studied 91 patients with cirrhosis and ascites identifying AKI within 48 h of hospital admission. The majority of patients had mild renal dysfunction (91% had stage 1 AKI). Patients with AKI had a hospital mortality rate of 52.7%. Presence of AKI conferred an OR of 2.6 for hospital mortality but quantifying the risk by stage was limited by the small numbers of patients with more severe AKI. Belcher et al29 prospectively studied 192 hospitalised cirrhotic patients with AKI in four centres in the USA. AKIN criteria were used to identify AKI, with 50 (26%) patients with AKI stage 1, 47 (24%) stage 2 and 95 (49%) stage 3. Baseline creatine was robustly defined as the most recent stable measurement prior to admission for the index hospitalisation within 1 year or if they had at least 5 days of stable values within the normal creatine range following admission. Overall mortality was 26% and severity of AKI was independently associated with mortality (adjusted OR=3.8, 95% CI 1.3 to 11.1), but the presence of AKI itself was not assessed as there was no control group without AKI. Tsient et al31 prospectively studied a total of 91 patients with cirrhosis and ascites recruited from hepatology outpatient clinics. Patients had bloods performed at 4–6 weekly intervals and AKI was defined by AKIN criteria if two samples within 48 h were available or if a 50% increase in serum creatine from the baseline visit was observed. If identified, AKI was treated with volume expansion of albumin (1 g/kg) for two consecutive days. In all, 49 patients suffered with 82 episodes of AKI, and again mortality was higher in those with AKI.
Our results add to these studies and confirm that AKI (as per AKIN criteria) is associated with mortality in patients with cirrhosis, even after adjustment for severity of liver disease and comorbid conditions. Although comparisons between studies are difficult, mortality rates in cirrhotics with AKI do appear to be higher than other reports of generalised hospital patients with AKI, suggesting that this patient group is particularly vulnerable when AKI develops.17 30 For contextual reference, the overall mortality of general hospitalised patients with AKI at our centre from September 2010 to August 2012 was 21.2% (stage 1, 15.2%; stage 2, 32.3%; stage 3, 33.5%). Despite potential differences between the demographics of USA and European cirrhotics, we observed mortality rates similar to those reported by Belcher et al.29 Although high (at over 30%), our observed mortality rates are lower than some historic studies.26 27 This may reflect our inclusion of patients with less severe AKI, an effect of using current diagnostic criteria; mortality rose to above 40% in those with AKI stage 3. However, it is important to recognise that even small acute rises in serum creatine are significant, with mortality in our patients with AKI stage 1 substantially elevated as compared with controls. The implications of relatively small changes in serum creatine often remain underappreciated in clinical practice, which may be partly addressed by wider usage of current AKI definitions.
Our observed mortality rate in the control group was low (3.8%). It is, however, similar to that reported in other studies of cirrhotic patients with ascites who did not sustain AKI.31 This may reflect the relatively young age of patients with cirrhosis, the low prevalence of comorbidity or a less severe acute illness without factors that may precipitate AKI.
Comparison between the groups with and without AKI showed they were well matched in terms of age, sex, cause and severity of liver disease. There were few differences in renal risk profile between the control group and those with cirrhosis and AKI, with the only differences in baseline creatine and diabetes. Although diabetes was not associated with increased mortality, it is possible that its presence may be a risk factor for developing AKI. The higher baseline creatine may reflect a gradual increase in creatine which may occur as a prodrome of renal dysfunction in patients with cirrhosis.33 Particular attention should therefore be paid defining baseline creatine in this group. In general patients, avoiding the use of results from the 7 days prior to hospital admission may avoid the use of falsely high reference creatine values,34 but in cirrhotic patients it is conceivable that this period should be extended due to the indolent increase sometimes in serum creatine prior to the acute presentation.
The modified Child–Turcotte–Pugh score is widely used as a simple descriptive or prognostic indicator in patients with cirrhosis.35 36 Unlike the model of end-stage liver disease score37 it does not include a marker of renal dysfunction, although it could be argued that the inclusion of bilirubin, ascites and encephalopathy are potential surrogate markers of renal injury.38 It is not surprising that increasing mortality was associated with increasing severity of liver disease in a stage-dependent manner as measured by the Child–Pugh class. It does, however, support that the scores were credibly calculated with a valid methodology.
Our study does have some potential weaknesses. The methodology for electronic identification of AKI employed by this study has been previously validated in a generalised hospital population with a false negative rate of 0.2%.30 However, in a population of patients with cirrhosis who are younger with lower than average baseline serum creatine values, it is possible that the initial step, which depends on a comparison with an estimated creatine value, may miss a small number of cases of AKI stage 1. In addition, the data presented are limited to information collected routinely as standard of care either in patient notes or on electronic record. Comorbidity data are based upon International Classification of Diseases – version 2010 (ICD-10) coding and therefore limited to these definitions. Thus, we are unable to report the impact of other variables, such as circulatory dysfunction, infection or inflammation. Further studies could evaluate whether changing AKIN creatine thresholds would be of added benefit in cirrhotics.
In conclusion, this study shows that AKI, as defined by the AKIN diagnostic criteria, is associated with increased mortality in patients with cirrhosis in a stage-dependent manner. Severe liver disease and AKI appear to be independent variables in predicting death in cirrhotic patients. The current methods for defining AKI appear to have clinical utility in patients with cirrhosis, and their wider use may help with improved recognition and earlier intervention for AKI, which has particular relevance in this vulnerable group.
What is already known about this subject.
Renal dysfunction in cirrhosis is common and confers a poor prognosis.
Previous definitions of renal dysfunction in patients with cirrhosis (eg, HRS definitions) have relied on creatinine thresholds.
AKIN criteria recognise that a small decline in renal function is associated with poor outcomes in general hospitalised patients.
What this study adds.
AKI, as defined by the AKIN diagnostic criteria, is associated with increased mortality in patients with cirrhosis in a stage-dependent manner.
Mortality rates in cirrhotics with AKI appear to be higher than other reports of generalised hospital patients with AKI.
Severe liver disease and AKI appear to be independent variables in predicting death in cirrhotic patients.
How might it impact on clinical practice in the foreseeable future.
AKIN criteria appear to have clinical utility in patients with cirrhosis.
Their wider use may help with improved recognition and earlier intervention for AKI which should improve patient outcomes.
Further work is needed to understand the mechanism and risk factors in developing renal dysfunction in patients with cirrhosis.
Footnotes
Contributors: NS and AA conceived the study and overall supervised conduct of the study. RS and NS contributed to study design, data collection, analysis and interpretation. NK contributed to data collection. CM contributed to data interpretation. All authors contributed to the drafting, revising of the article and approved the final version of the manuscript.
Competing interests: None.
Ethics approval: National Information Governance Board.
Provenance and peer review : Not commissioned; externally peer reviewed.
References
- 1.WHO Organization. WHO European Health for All database. 2012. http://www.euro.who.int/en/what-we-do/data-and-evidence/databases/european-health-for-all-database-hfa-db2 (accessed 2 Dec 2012).
- 2.Sheron N. A time to act: Improving liver health and outcomes in liver disease. London: British Society of Gastroenterology, 2009.
- 3.National End of Life Care Intelligence Network. Deaths from Liver Disease: Implications for end of life care in England, 2012. http://www.endoflifecare-intelligence.org.uk/resources/publications/deaths_from_liver_disease (accessed 9 April 2013). [Google Scholar]
- 4.du Cheyron D, Bouchet B, Parienti J, et al. The attributable mortality of acute renal failure in critically ill patients with liver cirrhosis. Intensive Care Med 2005;31:1693–9. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology 2008;48:2064–77. [DOI] [PubMed] [Google Scholar]
- 6.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44:217–31. [DOI] [PubMed] [Google Scholar]
- 7.Ginés P, Schrier RW. Renal failure in cirrhosis . N Engl J Med 2009;361:1279–90. [DOI] [PubMed] [Google Scholar]
- 8.Alessandria C, Ozdogan O, Guevara M, et al. MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver transplantation. Hepatology 2005;41:1282–9. [DOI] [PubMed] [Google Scholar]
- 9.Al Sibae MR, Cappell MS. Accuracy of MELD scores in predicting mortality in decompensated cirrhosis from variceal bleeding, hepatorenal syndrome, alcoholic hepatitis, or acute liver failure as well as mortality after non-transplant surgery or TIPS. Dig Dis Sci 2011;56:977–87. [DOI] [PubMed] [Google Scholar]
- 10.Arroyo V, Ginès P, Gerbes AL, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis: International Ascites Club. Hepatology 1996;23:164–76. [DOI] [PubMed] [Google Scholar]
- 11.Chen YC, Tsai MH, Ho YP, et al. Comparison of the severity of illness scoring systmes for critically ill cirrhotic patients with renal failure. Clin Nephrol 2004;61:111–18. [DOI] [PubMed] [Google Scholar]
- 12.Fang JT, Tsai MH, Tian YC, et al. Outcome predictors and new score of critically ill cirrhotic patients with acute renal failure. Nephrol Dial Transp 2008;23:1961–9. [DOI] [PubMed] [Google Scholar]
- 13.Cárdenas A, Ginès P, Uriz J, et al. Renal failure after upper gastrointestinal bleeding in cirrhosis: incidence, clinical course, predictive factors, and short-term prognosis. Hepatology 2001;34:671–6. [DOI] [PubMed] [Google Scholar]
- 14.Balasubramanian G, Al-ALy Z, Moiz A, et al. Early Nephrologist involvement in Hospital-Acquired Acute Kidney Injury: a pilot study. Am J Kidney Dis 2011;57:228–34. [DOI] [PubMed] [Google Scholar]
- 15.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellomo R, Ronco C, Kellum JA, et al. Acute Dialysis Quality Initiative workgroup Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uchino S, Bellomo R, Goldsmith D, et al. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 2006;34:1913–7. [DOI] [PubMed] [Google Scholar]
- 18.Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes agter acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009;53:961–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lafrance JP, Miller DR. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrology 2009;21:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchino S, Kellum JA, Bellomo R, et al. Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005;294:813–18. [DOI] [PubMed] [Google Scholar]
- 21.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int 2008;73:538–46. [DOI] [PubMed] [Google Scholar]
- 22.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrology 2005;16:3365–70. [DOI] [PubMed] [Google Scholar]
- 23.Orlando R, Floreani M, Padrini R, et al. Evaluation of measured and calculated creatinine clearances as glomerular filtration markers in different stages of liver cirrhosis. Clin Nephrol 1999;51:341–7. [PubMed] [Google Scholar]
- 24.Dimeski G, McWhinney B, Jones B, et al. Extent of bilirubin interference with Beckman creatinine methods. Ann Clin Biochem 2008;45:91–2. [DOI] [PubMed] [Google Scholar]
- 25.Sherman DS, Fish DN, Teitelbaum I. Assessing renal function in cirrhotic patients: problems and pitfalls. Am J Kidney Dis 2003;41:269–78. [DOI] [PubMed] [Google Scholar]
- 26.Jenq CC, Tsai MH, Tian YC, et al. RIFLE classification can predict short-term prognosis in critically ill cirrhotic patients. Intensive Care Med 2007;33:1921–30. [DOI] [PubMed] [Google Scholar]
- 27.Cholongitas E, Calvaruso V, Senzolo M, et al. RIFLE classification as predictive factor of mortality in patinets with cirrhosis admitted to intensive care unit. J Gastroenterol Hepatol 2009;24: 1639–47. [DOI] [PubMed] [Google Scholar]
- 28.Lopes JA, Melo MJ, Costa AC, et al. Acute kidney injury and in-hospital mortality in critically ill patients with cirrhosis: a cohort study. Gut 2012;61:655–6. [DOI] [PubMed] [Google Scholar]
- 29.Belcher JM, Garcia-Tsao G, Sanyal AJ, et al. TRIBE-AKI Consortium Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology 2012;57:753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selby NM, Crowley L, Fluck RJ, et al. Use of electronic results reporting to diagnose and montior AKI in hospitalized patients. Clin J Am Soc Nephrol 2012;7:533–40. [DOI] [PubMed] [Google Scholar]
- 31.Tsien CD, Rabie R, Wong F. Acute kidney injury in decompensated cirrhosis. Gut 2012;62:131–7. [DOI] [PubMed] [Google Scholar]
- 32.de Carvalho JR, Villela-Nogueira CA, Luiz RR, et al. Acute kidney injury network criteria as a predictor of hospital mortality in cirrhotic patients wih ascites. J Clin Gastroenterol, 2012;46:e21–6. [DOI] [PubMed] [Google Scholar]
- 33.Wong F, Nadim M, Kellum J, et al. Working party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut 2011;60:702–9. [DOI] [PubMed] [Google Scholar]
- 34.Siew E, Ikizler T, Matheny M, et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephron 2012;7:712–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Child C, Turcotte J. Surgery and portal hypertension. In: Child C. The liver and portal hypertension. Philadelphia: W. B. Saunders Co, 1964;1:1–85. [PubMed] [Google Scholar]
- 36.Pugh R, Murray-Lyon I, Dawson J, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646–9. [DOI] [PubMed] [Google Scholar]
- 37.Malinchoc M, Kamath P, Gordon F, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000;31:864–71. [DOI] [PubMed] [Google Scholar]
- 38.Durand F, Valla D. Assessment of the prognosis of cirrhosis: child–pugh versus MELD. J Hep 2005;42:S100–107. [DOI] [PubMed] [Google Scholar]


