Abstract
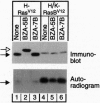
Benzodiazepine (BZA)-5B, a CAAX farnesyl-transferase inhibitor, was previously shown to block the farnesylation of H-Ras and to reverse the transformed morphology of Rat1 cells expressing oncogenic H-RasV12. Non-transformed Rat1 cells were not affected by BZA-5B, suggesting that they produce a form of Ras whose prenylation is not blocked by this compound. The likely candidate is K-RasB, which differs from H-Ras primarily in the terminal 24 amino acids. In the current study we examined the effect of BZA-5B on the prenylation of a chimeric oncogenic Ras protein designated H/K-RasBV12, consisting of the first 164 amino acids of H-RasV12 followed by the last 24 amino acids of K-RasB. BZA-5B failed to block the prenylation of this chimera and was thus unable to reverse the transformed morphology of Rat1 cells in which it was expressed. Another potent inhibitor of H-Ras farnesylation, L-739,749, also failed to block prenylation of H/K-RasBV12. Similar results were obtained in transfected cells expressing a widely used version of K-RasBV12 containing a 10-amino acid extension at its NH2 terminus. Neither BZA-5B nor L-739,749 reversed the transformed morphology of cells expressing H/K-RasBV12. The resistance of K-RasB to farnesyltransferase inhibition provides a likely explanation for the resistance of nontransformed cells to the growth inhibitory effects of BZA-5B and L-739,749.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres D. A., Goldstein J. L., Ho Y. K., Brown M. S. Mutational analysis of alpha-subunit of protein farnesyltransferase. Evidence for a catalytic role. J Biol Chem. 1993 Jan 15;268(2):1383–1390. [PubMed] [Google Scholar]
- Armstrong S. A., Hannah V. C., Goldstein J. L., Brown M. S. CAAX geranylgeranyl transferase transfers farnesyl as efficiently as geranylgeranyl to RhoB. J Biol Chem. 1995 Apr 7;270(14):7864–7868. doi: 10.1074/jbc.270.14.7864. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bos J. L. ras oncogenes in human cancer: a review. Cancer Res. 1989 Sep 1;49(17):4682–4689. [PubMed] [Google Scholar]
- Camp L. A., Hofmann S. L. Purification and properties of a palmitoyl-protein thioesterase that cleaves palmitate from H-Ras. J Biol Chem. 1993 Oct 25;268(30):22566–22574. [PubMed] [Google Scholar]
- Casey P. J. Protein lipidation in cell signaling. Science. 1995 Apr 14;268(5208):221–225. doi: 10.1126/science.7716512. [DOI] [PubMed] [Google Scholar]
- Casey P. J., Solski P. A., Der C. J., Buss J. E. p21ras is modified by a farnesyl isoprenoid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. J., Andres D. A., Goldstein J. L., Russell D. W., Brown M. S. cDNA cloning and expression of the peptide-binding beta subunit of rat p21ras farnesyltransferase, the counterpart of yeast DPR1/RAM1. Cell. 1991 Jul 26;66(2):327–334. doi: 10.1016/0092-8674(91)90622-6. [DOI] [PubMed] [Google Scholar]
- Glomset J. A., Farnsworth C. C. Role of protein modification reactions in programming interactions between ras-related GTPases and cell membranes. Annu Rev Cell Biol. 1994;10:181–205. doi: 10.1146/annurev.cb.10.110194.001145. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Stradley S. J., Reiss Y., Gierasch L. M. Nonfarnesylated tetrapeptide inhibitors of protein farnesyltransferase. J Biol Chem. 1991 Aug 25;266(24):15575–15578. [PubMed] [Google Scholar]
- Hancock J. F., Cadwallader K., Paterson H., Marshall C. J. A CAAX or a CAAL motif and a second signal are sufficient for plasma membrane targeting of ras proteins. EMBO J. 1991 Dec;10(13):4033–4039. doi: 10.1002/j.1460-2075.1991.tb04979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- James G. L., Brown M. S., Cobb M. H., Goldstein J. L. Benzodiazepine peptidomimetic BZA-5B interrupts the MAP kinase activation pathway in H-Ras-transformed Rat-1 cells, but not in untransformed cells. J Biol Chem. 1994 Nov 4;269(44):27705–27714. [PubMed] [Google Scholar]
- James G. L., Goldstein J. L., Brown M. S. Polylysine and CVIM sequences of K-RasB dictate specificity of prenylation and confer resistance to benzodiazepine peptidomimetic in vitro. J Biol Chem. 1995 Mar 17;270(11):6221–6226. doi: 10.1074/jbc.270.11.6221. [DOI] [PubMed] [Google Scholar]
- James G. L., Goldstein J. L., Brown M. S., Rawson T. E., Somers T. C., McDowell R. S., Crowley C. W., Lucas B. K., Levinson A. D., Marsters J. C., Jr Benzodiazepine peptidomimetics: potent inhibitors of Ras farnesylation in animal cells. Science. 1993 Jun 25;260(5116):1937–1942. doi: 10.1126/science.8316834. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Omer C. A., Conner M. W., Anthony N. J., Davide J. P., deSolms S. J., Giuliani E. A., Gomez R. P., Graham S. L., Hamilton K. Inhibition of farnesyltransferase induces regression of mammary and salivary carcinomas in ras transgenic mice. Nat Med. 1995 Aug;1(8):792–797. doi: 10.1038/nm0895-792. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Wilson F. R., Mosser S. D., Giuliani E., deSolms S. J., Conner M. W., Anthony N. J., Holtz W. J., Gomez R. P., Lee T. J. Protein farnesyltransferase inhibitors block the growth of ras-dependent tumors in nude mice. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):9141–9145. doi: 10.1073/pnas.91.19.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerner E. C., Qian Y., Hamilton A. D., Sebti S. M. Disruption of oncogenic K-Ras4B processing and signaling by a potent geranylgeranyltransferase I inhibitor. J Biol Chem. 1995 Nov 10;270(45):26770–26773. doi: 10.1074/jbc.270.45.26770. [DOI] [PubMed] [Google Scholar]
- Moores S. L., Schaber M. D., Mosser S. D., Rands E., O'Hara M. B., Garsky V. M., Marshall M. S., Pompliano D. L., Gibbs J. B. Sequence dependence of protein isoprenylation. J Biol Chem. 1991 Aug 5;266(22):14603–14610. [PubMed] [Google Scholar]
- Reiss Y., Brown M. S., Goldstein J. L. Divalent cation and prenyl pyrophosphate specificities of the protein farnesyltransferase from rat brain, a zinc metalloenzyme. J Biol Chem. 1992 Mar 25;267(9):6403–6408. [PubMed] [Google Scholar]
- Reiss Y., Goldstein J. L., Seabra M. C., Casey P. J., Brown M. S. Inhibition of purified p21ras farnesyl:protein transferase by Cys-AAX tetrapeptides. Cell. 1990 Jul 13;62(1):81–88. doi: 10.1016/0092-8674(90)90242-7. [DOI] [PubMed] [Google Scholar]
- Reiss Y., Stradley S. J., Gierasch L. M., Brown M. S., Goldstein J. L. Sequence requirement for peptide recognition by rat brain p21ras protein farnesyltransferase. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):732–736. doi: 10.1073/pnas.88.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling H. C., Bruenger E., Leining L. M., Buss J. E., Epstein W. W. Differential prenylation of proteins as a function of mevalonate concentration in CHO cells. Arch Biochem Biophys. 1993 Mar;301(2):210–215. doi: 10.1006/abbi.1993.1135. [DOI] [PubMed] [Google Scholar]
- Sepp-Lorenzino L., Ma Z., Rands E., Kohl N. E., Gibbs J. B., Oliff A., Rosen N. A peptidomimetic inhibitor of farnesyl:protein transferase blocks the anchorage-dependent and -independent growth of human tumor cell lines. Cancer Res. 1995 Nov 15;55(22):5302–5309. [PubMed] [Google Scholar]
- Yokoyama K., Goodwin G. W., Ghomashchi F., Glomset J. A., Gelb M. H. A protein geranylgeranyltransferase from bovine brain: implications for protein prenylation specificity. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5302–5306. doi: 10.1073/pnas.88.12.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]