Abstract
The relationship between Apolipoprotein E (ApoE) genotype and the risk of Alzheimer's disease (AD) is relatively well established in Caucasians, but less established in other ethnicities. To examine the association between ApoE polymorphism and the onset of AD in Chinese population, we searched the commonly used electronic databases between January 2000 and November 2013 for relevant studies. Total 20 studies, including 1576 cases and 1741 controls, were retrieved. The results showed statistically significant positive association between risk factor ε4 allele carriers and AD in Chinese population (OR = 3.93, 95% CI = 3.37–4.58, P < 0.00001). Genotype ApoE ε4/ε4 and ε4/ε3 have statistically significant association with AD as well (ε4/ε4: OR = 11.76, 95% CI = 6.38–21.47, P < 0.00001; ε4/ε3: OR = 3.08, 95% CI = 2.57–3.69, P < 0.00001). Furthermore, the frequency of the ApoE ε3 is lower in AD than that in the health controls, and the difference of ε3 allele is also statistically significant (OR = 0.42, 95% CI = 0.37–0.47, P < 0.00001). No significant heterogeneity was observed among all studies. This meta-analysis suggests that the subject with at least one ApoE ε4 allele has higher risk suffering from AD than controls in Chinese population. The results also provide a support for the protection effect of ApoE ε3 allele in developing AD.
Alzheimer's disease (AD) is a progressive and fatal brain disorder that causes memory loss, steady deterioration of cognition, and dementia1. It is the sixth leading cause of all deaths in the United States, and deaths increased by 66% between 2000 and 20082. Approximately 13% of people over the age of 65 years and 45% over the age of 85 years are estimated to have AD3. Therefore, discussing the risk factors and pathogenesis of AD, is of great significance for early detection, prevention and control of the susceptible population.
During the last two decades, researchers have found that Apolipoprotein E (ApoE) gene, located on chromosome 19, is closely associated with the onset of AD4,5. ApoE is a 299-aminoacid protein encoded by the ApoE gene and has a molecular mass of ~34 kDa6. It is a major cholesterol carrier that supports lipid transport and injury repair in the brain7. Three common polymorphisms in the ApoE gene, ε2, ε3, and ε4, including 6 genotypes, three homozygote (ε2/ε2, ε3/ε3, ε4/ε4) and three heterozygote (ε3/ε2, ε4/ε2, ε4/ε3)8, result in a single amino acid change in the ApoE protein. Differences between the three ApoE isoforms are limited to amino acid residues 112 and 1589. Of these, the ε3 allele is the most common, followed by ε4 and ε2, although these frequencies vary between populations. ApoE polymorphic alleles are the main genetic determinants of AD risk: individuals carrying the ε4 allele are at increased risk of AD compared with those carrying the more common ε3 allele, whereas the ε2 allele decreases risk10. Moreover, the ε4 allele is most highly associated with AD at a large range of ages and in all ethnic groups; its presence is related with increased risk of cerebral amyloid angiopathy and age-associated cognitive decline during normal ageing11,12; It is also associated with hyperlipidaemia and hypercholesterolaemia, which lead to atherosclerosis, coronary heart disease and stroke13. The effects of ApoE genotype on risk of these diseases are likely to be mediated by differential effects of ApoE on amyloid-β accumulation in the brain and its vasculature14,15. Response to treatment for AD might differ according to ApoE genotype16.
Although numerous studies have demonstrated the association, inconsistency was presented for different allele frequencies among study populations, particularly in different ethnic and geographical groups. The purpose of conducting this meta-analysis is to reduce heterogeneity and summarize the published evidence on the prevalence of the ApoE polymorphism among patients diagnosed with AD in Chinese population.
Results
Study selection and characteristics
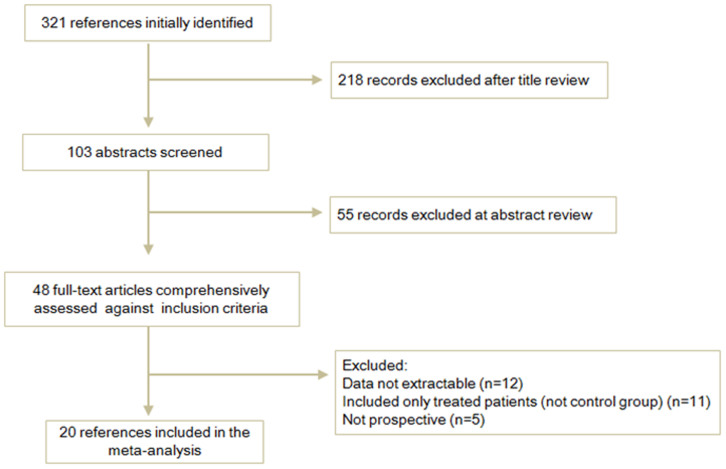
The electronic database search identified 312 references. Of those, 218 records excluded after title review and 103 articles were judged potentially relevant. Following abstracts screened for relevance, 48 full-text articles comprehensively assessed against inclusion criteria. Overall, the initial search with the keywords and the subject terms identified 20 publications, including 1576 cases and 1741 controls that met the inclusion criteria and were eligible for review. Figure 1 shows the study flow.
Figure 1. Flow chart of literature screening.
Of the 20 reports focusing on the relationship between ApoE polymorphism and AD, 18 were from mainland17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34, two were from Taiwan35,36. The distribution of the genotypes in the control group was consistent with Hardy-Weinberg equilibrium (HWE). The detailed characteristics of the included studies were shown in Table 1. The distributions of genotypes in the individual studies were presented in Table 2.
Table 1. Main characteristics of the eligible studies.
| Total no. of | Mean-age | |||||
|---|---|---|---|---|---|---|
| First author-published year | Geographical location | cases | controls | diagnostic criteria | Cases | Controls |
| Zhu-2000 | Shandong | 36 | 36 | NINCDS-ADRDA | - | - |
| Huang-2001 | Guangzhou | 41 | 85 | DSM-III-R | 80.8 | 59.6 |
| Jia-2002 | Beijing | 58 | 60 | NINCDS-ADRDA | 68.6 ± 7.6 | 65.9 ± 8.5 |
| Huang H-2002 | Taiwan | 99 | 96 | NINCDS-ADRDA | 76.3 ± 6.9 | 72.2 ± 6.9 |
| Bi-2002 | Haerbin | 42 | 40 | NINCDS-ADRDA | 70.4 ± 6.6 | 68.1 ± 4.3 |
| Zhou-2003 | Wulumuqi | 51 | 52 | NINCDS-ADRDA | 74.1 ± 9.7 | 69.4 ± 11.4 |
| Chen-2003 | Beijing/Shanxi | 160 | 195 | NINCDS-ADRDA | 69.4 ± 9.5 | 69.8 ± 7.8 |
| Zhang-2004 | Shandong | 32 | 40 | CCMD-2-R | 74.1 ± 7.3 | 63.9 ± 7.3 |
| He-2005 | Beijing | 27 | 67 | NINCDS-ADRDA | 82.9 ± 7.9 | - |
| Wang-2006 | Taiwan | 151 | 161 | DSM-IV | 74.8 ± 7.9 | 62.5 ± 8.7 |
| Li-2006 | Guizhou | 30 | 30 | DSM-IV | 70.2 ± 11.6 | 71.4 ± 10.5 |
| Yang J-2008 | Yunnan | 58 | 96 | NINCDS-ADRDA | 74.0 ± 8.48 | 74.2 ± 4.72 |
| Yang L-2008 | Henan | 102 | 98 | NINCDS-ADRDA | 78.5 ± 7.3 | 76.5 ± 9.3 |
| Wu-2009 | Shanghai | 262 | 118 | NINCDS-ADRDA | 76.91 ± 5.10 | 60.72 ± 4.88 |
| Duan-2009 | Henan | 32 | 76 | DSM-IV-R | 70.6 ± 9.8 | 63.5 ± 6.7 |
| Mai-2010 | Guangdong | 88 | 97 | DSM-IV-R | 79.6 ± 9.2 | 79.7 ± 8.6 |
| Jiang-2010 | Guangxi | 79 | 156 | NINCDS-ADRDA | 72.8 ± 9. 5 | 71.2 ± 9. 3 |
| Zhou C-2012 | Chongqing | 68 | 72 | DSM-IV-TR | 70.6 ± 6. 8 | 71.2 ± 6. 6 |
| Lv-2012 | Shanghai | 100 | 106 | NINCDS-ADRDA | 77.0 ± 4. 8 | 79.5 ± 5. 0 |
| Dong-2013 | Liaoning | 60 | 60 | NINCDS-ADRDA | - | - |
Table 2. Distribution of genotypes in the individual studies.
| First author | cases | controls | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ε4/ε4 | ε3/ε3 | ε2/ε2 | ε4/ε3 | ε3/ε2 | ε4/ε2 | ε4/ε4 | ε3/ε3 | ε2/ε2 | ε4/ε3 | ε3/ε2 | ε4/ε2 | ||
| Zhu | 2000 | 3 | 16 | 0 | 12 | 5 | 0 | 0 | 28 | 0 | 4 | 4 | 0 |
| Huang | 2001 | 4 | 20 | 1 | 6 | 7 | 3 | 0 | 62 | 0 | 9 | 0 | 4 |
| Jia | 2002 | 1 | 29 | 0 | 15 | 7 | 6 | 1 | 47 | 0 | 4 | 8 | 0 |
| Huang | 2002 | 6 | 53 | 5 | 23 | 9 | 3 | 0 | 66 | 3 | 15 | 12 | 0 |
| Bi | 2002 | 2 | 20 | 0 | 15 | 2 | 3 | 0 | 28 | 1 | 6 | 3 | 2 |
| Zhou | 2003 | 1 | 26 | 3 | 19 | 2 | 0 | 0 | 35 | 4 | 4 | 9 | 0 |
| Chen | 2003 | 10 | 85 | 0 | 47 | 11 | 7 | 0 | 137 | 0 | 26 | 29 | 3 |
| Zhang | 2004 | 1 | 18 | 0 | 9 | 2 | 2 | 0 | 32 | 0 | 3 | 4 | 1 |
| He | 2005 | 0 | 13 | 0 | 9 | 4 | 1 | 0 | 55 | 0 | 5 | 5 | 0 |
| Wang | 2006 | 13 | 75 | 0 | 54 | 7 | 2 | 0 | 120 | 1 | 27 | 11 | 2 |
| Li | 2006 | 2 | 17 | 0 | 9 | 1 | 1 | 0 | 23 | 0 | 1 | 2 | 1 |
| Yang L | 2008 | 10 | 44 | 1 | 35 | 5 | 7 | 1 | 82 | 0 | 8 | 6 | 1 |
| Yang J | 2008 | 1 | 40 | 0 | 14 | 3 | 0 | 0 | 75 | 0 | 8 | 11 | 2 |
| Wu | 2009 | 30 | 118 | 0 | 102 | 12 | 1 | 1 | 77 | 2 | 23 | 13 | 2 |
| Duan | 2009 | 5 | 8 | 0 | 12 | 4 | 3 | 1 | 53 | 1 | 9 | 10 | 2 |
| Mai | 2010 | 6 | 45 | 2 | 18 | 14 | 3 | 0 | 65 | 0 | 12 | 16 | 4 |
| Jiang | 2010 | 2 | 47 | 0 | 20 | 6 | 4 | 0 | 107 | 0 | 17 | 31 | 1 |
| Zhou C | 2012 | 5 | 37 | 0 | 17 | 7 | 2 | 0 | 49 | 0 | 9 | 11 | 3 |
| Lv | 2012 | 11 | 44 | 2 | 33 | 10 | 0 | 0 | 76 | 2 | 15 | 12 | 1 |
| Dong | 2013 | 4 | 27 | 0 | 23 | 3 | 2 | 1 | 44 | 0 | 9 | 6 | 0 |
Association between ApoE allele and AD
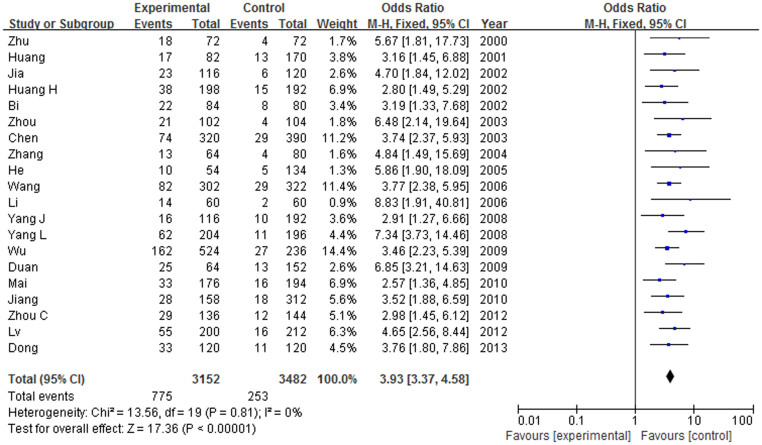
The results of each allele and genotypes of ApoE in this meta-analysis were listed in Table 3. The heterogeneity between studies was not significant excepting the allele ε2. The fixed effect model or the random effect model was employed for calculating the pooled OR. Overall, this meta-analysis showed that the frequency of ApoE ε4 allele is higher in AD than that in the health controls, and demonstrated statistically significant positive association between risk factor ε4 allele carriers and AD in Chinese population (OR = 3.93, 95% CI = 3.37–4.58, P < 0.00001), as shown in Figure 2. The frequency of the ApoE ε3 is lower in AD than that in the health controls and the difference is also statistically significant (OR = 0.42, 95% CI = 0.37–0.47, P < 0.00001), implying the protection effect of ε3 allele in developing AD. No significant association was found between ApoE ε2 allele and AD (OR = 0.93, 95% CI = 0.66–1.29, P = 0.65) in a random-effect model.
Table 3. Meta-analysis of Apolipoprotein E gene polymorphism in Alzheimer's disease.
| Fix-effect model | Random-effect model | |||||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | Ph | I2 | OR (95% CI) | P | Ph | I2 | |
| Allele | ||||||||
| ApoE ε2 | 0.88 (0.73, 1.07) | 0.20 | 0.0002 | 61% | 0.93 (0.66, 1.29) | 0.65 | 0.0002 | 61% |
| ApoE ε3 | 0.42 (0.37, 0.47) | <0.00001 | 0.07 | 34% | 0.42 (0.36, 0.49) | <0.00001 | 0.07 | 34% |
| ApoE ε4 | 3.93 (3.37, 4.58) | <0.00001 | 0.81 | 0% | 3.89 (3.33, 4.54) | <0.00001 | 0.81 | 0% |
| Genotype | ||||||||
| ApoE ε4/ε4 | 11.71 (6.38, 21.47) | <0.00001 | 0.98 | 0% | 9.84 (5.28, 18.34) | <0.00001 | 0.98 | 0% |
| ApoE ε3/ε3 | 0.39 (0.33, 0.45) | <0.00001 | 0.25 | 16% | 0.38 (0.33, 0.45) | <0.00001 | 0.25 | 16% |
| ApoE ε2/ε2 | 1.06 (0.55, 2.06) | 0.86 | 0.60 | 0% | 1.09 (0.52, 2.27) | 0.83 | 0.60 | 0% |
| ApoE ε4/ε3 | 3.08 (2.57, 3.69) | <0.00001 | 0.06 | 35% | 3.16 (2.48, 4.02) | <0.00001 | 0.06 | 35% |
| ApoE ε3/ε2 | 0.67 (0.53, 0.85) | 0.0008 | 0.18 | 23% | 0.66 (0.49, 0.88) | 0.005 | 0.18 | 23% |
| ApoE ε4/ε2 | 2.10 (1.35, 3.26) | 0.001 | 0.32 | 11% | 1.92 (1.13, 3.28) | 0.02 | 0.32 | 11% |
Ph, Pheterogeneity.
Figure 2. Forest plot on the association between ε4 allele carriers and AD in Chinese population.
Association between ApoE genotype and AD
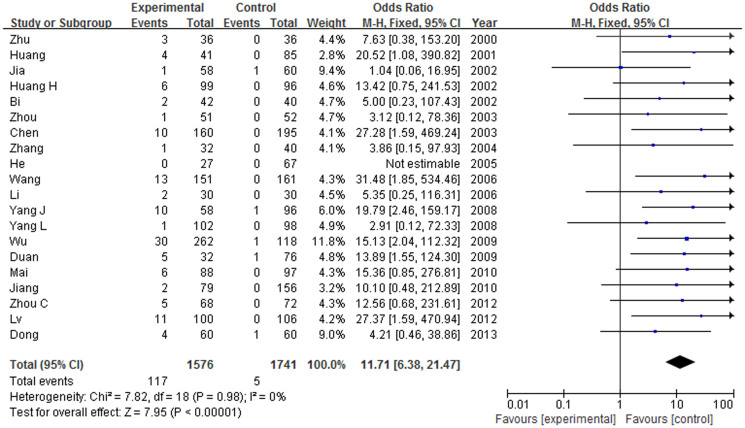
As shown in table 3, the heterogeneity between studies was not significant (I2 < 50%) and the fixed effect model was used for calculating the pooled OR. ApoE ε4/ε4 and ApoE ε4/ε3 have statistically significant association with AD (ε4/ε4: OR = 11.76, 95% CI = 6.38–21.47, P < 0.00001; ε4/ε3: OR = 3.08, 95% CI = 2.57–3.69, P < 0.00001). Figure 3 showed the association between ε4/ε4 and AD in Chinese population. Genotype ε3/ε3 also has significant association with AD (OR = 0.39, 95% CI = 0.33–0.45, P < 0.00001). ApoE ε3/ε2 and ApoE ε4/ε2 have slight association with AD. There is no association between ε2/ε2 and AD.
Figure 3. Forest plot on the association between ε4/ε4 genotype and AD in Chinese population.
Sensitivity analysis and publication bias
For this meta-analysis, the influence of a single study on the overall meta-analysis estimate was investigated by omitting one study at a time, respectively. The risk ratio was not significantly influenced by omitting any single study.
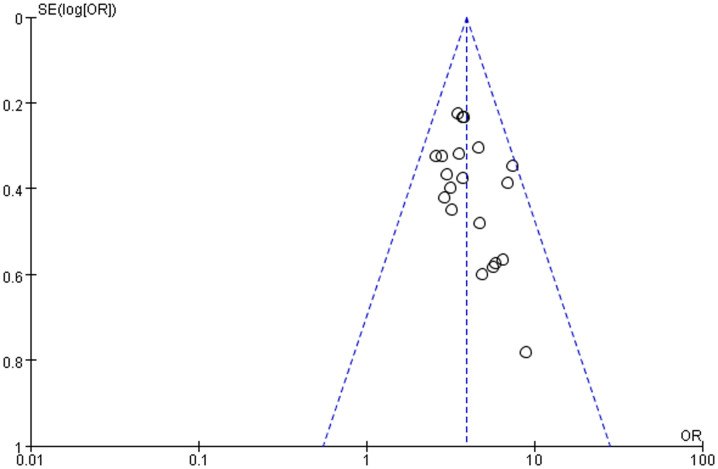
The distribution of the ORs from individual studies in relation to their respective standard deviation in funnel plot, as shown in Figure 4 and Figure 5. The funnel revealed no evidence of asymmetry. Thus, there was no possibility of publication bias risk in the meta-analysis.
Figure 4. Funnel plot on the association between ε4 allele carriers and AD.
Figure 5. Funnel plot on the association between ε4/ε4 genotype and AD.
Discussion
ApoE gene, known to mediate the regulation of cholesterol and triglyceride metabolism, is immunochemically localized to the senile plaques, vascular amyloid, and neurofibrillary tangles of AD37,38. In 1993, Strittmatter et al. for the first time demonstrated that there was a highly significant association of ApoE ε4 allele and late-onset familial AD39. Subsequently, most studies reported gene frequency of ApoE ε4 was significantly increased in sporadic AD than in controls. Sando et al. proved that ApoE ε4 is a very strong risk factor for AD in the population of central Norway, and lowers age at onset of late onset AD (LOAD) significantly40; Rhinn et al. identified an ApoE ε4 associated molecular pathway that promotes LOAD41; Genin et al. demonstrated that ApoE ε4 is a risk factor not only for late-onset but for early-onset AD as well42. Together, these results urge a reappraisal of the impact of ApoE in AD.
Previous meta-analysis demonstrated that ApoE ε4 genotype prevalence varies among AD patients by region and within each country43: the highest estimates were in Northern Europe; the lowest estimates were in Asia and Southern Europe. To further explore and examine the association of prevalence district of ApoE genotype and AD risk, we conducted this meta-analysis only in Chinese population. Overall, our results showed that the risk of developing AD in ε4 allele carriers was 3.93-fold higher than individuals without ε4 allele. The risk of developing AD in individuals with ε4/ε4 genotype was 11.76-fold higher than individuals without ε4/ε4 genotype. There probably exists a dose-dependent association between the number of ApoE ε4/ε4 allele and the risk of AD in Chinese population. Furthermore, the frequency of the ApoE ε3 is lower in AD than that in the health controls and the difference is also statistically significant, implying the protection effect of ε3 allele in developing AD. No association was found between ε2 allele and AD risk.
The ε4 allele of ApoE is the “risk” variant for several phenotypes compared with ε3 (“neutral”), and ε2 (generally considered “protective”, although less consistently). The ApoE gene confers differential susceptibility to AD etiology depending on the combination of the 3 alleles as well as the age and ethnicity of the person. The ε4 allele of ApoE is the strongest genetic risk factor for the development of AD. Although multiple genetic and environmental risk factors are involved in LOAD pathogenesis, overall impairment in Aβ clearance is probably a major contributor to disease development44. Accumulation of amyloid-β (Aβ) is hypothesized to initiate synaptic and neuronal dysfunction that ultimately lead to neuronal cell death in AD, and several lines of evidence strongly suggest that the differential effects of ApoE isoforms on Aβ aggregation and/or clearance plays a major role in AD pathogenesis45,46. The ApoE isoforms could influence the risk for AD via other mechanisms as well47. Relative to the common ε3 allele, possession of ε4 increases disease risk and decreases age at onset in a dose-dependent manner48. In contrast, possession of the ε2 allele may confer protection against AD, as carriers of this allele are less likely to develop the disease than ε3 homozygotes49. The ApoE ε4 allele is associated with greater accumulation of both Aβ plaques and neurofibrillary tangles than the ε3 allele50, while carriers of the ε2 allele typically develop less AD-related pathology than both ε4 carriers and ε3 homozygotes. Pomara et al. showed a potential effect of the ApoE ε2 allele and of family history of Alzheimer's disease on brain amyloid-β in normal elderly51. Hostage et al. confirmed and extended prior data on the opposing effects of the ApoE ε4 and ε2 alleles on hippocampal morphology across the spectrum of cognitive aging48. This general pattern holds for many physiological phenomena influenced by ApoE genotype, and potentially related to AD, including measures of synaptic plasticity and repair52, antioxidant properties, and certain immune responses53, and cholesterol levels54.
Currently, genetic testing of ApoE ε4 carrier status is not routinely considered in clinical practice. As ApoE genotype determines AD risk, and ApoE has crucial roles in cognition55,56, ApoE might offer an attractive alternative target for AD therapy. ApoE genotype status could be included in clinical trial enrolment criteria, as some therapies might be effective only in specific ApoE genotypes. In addition, ApoE is a crucial regulator of the innate immune system, with ApoE ε4 promoting pro-inflammatory responses that could exacerbate AD pathogenesis.
Several limitations were presented in this meta-analysis. Firstly, in AD group of retrieved case-control studies, there may exist mixed dementia. It might increase the apparent association of ApoE with AD, since ApoE ε4 allele is associated with dementia. Secondly, ApoE polymorphism may interact with other known and unknown risk factors which should be considered. Thirdly, the selected studies may have more subject to bias and artifact than prospective studies.
In conclusion, our meta-analysis suggests that ApoE ε4 carrier is associated with AD and provide a support for the protection effect of ApoE ε3 allele in developing AD in Chinese population. Although ApoE polymorphism is a well-studied genetic risk factor for developing AD, in some regions most patients do not carry this genotype. Therefore, additional research with well-designed and large sample sizes is needed to be able to understand both other genetic and environmental risk factors in the future.
Methods
Identification and eligibility of relevant studies
We conducted a comprehensive literature search using the electronic database of PubMed, Wanfang database and CNKI (China National Knowledge Infrastructure) for relevant articles assessing the association of ApoE polymorphism and AD in Chinese population from January 2000 to November 2013. The Medical Subject Heading (MeSH) terms “Alzheimer's disease”, “AD”, “ApoE”, “Apolipoprotein E”, and “polymorphism” were employed as the searching words. The equivalent Chinese terms were used in the Chinese databases. All studies matching the eligibility criteria were retrieved, and references were checked for other relevant publications.
Criteria for article screening
Studies eligible for inclusion in this meta-analysis must meet the following criteria: 1) case-control or cohort study; 2) measure the relationship between ApoE polymorphism and AD; 3) clinical diagnosis of AD based on standards of the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer's Disease and Related Disorders Association (NINCDS/ADRDA) work group57; 4) the results were expressed as odds ratio (OR) and corresponding 95 percent confidence interval (95% CI); 5) genotype distribution of control for a certain polymorphism must be in HWE; and 6) when the same authors reported two or more publications on possibly the same patient populations, only the most recent or complete study was included into this meta-analysis.
Quality assessment and data extraction
Two investigators independently extracted data and reached a consensus on all of the items. Any disagreement was resolved by discussing with the third expert. Data retrieved from the reports included first author, publication year, demographics, number of cases and controls, distribution of genotypes, and the diagnosis criteria of AD.
Statistical analysis
The odd ratio (OR) with 95% confidence intervals (95% CI) was used to assess the relationship of ApoE polymorphism and AD. The significance of the pooled OR was determined by the Z test, and a P value less than 0.05 was considered significant. The heterogeneity for the included articles was evaluated using Cochran's Q test and I2 statistics. P-value less than 0.05 and I2 less than 50% were considered to be statistically significant. When a significant heterogeneity existed across the included studies (I2 > 50%), the random effect model was used58; When there was no significant heterogeneity across the included studies (I2 < 50%), the fixed effect model was used58. To assess whether our results were substantially influenced by the presence of any individual study, we conducted a sensitivity analysis by systematically removing each study and recalculating the significance of the result. Begg's funnel plot was performed to examine the publication bias. Analyses were carried out using the Review manager 5.2 (The Cochrane Collaboration). All tests were two sided.
Author Contributions
Conceived and designed the study: J.Q.Z. and F.W.; Performed the experiments: M.Y.L., C.B., J.Q.Z. and F.W.; Contributed material/analysis tools: M.Y.L., J.Q.Z. and F.W.; Statistical analyses and paper writing, revising: M.Y.L., C.B., J.Q.Z. and F.W.
Acknowledgments
This study was supported by the National Science Foundation of China (NSFC, No. 81171035).
References
- Selkoe D. J. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev 81, 741–766 (2001). [DOI] [PubMed] [Google Scholar]
- Thies W. & Bleiler L. 2011 Alzheimer's disease facts and figures. Alzheimers Dement 7, 208–244 (2011). [DOI] [PubMed] [Google Scholar]
- Alzheimer's A. 2012 Alzheimer's disease facts and figures. Alzheimers Dement 8, 131–168 (2012). [DOI] [PubMed] [Google Scholar]
- Siest G. et al. Apolipoprotein E: an important gene and protein to follow in laboratory medicine. Clin Chem 41, 1068–1086 (1995). [PubMed] [Google Scholar]
- Levy-Lahad E. & Bird T. D. Genetic factors in Alzheimer's disease: a review of recent advances. Ann Neurol 40, 829–840 (1996). [DOI] [PubMed] [Google Scholar]
- Mahley R. W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240, 622–630 (1988). [DOI] [PubMed] [Google Scholar]
- Mahley R. W. & Rall S. C. Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet 1, 507–537 (2000). [DOI] [PubMed] [Google Scholar]
- Zannis V. I. et al. Proposed nomenclature of apoE isoproteins, apoE genotypes, and phenotypes. J Lipid Res 23, 911–914 (1982). [PubMed] [Google Scholar]
- Morrow J. A. et al. Differences in stability among the human apolipoprotein E isoforms determined by the amino-terminal domain. Biochemistry 39, 11657–11666 (2000). [DOI] [PubMed] [Google Scholar]
- Hauser P. S. & Ryan R. O. Impact of apolipoprotein E on Alzheimer's disease. Curr Alzheimer Res 10, 809–817 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghebremedhin E. et al. Gender and age modify the association between APOE and AD-related neuropathology. Neurology 56, 1696–1701 (2001). [DOI] [PubMed] [Google Scholar]
- Millar K., Nicoll J. A., Thornhill S., Murray G. D. & Teasdale G. M. Long term neuropsychological outcome after head injury: relation to APOE genotype. J Neurol Neurosurg Psychiatry 74, 1047–1052 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahoz C. et al. Apolipoprotein E genotype and cardiovascular disease in the Framingham Heart Study. Atherosclerosis 154, 529–537 (2001). [DOI] [PubMed] [Google Scholar]
- Kim J. et al. Anti-apoE immunotherapy inhibits amyloid accumulation in a transgenic mouse model of Abeta amyloidosis. J Exp Med 209, 2149–2156 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese P. B. et al. ApoE influences amyloid-beta (Abeta) clearance despite minimal apoE/Abeta association in physiological conditions. Proc Natl Acad Sci U S A 110, E1807–1816 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claxton A. et al. Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer's disease. J Alzheimers Dis 35, 789–797 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Zhang J. & Zhang Z. Apolipoprotein E Gene Polymorphisms and Alzheimer's Disease. J G Genomics 30, 1167–1170 (2003). [PubMed] [Google Scholar]
- Zhang Y., Liu Z. & Du Y. Association between Apolipoprotein E gene polymorphism and Vascular dementia and Alzheimer's disease. Shandong Med J 22, 1–2 (2004). [Google Scholar]
- Mai Y., Luo Y., Gu H., Zhang L. & LIN J. Association between Interleukin-8 and Apolipoprotein E gene polymorphism and late-onset Alzheimer's disease. J sun yat-sen uni 1, 118–121 (2010). [Google Scholar]
- Jia J., Zhang J., Xu M. & Zhou W. Association between the apolipoprotein E gene polymorphism and the genetic sensitivity of sporadic Alzheimer's disease. Chin J Geriatrics 4, 7–9 (2002). [Google Scholar]
- Zhu J., Xu W. & Gao Y. The association of apolipoprotein E and Alzheimer's disease. Practical Geriatrics 1, 23–25 (2000). [Google Scholar]
- Jiang W., Lv Z. & Tan L. Correlation between apolipoprotein E gene polymorphism s and Alzheimer's disease and vascular dementia. J Clin Neurology 6, 408–410 (2010). [Google Scholar]
- He S. R., Liu D. G., Wang S. & Xia Y. J. Expression of apolipoprotein E in Alzheimer's disease and its significance. Chin J Pathology 34, 556–560 (2005). [PubMed] [Google Scholar]
- Zhou C., Xu J. & He J. Genetic correlation study between apolipoprotein E gene and Alzheimer's disease. Med J NDFNC 4, 244–246 (2003). [Google Scholar]
- Huang S., Lu Y. & Chen S. Inquiring relation between Apolipoprotein E Gene polymorphysm and the Alzheimer's disease. J M Clin Med Bio 1, 9–11 (2001). [Google Scholar]
- Li G., Zhao M., Zuo L., Liu F. & Kuang S. Polymorphism in the Apolipoprotein E Gene and Its Association with Sporadic Alzheimer Disease. J Guiyang Med 1, 24–26 (2006). [Google Scholar]
- Bi S. et al. The Relationship between ApoE Genotype, Serum Total Cholesterol Level, and Alzheimer's disease. Chin J neuroimmunology 1, 13–16 (2002). [Google Scholar]
- Wu P., Guo Q., Chen M., Zhou Y. & Hong Z. Relationship Between Apolipoprotein E Gene Polymorphism and Early-onset Alzheimer' s Disease. Chin J Clin Neurosci 3, 1168–1170 (2009). [Google Scholar]
- Zhou C., Cheng X. & Xia X. Relationship between Apolipoprotein E Polymorphism and Alzheimer's Disease in Chongqing. J Chin GP 27, 3115–3117 (2012). [Google Scholar]
- Yang J., Yu F. & Wang Y. Study of the polymorphisms of APOE gene in Alzheimer's disease and vascular dementia. Neuro Dis Mental H 2, 138–140 (2008). [Google Scholar]
- Dong X. The study of the relationship between AD and the ApoE polymorphism. Chin H Care Nutrition 2, 523–524 (2013). [Google Scholar]
- Yang L. Study on the association between Apolipoprotein E gene polymorphisms and sporadic Alzheimer's disease. Chin Mod Doctor 24, 89–90 (2008). [Google Scholar]
- Duan G., Guo L. & Yang L. Study on the Association of Apolipoprotein E-gene Polymorphism with Demented Patients. J Henan Med C 6, 559–561 (2009). [Google Scholar]
- Lv X. & Zhong Y. Study on the relationship between apolipoprotein E gene polymorphism and mild cognitive impairment and Alzheimer's disease. Chin J Gerontology 5, 917–919 (2012). [Google Scholar]
- Huang H. M., Kuo Y. M., Ou H. C., Lin C. C. & Chuo L. J. Apolipoprotein E polymorphism in various dementias in Taiwan Chinese population. J Neural Transm 109, 1415–1421 (2002). [DOI] [PubMed] [Google Scholar]
- Wang H. K. et al. Apolipoprotein E, angiotensin-converting enzyme and kallikrein gene polymorphisms and the risk of Alzheimer's disease and vascular dementia. J Neural Transm 113, 1499–1509 (2006). [DOI] [PubMed] [Google Scholar]
- Saunders A. M. et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology 43, 1467–1472 (1993). [DOI] [PubMed] [Google Scholar]
- Schmechel D. E. et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A 90, 9649–9653 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter W. J. et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A 90, 1977–1981 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando S. B. et al. APOE epsilon 4 lowers age at onset and is a high risk factor for Alzheimer's disease; a case control study from central Norway. BMC Neurol 8, 9 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhinn H. et al. Integrative genomics identifies APOE epsilon4 effectors in Alzheimer's disease. Nature 500, 45–50 (2013). [DOI] [PubMed] [Google Scholar]
- Genin E. et al. APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Mol Psychiatry 16, 903–907 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A. et al. Prevalence of apolipoprotein E4 genotype and homozygotes (APOE e4/4) among patients diagnosed with Alzheimer's disease: a systematic review and meta-analysis. Neuroepidemiology 38, 1–17 (2012). [DOI] [PubMed] [Google Scholar]
- Mawuenyega K. G. et al. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science 330, 1774 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie R. M. et al. Apolipoprotein E4 effects in Alzheimer's disease are mediated by synaptotoxic oligomeric amyloid-beta. Brain 135, 2155–2168 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Basak J. M. & Holtzman D. M. The role of apolipoprotein E in Alzheimer's disease. Neuron 63, 287–303 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano J. M. et al. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med 3, 89ra57 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostage C. A., Roy Choudhury K., Doraiswamy P. M., Petrella J. R. & Alzheimer's Disease Neuroimaging I. Dissecting the gene dose-effects of the APOE epsilon4 and epsilon2 alleles on hippocampal volumes in aging and Alzheimer's disease. PLoS One 8, e54483 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlau D. J., Corrada M. M., Head E. & Kawas C. H. APOE epsilon2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology 72, 829–834 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman E. M. et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci U S A 106, 6820–6825 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomara N. & Bruno D. Potential effects of the APOE epsilon2 allele and of family history of Alzheimer's disease on brain amyloid-beta in normal elderly. Proc Natl Acad Sci U S A 108, E1007; author reply E1008 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofre-Monseny L., Minihane A. M. & Rimbach G. Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol Nutr Food Res 52, 131–145 (2008). [DOI] [PubMed] [Google Scholar]
- Smith J. D. Apolipoproteins and aging: emerging mechanisms. Ageing Res Rev 1, 345–365 (2002). [DOI] [PubMed] [Google Scholar]
- Jack C. R. Jr et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain 132, 1355–1365 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G. et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34, 939–944 (1984). [DOI] [PubMed] [Google Scholar]
- Kim S. M. et al. Regional cerebral perfusion in patients with Alzheimer's disease and mild cognitive impairment: effect of APOE epsilon4 allele. Neuroradiology 55, 25–34 (2013). [DOI] [PubMed] [Google Scholar]
- DerSimonian R. & Laird N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–188 (1986). [DOI] [PubMed] [Google Scholar]
- Mantel N. & Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22, 719–748 (1959). [PubMed] [Google Scholar]