Abstract
Objectives:
This study compares very late outcomes following primary percutaneous coronary intervention for ST-elevation myocardial infarction (STEMI) with stenting versus balloon angioplasty (BA).
Background:
Stenting compared with BA for STEMI improves outcomes at 6–12 months, but comparisons beyond 6–12 months have not been studied. Recent studies have shown that stent thrombosis (ST) continues to increase beyond 3–5 years and may be higher with drug-eluting stents (DES) than bare metal stents (BMS). We hypothesized that there may be a very late hazard with stenting versus BA due to very late ST.
Methods:
From 1994 to 2010 consecutive patients with STEMI treated with BA (n = 601) or stenting (n = 1,594) were prospectively enrolled in our registry and followed for 1–16 years.
Results:
Patients treated with BA were older, were more often female, had more three-vessel disease, and had smaller vessels. Stented patients had trends for less stent/lesion thrombosis (ST/LT) and target vessel (TV) reinfarction at 1 year. In landmark analyses >1 year, stented patients had more very late ST/LT (6.1% vs. 2.9%, P = 0.002) and more TV reinfarction (7.9% vs. 3.1%, P < 0.001) which remained significant after adjusting for baseline risk. The greatest differences in very late outcomes were between DES and BA, but there were also significant differences between BMS and BA.
Conclusions:
There appears to be a very late hazard with stenting versus BA for STEMI. These data should encourage new strategies for prevention of very late ST with both BMS and DES including the development of bioabsorbable polymers and stent platforms.
Introduction
Coronary stenting has become the default strategy with primary percutaneous coronary intervention (PCI) for ST-elevation myocardial infarction (STEMI). This is based on data showing that stenting compared with balloon angioplasty alone (BA) reduces angiographic restenosis and reocclusion of the infarct artery and reduces the need for target vessel (TV) revascularization at 6–12 months.1-6 However, long-term outcomes beyond 6–12 months comparing stenting with BA have not been evaluated.
Several studies have shown that the cumulative frequency of stent thrombosis (ST) following stenting with both bare metal stents (BMS) and drug-eluting stents (DES) for STEMI continues to increase beyond 3–5 years and that the frequency of very late ST may be higher with early-generation DES.7-11 Because of these findings, we hypothesized that there may be a very late hazard with stenting compared with BA alone due to very late ST.
We have prospectively enrolled consecutive STEMI patients treated with primary PCI from 1994, when stents were first used in the treatment of STEMI, to the present time, and we have obtained long-term follow-up. This has provided a unique opportunity to compare long-term outcomes with BA versus stenting for STEMI. The purpose of this study is to evaluate the hypothesis that there may be a late hazard with stenting versus BA due to very late ST.
Methods
Study Population and Treatment Protocol
The study population consists of 2,195 consecutive patients with STEMI treated with BA (n = 601) or stenting (n = 1,594) at our institution from 1994 through 2010 who had successful PCI (TIMI 2–3 flow and residual stenosis ≤50% post-PCI) and did not have STEMI due to ST. Patients were included in our registry if they had electrocardiographic ST-segment elevation ≥1 mm in ≥2 contiguous leads or new left bundle branch block, symptoms of <12 hours duration (>12 hours for persistent ischemic symptoms or hemodynamic compromise), and were treated with primary PCI. Patients were treated with contemporary standards of care for primary PCI. In the early years, this included antithrombotic therapy with aspirin and unfractionated heparin. In the middle years, aspirin, ticlopidine or clopidogrel, unfractionated heparin, and glycoprotein IIb/IIIa platelet inhibitors were used. In recent years, aspirin, clopidogrel, and bivalirudin were used, usually without glycoprotein IIb/IIIa platelet inhibitors. From 1994 to 1995, stents were used infrequently. From 1996 to 1999, stents were used primarily in clinical trials in which patients were randomized to stents versus BA. Outside of clinical trials and after 1999, stents were used at the discretion of the operator generally according to the following inclusion and exclusion criteria: (1) vessel size ≥2.25 mm and ≤4.0 mm, (2) expected ability to deliver and deploy the stent, (3) not a left main lesion, and (4) not multivessel disease expected to require surgery during the index hospitalization. BMS were used exclusively from 1994 to 2003 and DES or BMS were used from 2003 to 2010 at the operator’s discretion. Of 1,594 patients who received stents, 1,165 received BMS, 421 received DES, and 8 received mixed BMS and DES. Of the 421 patients who received DES, 338 were early-generation DES (sirolimus-eluting stents [SES] [n = 117], paclitaxel-eluting stents [PES] [n = 207], zotarolimus-eluting stents, fast release [ZES] [n = 11], or mixed early-generation DES [n = 3]) and 83 were new-generation DES (all everolimus-eluting stents [EES]).
Data Collection, Clinical Follow-Up, and Definitions
Patients were enrolled prospectively into the database from 1994 through 2010. Procedural data were assessed and entered by the interventional cardiologist at the time of the PCI. Hospital data and posthospital data were obtained from hospital and office chart reviews by clinical nurse coordinators, and this was supplemented with telephone follow-up. Deaths were also sought through the social security death index, in which case the cause of death was determined by death certificates. All deaths, cardiac versus noncardiac, reinfarctions, and STs or lesion thromboses (LTs) were adjudicated by one of the investigators.
ST was defined as definite ST according to the Academic Research Consortium definition.12 Definite ST occurred when there was an acute coronary syndrome with angiographic confirmation of thrombus within the stent with partial or total occlusion of the stent. In patients who received BA only, LT was defined similarly to ST. Definite LT occurred when there was an acute coronary syndrome with angiographic confirmation of thrombus at the prior BA site with partial or total occlusion of the coronary artery. If there was uncertainty by the operator whether definite ST or LT occurred, angiograms were reviewed by one of the investigators. In-hospital reinfarction was defined as occurring when there were recurrent ischemic symptoms associated with re-elevation of the cardiac markers or documented occlusion of the infarct artery. Posthospital reinfarction was defined as occurring when there was rehospitalization for ischemic symptoms associated with elevation of the cardiac markers. TV reinfarction was defined as occurring when there was angiographic confirmation that the culprit lesion responsible for the reinfarction was located in the TV. The primary outcomes of this study were very late mortality, very late reinfarction, very late TV reinfarction, and very late ST/LT (all landmark analyses >1 year). Secondary outcomes were overall (non-landmark analyses) mortality, reinfarction, TV reinfarction, and ST/LT.
Statistical Analyses
Statistical comparisons of categorical variables were performed using the chi-squared or Fisher’s exact test, as appropriate, and comparisons of continuous variables were made with the Mann–Whitney U-test. Late clinical outcomes were assessed by Kaplan–Meier estimates, and comparisons were made using log-rank statistics. Landmark Kaplan–Meier estimates of outcomes were performed at 0–1 year and at >1 year in patients who were event-free at 1 year. Cox proportional hazards regression models were used to adjust for differences in baseline variables when comparing outcomes with stenting versus BA. The following variables were entered into the Cox regression models: age, gender, diabetes, prior infarction, cardiogenic shock, infarct-related artery, three-vessel coronary disease, TIMI flow prior to PCI, vessel size, GPI use, reperfusion time, and treatment with stent versus BA. Backward elimination at alpha = 0.05 was used, and stent versus BA was retained in all models. All analyses were performed with SPSS 19.0 (IBM Incorporated, Armonk, NY, USA) and SAS 9.2 (SAS Institute, Cary, NC, USA) software.
Results
From 1994 through 2010, 2,195 consecutive patients undergoing successful BA (n = 601) or stenting (n = 1,594) for STEMI who did not have STEMI due to ST were enrolled in our database and followed prospectively for 1–16 years. Clinical follow-up was complete or out to at least 2 years in 86.2% of patients with a median follow-up time of 4.7 years. The number of patients treated with BA, BMS, and DES by year is shown in Table 1. Thienopyridines were not indicated in STEMI patients treated with BA early in our study, and consequently thienopyridine use at hospital discharge was much more frequent in stented versus BA patients (92.5% vs. 23.0%, P < 0.001). The use of thienopyridines at hospital discharge remained relatively constant in stented patients throughout the study period.
Table 1.
Stent and Balloon Angioplasty Use by Year
| Year | Balloon Angioplasty |
Stent | BMS | DES | Total Patients |
|---|---|---|---|---|---|
| 1994 | 131 | 1 | 1 | 0 | 132 |
| 1995 | 106 | 27 | 27 | 0 | 133 |
| 1996 | 81 | 72 | 72 | 0 | 153 |
| 1997 | 59 | 77 | 77 | 0 | 136 |
| 1998 | 52 | 64 | 64 | 0 | 116 |
| 1999 | 36 | 66 | 66 | 0 | 102 |
| 2000 | 26 | 95 | 95 | 0 | 121 |
| 2001 | 26 | 98 | 98 | 0 | 124 |
| 2002 | 9 | 114 | 114 | 0 | 123 |
| 2003 | 10 | 103 | 94 | 9 | 113 |
| 2004 | 12 | 128 | 49 | 79 | 140 |
| 2005 | 12 | 154 | 49 | 105 | 166 |
| 2006 | 12 | 124 | 50 | 74 | 136 |
| 2007 | 5 | 111 | 84 | 27 | 116 |
| 2008 | 7 | 132 | 88 | 44 | 139 |
| 2009 | 5 | 105 | 70 | 35 | 110 |
| 2010 | 12 | 123 | 67 | 56 | 135 |
| Total | 601 | 1,594 | 1,165 | 429 | 2,195 |
BMS, bare metal stent; DES, drug-eluting stent.
Baseline Clinical and Angiographic Variables
Patients treated with BA versus stenting were older, were more often female, had more hypertension, had more hyperlipidemia, and were less often smokers (Table 2). BA patients had more three-vessel coronary disease, higher ejection fractions, a higher frequency of infarction in the distribution of the left anterior descending and circumflex arteries and less infarction in the distribution of the right coronary artery, more frequent total occlusion of the infarct artery (TIMI 0–1 flow) on initial angiography, smaller vessel size (<3.0 mm), less glycoprotein IIb/IIIa platelet inhibitor use, less bivalirudin use, and longer door-to-balloon times and reperfusion times (Table 2).
Table 2.
Baseline Variables: Stent vs. Balloon Angioplasty
| Balloon Angioplasty (n = 601) |
Stent (n = 1,594) |
||||
|---|---|---|---|---|---|
| Median or No. | (IQR) or % | Median or No. | (IQR) or % | P-Value | |
| Clinical variables | |||||
| Age, years (median [IQR]) | 61 | (50, 70) | 58 | (50, 69) | 0.012 |
| Age ≥70 years | 160 | 26.6% | 364 | 22.8% | 0.064 |
| Women | 212 | 35.3% | 490 | 30.7% | 0.042 |
| Diabetes | 95 | 15.8% | 253 | 15.9% | 0.97 |
| Current smoker | 275 | 45.8% | 826 | 51.8% | <0.001 |
| Prior infarction | 85 | 14.1% | 183 | 11.5% | 0.089 |
| Hypertension | 313 | 52.1% | 748 | 46.9% | 0.019 |
| Hyperlipidemia (on medication) | 265 | 44.1% | 536 | 33.6% | <0.001 |
| Prior bypass surgery | 26 | 4.3% | 76 | 4.8% | 0.66 |
| Anterior infarction | 231 | 38.4% | 570 | 35.8% | 0.25 |
| Cardiogenic shock | 36 | 6.0% | 103 | 6.5% | 0.69 |
| Creatinine (mg/dl) | 1.0 | (0.8, 1.2) | 1.0 | (0.8, 1.2) | 0.76 |
| Creatinine clearance <60 cc/min/1.73 m2* | 104 | 23.6% | 316 | 24.2% | 0.81 |
| Angiographic and procedural | |||||
| Variables | |||||
| 3 Vessel coronary disease | 190 | 31.6% | 364 | 22.8% | <0.001 |
| Ejection fraction % (median [IQR]) | 52 | (43, 60) | 50 | (40, 58) | 0.005 |
| Left ventricular ejection fraction <40% | 98 | 16.3% | 294 | 18.4% | 0.35 |
| Infarct vessel | 0.004 | ||||
| Left main/left anterior descending | 229 | 38.1% | 564 | 35.4% | |
| Circumflex | 107 | 17.8% | 206 | 12.9% | |
| Right coronary artery | 251 | 41.8% | 786 | 49.3% | |
| Vein graft | 14 | 2.3% | 38 | 2.4% | |
| TIMI 0–1 flow pre-PCI | 483 | 80.4% | 1,187 | 74.5% | 0.004 |
| Infarct vessel diameter (<3.0 mm) | 211 | 35.1% | 347 | 21.8% | <0.001 |
| Glycoprotein IIb/IIIa inhibitor used | 190 | 31.6% | 1,050 | 65.9% | <0.001 |
| Bivalirudin used | 29 | 4.8% | 344 | 21.6% | <0.001 |
| Reperfusion time ≤2 hours | 34 | 5.7% | 142 | 8.9% | 0.012 |
| Reperfusion time, hours (median [IQR]) | 4.2 | (3.1, 6.3) | 3.9 | (2.8, 6.0) | 0.002 |
| Door-to-balloon time, hours (median [IQR]) | 2.3 | (1.6, 3.3) | 2.0 | (1.3, 2.9) | <0.001 |
Cockroft-Gault formula. IQR, interquartile range; PCI, percutaneous coronary intervention.
Clinical Outcomes
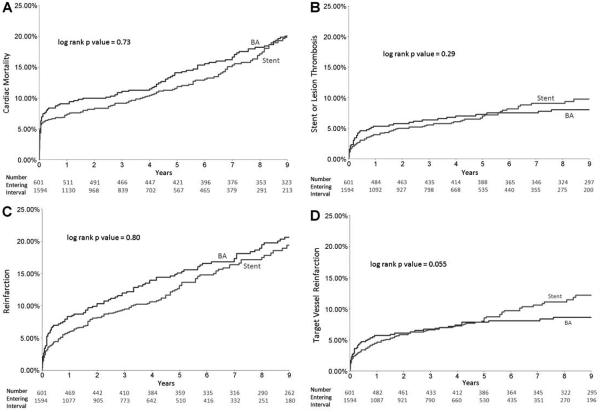
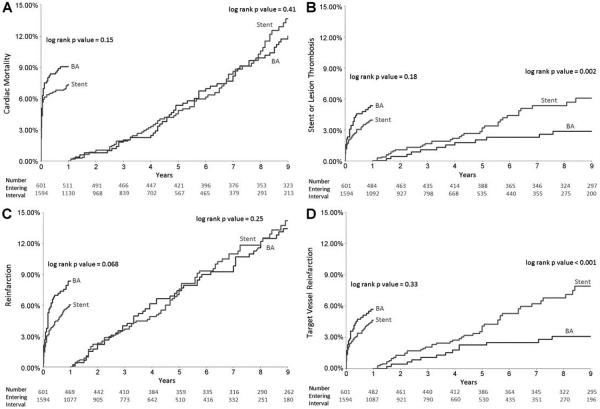
There were no significant differences in overall Kaplan–Meier cumulative event rates comparing stenting with BA for cardiac mortality, ST/LT, reinfarction, or TV reinfarction (Fig. 1A–D). Landmark analyses from 0 to 1 year showed trends for lower reinfarction rates with stenting versus BA (6.0% vs. 8.3%, P = 0.068) but no significant differences in cardiac mortality, ST/LT, or TV reinfarction (Fig. 2A–D). Landmark analyses at greater than 1 year showed that patients treated with stenting compared with BA had significantly higher frequencies of ST/LT (6.1% vs. 2.9% from 1 to 9 years, P = 0.002) and TV reinfarction (7.9% vs. 3.1% from 1 to 9 years, P < 0.001), but there were no significant differences in cardiac mortality or total reinfarction (Fig. 2A–D).
Figure 1.
Kaplan–Meier estimates of event rates in patients treated with stenting versus balloon angioplasty (BA) for STEMI: (A) cardiac mortality, (B) stent or lesion thrombosis, (C) reinfarction, and (D) target vessel reinfarction. There were no significant differences in any of the outcomes comparing stenting versus balloon angioplasty.
Figure 2.
Landmark analyses showing Kaplan–Meier estimates of event rates at 0–1 year and >1 year in patients treated with stenting versus balloon angioplasty (BA) for STEMI: (A) cardiac mortality, (B) stent or lesion thrombosis (ST/LT), (C) reinfarction, and (D) target vessel reinfarction. Patients treated with stents had greater frequency of ST/LT and target vessel reinfarction compared with BA after 1 year.
There were no significant differences in the frequency of ST/LT or TV reinfarction from 0 to 1 year between DES and BA or between BMS and BA. Landmark analyses at >1 year comparing DES with BA showed that DES had significantly higher rates of ST/LT (7.0% vs. 1.8% from 1 to 5 years, P = 0.001) and TV reinfarction (8.1% vs. 2.3% from 1 to 5 years, P < 0.001) (Fig. 3A and B). Similar analyses comparing BMS with BA showed BMS had significantly higher rates of ST/LT (5.0% vs. 2.9% from 1 to 9 years, P = 0.016) and TV reinfarction (6.7% vs. 3.1% from 1 to 9 years, P = 0.001) (Fig. 3A and B).
Figure 3.
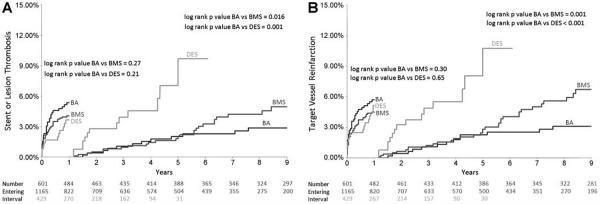
Landmark analyses showing Kaplan–Meier estimates of event rates at 0–1 year and >1 year in patients treated with DES, BMS, and balloon angioplasty (BA) for STEMI: (A) stent or lesion thrombosis (ST/LT), (B) target vessel reinfarction. Patients treated with both DES and BMS had greater frequency of ST/LT and target vessel reinfarction after 1 year compared with BA.
Multivariable Analyses
After adjusting for differences in baseline variables using Cox proportional hazard regression models, there were no significant differences in overall cardiac mortality, reinfarction, TV reinfarction, and ST/LT between stents and BA (Table 3). There also were no significant differences between stenting and BA in landmark analyses from 0 to 1 year for cardiac mortality, reinfarction, TV reinfarction, and ST/LT. In landmark analyses after 1 year, there were significantly higher adjusted frequencies of ST/LT and TV reinfarction with stenting compared with BA, but there were no significant differences in cardiac mortality or total reinfarction (Table 3). In subgroup analyses, there were higher adjusted frequencies of ST/LT and TV reinfarction after 1 year (landmark analyses) comparing DES with BA and BMS with BA (Table 3).
Table 3.
Outcomes Following Primary PCI with Stent vs. Balloon Angioplasty by Cox Regression
| Overall Outcomes |
Landmark Outcomes (0–1 Year) |
Landmark Outcomes (>1 year) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-Value | HR | 95% CI | P-Value | HR | 95% CI | P-Value | |
| Stent vs. balloon angioplasty | |||||||||
| Unadjusted outcomes | |||||||||
| Cardiac mortality | 0.96 | 0.76–1.21 | 0.73 | 0.79 | 0.57–1.09 | 0.15 | 1.14 | 0.84–1.55 | 0.41 |
| Reinfarction | 0.97 | 0.76–1.24 | 0.80 | 0.71 | 0.50–1.03 | 0.069 | 1.20 | 0.88–1.65 | 0.25 |
| Target vessel reinfarction | 1.41 | 0.99–2.00 | 0.056 | 0.81 | 0.53–1.24 | 0.33 | 3.00 | 1.68–5.34 | <0.001 |
| Stent/lesion thrombosis | 1.22 | 0.84–1.77 | 0.30 | 0.74 | 0.47–1.16 | 0.19 | 2.63 | 1.40–4.95 | 0.003 |
| Adjusted outcomes | |||||||||
| Cardiac mortality | 1.11 | 0.87–1.42 | 0.39 | 0.85 | 0.61–1.21 | 0.37 | 1.16 | 0.85–1.58 | 0.36 |
| Reinfarction | 0.99 | 0.77–1.27 | 0.93 | 0.76 | 0.52–1.10 | 0.15 | 1.07 | 0.76–1.49 | 0.71 |
| Target vessel reinfarction | 1.41 | 0.99–2.01 | 0.061 | 0.85 | 0.53–1.32 | 0.46 | 2.85 | 1.60–5.07 | <0.001 |
| Stent/lesion thrombosis | 1.12 | 0.75–1.67 | 0.59 | 0.79 | 0.50–1.25 | 0.31 | 2.42 | 1.28–4.56 | 0.006 |
| BMS vs. balloon angioplasty | |||||||||
| Unadjusted outcomes | |||||||||
| Cardiac mortality | 1.02 | 0.81–1.29 | 0.85 | 0.90 | 0.64–1.26 | 0.54 | 1.14 | 0.83–1.56 | 0.41 |
| Reinfarction | 0.90 | 0.69–1.16 | 0.40 | 0.67 | 0.45–0.99 | 0.045 | 1.09 | 0.79–1.52 | 0.60 |
| Target vessel reinfarction | 1.29 | 0.90–1.86 | 0.17 | 0.79 | 0.50–1.24 | 0.30 | 2.62 | 1.44–4.76 | 0.002 |
| Stent/lesion thrombosis | 1.14 | 0.78–1.68 | 0.50 | 0.77 | 0.48–1.23 | 0.27 | 2.22 | 1.15–4.30 | 0.018 |
| Adjusted outcomes | |||||||||
| Cardiac mortality | 1.18 | 0.92–1.50 | 0.20 | 1.09 | 0.74–1.59 | 0.67 | 1.19 | 0.87–1.63 | 0.28 |
| Reinfarction | 0.91 | 0.70–1.18 | 0.48 | 0.75 | 0.50–1.12 | 0.16 | 0.98 | 0.69–1.39 | 0.93 |
| Target vessel reinfarction | 1.22 | 0.85–1.76 | 0.29 | 0.86 | 0.53–1.37 | 0.52 | 2.46 | 1.35–4.47 | 0.003 |
| Stent/lesion thrombosis | 1.06 | 0.72–1.56 | 0.79 | 0.80 | 0.49–1.30 | 0.37 | 1.99 | 1.03–3.86 | 0.042 |
| DES vs. balloon angioplasty | |||||||||
| Unadjusted outcomes | |||||||||
| Cardiac mortality | 0.57 | 0.36–0.91 | 0.018 | 0.46 | 0.26–0.81 | 0.007 | 1.01 | 0.45–2.26 | 0.99 |
| Reinfarction | 1.18 | 0.81–1.70 | 0.39 | 0.84 | 0.51–1.38 | 0.50 | 1.85 | 1.07–3.21 | 0.028 |
| Target vessel reinfarction | 1.48 | 0.93–2.36 | 0.10 | 0.87 | 0.48–1.57 | 0.65 | 4.14 | 1.85–9.27 | 0.001 |
| Stent/lesion thrombosis | 1.24 | 0.73–2.06 | 0.41 | 0.66 | 0.33–1.28 | 0.22 | 3.96 | 1.68–9.35 | 0.002 |
| Adjusted outcomes | |||||||||
| Cardiac mortality | 0.73 | 0.46–1.17 | 0.19 | 0.64 | 0.36–1.14 | 0.13 | 1.17 | 0.52–2.63 | 0.71 |
| Reinfarction | 0.98 | 0.66–1.45 | 0.92 | 0.78 | 0.48–1.29 | 0.34 | 1.40 | 0.78–2.52 | 0.26 |
| Target vessel reinfarction | 1.25 | 0.75–2.06 | 0.39 | 0.86 | 0.48–1.56 | 0.63 | 4.27 | 1.91–9.55 | <0.001 |
| Stent/lesion thrombosis | 1.17 | 0.70–1.96 | 0.55 | 0.64 | 0.32–1.25 | 0.64 | 3.48 | 1.47–8.21 | 0.004 |
HR, hazard ratio; CI, confidence interval; BMS, bare metal stent.
Discussion
The major findings of our study are (1) that stenting compared with BA alone for STEMI is associated with a higher incidence of very late ST/LT and TV reinfarction after the first year, and (2) that the differences in very late ST/LT and TV reinfarction are greatest when comparing DES with BA, but there are also significant differences when comparing BMS with BA.
As far as we know, our data are the only data available comparing late clinical outcomes in STEMI patients treated with stenting versus BA alone. Although our results are subject to many potential biases and are not conclusive, they are very provocative and could have important implications. If our results are valid, this would imply that there is a late hazard with the use of both DES and BMS for STEMI compared with BA alone, and would support work already underway for the prevention of very late ST, including the development of new-generation DES with more biocompatible polymers, the development of bioabsorbable polymers, and the development of bioabsorbable stent platforms. New-generation DES (EES) have already shown a remarkable improvement in clinical outcomes compared with first-generation DES and BMS.13,14 Palmerini et al.13 reported a large meta-analysis of 49 randomized trials including over 50,000 patients undergoing elective PCI and PCI for acute coronary syndromes including STEMI and found that new-generation EES had substantially lower ST rates at 2 years compared with PES or BMS. This study suggests a paradigm shift that new-generation DES may have a safety advantage rather than a hazard compared with BMS. Räber et al.14 published data from a multicenter registry describing a cohort of over 12,000 patients treated with new-generation EES or early-generation DES and found significantly less ST at 4-year follow-up with EES. In the subgroup of patients with STEMI, the frequency of ST at 4 years with EES was significantly less than that with SES or PES.14
The development of stents with bioabsorbable polymers and bioabsorbable platforms holds promise for further reduction of very late ST. The major advantages of stents are prevention of acute occlusion by scaffolding intimal tissue flaps, prevention of recoil and early negative remodeling (which contribute to restenosis), and prevention of intimal hyperplasia with the use of antiproliferative drugs to prevent restenosis. Since scaffolding to prevent recoil and negative remodeling is only needed for several months after implant, absorption of the stent platform after several months should not cause problems with recoil and may eliminate the disadvantages of the long-term presence of the metal stent.15 It is hoped that absorption of the stent platform will prevent chronic inflammation in the vessel wall, allow for late positive remodeling, restore more normal endothelial function, and result in less late restenosis, less ST, and less neo-atherosclerosis.15 Studies with the bioabsorbable EES have documented full resorption of the polymeric scaffold struts and return of normal endothelial function at 2 years.16 Other potential benefits remain to be proven.
Limitations
Our study has a number of important limitations. This is an observational study spanning 16 years during which time treatment strategies with primary PCI for STEMI have shown considerable evolution, and this has created biases that can affect our outcomes. Adjunctive treatments, including the development of new anticoagulant and new antiplatelet therapies, have changed over the study period. The use of glycoprotein IIb/IIIa platelet inhibitors and bivalirudin during PCI and thienopyridines at hospital discharge was more frequent in stented patients and could affect outcomes, although these therapies are less likely to affect outcomes beyond the first year. BA was selected for patients with smaller vessels and lesions not suitable for stenting, for patients not compliant with dual antiplatelet therapy, and for patients thought to require bypass surgery. Both stents and balloons have evolved and changed over the study period, and this could bias comparisons between the two groups. Long-term follow-up was not available in 14% of our patients and this could have created bias in comparison of outcomes between patients treated with stent versus BA. Most DES used in this study were early-generation DES and our results may not be applicable to new-generation DES, which have shown substantial reductions in the frequency of ST. Finally, we do not have complete data on compliance with dual antiplatelet therapy which is an important determinant of ST and possibly a determinant of LT.
Conclusions
Although stents have clear short-term advantages over BA in patients with STEMI by preventing abrupt occlusion, reducing angiographic restenosis and reocclusion of the infarct artery, and reducing the need for TV revascularization, our data suggest there may be a very late hazard with stenting. In this observational study, stenting with both BMS and DES compared with BA was associated with an increased incidence of very late ST/LT and TV reinfarction. Our study has many potential biases, but our data suggest that the continued presence of a metal stent in the infarct artery, with or without an associated polymer coating, may predispose to very late adverse events. Our data should support new strategies that are currently being evaluated for the prevention of very late ST with both BMS and DES, including the development of new-generation DES with more bio-compatible polymers and the development of bio-absorbable polymers and stent platforms.
Acknowledgments
LeBauer Charitable Research Foundation
NIEHS
This study was supported by an unrestricted grant from the LeBauer Charitable Research Foundation and by the intramural program of the National Institute of Environmental Health Sciences (NIEHS), Research Triangle Park, NC.
Footnotes
Thomas Stuckey is on an advisory board and has received speaker honoraria from Boston Scientific Medical.
References
- 1.Grines CL, Cox DA, Stone GW, et al. Coronary angioplasty with or without stent implantation for acute myocardial infarction. N Engl J Med. 1999;341:1949–1956. doi: 10.1056/NEJM199912233412601. [DOI] [PubMed] [Google Scholar]
- 2.Stone GW, Grines CL, Cox DA, et al. Comparison of angioplasty with stenting, with or without abciximab, in acute myocardial infarction. N Engl J Med. 2002;346:957–966. doi: 10.1056/NEJMoa013404. [DOI] [PubMed] [Google Scholar]
- 3.Suryapranata H, van’t Hof AWJ, Hoorntje JCA, et al. Randomized comparison of coronary stenting with balloon angioplasty in selected patients with acute myocardial infarction. Circulation. 1998;97:2502–2505. doi: 10.1161/01.cir.97.25.2502. [DOI] [PubMed] [Google Scholar]
- 4.Rogriquez A, Bernardi V, Fernandez M, et al. In-hospital and late results of coronary stents versus conventional balloon angioplasty in acute myocardial infarction (GRAMI trial) Am J Cardiol. 1998;81:1286–1291. doi: 10.1016/s0002-9149(98)00154-4. [DOI] [PubMed] [Google Scholar]
- 5.Antoniucci D, Santoro GM, Bolognese L, et al. A clinical trial comparing primary stenting of the infarct-related artery with optimal primary angioplasty for acute myocardial infarction: Results from the Florence Randomized Elective Stenting in Acute Coronary Occlusions (FRESCO) trial. J Am Coll Cardiol. 1998;31:1234–1239. doi: 10.1016/s0735-1097(98)00097-7. [DOI] [PubMed] [Google Scholar]
- 6.Maillard L, Hamon M, Khalife K, et al. A comparison of systematic stenting and conventional balloon angioplasty during primary percutaneous transluminal coronary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2000;35:1729–1736. doi: 10.1016/s0735-1097(00)00612-4. [DOI] [PubMed] [Google Scholar]
- 7.Kukreja N, Onuma Y, Garcia-Garcia HM, et al. The risk of stent thrombosis in patients with acute coronary syndromes treated with bare-metal and drug-eluting stents. J Am Coll Cardiol Intv. 2009;2:534–541. doi: 10.1016/j.jcin.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Stone GW, Witzenbichler B, Guagliumi G, et al. Heparin plus a glycoprotein IIb/IIIa inhibitor versus bivalirudin monotherapy and paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction (HORIZONS-AMI): Final 3-year results from a multicenter randomized controlled trial. Lancet. 2011;377:2193–2204. doi: 10.1016/S0140-6736(11)60764-2. [DOI] [PubMed] [Google Scholar]
- 9.Vink MA, Dirksen MT, Suttorp MJ, et al. 5-Year follow-up after primary percutaneous coronary intervention with a paclitaxel-eluting stent versus a bare-metal stent in acute ST-segment elevation myocardial infarction. J Am Coll Cardiol Interv. 2011;4:24–29. doi: 10.1016/j.jcin.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Brodie B, Pokharel Y, Fleishman N, et al. Very late stent thrombosis after primary percutaneous coronary intervention with bare-metal and drug-eluting stents for ST-segment elevation myocardial infarction. J Am Coll Cardiol Interv. 2011;4:30–38. doi: 10.1016/j.jcin.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Luca G, Dirksen MT, Spaulding C, et al. Drug-Eluting Stent in Primary Angioplasty (DESERT) Cooperation. Drug-eluting vs bare-metal stents in primary angioplasty: A pooled patient-level meta-analysis of randomized trials. Arch Intern Med. 2012;172(8):611–621. doi: 10.1001/archinternmed.2012.758. [DOI] [PubMed] [Google Scholar]
- 12.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials. A case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 13.Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Stent thrombosis with drug-eluting and bare-metal stents: Evidence from a comprehensive network meta-analysis. Lancet. 2012;379(9824):1393–1402. doi: 10.1016/S0140-6736(12)60324-9. [DOI] [PubMed] [Google Scholar]
- 14.Räber L, Magro M, Stefanini GG, et al. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: A prospective cohort study. Circulation. 2012;125:1110–1121. doi: 10.1161/CIRCULATIONAHA.111.058560. [DOI] [PubMed] [Google Scholar]
- 15.Ormiston JA, Serruys PWS. Bioabsorbable coronary stents. Circ Cardiovasc Intervent. 2009;2:255–260. doi: 10.1161/CIRCINTERVENTIONS.109.859173. [DOI] [PubMed] [Google Scholar]
- 16.Serruys PW, Ormiston JA, Onuma Y, et al. A bio-absorbable everolimus-eluting coronary stent system (ABSORB): 2-Year outcomes and results from multiple imaging methods. Lancet. 2009;373:897–910. doi: 10.1016/S0140-6736(09)60325-1. [DOI] [PubMed] [Google Scholar]