Abstract
The intestinal tract is home to nematodes as well as commensal bacteria (microbiota), which have coevolved with the mammalian host. The mucosal immune system must balance between an appropriate response to dangerous pathogens and an inappropriate response to commensal microbiota that may breach the epithelial barrier, in order to maintain intestinal homeostasis. IL-22 has been shown to play a critical role in maintaining barrier homeostasis against intestinal pathogens and commensal bacteria. Here we review the advances in our understanding of the role of IL-22 in helminth infections, as well as in response to commensal and pathogenic bacteria of the intestinal tract. We then consider the relationship between intestinal helminths and gut microbiota and hypothesize that this relationship may explain how helminths may improve symptoms of inflammatory bowel diseases. We propose that by inducing an immune response that includes IL-22, intestinal helminths may enhance the mucosal barrier function of the intestinal epithelium. This may restore the mucosal microbiota populations from dysbiosis associated with colitis and improve intestinal homeostasis.
Keywords: Helminth therapy, Microbiota, IL-22, Inflammatory bowel diseases, Mucosal Immunity
1. Introduction
Our mucosal immune system plays an essential role in maintaining intestinal homeostasis with commensal bacteria and other organisms. Gastrointestinal helminths have coevolved with the mammalian immune system similarly to the gut microbiota. Just as commensal bacteria can shape mammalian immunity, helminths exert immune regulatory effects on their mammalian hosts. However, the relationship between helminths and gut microbiota is still unclear. This relationship becomes particularly important in the context of inflammatory bowel disease (IBD), which is generally considered to result from an aberrant response of the mucosal immune response against gut microbiota and is associated with microbial dysbiosis. Treatment with helminths is now in clinical trials for IBD but the mechanism by which they may improve the symptoms of IBD is not well understood. Recent evidence has suggested a role for the cytokine, IL-22, during helminth infection and in maintaining mucosal barrier function. IL-22 may therefore play an important role in the relationship between the mammalian immune response, gut microbiota and helminth infections.
2. IL-22 regulates barrier immunity and intestinal injury
IL-22 is a member of the IL-10 cytokine family which also includes IL-19, IL-20, IL-24 and IL-26 (Pestka et al., 2004). IL-22 signals through a heterodimeric receptor complex consisting of two subunits, IL-22R1 and IL-10R2 (Witte et al., 2010; Sonnenberg et al., 2011; Zenewicz and Flavell, 2011; Mizoguchi, 2012). While IL-10R2 is ubiquitously expressed on virtually all cell types, IL-22R1 expression is limited to the surfaces of non-hematopoietic cells such as epithelial cells, hepatocytes and keratinocytes (Witte et al., 2010; Sonnenberg et al., 2011; Zenewicz and Flavell, 2011; Mizoguchi, 2012). This restricted expression of IL-22R1 on non-hematopoietic cells thus allows IL-22 to specifically target innate cell populations within such tissues as the gastrointestinal tract, liver, skin, kidneys and lungs (Tachiiri et al., 2003; Wolk et al., 2004).
IL-22 can be produced by a wide variety of innate and adaptive immune cells including CD4+ T cells, most notably TH17 and TH22 cells, CD8+ T cells, natural killer (NK) cells, γδ T cells, lymphoid tissue inducer (LTi) cells and innate lymphoid cells (ILCs) (Wolk et al., 2010; Zenewicz and Flavell, 2011). Upon binding to the IL-22R1 and IL-10R2 receptor complex, IL-22 produced from these cells activates the receptor-associated Janus kinases, Jak1 and Tyk2, resulting in tyrosine phosphorylation of STAT3 and to a lesser extent STAT1 and STAT5 (Nagalakshmi et al., 2004; Pestka et al., 2004). This in turn allows IL-22 to induce various tissue-specific genes including those encoding proteins involved in antimicrobial defense, cellular differentiation and mucin production (Wolk et al., 2006; Zenewicz and Flavell, 2011).
These effects of IL-22 are particularly important in regulating inflammatory responses within the intestine through the production of antimicrobial peptides, the enhancement of epithelial regeneration and the regulation of wound repair. Recent studies have thus looked at possible protective roles for IL-22 in IBD using several mouse models of colitis induced by dextran sulfate sodium (DSS) as well as TH1- and TH2-mediated colitis. In DSS-induced colitis, DSS given in the drinking water of mice causes disruption of the intestinal epithelial layer, leading to inflammation and colitis within 1 week (Strober et al., 2002). IL-22 knockout mice or wild-type (WT) mice treated with neutralizing anti-IL-22 antibodies exhibit increased epithelial damage and inflammation in the colon, more severe weight loss and impaired recovery from DSS-induced acute colonic injury (Sugimoto et al., 2008; Zenewicz et al., 2008; Pickert et al., 2009; Neufert et al., 2010).
In a TH1 cytokine-mediated model of colitis, IL-22 expression by CD4+ T cells was shown to be critical for limiting disease severity (Zenewicz et al., 2008). Sugimoto et al. (2008) used a model of TH2-mediated chronic colitis and found that the severity of colitis was attenuated in the colons of mice receiving supplemental IL-22. IL-22 gene delivery mediated STAT3 activation specifically within colonic epithelial cells and enhanced mucus production and goblet cell restitution, thus reinforcing the mucus barrier function within the intestine. These mouse models of IBD indicate that IL-22 plays a protective role in IBD through its ability to enhance innate epithelial defenses and mucosal barrier integrity.
3. IL-22 responses to bacterial pathogens and the gut microbiota
In addition to maintaining the mucosal barrier function in the gastrointestinal tract, IL-22 induces genes that encode proteins involved in antimicrobial defense, thus suggesting a role for IL-22 in the innate immunity against extracellular bacteria. IL-22 has been shown to regulate the expression of antimicrobial peptides such as the β -defensin family proteins (β -defensins BD2 and BD3), S100 family proteins (S100A7, S100A8 and S100A9), the Reg family proteins (RegIII α, RegIII β and RegIIIγ) and lipocalin-2 (Aggarwal et al., 2001; Wolk et al., 2004, 2006; Zheng et al., 2008). IL-22 serves as a protective and proinflammatory mediator in the host’s inflammatory response against intestinal bacterial infections; however, this seems to be dependent upon the specific type of pathogen.
Zheng et al. (2008) showed that IL-22 was essential for host protective immunity against infection with the Gram-negative enteric bacteria, Citrobacter rodentium, as infected IL-22 knockout mice had increased bacterial burden, intestinal epithelial damage and mortality due to a defective induction of the Reg family proteins. IL-22 also plays a protective role in systemic infection with Salmonella enterica. IL-23-dependent IL-22 was required for both liver cell survival and pathogen defense against systemic Salmonella infection in mice, especially when coupled with a diminished production of IL-12 (Schulz et al., 2008). In addition to protecting against intestinal bacterial pathogens, IL-22 serves a protective role in intestinal fungal infections with Candida albicans. IL-22 knockout mice infected with C. albicans hyphae intragastrically had an increased fungal burden and showed signs of mucosal hyperplasia in the stomach and colon compared with infected WT mice (De Luca et al., 2010). Hence IL-22 serves as a protective mediator in regulating inflammatory responses and mucosal barrier integrity in a variety of intestinal infections.
However, IL-22 seems to promote intestinal inflammation after oral infection with Toxoplasma gondii (Munoz et al., 2009; Wilson et al., 2010). Infected IL-22 knockout mice and mice whose IL-22 was neutralized with an anti-IL-22 monoclonal antibody developed significantly less intestinal pathology and had reduced weight loss and mortality, despite having similar parasite burdens to those of infected WT mice. Perhaps the strongly biased TH1 cytokine environment of T. gondii infection may explain this difference.
As noted above, IL-22 is produced by TH17 cells which are regulated by the intestinal microbiota. In contrast to IL-17, which induces neutrophil responses at inflammatory sites (Korn et al., 2009), IL-22 may be more important in tissue repair during mucosal immunity. Regardless, there is a close relationship between the gut microbiota and IL-22 producing cells. Most notably, it was recently shown that IL-22 producing innate lymphocytes were important in preventing the systemic dissemination of a commensal bacteria that could cause systemic inflammation (Sonnenberg et al., 2012). When Sonnenberg et al. (2012) treated Rag1−/ − mice with a neutralizing antibody to IL-22, bacteria could be cultured from the spleen and the liver of these mice, together with signs of systemic inflammation and increased levels of lipopolysaccharide (LPS). The disseminating commensal bacteria was then identified to be Alcaligenes sp. Hence, IL-22 plays an important role in the mucosal firewall separating our intestinal tract from our gut microbiota, which would be consistent with a protective role against IBD. Under homeostatic conditions, live bacteria are sampled by dendritic cells that carry them to the mesenteric lymph nodes, but do not disseminate systemically to secondary lymphoid tissues (Hooper, 2012) indicating that a mesenteric firewall may act in concert with a mucosal firewall to compartmentalize gut bacteria.
4. IL-22 during helminth infection and treatment of intestinal diseases
IL-22 has been shown to be upregulated within the gastrointestinal tract following infection with certain intestinal nematodes. Broadhurst et al. (2010) described a case study of an individual who was self-infected with the whipworm, Trichuris trichuria, in order to treat his symptoms of ulcerative colitis. Infection with T. trichuria ameliorated the patient’s disease activity and this effect was associated with an increased expression of IL-22 and TH2 cytokines within the gastrointestinal tract. It was hypothesized that the activation of a TH2 and IL-22 response by T. trichuria allowed for the expulsion of this parasite through the increased turnover of intestinal epithelial cells, mucus hypersecretion and goblet cell hyperplasia (Finkelman et al., 2004; Artis and Grencis, 2008; Sonnenberg et al., 2011).
Additional evidence that IL-22 is induced in the human intestinal mucosa by helminth infection comes from celiac disease trials with the hookworm, Necator americanus, in Queensland, Australia (Daveson, 2011; McSorley, 2011; Gaze, 2012). McSorley et al. (2011) showed that intestinal biopsies from patients infected with N. americanus larvae showed upregulation of IL-22 mRNA levels after restimulation with N. americanus excretory/secretory proteins (NaES) in vitro. Intestinal biopsies taken prior to hookworm infection did not show increased IL-22 expression upon NaES stimulation. However, the mechanisms underlying these IL-22 responses have not been further examined in clinical studies. Thus, even though helminth infections have the ability to induce IL-22 producing cells in humans, there is still no direct evidence that IL-22 is functionally important during helminth infection and a putative role for IL-22 in humans is based on correlative evidence only.
A more direct causal role for IL-22 in helminth induced immune regulation would have to be determined in mouse models. In mouse models however, whereby the activity of IL-22 can be eliminated through the use of IL-22 deficient animals and through neutralizing antibodies to IL-22, infection with the tissue dwelling helminth, Schistosoma mansoni, led to a similar phenotype as control animals (Wilson et al., 2010). There were similar worm and tissue egg burdens in IL-22−/− mice as that of infected WT mice. Both groups also developed nearly identical hepatic and intestinal granulomas and succumbed to similar weight loss and survival rates, suggesting that IL-22 played little to no role in the pathogenesis of schistosomiasis. While blocking IL-22 has no effect on schistosome pathogenesis, helminth infections that colonize the gastrointestinal tract have not been investigated using these mouse models.
5. Gut microbiota and inflammatory bowel diseases
The IBD, Crohn’s disease and ulcerative colitis, are thought to be driven by an abnormal inflammatory response to the intestinal microbiota (Kaser et al., 2010). However, since dysbiosis of the microbiota is also a feature of IBD, it is quite difficult to determine whether there is an inflammatory response to abnormal bacteria, or if an abnormal inflammatory response is altering the microbial communities. As an environmental factor, gut microbiota could be linked to genetic predispositions that alter the interactions between our microbial communities and ourselves. The first major susceptibility gene for Crohn’s disease is a receptor for bacterial peptidoglycan, known as NOD2 (or CARD15) (Hugot et al., 2001; Ogura et al., 2001) and another susceptibility gene, ATG16L1, is critical for autophagy (Hampe et al., 2007). The intestinal microbiota may also lead to a dysregulation of intestinal lymphoid cell subsets such as TH17 cells and innate lymphocytes which are important in regulating mucosal immunity.
While there have been numerous studies investigating stool samples and mucosa associated bacteria in IBD patients, there has been a lack of consensus and reproducibility between studies (Sun et al., 2011). While broad changes, such as the expansion of the Proteobacteria phylum in IBD patients, have been observed (Mukhopadhya et al., 2012), more specific associations that are reproducibly identified have been few. While the identification of causative microbiota species that can trigger IBD is underway, a generalizable theme that has emerged to date is that the diversity of microbial communities is significantly reduced in IBD (Cho and Blaser, 2012). There have also been repeated observations that specific bacterial taxa are depleted in IBD patients, most notably that Faecalibacterium prausnitzii, a member of the Lachnospiraceae family, is reduced in the intestinal mucosa (Sun et al., 2011).
6. Effects of helminth infections on the gut microbiota
While we now have an in-depth understanding of the healthy human microbiota from individuals of the developed world, the fecal microbial communities of residents of developing countries are extremely different to residents from the developed world (De Filippo et al., 2010; Yatsunenko et al., 2012). The genus Prevotella was found to be more common in the fecal microbiota of children in developing countries compared with Europe and the USA (De Filippo et al., 2010). Notably, the helminth infection status of the individuals sampled in the developing countries was not discussed in these studies. While the assumption is that diet contributes most significantly to differences between the developing and developed worlds, it is conceivable that helminth infections may also have a substantial impact on the human microbiome. There is certainly evidence from animal studies that helminths can alter the gut microbiota.
In mice the nematode parasite, Heligmosomoides polygyrus, was found to alter gut microbiota of healthy mice (Walk et al., 2010). Heligmosomoides polygyrus typically establishes a chronic infection in the intestinal lumen during primary infection (Anthony et al., 2007), which has been shown to inhibit colitis (Elliott et al., 2004; Hang et al., 2010). Notably, members of the Lactobacillaceae family were increased after infection in two independent experiments (Walk et al., 2010). Interestingly, early experiments with germ-free mice had found that fewer adult worms were recovered from these mice, which was associated with increased eosinophilia, granulomas and thickening of the small intestinal wall (Wescott, 1968). It is possible that commensal bacteria help to reduce the inflammatory response against the worms, or that H. polygyrus requires commensal bacteria to develop appropriately. Another example of such a synergistic relationship was shown recently for another nematode, Trichuris muris, which utilizes the cecal microbiota to provide the right environmental cues for it to remove the plugs from its ova and enable hatching and exit of the larvae (Hayes et al., 2010). Whether or not T. muris also alters the intestinal microbiota to promote expansion of bacterial taxa that can enable hatching and in order to promote mating opportunities will be interesting to determine.
Trichuris suis ova (TSO) have been used in clinical trials to treat IBD patients (Elliott and Weinstock, 2011; Wolff et al., 2012). While the effects of TSO on the human intestinal microbiota has yet to be characterized, a recent study investigated the effects of T. suis infection on the intestinal microbiota of pigs (Li et al., 2012) and another study investigated the relationship between the mucosal immune response, worm burden and alterations in microbial compositions (Wu et al., 2012). Mucispirillum bacteria, which colonizes mucus, were substantially increased in infected animals (Li et al., 2012). Interestingly, shotgun sequencing indicated that T. suis infection reduces carbohydrate metabolism, coinciding with reductions in Ruminococcus bacterium that are cellulolytic (Li et al., 2012). Pigs analyzed at a later time point (53 days) also had reduced Ruminococcus (and Fibrobacter) (Wu et al., 2012). At this time point, some pigs were cleared of adult worms, whereas others were still colonized, enabling the comparison of infected pigs that retained worms with pigs that had cleared worms (Wu et al., 2012). The presence of a heavy parasite burden was associated with increased expression of inflammatory genes, arg1, cxcr2, c3ar1, il6, muc5ac and ptgs2, although significant changes in the microbiota occurred regardless of worm status (Wu et al., 2012). It was suggested that persistent changes of the microbiota occurred from the initial infection (Wu et al., 2012). Of note, Campylobacter was much more common in worm bearing pigs, consistent with previous studies showing that T. suis is associated with exacerbation of campylobacteriosis (Mansfield et al., 2003). The effects of a nematode parasite, Ostertagia ostertagi, on cattle has also been investigated but in that case infection led to minimal changes to the microbiota (Li et al., 2011). However, this parasite infects the abomasum, which has a very low pH environment compared with the colon and it is possible that the abomasal microbiota are less sensitive to the mucosal immune responses elicited by these helminths. The assumption here would be that the mucosal immune responses to helminth infections are responsible for these alterations of the microbiota, which has still not been clearly demonstrated.
7. Treatment of autoimmune diseases through acute versus chronic helminth infections
Helminth infections generally trigger a TH2 type immune response, which can be protective in terms of parasite expulsion (Anthony et al., 2007; Patel et al., 2009) as well as protective against tissue damage to the host (Allen and Wynn, 2011). However, many helminths are able to establish persistent and chronic infections in their hosts and evade expulsion, in which case they establish an immune regulatory network that reduces inflammatory damage to both the host and parasite (van Riet et al., 2007; Taylor et al., 2012). This immune regulatory network has been suggested to be a major mechanism acting in the context of a therapeutic helminth infection; however it should only be established during chronic infection and not acute infection. Nonetheless, acute infections could also lead to long lasting effects such as persistent alterations in the microbiota (Wu et al., 2012). A detailed characterization of the glycoconjugate components during acute and chronic T. muris infection revealed a striking increase in thickness of the glycocalyx, especially during acute infection (Hasnain et al., 2011). This likely has profound effects on the mucosa-associated microbiota, which has yet to be investigated. It is therefore conceivable that repeated acute infections might also lead to the establishment of a microbiota associated immune regulatory network similar to a chronic infection.
This distinction between the effects of acute versus chronic infection has implications for the utilization of TSO as a therapeutic agent. The advantage of not infecting human subjects with T. suis and establishing patency also means that chronic infection is never established. While TSO has been shown to be efficacious in the treatment of patients with IBD, the mechanism of action is still unclear. The large doses (2,500 eggs) typically given to patients in clinical trials should elicit an acute response that is generally repeated every 2 weeks. While a TH2 response is clearly elicited in terms of an increased eosinophilia in the blood (Bager et al., 2010; Fleming et al., 2011), there is less evidence for increased numbers of CD4+Foxp3+ regulatory T cells (Fleming et al., 2011). Nonetheless, systemic effects of TSO were implicated in a small study showing that in multiple sclerosis patients, there is a decrease in the number of magnetic resonance imaging (MRI)-detected lesions after 3 months of treatment, followed by an increase in lesions 2 months after treatment had ended (Fleming et al., 2011). This indicates that a protective immune regulatory network may be established despite the lack of a chronic infection. Since TSO is given every 2 weeks, the repeated infections may be equivalent to a chronic infection in terms of establishing an immune regulatory network. It will be interesting to determine in the future whether long term TSO treated patients will mimic the effects of chronic infection in endemic populations that have a well characterized depressed immune reactivity to parasite antigens in peripheral T cell responses.
Several other clinical trials are now under way for a number of clinical indications that should inform whether the effects of TSO will be limited to the intestinal tract or may influence multiple other organ systems (Jouvin and Kinet, 2012; Wolff et al., 2012). One strong possibility could be that TSO may have different immunological effects on different people, just as there is a tremendous diversity of parasite burdens (and associated pathologies) in an endemic population that is heavily exposed to helminth infections (Hotez et al., 2008). Different people will also have very different microbial communities. The identification of biomarkers that provide indicators to the responsiveness of individuals to helminth treatment or infections would be a powerful tool to target TSO treatment to those most likely to respond, as well as to identify individuals in an endemic community that would be most at risk for developing severe pathologies from a heavy parasite burden.
8. Could protective immunity triggered by intestinal helminths treat colitis by reversing dysbiosis of the gut microbiota?
We recently proposed a model whereby Trichuris infection/treatment activates TH2 responses and IL-22 production, which could then alter the intestinal epithelium, goblet cells and mucus layer. This could in turn change the composition of the mucosal microbiota, leading to protection against IBD (Wolff et al., 2012). In support of this model, we have recent data (Broadhurst, et al., In Press) from macaques suffering from idiopathic colitis. By characterizing the mucosal microbiota in these monkeys, we found that there was reduced diversity and an increased proportion of bacteria from the phyla Cyanobacteria and Proteobacteria, consistent with IBD patients. After infection with T. trichiura, the diversity of the mucosal microbiota was restored and microbial communities were more similar to control unaffected animals. We are currently embarking on an interventional trial on ulcerative colitis patients to determine whether this hypothesis will be supported by data from human subjects.
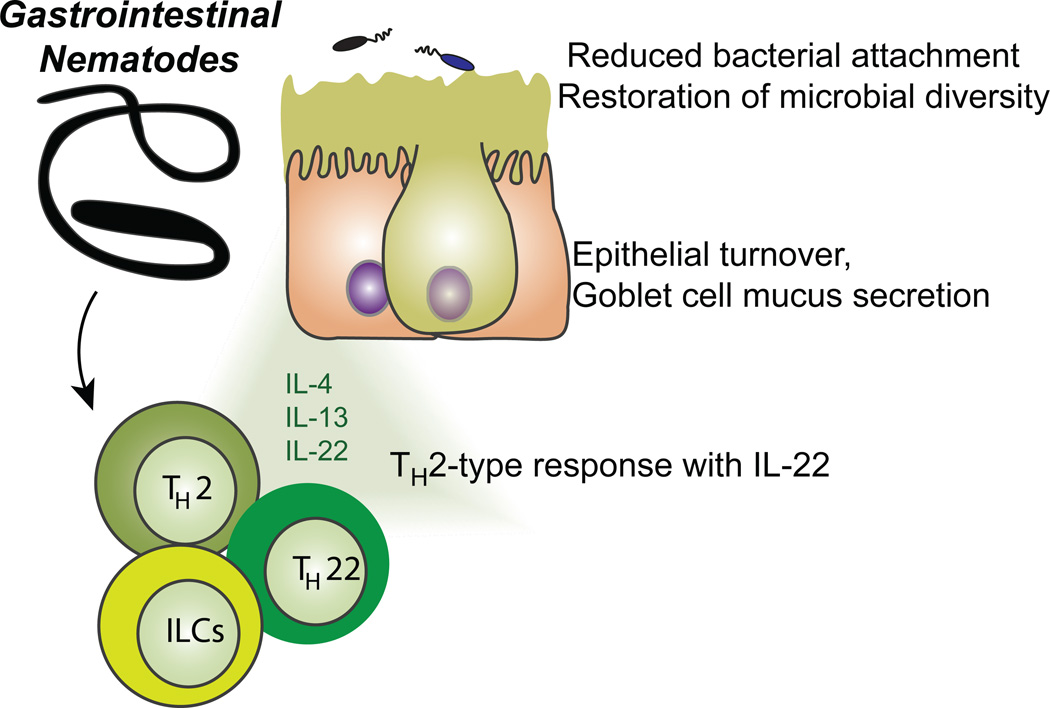
Fig. 1.
A model for gastrointestinal helminth infection stimulating the production of IL-22 by innate lymphoid cells (ILCs) and TH22 cells, in combination with the TH2 cytokines, IL-4 and IL-13, which will stimulate the increased turnover and proliferation of intestinal epithelial cells as well as mucus production by goblet cells. This will reduce the attachment of bacteria to the epithelial cells, especially from the phylum Proteobacteria, and restore the microbial community diversity that was reduced as a result of dysbiosis during intestinal inflammation.
Highlights.
IL-22 helps maintain barrier homeostasis against intestinal pathogens and commensal bacteria.
The role of IL-22 in helminth infections and in response to commensal and pathogenic bacteria is reviewed.
The relationship between intestinal helminths, gut microbiota and inflammatory bowel diseases is explored.
IL-22 induced by helminths may enhance mucosal barrier function and restore the microbiota populations from dysbiosis.
Acknowledgements
Research in the Loke Laboratory is supported by grants from the National Institutes of Health, USA (1R01AI093811 1R21AI094166) and from the Broad Medical Research Program Inflammatory Bowel Disease Program, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal S, Xie MH, Maruoka M, Foster J, Gurney AL. Acinar cells of the pancreas are a target of interleukin-22. J Interferon Cytokine Res. 2001;21:1047–1053. doi: 10.1089/107999001317205178. [DOI] [PubMed] [Google Scholar]
- Allen JE, Wynn TA. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Rutitzky LI, Urban JF, Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nature Rev. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artis D, Grencis RK. The intestinal epithelium: sensors to effectors in nematode infection. Mucosal Immunol. 2008;1:252–264. doi: 10.1038/mi.2008.21. [DOI] [PubMed] [Google Scholar]
- Bager P, Arnved J, Ronborg S, Wohlfahrt J, Poulsen LK, Westergaard T, Petersen HW, Kristensen B, Thamsborg S, Roepstorff A, Kapel C, Melbye M. Trichuris suis ova therapy for allergic rhinitis: a randomized, double-blind, placebo-controlled clinical trial. J Allergy Clin Immunol. 2010;125:123–130. doi: 10.1016/j.jaci.2009.08.006. e121-123. [DOI] [PubMed] [Google Scholar]
- Broadhurst MJ, Leung JM, Kashyap V, McCune JM, Mahadevan U, McKerrow JH, Loke P. IL-22+ CD4+ T cells are associated with therapeutic Trichuris trichiura infection in an ulcerative colitis patient. Sci Transl Med. 2010;2:60ra88. doi: 10.1126/scitranslmed.3001500. [DOI] [PubMed] [Google Scholar]
- Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A, Zelante T, D'Angelo C, Zagarella S, Fallarino F, Spreca A, Iannitti RG, Bonifazi P, Renauld JC, Bistoni F, Puccetti P, Romani L. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol. 2010;3:361–373. doi: 10.1038/mi.2010.22. [DOI] [PubMed] [Google Scholar]
- Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF, Jr, Weinstock JV. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur. J. Immunol. 2004;34:2690–2698. doi: 10.1002/eji.200324833. [DOI] [PubMed] [Google Scholar]
- Elliott DE, Weinstock JV. Helminth-host immunological interactions: prevention and control of immune-mediated diseases. Ann. New York Acad. Sci. 2011;1247:83–96. doi: 10.1111/j.1749-6632.2011.06292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, Schopf L, Urban JF., Jr Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139–155. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- Fleming JO, Isaak A, Lee JE, Luzzio CC, Carrithers MD, Cook TD, Field AS, Boland J, Fabry Z. Probiotic helminth administration in relapsing-remitting multiple sclerosis: a phase 1 study. Mult Scler. 2011;17:743–754. doi: 10.1177/1352458511398054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaze S, McSorley HJ, Daveson J, Jones D, Bethony JM, Oliveira LM, Speare R, McCarthy JS, Engwerda CR, Croese J, Loukas A. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog. 2012;8:e1002520. doi: 10.1371/journal.ppat.1002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, Albrecht M, Mayr G, De La Vega FM, Briggs J, Gunther S, Prescott NJ, Onnie CM, Hasler R, Sipos B, Folsch UR, Lengauer T, Platzer M, Mathew CG, Krawczak M, Schreiber S. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nature Genetics. 2007;39:207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- Hang L, Setiawan T, Blum AM, Urban J, Stoyanoff K, Arihiro S, Reinecker HC, Weinstock JV. Heligmosomoides polygyrus infection can inhibit colitis through direct interaction with innate immunity. J Immunol. 2010;185:3184–3189. doi: 10.4049/jimmunol.1000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasnain SZ, Thornton DJ, Grencis RK. Changes in the mucosal barrier during acute and chronic Trichuris muris infection. Parasite Immunol. 2011;33:45–55. doi: 10.1111/j.1365-3024.2010.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes KS, Bancroft AJ, Goldrick M, Portsmouth C, Roberts IS, Grencis RK. Exploitation of the intestinal microflora by the parasitic nematode Trichuris muris. Science. 2010;328:1391–1394. doi: 10.1126/science.1187703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J. Clin. Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- Jouvin MH, Kinet JP. Trichuris suis ova: Testing a helminth-based therapy as an extension of the hygiene hypothesis. J Allergy Clin Immunol. 2012;130:3–10. doi: 10.1016/j.jaci.2012.05.028. [DOI] [PubMed] [Google Scholar]
- Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Li RW, Wu S, Li W, Huang Y, Gasbarre LC. Metagenome plasticity of the bovine abomasal microbiota in immune animals in response to Ostertagia ostertagi infection. PLoS One. 2011;6:e24417. doi: 10.1371/journal.pone.0024417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RW, Wu S, Li W, Navarro K, Couch RD, Hill D, Urban JF., Jr Alterations in the porcine colon microbiota induced by the gastrointestinal nematode Trichuris suis. Infect. Immun. 2012;80:2150–2157. doi: 10.1128/IAI.00141-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield LS, Gauthier DT, Abner SR, Jones KM, Wilder SR, Urban JF. Enhancement of disease and pathology by synergy of Trichuris suis and Campylobacter jejuni in the colon of immunologically naive swine. Am. J. Trop. Med. Hyg. 2003;68:70–80. [PubMed] [Google Scholar]
- McSorley HJ, Gaze S, Daveson J, Jones D, Anderson RP, Clouston A, Ruyssers NE, Speare R, McCarthy JS, Engwerda CR, Croese J, Loukas A. Suppression of inflammatory immune responses in celiac disease by experimental hookworm infection. PLoS One. 2011;6:e24092. doi: 10.1371/journal.pone.0024092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A. Healing of intestinal inflammation by IL-22. Inflamm Bowel Dis. 2012;18:1777–1784. doi: 10.1002/ibd.22929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhya I, Hansen R, El-Omar EM, Hold GL. IBD-what role do Proteobacteria play? Nat Rev Gastroenterol Hepatol. 2012;9:219–230. doi: 10.1038/nrgastro.2012.14. [DOI] [PubMed] [Google Scholar]
- Munoz M, Heimesaat MM, Danker K, Struck D, Lohmann U, Plickert R, Bereswill S, Fischer A, Dunay IR, Wolk K, Loddenkemper C, Krell HW, Libert C, Lund LR, Frey O, Holscher C, Iwakura Y, Ghilardi N, Ouyang W, Kamradt T, Sabat R, Liesenfeld O. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med. 2009;206:3047–3059. doi: 10.1084/jem.20090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagalakshmi ML, Rascle A, Zurawski S, Menon S, de Waal Malefyt R. Interleukin-22 activates STAT3 and induces IL-10 by colon epithelial cells. Int Immunopharmacol. 2004;4:679–691. doi: 10.1016/j.intimp.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Neufert C, Pickert G, Zheng Y, Wittkopf N, Warntjen M, Nikolaev A, Ouyang W, Neurath MF, Becker C. Activation of epithelial STAT3 regulates intestinal homeostasis. Cell Cycle. 2010;9:652–655. doi: 10.4161/cc.9.4.10615. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nunez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- Patel N, Kreider T, Urban JF, Jr, Gause WC. Characterisation of effector mechanisms at the host:parasite interface during the immune response to tissue-dwelling intestinal nematode parasites. Int. J. Parasitol. 2009;39:13–21. doi: 10.1016/j.ijpara.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, Ouyang W, Neurath MF, Becker C. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz SM, Kohler G, Schutze N, Knauer J, Straubinger RK, Chackerian AA, Witte E, Wolk K, Sabat R, Iwakura Y, Holscher C, Muller U, Kastelein RA, Alber G. Protective immunity to systemic infection with attenuated Salmonella enterica serovar enteritidis in the absence of IL-12 is associated with IL-23-dependent IL-22, but not IL-17. J Immunol. 2008;181:7891–7901. doi: 10.4049/jimmunol.181.11.7891. [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, Tardif MR, Sathaliyawala T, Kubota M, Farber DL, Collman RG, Shaked A, Fouser LA, Weiner DB, Tessier PA, Friedman JR, Kiyono H, Bushman FD, Chang KM, Artis D. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Nava GM, Stappenbeck TS. Host genetic susceptibility, dysbiosis, and viral triggers in inflammatory bowel disease. Curr Opinion Gastroenterol. 2011;27:321–327. doi: 10.1097/MOG.0b013e32834661b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachiiri A, Imamura R, Wang Y, Fukui M, Umemura M, Suda T. Genomic structure and inducible expression of the IL-22 receptor alpha chain in mice. Genes Immun. 2003;4:153–159. doi: 10.1038/sj.gene.6363934. [DOI] [PubMed] [Google Scholar]
- Taylor MD, van der Werf N, Maizels RM. T cells in helminth infection: the regulators and the regulated. Trends Immunol. 2012;33:181–189. doi: 10.1016/j.it.2012.01.001. [DOI] [PubMed] [Google Scholar]
- van Riet E, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology. 2007;212:475–490. doi: 10.1016/j.imbio.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Walk ST, Blum AM, Ewing SA, Weinstock JV, Young VB. Alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus. Inflamm Bowel Dis. 2010;16:1841–1849. doi: 10.1002/ibd.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wescott RB. Experimental Nematospiroides dubius infection in germfree and conventional mice. Exp. Parasitol. 1968;22:245–249. doi: 10.1016/0014-4894(68)90099-4. [DOI] [PubMed] [Google Scholar]
- Wilson MS, Feng CG, Barber DL, Yarovinsky F, Cheever AW, Sher A, Grigg M, Collins M, Fouser L, Wynn TA. Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth infections. J Immunol. 2010;184:4378–4390. doi: 10.4049/jimmunol.0903416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte E, Witte K, Warszawska K, Sabat R, Wolk K. Interleukin-22: a cytokine produced by T, NK and NKT cell subsets, with importance in the innate immune defense and tissue protection. Cytokine Growth Factor Rev. 2010;21:365–379. doi: 10.1016/j.cytogfr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Wolff MJ, Broadhurst MJ, Loke P. Helminthic therapy: improving mucosal barrier function. Trends Parasitol. 2012;28:187–194. doi: 10.1016/j.pt.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, Volk HD, Sterry W, Sabat R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–1323. [Google Scholar]
- Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol. 2010;32:17–31. doi: 10.1007/s00281-009-0188-x. [DOI] [PubMed] [Google Scholar]
- Wu S, Li RW, Li W, Beshah E, Dawson HD, Urban JF., Jr Worm burden-dependent disruption of the porcine colon microbiota by Trichuris suis infection. PLoS One. 2012;7:e35470. doi: 10.1371/journal.pone.0035470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz LA, Flavell RA. Recent advances in IL-22 biology. Int Immunol. 2011;23:159–163. doi: 10.1093/intimm/dxr001. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]