Abstract
IMPORTANCE
Severe obesity (Body Mass Index, BMI ≥35) is associated with a broad range of health risks. Bariatric surgery induces weight loss and short-term health improvements but little is known about long term outcomes of these operations.
OBJECTIVE
Report 3 year change in weight and select health parameters following common bariatric surgical procedures.
DESIGN and SETTING
The Longitudinal Assessment of Bariatric Surgery (LABS) Consortium is a multi-center observational cohort study at ten hospitals in six geographically diverse clinical centers in the United States.
PARTICIPANTS and EXPOSURE
Adults undergoing first-time bariatric surgical procedure as part of routine clinical care by participating surgeons were recruited between 2006 and 2009 and followed until September 2012. Participants completed research assessments utilizing standardized and detailed data collection prior to surgery and 6 months, 12 months, and then annually post-surgery.
MAIN OUTCOMES
Three years following Roux-en-Y gastric bypass (RYGB) or laparoscopic adjustable gastric banding (LAGB) we assessed percent weight change from baseline and the percentage of patients with diabetes achieving HbA1c <6.5 or FPG < 126 mg/dL without pharmacologic therapy. Dyslipidemia or hypertension resolution at 3 years was also assessed.
RESULTS
At baseline, participants (n=2458) ranged in age from 18 to 78 years, 79% were women, the median BMI was 45.9 (interquartile range (IQR) 41.7–51.5) kg/m2 and median baseline weight was 129 (115, 147) kg. 1738 participants underwent RYGB, 610 LAGB, and 110 other procedures. At baseline, 774 (33%) had diabetes, 1252 (63%) dyslipidemia, and 1601 (68%) hypertension. 3 years post-surgery, the median actual weight loss for RYGB participants was 41 (IQR: 31, 52) kg corresponding to percent of baseline weight lost of 31.5% (IQR: 24.6%–38.4%). For LAGB participants, actual weight loss was 20 (IQR: 10, 29) kg which results in 15.9% (IQR: 7.8%–23.0%) weight loss. The majority of weight loss was evident one year post-surgery for both procedures. Five distinct weight change trajectory groups were identified for each procedure. 216(67.5%) of RYGB and 28(28.6%) of LAGB participants who had diabetes at baseline experienced partial remission at three years. The incidence of diabetes was 0.9% after RYGB and 3.2% following LAGB. Dyslipidemia resolved in 237 (61.9%) of RYGB and 39 (27.1%) of LAGB participants, remission of hypertension occurred in 269(38.2%) and 43(17.4%) of RYGB and LAGB participants, respectively.
CONCLUSIONS and RELEVANCE
Among patients with severe obesity, there was substantial weight loss 3 years following bariatric surgery with the majority experiencing maximum weight change during the first year. However, there was variability in the amount and trajectories of weight loss, and in diabetes, blood pressure, and lipid outcomes. NCT00465829, ClinicalTrials.gov
INTRODUCTION
Bariatric surgery results in large, sustained weight loss in severely obese populations. Although generally accepted as the most effective means for inducing weight loss in very heavy patients, few studies exist reporting outcomes longer than two years after the surgery was performed. Long term outcomes studies that do exist are mostly case series, from limited geographical areas, or report surgical procedures no longer performed.1–5 For example, high quality long term outcomes from the Swedish Obesity Study are well described but most of the participants underwent a vertical banded gastroplasty procedure, an operations no longer used.6–8 Six year follow up after Roux-en-Y gastric bypass (RYGB) was reported but these data may not generalize since all the patients are from one surgical practice in Utah.9 Even though surgically-induced weight loss is much more effective than non-surgical treatments for seriously obese patients, surgery is still not universally accepted because of incomplete knowledge of long term outcomes from the procedures.
The Longitudinal Assessment of Bariatric Surgery (LABS) Consortium was formed to acquire long-term data on the safety, effectiveness, and durability of bariatric surgical procedures currently performed in the United States using standardized data collection practices. LABS is a multi-center observational, cohort study with standardized and detailed data collection protocols. LABS has three phases; LABS-1, LABS-2, and LABS-3.10 The 30-day safety of bariatric surgery was reported in LABS-1.11 LABS-2 focuses on longer term safety, outcomes, and durability of health changes. The major priorities for LABS-2 were to determine weight, medical, surgical, and behavioral outcomes, including incidence and remission of co-morbid conditions, and to evaluate patient, procedure, and other characteristics that were associated with these outcomes. LABS-3 were two sub-studies that examined mechanisms of diabetes change and psychosocial aspects in more detail.
We now report the major clinical outcomes from LABS-2 including 3-year weight change from baseline and diabetes, lipid and hypertension outcomes following Roux-en-Y Gastric Bypass (RYGB) and Laparoscopic Adjustable Gastric Banding (LAGB).
METHODS
PARTICIPANTS
LABS-2 participants were at least 18 years old and underwent first-time bariatric procedures with a surgeon participating in the LABS consortium at one of 10 hospitals at six clinical centers in the U.S.12 All participants provided written informed consent and underwent surgery between March 2006 and April 2009. Follow-up for this report ended September 2012. Socio- demographics were self-reported. Race was considered missing for participants not self-reporting their race or ethnicity as at least one of the following: White or Caucasian, Black or African-American, Asian, American Indian or Alaska Native, Native Hawaiian or Latina.
Participants whose initial bariatric surgery was subsequently revised or reversed are included in the three year results as they represent the natural history of the participant’s post-surgical course.
The institutional review boards at each center approved the protocol and consent forms. LABS is registered at ClinicalTrials.gov (NCT00465829).
ASSESSMENTS AND OUTCOME MEASURES
LABS-certified trained personnel collected study outcome data. Weights and other clinical data were collected within 30 days before surgery. If the original operation date had to be changed, biospecimen samples collected within 180 days of the bariatric operation were used. At 6 months after surgery, a brief clinical assessment was performed with a complete evaluation performed annually after surgery.
Weight change is reported as the percent change from baseline.10,13,14 During in-person follow-up visits, weight was measured using a standard protocol (“protocol” weight) on a study-purchased standard scale (Tanita® Body Composition Analyzer, model TBF-310).10 If a protocol weight was not obtained, weight was measured by research or medical personnel on a non-study scale and is referred to as a “clinical weight”. If neither a protocol nor clinical weight was available, a patient self-reported weight was used. Differences between measured and self-reported weights in this cohort were small, and did not systematically differ by measured BMI or degree of postoperative weight change. The average degree of under-reporting by self-report was 0.7 kg for women and 1.0 kg for men.15 Weights of women in their 2nd or 3rd trimester of pregnancy and those up to 6-months postpartum were excluded from analyses (n=38 RYGB, n=13 LAGB).
Diabetes was defined as currently taking diabetes medication or having glycated hemoglobin (HbA1c) ≥6.5%, or if HbA1c was not available, then fasting plasma glucose ≥126 mg/dL as measured by a central laboratory subcontracted by the LABS consortium to provide results for participants, regardless of where they were recruited.16 Participants reporting having Polycystic Ovarian Syndrome who did not meet laboratory criteria for diabetes and were not taking a diabetes medication other than Metformin were not considered to have diabetes.12
Hypertension was defined as having systolic blood pressure of at least 140 mm Hg or diastolic blood pressure of at least 90 mm Hg from a single measurement, or taking an anti-hypertensive medication when evaluated.12
Dyslipidemia was defined as either low density lipoprotein(LDL)≥160 mg/dL, high density lipoprotein(HDL)< 40 mg/dL, fasting triglycerides ≥200 mg/dL, or currently taking a lipid-lowering medication.12 Hyperlipidemia was defined as currently taking a lipid lowering medication orLDL ≥160 mg/dL. Low HDL was defined as <40 mg/dL and high triglycerides as fasting level of ≥200 mg/dL.12
Deaths were adjudicated by committee using all available information (e.g. death certificate, medical record, autopsy report). Subsequent bariatric procedures were identified by medical record review or participant self-report. Deaths and subsequent bariatric procedures within 3 years of the initial bariatric surgery are reported.
STATISTICAL ANALYSIS
Descriptive statistics summarize baseline characteristics for each procedure. Frequencies and percentages are reported for categorical data. Medians, 25th and 75th percentiles, and IQR (difference between the 25th and 75th percentiles) are reported for continuous data.
The median and IQR of the observed percent weight change and modeled percent weight change from a normal mixed model with only an intercept and indicators for time after surgery, are reported separately for RYGB and LAGB at each follow-up time point. The modeled values are used to account for missing weights due to missed visits.
Growth mixture models17 were used to estimate weight change trajectories for each participant and to classify participants with similar modeled trajectories into groups. We required that the smallest trajectory group included at least 1% of the sample. With this constraint, the best fitting model was determined using the Bayesian information criterion (BIC), The modeled trajectories of each group are plotted with bars indicating the IQR of the observed percent weight change for the participants within a group. Separate trajectories were derived for participants undergoing RYGB and LAGB.
Normal mixed models and growth mixture models assume weights are missing at random. To address this assumption, the baseline characteristics of participants with follow-up weights at every time point were compared to participants missing at least one follow-up weight. Statistical significance was tested using the Wilcoxon rank sum test for continuous data and Pearson’s chi-square test for categorical data. Furthermore, normal mixed models were used to test whether those missing weight at a particular time point differed significantly from those with weight recorded with respect to percent weight change at the preceding and the next time point. With lack of statistically significant results of percentage weight change at time points when they were available for those with missing weights, the assumption that data were missing at random was considered reasonable.
The percentage of participants with a comorbidity at baseline who did not have the comorbidity at year three was used to determine remission rates. The incidence rate was defined as the percentage of participants without the comorbidity at baseline who newly developed the comorbidity at year three.
To determine the similarity of patients missing outcomes data at year 3 to those having the outcomes information, baseline characteristics of participants a were compared using the Wilcoxon rank sum test for continuous data and Pearson’s chi-square test for categorical data. In addition, the prevalence of each comorbidity at baseline and at years 1 and 2 was compared between participants with known and missing comorbidity data at year three using a logistic model adjusting for baseline characteristics found to be significantly associated with missing three year data.
Weighted percentages for incidence and remission were calculated using inverse proportional weighting to account for missing clinical information.18 Participants with comorbidity information at both baseline and year three were weighted by the inverse of the probability of having follow-up data at year three. These probabilities were estimated separately for remission and incidence percentages using a logistic regression model adjusting for age and site, stratified by procedure. In the weighted estimates, the individuals with complete data represent themselves as well as similar participants who had missing data.
The rates of death and of subsequent bariatric procedures, respectively, were estimated by dividing the number of events known to occur within 3 years of the initial bariatric surgery by the number of person-years of observation.. We report the rates per 300 person-years which corresponds to the number of events expected if 100 people were followed for 3 years. Exact confidence intervals for rates were constructed using the Poisson distribution.
Analyses were conducted using SAS (version 9.2), R (version 2.15.2) and Mplus (version 7). All reported P-values are two-sided. P-values less than 0.05 are considered to be statistically significant.
RESULTS
This report includes the 1738 participants who underwent RYGB surgery and the 610 participants who underwent an LAGB procedure as their first bariatric operation. The 110 participants who underwent the less commonly performed procedures are not included.
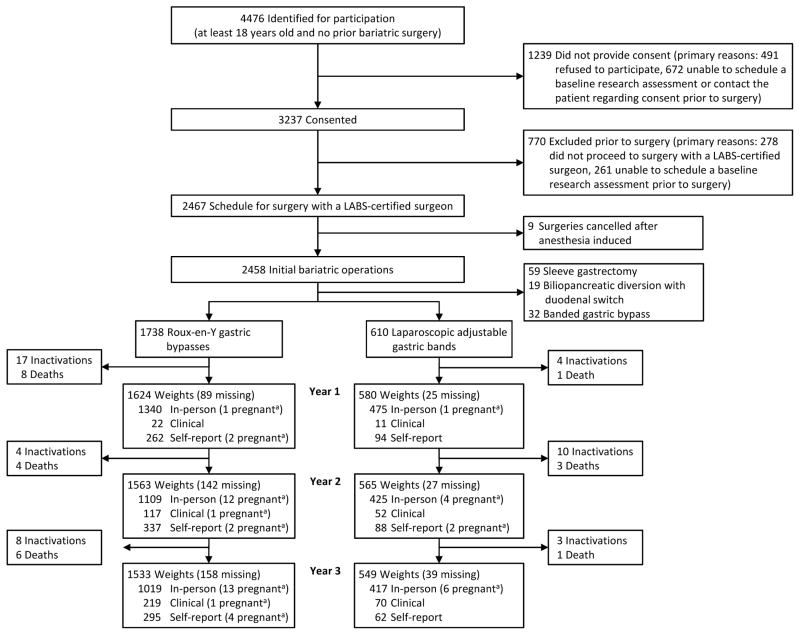
As shown in Figure 1, three years post-surgery, weight was obtained for 91% (91% RYGB and 93% LAGB) of the 2279 active participants (23 participants died and 46 were withdrawn from the study, 41 due to participant request). Weights from research visits were available for 66% of the RYGB participants and 76% of the LAGB participants at year three (Figure 1). Across all 3 annual follow-up time points, 72% of weights for RYGB and 73% of weights for LAGB were at research visits.
Figure 1.
Recruitment, follow-up, and types of weight measurements
aWeights of women in their 2nd or 3rd trimester and those up to 6-months postpartum were excluded from analyses.
Details of the baseline characteristics of the LABS-2 cohort have been published.12 Briefly, the median age is 46 years (range 18–78) and the median BMI is 46 kg/m2 (range 33–94), the majority of participants are female (79%), and 14% are non-Caucasian. Table 1 presents select baseline characteristics of LABS-2 participants stratified by surgical procedure.
Table 1.
Baseline characteristics of the LABS-2 cohort by procedure
| Overall (n = 2458) | Roux-en-Y Gastric Bypass (n = 1738) | Laparoscopic Adjustable Gastric Band (n = 610) | Sleeve Gastrectomy (n = 59) | BPDS (n = 19) | Banded Gastric Bypassa (n = 32) | |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| Median (Q1, Q3) | 46 (37, 54) | 45 (37, 54) | 48 (37, 56) | 48 (36, 55) | 39 (35, 46) | 48 (40, 54) |
| Range | 18 to 78 | 19 to 75 | 18 to 78 | 21 to 73 | 26 to 60 | 21 to 69 |
| Weight (kg) | ||||||
| Median (Q1, Q3) | 129 (115, 147) | 131 (116, 150) | 123 (111, 139) | 158 (134, 180) | 136 (123, 151) | 136 (116, 157) |
| Range | 75 to 290 | 75 to 240 | 85 to 246 | 82 to 290 | 110 to 192 | 97 to 227 |
| Body mass index (kg/m2) | ||||||
| Median (Q1, Q3) | 45.9 (41.7, 51.5) | 46.6 (42.4, 51.9) | 43.9 (40.4, 48.0) | 57.7 (46.8, 64.1) | 50.0 (44.9, 52.3) | 49.2 (42.5, 54.1) |
| Range | 33.0 to 94.3 | 33.7 to 81.0 | 33.0 to 87.3 | 35.5 to 94.3 | 37.9 to 62.6 | 36.2 to 76.0 |
| Sex - n (%) | ||||||
| Female | 1931 (78.6) | 1389 (79.9) | 465 (76.2) | 39 (66.1) | 14 (73.7) | 24 (75.0) |
| Male | 527 (21.4) | 349 (20.1) | 145 (23.8) | 20 (33.9) | 5 (26.3) | 8 (25.0) |
| Raceb - n (%) | ||||||
| White | 2102 (86.4) | 1463 (85.1) | 543 (89.6) | 47 (82.5) | 18 (94.7) | 31 (100.0) |
| Black | 256 (10.5) | 196 (11.4) | 51 (8.4) | 8 (14.0) | 1 (5.3) | 0 (0.0) |
| Other | 75 (3.1) | 61 (3.5) | 12 (2.0) | 2 (3.5) | 0 (0.0) | 0 (0.0) |
| Ethnicityb - n (%) | ||||||
| Hispanic | 119 (4.8) | 85 (4.9) | 26 (4.3) | 6 (10.2) | 0 (0.0) | 2 (6.2) |
| Non-Hispanic | 2337 (95.2) | 1652 (95.1) | 583 (95.7) | 53 (89.8) | 19 (100.0) | 30 (93.8) |
| Diabetesb – n (%) | 774 (33.4) | 583 (35.4) | 164 (28.8) | 15 (28.8) | 7 (38.9) | 5 (17.2) |
| Dyslipidemiab– n (%) | 1252 (63.4) | 901 (64.4) | 291 (60.9) | 33 (64.7) | 9 (52.9) | 18 (64.3) |
| Hyperlipidemiab – n (%) | 725 (36.6) | 515 (36.7) | 177 (36.7) | 22 (43.1) | 5 (29.4) | 6 (21.4) |
| Low HDLb – n (%) | 883 (37.5) | 648 (38.8) | 194 (33.3) | 21 (37.5) | 5 (27.8) | 15 (50.0) |
| High Triglyceridesb – n (%) | 462 (22.9) | 339 (23.8) | 103 (21.1) | 12 (21.8) | 5 (27.8) | 3 (10.7) |
| Hypertensionb – n (%) | 1601 (67.5) | 1159 (68.9) | 367 (62.7) | 44 (80.0) | 10 (52.6) | 21 (67.7) |
Banded gastric bypass, is a gastric bypass with a non-adjustable band
Missing data: 25 race; 2 ethnicity; 144 diabetes; 484 dyslipidemia; 476 hyperlipidemia; 101 low HDL; 444 high triglycerides; 86 hypertension BPDS is biliopancreatic diversion with duodenal switch
Q1 is the 25th percentile, Q3 is the 75th percentile
Weight Change
Weight was obtained at all four follow-up time points for 1365 (79%) RYGB participants and 521 (85%) LAGB participants. Participants with a weight at every time point were significantly older (RYGB median (IQR) age 46 (38, 55) years; LAGB median age 48 (38, 57) years) compared to participants missing at least one follow-up weight(RYGB median age 41 (34, 49) years, P<0.001; LAGB median age 44 (33, 53) years, P=0.01). RYGB participants with a weight at every time point had a significantly lower baseline BMI (median BMI 46.2(42.2, 51.7) kg/m2) and were more likely to be Caucasian (86%) than those missing at least one follow-up weight (median BMI 48.0(43.0, 52.9) kg/m2, P=0.002; 80% Caucasian, P=0.01), however these differences in BMI are small. The percentage of RYGB and LAGB participants with complete weight data also varied significantly by clinical center (P<0.001). For RYGB and LAGB the percent weight change preceding a missed visit was not significantly different from those without a missed visit. Similarly, the percent weight change following a missed visit was not significantly different from those without a missed visit (all P>0.05).
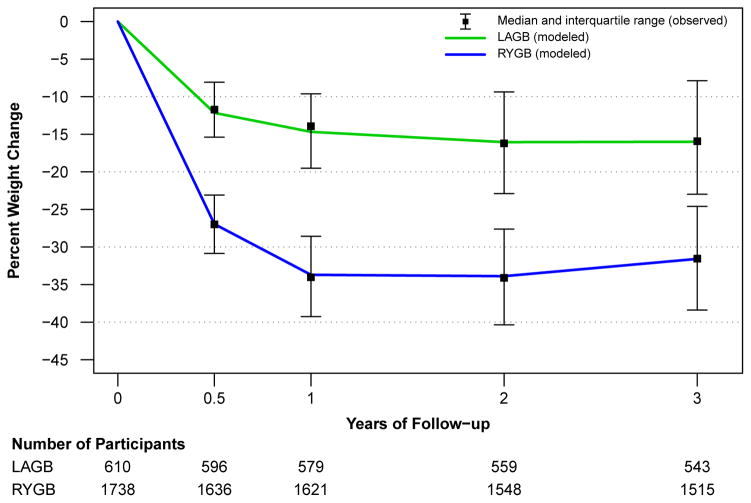
Figure 2 depicts both observed and modeled percent weight change by procedure and by time point. The modeled weight change (continuous lines) for both RYGB and LAGB show a close approximation to the observed medians at each time point. The IQR for observed weight change shows that there is substantial variability in weight change for participants at each time point, e.g., at year three one-quarter of RYGB participants lost less than 25% of their baseline weight whereas one-quarter lost more than 38% of their baseline weight. At three years post-surgery the observed median (IQR) percent weight loss for participants who underwent RYGB is 31.5% (24.6%, 38.4%) of baseline weight and 15.9% (7.9%, 23.0%) for LAGB. The actual median (IQR) three year weight loss was 41 (31, 52) kg for RYGB and 20 (10, 29) kg for LAGB. As a group, participants experienced most of their total weight change in the first year after surgery. From years two to three modeled LAGB weight loss remained stable while RYGB weight loss began to demonstrate some modest erosion from 2 year levels.
Figure 2.
Observed and modeled percent weight change by time point
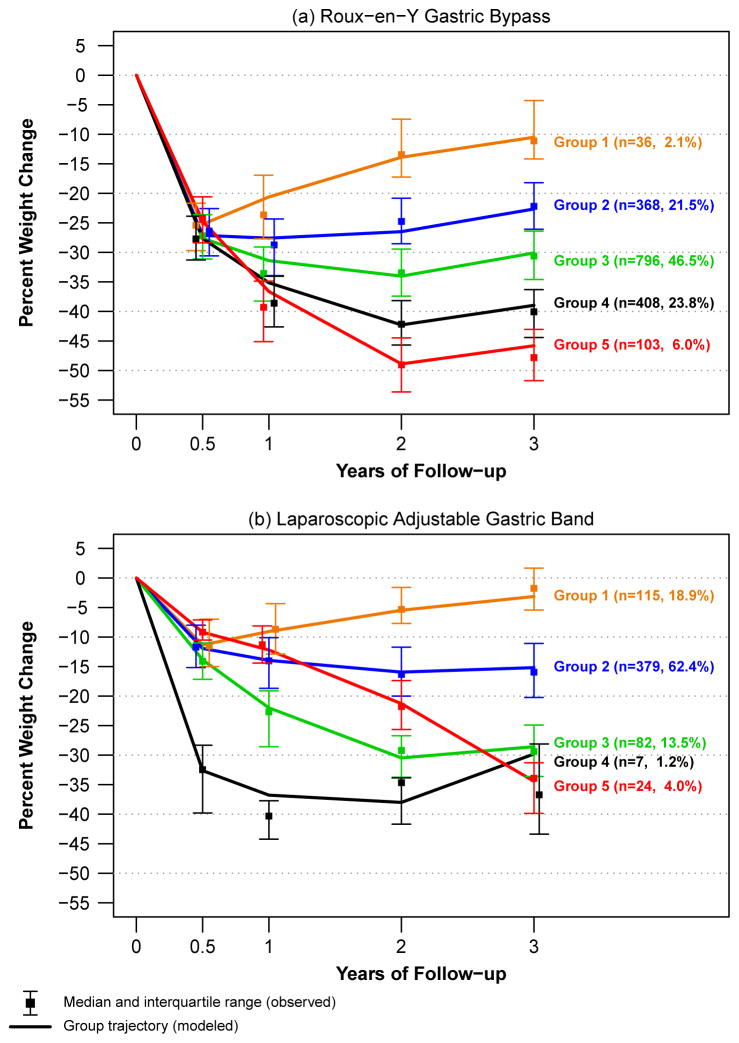
To evaluate common patterns of weight change from baseline to three years among participants by procedure, 5 weight change trajectory groups were identified (Figure 3).
Figure 3.
Percent weight change trajectories
The 5 RYGB trajectories (Figure 3a) all show initial weight loss within the first 6 months after surgery. After 6 months, on average the participants in group 1 (2%) begin to re-gain weight while those in groups 3, 4 and 5 continue to lose weight through two years. After two years of follow-up, the trajectories for groups 2, 3, 4 and 5 begin to demonstrate some weight regain. However, in 76% of the RYGB participants represented (groups 3 – 5) the weight regain is small compared with the overall loss.
Figure 3b shows 5 weight change trajectory groups for those who underwent LAGB. Almost two-thirds (62%) of the participants are included in group 2 which shows the greatest average weight loss occurring in the first 6 months then a slowing of weight loss and stabilization after two years. LAGB participants in group 1, the second most common trajectory (19%), experienced weight loss, on average, only during the first 6 months of follow-up, gaining weight, on average through year three. The group 5 trajectory (4%) shows continual weight loss throughout the three year period while group 3 (14%) shows steady weight loss for the first two years then slight weight regain from years two to three. Group 4 includes only 7 participants who experienced substantial weight loss through year two with an increase in weight afterward.
Comorbid conditions
Participants with missing comorbidity data at year three were significantly younger than participants with known three year comorbidity data. The percentage of participants with missing comorbidity data varied significantly by site. Percent weight change at year three was not significantly associated with missing three year data for any of the comorbidities. Adjusting for age and site, the prevalence of each comorbid condition prior to year 3 did not differ significantly between participants with known and unknown three year comorbidity data. Table 2 shows both observed and “weighted” remission and incidence of several important co-morbid health conditions at three years following surgery. Only observed rates are reported in the text since observed and weighted values are similar.
Table 2.
Observed and weighted remission and incident rates three years after bariatric surgery by procedure
| Roux-en-Y Gastric Bypass (n=1691a)
|
Laparoscopic Adjustable Gastric Band (n=588a)
|
|||||
|---|---|---|---|---|---|---|
| Observedb
|
Weightedc
|
Observedb
|
Weightedc
|
|||
| count/n (%) | % (95% CI) | count/n (%) | % (95% CI) | |||
| Diabetes | ||||||
| Remission | 216/320 | (67.5) | 68.9 (64.9, 72.5) | 28/98 | (28.6) | 29.4 (22.8, 37.0) |
| Incidence | 5/560 | (0.9) | 0.8 (0.4, 1.6) | 8/247 | (3.2) | 3.2 (1.8, 5.5) |
| Dyslipidemia | ||||||
| Remission | 237/383 | (61.9) | 61.6 (58.2, 64.8) | 39/144 | (27.1) | 25.9 (21.0, 31.5) |
| Incidence | 7/221 | (3.2) | 3.2 (2.0, 5.3) | 15/94 | (16.0) | 16.9 (11.9, 23.3) |
| Hyperlipidemia | ||||||
| Remission | 151/253 | (59.7) | 58.9 (54.4, 63.2) | 22/97 | (22.7) | 23.5 (17.7, 30.7) |
| Incidence | 9/353 | (2.5) | 2.4 (1.5, 3.7) | 21/143 | (14.7) | 14.3 (10.6, 18.9) |
| Low HDL (less than 40 mg/dl) | ||||||
| Remission | 292/341 | (85.6) | 86.5 (83.6, 89.0) | 76/113 | (67.3) | 65.9 (58.6, 72.4) |
| Incidence | 9/616 | (1.5) | 1.4 (0.9, 2.4) | 10/266 | (3.8) | 3.5 (2.0, 5.9) |
| High Triglycerides (200 mg/dl or higher) | ||||||
| Remission | 139/162 | (85.8) | 83.9 (79.4, 87.6) | 36/58 | (62.1) | 60.4 (50.1, 69.9) |
| Incidence | 8/495 | (1.6) | 1.4 (0.8, 2.3) | 14/206 | (6.8) | 7.0 (4.8, 10.1) |
| Hypertension | ||||||
| Remission | 269/705 | (38.2) | 41.0 (38.1, 43.9) | 43/247 | (17.4) | 18.8 (15.0, 23.2) |
| Incidence | 39/309 | (12.6) | 12.6 (10.0, 15.8) | 27/149 | (18.1) | 17.3 (12.7, 23.2) |
Active participants at year three; participants not included in the denominator for remission or incidence are missing three year comorbidity data.
Observed percentages for participants with comorbidity data at baseline and year 3.
Model estimates from weighting the participants with comorbidity data at baseline and year 3 by the inverse of the estimated probability that the participant has comorbidity data at year 3 adjusting for age and site.
Remission is the percentage of participants with the comorbidity at baseline who did not have the comorbidity at year 3. Incidence is the percentage of participants without the comorbidity at baseline who did have the comorbidity at year 3. CI stands for confidence interval
Three years following surgery, the percentages of participants experiencing at least partial diabetes remission were 67.5% and 28.6% for RYGB and LAGB, respectively, while the percentages of participants with new onset (incident) diabetes were 0.9% and 3.2% for RYGB and LAGB, respectively.
Dyslipidemia was in remission for 61.9% RYGB and 27.1% LAGB at three years. A new diagnosis of dyslipidemia was made in 3.2% of RYGB and 16.0% of LAGB patients. Changes in hyperlipidemia were similar. Low HDL remitted in 85.6% and 67.3% for RYGB and LAGB groups and high triglycerides 85.8% and 62.1%, respectively. Hypertension was in remission at three years in 38.2% RYGB and 17.4% LAGB and incidence was 12.6% RYGB and 18.1% LAGB over three years.
Deaths and subsequent bariatric procedures within 3 years of initial bariatric surgery are detailed in Table 3. Following RYGB, 16 deaths were reported, with 3 occurring in the immediate 30 day post-surgical period. There were 2 RYGB revision procedures and 2 RYGB reversal procedures over the 3 years.
Table 3.
Deaths and subsequent bariatric surgical procedures within 3 years
| Roux-en-Y Gastric Bypass (n = 1738) | Count | Ratea (95% CI) |
|---|---|---|
| Deaths | 16 | 0.9 (0.5, 1.5) |
| Within 30-days of surgery | 3 | 0.2 (0.04, 0.5) |
| Sepsis | 1 | |
| Cardiovascular disease | 1 | |
| Pulmonary embolism | 1 | |
| More than 30-days after surgery | 13 | 0.8 (0.4, 1.3) |
| Bowel obstruction | 1 | |
| Sepsis | 1 | |
| Respiratory failure | 1 | |
| Cardiovascular disease | 3 | |
| Suicide/substance abuse | 2 | |
| Cancer | 1 | |
| Indeterminate after adjudication | 4 | |
| Subsequent Bariatric Surgery Procedures | 4 | 0.3 (0.1, 0.9) |
| Revision | 2 | |
| Reversal | 2 |
| Laparoscopic Adjustable Gastric Band (n = 610) | Count | Ratea (95% CI) |
|---|---|---|
| Deaths | 5 | 0.8 (0.3, 1.9) |
| Within 30-days of surgery | 0 | 0 (0, 0.6) |
| More than 30-days after surgery | 5 | 0.8 (0.3, 1.9) |
| Organ failure | 2 | |
| Respiratory failure | 1 | |
| Cancer | 1 | |
| Indeterminate after adjudication | 1 | |
| Subsequent Bariatric Surgery Procedures | 77 | 17.5 (13.8, 21.9) |
| Band replacement | 7 | |
| Port revision | 19 | |
| Revision other than port or band replacement | 10 | |
| Band removal | 21 | |
| Revision to another bariatric procedure | 20 |
Estimated number of events per 300 person-years (i.e. estimated number of events if 100 participants were followed for three years).
For LAGB there were 5 deaths with none occurring during the 30 day post-surgical period. There were 77 subsequent bariatric procedures among the 610 LAGB participants over the 3 year period, the majority for band removal, revision to another bariatric procedure, or port revision.
DISCUSSION
In 3-year follow-up after bariatric surgery, substantial weight loss was observed with most of the change occurring during the first year. Compared to the very modest weight loss resulting from lifestyle intervention, at 3 years, weight loss from bariatric surgery was substantial: 31.5% of baseline weight for RYGB and 15.9% for LAGB.19,20 There was great variability in the amount and trajectory of weight loss, as well as in diabetes, blood pressure, and lipid outcomes. Variability in weight change also indicates a potential opportunity to improve patient selection and education prior to operation as well as enhance support for continued adherence to lifestyle adjustments in the post-operative years.
Weight loss from RYGB in LABS-2 was similar to that observed in the Utah obesity study.9 Since our patient population was more diverse than the Utah patients and our results were from a large number of surgeons and surgical centers, long term, sustained weight loss can be expected from RYGB and the Utah results are likely generalizable. Our RYGB results were also similar to those observed for RYGB in the Swedish Obesity Study even though their patients were much more homogenous than ours.6 Weight loss associated with the LAGB procedure was less than anticipated in our study and less than what would be expected based on published series.21–23 These studies reported 20–25% initial weight loss with weight loss continuing through and then reaching a plateau at 3 years. LABS LAGB participants experienced different weight loss trajectories than previously reported, with less than 20% of participants experiencing much weight loss after the first year. The differences between our results and those of prior publications and the extreme heterogeneity in outcomes we found highlight the need to better understand factors contributing to individual differences in weight loss results.
Health improvements in diabetes, blood pressure, and lipids also showed variability in response. The diabetes partial remission rate for RYGB in LABS-2 (67.5%) is comparable to the 6 year remission rate in the Utah Obesity Study (62%) but is somewhat lower than the 75–80% remission rates observed in other retrospective reviews1–5,24,25 and randomized controlled single-site studies/trials of RYGB with shorter (1–2 year) follow-up periods.26,27 This is perhaps due to at least two factors including the duration of follow-up and the definition of diabetes and diabetes remission that were utilized (quite similar in LABS-2 and The Utah Obesity Study) as well as other differences in the nature of subject follow-up (standardized vs. clinical vs. administrative data collection). The partial remission rate of diabetes among LAGB participants in LABS-2, 28.6%, is considerably lower than the 73% rate after 2 years as reported by Dixon et al., although participants in that trial had mild diabetes by study design and experienced more consistent weight loss.28 In contrast, the LABS-2 participants were not selected based on diabetes status but recruited from all eligible and consenting participants for bariatric surgery at the clinical sites and represent a group of participants with diabetes over the entire spectrum of disease severity. The range of disease severity and the relatively modest weight change of LAGB participants in LABS-2 likely account for the lower rates of diabetes improvement.
The RYGB rates of hypertension remission (38.2%) in LABS-2 are consistent with the SOS remission rates of all surgery participants of 34% and 19% at 2 and 10 years of follow-up, respectively. The Utah Obesity Study of RYGB reported hypertension remission of 53% and 42% at 2 and 6 years follow-up, slightly better than that observed in LABS-2.9 The remission of hypertension is a complex measurement that requires frequent monitoring and longer-term follow-up as many hypertensive participants experience a period of remission while not receiving medication, but many eventually relapse.29 In addition, the relapse in blood pressure after surgical weight loss appears to be more related to aging and recent small weight increases than to baseline weight or the type of initial weight loss post procedure.30 Among the LAGB participants, remission rates of hypertension are lower than previously reported, again perhaps due to either initial disease severity or the more modest weight loss.31,32
Remission of any dyslipidemia in LABS-2 was 61.9% for RYGB and 27.1% for LAGB, consistent with prior studies for RYGB but lower than other LAGB reports.33,34 Hyperlipidemia was in remission in 59.7% of RYGB in LABS-2 consistent with Adams et al. 2 and 6 year rates of 57% and 53%, respectively, for RYGB. High triglyceride levels improved after both RYGB and LAGB in LABS-2 with remission in 85.8% and 62.1%, respectively. High resolution rates were also observed for triglycerides in both the Utah and SOS studies6,7,9 at the reported time points.
The primary strength of the LABS-2 study is that data were collected on a large sample using standardized definitions and procedures by trained evaluators in a, multicenter geographically diverse cohort. These factors should make the results of the LABS-2 study more generalizable to clinical practice.10 In addition, longer term and detailed complication data including deaths as well as surgical revisions and reversals are also collected in LABS-2. These events as reported to date (Table 3) show mortality rates of 1% or less for both RYGB and LAGB and subsequent bariatric procedures more common among LAGB participants at three years.
Conclusions regarding the long term efficacy of bariatric surgery are limited because many studies have incomplete long-term follow-up rates, with completeness of data collection declining below 50% after one to two years.5,35 As a longitudinal study, LABS-2 has a strong emphasis on retention with resources devoted to obtaining high ascertainment rates.36 A strength of the current study is that it reports follow-up weights in over 90%of active participants at three years. Despite its commitment, LABS-2 did not accomplish complete in person follow-up required for measures of co-morbid health outcomes. However, the results presented here are based on a thorough analysis of the missing data to provide an estimate of co-morbid health at three years post RYGB and LAGB. This highlights one of the significant challenges to large, multi-center clinical observational studies in bariatric surgery5,36 and stresses the need for sustained efforts to maximize retention and follow-up.
Another weakness to consider in interpreting differences in weight loss and comorbidity improvement between procedures is that as an observational study, patients underwent specific procedures based on clinical decision-making, including patient or surgeon preference. Thus, baseline differences, both measured and unmeasured, between patients undergoing differing procedures may have had an impact on both weight and health outcomes.
In conclusion, LABS-2 data confirm in a heterogeneous population with a high degree of follow up that RYGB and LAGB were associated with significant weight and health improvements at three-years following surgery. Reduction in weight and improvements in comorbid conditions with LAGB were less than reported in previous studies and not as large as those seen with RYGB. Longer-term follow up of this cohort will determine the durability of these improvements over time and factors associated with variability in effect.
Acknowledgments
Funding/Support: LABS-2 was a cooperative agreement funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Grant numbers: Data Coordinating Center – U01 DK066557; Columbia University Medical Center – U01-DK66667 (in collaboration with Cornell University Medical Center CTRC, Grant UL1-RR024996); University of Washington – U01-DK66568 (in collaboration with CTRC, Grant M01RR-00037); Neuropsychiatric Research Institute – U01-DK66471; East Carolina University – U01-DK66526; University of Pittsburgh Medical Center – U01-DK66585 (in collaboration with CTRC, Grant UL1-RR024153); Oregon Health & Science University – U01-DK66555.
Role of the Sponsor: The NIDDK scientists contributed to the design and conduct of the study, which included collection, and management of data. The project scientist from the NIDDK served as a member of the steering committee, along with the principal investigator from each clinical site and the data coordinating center. The data coordinating center housed all data during the study and performed data analyses according to a prespecified plan developed by the data coordinating center biostatistician and approved by the steering committee and independent data and safety monitoring board. The decision to publish was made by the Longitudinal Assessment of Bariatric Surgery-2 steering committee, with no restrictions imposed by the sponsor. As a coauthor, an NIDDK scientist contributed to the interpretation of the data and preparation, review, or approval of the manuscript.
Footnotes
Authors Contributions: Drs. Courcoulas and Christian had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Courcoulas, Christian, Belle
Acquisition of data: Courcoulas, Flum, Pories, Patterson.
Analysis and interpretation of data: Christian, Belle, Courcoulas, Yanovski
Drafting of the manuscript: Courcoulas
Critical revision of the manuscript for important intellectual content: Courcoulas, Christian, Belle, Berk, Flum, Garcia, Horlick, Kalarchian, King, Mitchell, Patterson, Pender, Pomp, Pories, Thirlby, Yanovski, Wolfe
Statistical analysis: Christian, King, Belle
Obtained funding: Courcoulas, Mitchell, Flum, Berk
Study supervision: Courcoulas, Belle, Pomp, Wolfe
Conflict of Interest Disclosures: Dr. Courcoulas has received research grants from Allergan Pfizer, Covidien, EndoGastric Solutions, Nutrisystem and is on the Scientific Advisory Board of Ethicon J & J Healthcare System. Dr. Flum has received research grants from Covidien and Sanofi-Aventis. Dr. Kalarchian has received a research grant from Nutrisystem and has received PI support from the Obesity Society for the Use of Nutrisystem after Bariatric Surgery. Dr. Mitchell has received a research grant form Shire Pharmaceuticals. Dr. Patterson is a consultant for the manufacturer of the Lap-band (trademark), Allergan Health, a company that may have a commercial interest in the results of this research. This potential conflict of interest has been reviewed and managed by OHSU. Dr. Pender has received research grants from Glaxo Smith Kline and Covidien. Dr. Pories has received research grants from Ethicon and GlaxoSmithKline. Dr. Wolfe is a Consultant and Advisor for Covidien, Ethicon, Crospon, Viudico, Medtronics and has received a research grant from Enteromedics. Drs. Belle, Berk, Christian, Garcia, Horlick, King, Pomp, Thirlby, and Yanovski have no disclosures to report.
Contributor Information
Anita P. Courcoulas, University of Pittsburgh Medical Center, Department of Surgery.
Nicholas J. Christian, University of Pittsburgh Graduate School of Public Health, Department of Epidemiology.
Steven H. Belle, University of Pittsburgh Graduate School of Public Health, Department of Epidemiology.
Paul D. Berk, Columbia University Medical Center, Department of Medicine.
David R. Flum, University of Washington, Department of Surgery.
Luis Garcia, University of North Dakota School of Health and Sciences, Department of Surgery.
Mary Horlick, National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition.
Melissa A. Kalarchian, University of Pittsburgh, Department of Psychiatry, School of Medicine.
Wendy C. King, University of Pittsburgh Graduate School of Public Health, Department of Epidemiology.
James E. Mitchell, Neuropsychiatric Research Institute, University of North Dakota School of Medicine and Health Sciences, Department of Neuroscience.
Emma J. Patterson, Legacy Good Samaritan Medical Center, Department of Surgery.
John R. Pender, East Carolina University, Department of Surgery.
Alfons Pomp, Weill Cornell Medical Center, Department of Surgery.
Walter J. Pories, East Carolina University, Department of Surgery, Brody School of Medicine.
Richard C. Thirlby, Virginia Mason Medical Center, Department of Surgery.
Susan Z. Yanovski, National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition.
Bruce M. Wolfe, Oregon Health & Science University, Department of Surgery.
References
- 1.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004 Oct 13;292(14):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 2.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995 Sep;222(3):339–350. doi: 10.1097/00000658-199509000-00011. discussion 350–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Favretti F, Segato G, Ashton D, et al. Laparoscopic adjustable gastric banding in 1,791 consecutive obese patients: 12-year results. Obes Surg. 2007 Feb;17(2):168–175. doi: 10.1007/s11695-007-9043-0. [DOI] [PubMed] [Google Scholar]
- 4.Sugerman HJ, Wolfe LG, Sica DA, Clore JN. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg. 2003 Jun;237(6):751–756. doi: 10.1097/01.SLA.0000071560.76194.11. discussion 757–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higa K, Ho T, Tercero F, Yunus T, Boone KB. Laparoscopic Roux-en-Y gastric bypass: 10-year follow-up. Surg Obes Relat Dis. 2011 Jul-Aug;7(4):516–525. doi: 10.1016/j.soard.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004 Dec 23;351(26):2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 7.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007 Aug 23;357(8):741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 8.Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012 Aug 23;367(8):695–704. doi: 10.1056/NEJMoa1112082. [DOI] [PubMed] [Google Scholar]
- 9.Adams TD, Davidson LE, Litwin SE, et al. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012 Sep 19;308(11):1122–1131. doi: 10.1001/2012.jama.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belle SH, Berk PD, Courcoulas AP, et al. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surg Obes Relat Dis. 2007 Mar-Apr;3(2):116–126. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Longitudinal Assessment of Bariatric Surgery C. Flum DR, Belle SH, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009 Jul 30;361(5):445–454. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belle SH, Berk PD, Chapman WH, et al. Baseline characteristics of participants in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study [published online ahead of print March 7, 2013] Surg Obes Relat Dis. doi: 10.1016/j.soard.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belle SH, Berk PD, Courcoulas AP, et al. Reporting weight change: standardized reporting accounting for baseline weight [published online ahead of print December 14, 2012.] Surg Obes Relat Dis. doi: 10.1016/j.soard.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatoum IJ, Kaplan LM. Advantages of percent weight loss as a method of reporting weight loss after Roux-en-Y gastric bypass. Obesity. 2013 Aug;21(8):1519–1525. doi: 10.1002/oby.20186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christian NJ, King WC, Yanovski S, et al. Validity of Self-Reported Weights Following Bariatric Surgery in the LABS-2 Cohort. Manuscript submitted for publication 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes care. 2009 Nov;32(11):2133–2135. doi: 10.2337/dc09-9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Wang X. Structural Equation Modeling: Applications using Mplus. Hoboken, NJ: John Wiley & Sons; 2012. [Google Scholar]
- 18.Kenward MG, Molenberghs G. Missing Data in Clinical Studies. Hoboken, NJ: John Wiley& Sons; 2007. [Google Scholar]
- 19.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes care. 2007 Jun;30(6):1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wadden TA, Neiberg RH, Wing RR, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity. 2011 Oct;19(10):1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien PE, McPhail T, Chaston TB, Dixon JB. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006 Aug;16(8):1032–1040. doi: 10.1381/096089206778026316. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien PE, MacDonald L, Anderson M, Brennan L, Brown WA. Long-term outcomes after bariatric surgery: fifteen-year follow-up of adjustable gastric banding and a systematic review of the bariatric surgical literature. Ann Surg. 2013 Jan;257(1):87–94. doi: 10.1097/SLA.0b013e31827b6c02. [DOI] [PubMed] [Google Scholar]
- 23.Alhamdani A, Wilson M, Jones T, et al. Laparoscopic adjustable gastric banding: a 10-year single-centre experience of 575 cases with weight loss following surgery. Obes Surg. 2012 Jul;22(7):1029–1038. doi: 10.1007/s11695-012-0645-9. [DOI] [PubMed] [Google Scholar]
- 24.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003 Oct;238(4):467–484. doi: 10.1097/01.sla.0000089851.41115.1b. discussion 484–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wittgrove AC, Clark GW. Laparoscopic gastric bypass, Roux-en-Y- 500 patients: technique and results, with 3–60 month follow-up. Obes Surg. 2000 Jun;10(3):233–239. doi: 10.1381/096089200321643511. [DOI] [PubMed] [Google Scholar]
- 26.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012 Apr 26;366(17):1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012 Apr 26;366(17):1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 28.Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008 Jan 23;299(3):316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 29.Dannenberg AL, Kannel WB. Remission of hypertension. The ‘natural’ history of blood pressure treatment in the Framingham Study. JAMA. 1987 Mar 20;257(11):1477–1483. doi: 10.1001/jama.257.11.1477. [DOI] [PubMed] [Google Scholar]
- 30.Sjostrom CD, Peltonen M, Sjostrom L. Blood pressure and pulse pressure during long-term weight loss in the obese: the Swedish Obese Subjects (SOS) Intervention Study. Obes Res. 2001 Mar;9(3):188–195. doi: 10.1038/oby.2001.20. [DOI] [PubMed] [Google Scholar]
- 31.Dixon JB, O’Brien PE. Health outcomes of severely obese type 2 diabetic subjects 1 year after laparoscopic adjustable gastric banding. Diabetes care. 2002 Feb;25(2):358–363. doi: 10.2337/diacare.25.2.358. [DOI] [PubMed] [Google Scholar]
- 32.Ponce J, Haynes B, Paynter S, et al. Effect of Lap-Band-induced weight loss on type 2 diabetes mellitus and hypertension. Obes Surg. 2004 Nov-Dec;14(10):1335–1342. doi: 10.1381/0960892042583932. [DOI] [PubMed] [Google Scholar]
- 33.Benaiges D, Flores-Le-Roux JA, Pedro-Botet J, et al. Impact of restrictive (sleeve gastrectomy) vs hybrid bariatric surgery (Roux-en-Y gastric bypass) on lipid profile. Obes Surg. 2012 Aug;22(8):1268–1275. doi: 10.1007/s11695-012-0662-8. [DOI] [PubMed] [Google Scholar]
- 34.Dixon JB, O’Brien PE. Changes in comorbidities and improvements in quality of life after LAP-BAND placement. Am J Surg. 2002 Dec;184(6B):51S–54S. doi: 10.1016/s0002-9610(02)01181-9. [DOI] [PubMed] [Google Scholar]
- 35.Garb J, Welch G, Zagarins S, Kuhn J, Romanelli J. Bariatric surgery for the treatment of morbid obesity: a meta-analysis of weight loss outcomes for laparoscopic adjustable gastric banding and laparoscopic gastric bypass. Obes Surg. 2009 Oct;19(10):1447–1455. doi: 10.1007/s11695-009-9927-2. [DOI] [PubMed] [Google Scholar]
- 36.Gourash WF, Ebel F, Lancaster K, et al. Longitudinal Assessment of Bariatric Surgery (LABS): Retention Strategy and Results at 24 Months. Surg Obes Relat Dis. doi: 10.1016/j.soard.2013.02.012. published online ahead of print March 15, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]