Abstract
Background
Development of targeted therapies for medullary thyroid cancer (MTC) has focused on inhibition of the RET (Rearranged during Transfection) proto-oncogene. Akt has been shown to be a downstream target of RET via the key mediator phosphoinositide-3-kinase. MK-2206 is an orally administered allosteric Akt inhibitor that has exhibited minimal toxicity in phase 1 trials. We explored the anti-tumor effects of this compound in MTC.
Methods
Human MTC-TT cells were treated with MK-2206 (0–20 µM) for 8 days. Assays for cell viability were performed at multiple time points with 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). The mechanism of action, mechanism of growth inhibition, and production of neuroendocrine tumor markers were assessed with Western blot analysis.
Results
MK-2206 suppressed MTC cell proliferation in a dose-dependent manner (p≤0.001). Levels of Akt phosphorylated at serine 473 declined with increasing doses of MK-2206, indicating successful Akt inhibition. The apoptotic proteins cleaved poly (ADP-ribose) polymerase (PARP) and cleaved caspase-3 increased in a dose-dependent manner with MK-2206, while the apoptosis inhibitor survivin was markedly reduced. Importantly, the anti-tumor effects of MK-2206 were independent of RET inhibition, as the levels of RET protein were not blocked.
Conclusions
MK-2206 significantly suppresses MTC proliferation without RET inhibition. Given its high oral bioavailability and low toxicity profile, phase II studies with this drug alone or in combination with RET inhibitors are warranted.
Keywords: MK-2206, Akt Inhibitor, Medullary Thyroid Cancer, Neuroendocrine Tumors, Phosphotidylinositol 3-Kinase/Akt Pathway, RET
Introduction
Medullary thyroid cancer (MTC) is a neuroendocrine tumor (NET) that arises from the calcitonin-producing parafollicular C-cells in the thyroid. MTC accounts for 5–10% of all thyroid cancers and carries a worse prognosis than other differentiated thyroid cancers with an overall 10-year survival rate of 75–85% [1, 2]. Early surgical intervention is the only curative option, but even with surgery, approximately 50% of patients have persistent or recurrent disease [3]. For this reason, targeted therapies are being investigated for systemic treatment of MTC.
Several signaling pathways, including Notch1/Hairy Enhancer of Split-1 (HES-1)/acheate-scute complex like-1 (ASCL1), glycogen synthase kinase-3β (GSK-3β), Raf-1/MEK/extracellular signal-regulated kinase (ERK), and phosphatidylinositol 3-kinase (PI3K)/Akt, have been shown to play important roles in regulation of neuroenodcrine tumors, including MTC [4–13]. Dominant activating mutations of RET (Rearranged during Transfection) proto-oncogene have been identified in MTC, and constitutively active RET kinases activate the PI3K/Akt pathway [14]. In addition to RET-induced activation, hyperactivation of the PI3K/Akt pathway can occur with loss of expression of the tumor suppressor phosphatase and tensin homologue (PTEN) or activated p21 [15, 16]. Recent studies show that targeted inhibition of the PI3K/Akt pathway causes growth suppression in solid non-neuroendocrine tumors with the PI3K and mammalian target of rapamycin (mTOR) inhibitor GDC-0980 [17] and in MTC with the PI3K inhibitor LY294002 [12]. Unfortunately, LY294002 was shown to have poor pharmacological properties in vivo [18], making it ineffective in tumor treatment.
A novel Akt-specific inhibitor, MK-2206, was evaluated first in combination with selected growth factor receptor inhibitors and then as a single treatment in multiple tumor cell lines, and was shown to successfully inhibit tumor cell growth and migration [19–21]. In a phase I clinical trial, treatment with this oral allosteric Akt inhibtor resulted in decreased activated Akt in tumor biopsies with a tolerable adverse effect profile [22]. However, previous studies have not evaluated treatment of NETs with MK-2206 in vitro. Based on our previous experience with PI3K inhibition in MTC, we proposed in the current study to evaluate MK-2206 treatment of MTC cells. In this study, we demonstrate that MK-2206 effectively inhibits Akt phosphorylation in MTC cells, and through inhibition of this pathway reduces tumor cell growth. We show that this growth suppression is due to activation of the apoptosis pathway, with cleavage of caspase-3 and poly (ADP-ribose) polymerase (PARP). Inhibition of Akt by MK-2206 also results in decreased production of neuroendocrine tumor markers achaete-scute complex-like 1 (ASCL1) and chromogranin A (CgA). Finally, we show that these effects are not related to any inhibition of the RET protein.
Materials and Methods
Cell Culture
Human MTC-TT cells were obtained from the American Type Culture Collection and maintained in RPMI 1640 (Invitrogen Life Technologies, Carlsbad, CA) with 16% Fetal Bovine Serum (Sigma, St. Louis, MO), 100 IU/mL penicillin, and 100 µg/mL streptomycin (Invitrogen), incubated in a humidified atmosphere of 5% CO2 in air at 37°C.
Cell Proliferation Assay
MTC-TT cell viability and proliferation was measured using the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay as previously described [13]. Briefly, cells were seeded into 24-well culture plates and incubated overnight to allow cell adhesion. Cultures were treated with MK-2206 (0–20 µM) (Merck Corporation, Whitehouse Station, NJ), and treatment media was subsequently changed every 2 days. MTT assays were performed every 2 days for 8 days. To perform the MTT assays, treatment media was removed from the plates, and they were then incubated in 250 µl of serum-free RPMI 1640 with 0.5 mg/ml MTT (Sigma) for 3.5 hours. After this incubation, 750 µl of dimethyl sulfoxide (DMSO) (Fischer Scientific, Pittsburg, PA) was added to each well and mixed for 5 minutes. The absorbance at 540 nm was measured in each well with a spectrophotometer (µQuant, Bio-Tek Instruments, Winooski, VT); average absorbance and percent growth compared to controls were calculated.
Immunoblot Analysis
MTC-TT cells were plated onto 100-mm dishes, and incubated overnight. Growth media was then removed and replaced with fresh media containing various concentrations of MK-2206 (0–10 µmol/L). Control cells were treated with DMSO, as this was the solvent used for MK-2206. Plates were incubated for four days, with treatment media changed after two days. Total cellular proteins were isolated and protein concentrations were determined using a bicinchoninic acid assay (Thermo Scientific, Waltham, MA) with standard protocol [10]. Denatured protein extracts (30 µg) were resolved on 7.5%, 10%, or 12% SDS-PAGE and Western blot was carried out as previously described [23]. Proteins were then transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA), blocked in milk (5% nonfat dry milk and 0.05% Tween 20 in 1X phosphate buffered saline), and incubated with primary and secondary antibodies as previously described [23]. The following primary antibody dilutions were used: MASH for ASCL1 (1:2000; BD Pharmingen, San Diego, CA), CgA (1:3000; Zymed Laboratories, San Francisco, CA), G3PDH (1:3000; Trevigen, Gaithersburg, MD), pRETY1062 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), and pAktS473 (1:1000), pAktTh308 (1:1000), Akt (1:1000), poly-ADP ribose polymerase (PARP) (1:1000), cleaved PARP (1:2000), cleaved caspase-3 (1:1000), survivin (1:1000), Cyclin D1 (1:1000), pRETY905 (1:1000), and RET (1:1000) (Cell Signaling Technology, Beverly, MA). Membranes were then incubated in appropriate amounts of horseradish peroxidase goat anti-rabbit or anti-mouse antibody (Cell Signaling Technology), which were detected using Immun-star (Biorad), Supersignal West Dura, Pico, or Femto (Pierce Protein Research Products, Rockford, IL) kits according to the manufacturer’s instructions.
Statistical Analysis
Continuous variables were compared using one-way analysis of variance (ANOVA) with Bonferroni correction for between-group comparisons (IBM SPSS, version 20.0; SPSS Inc., Chicago, IL). A p-value less than 0.05 was considered significant.
Results
MK-2206 Suppresses Growth in MTC Cells
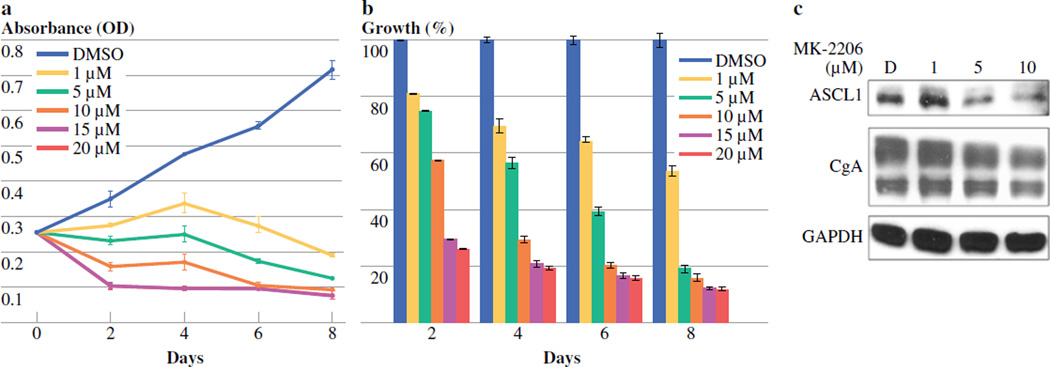
MTC-TT cells were treated with varying doses of MK-2206 (0–20 µM) for 2, 4, 6, and 8 days. MTT assays at each of these time points demonstrated a dose-dependent decrease in MTC-TT cell growth (Figure 1a,b). Compared to the control, 2 days of treatment with MK-2206 significantly reduced cell growth at 1 µM, 5 µM, 10 µM, 15 µM, and 20 µM, by 19, 25, 43, 71, and 74%, respectively (p < 0.001). This dose-dependent growth suppression continued at all time points (Figure 1b). Statistical analysis showed that the growth inhibition was significant at all time points. The IC50 doses for 2 and 4 days of treatment were 7 and 4 µM, respectively, so the maximum dose for all following experiments was set at 10 µM.
Figure 1. Treatment with MK-2206 inhibits medullary thyroid cancer cell (MTC-TT) growth and neuroendocrine tumor marker production.
MTT cellular viability assay demonstrates a dose-dependent reduction in MTC-TT cell growth over 8 days. Western blot analysis shows an associated decrease in expression of neuroendocrine tumor markers achaete-scute complex-like 1 (ASCL1) and chromogranin A (CgA). MTC-TT cells were plated into 24-well culture plates, incubated overnight, and then treated with MK-2206 (0–20µM) in standard cell media. Dimethyl sulfoxide (DMSO) was used as a treatment control. A) Absorbance values for MTT assays at each time point. Error bars represent standard error of the mean. B) Percentage reduction in growth at each time point. Error bars represent standard error of the percentage mean. One-way analysis of variance with Bonferroni correction demonstrated that all doses significantly reduced growth compared to DMSO control at all time points, p≤0.001. C) Protein lysates isolated after 4 days of treatment with MK-2206 (0–10 µM) show a significant dose-dependent decrease in production of ASCL1 and a marginal decrease in CgA. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control.
MK-2206 Suppresses Production of MTC Tumor Markers
In conjunction with growth suppression, we hypothesized that MK-2206 treatment would result in decreased expression of neuroendocrine tumor markers in MTC cells. We have previously shown that ASCL1 is highly expressed in many neuroendocrine tumor (NET) cells, and that in MTC cells it is important for determining neuroendocrine cell fate and hormone production [11, 24, 25]. CgA is a secretory glycoprotein highly expressed in neuroendocrine cells, and changes in CgA expression levels correspond with changes in other NET markers [10, 26]. We demonstrated in earlier studies that inhibition of the PI3K/Akt pathway reduced production of CgA in MTC cells [12]. We examined the effect of MK-2206 treatment on levels of ASCL1 and CgA in these cells using Western blot analysis. After 4 days of treatment with MK-2206, both of these tumor markers showed a clear dose-dependent decrease in expression levels (Figure 1c). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control to ensure equal protein quantity was studied across doses.
MK-2206 Inhibits Phosphorylation of Akt
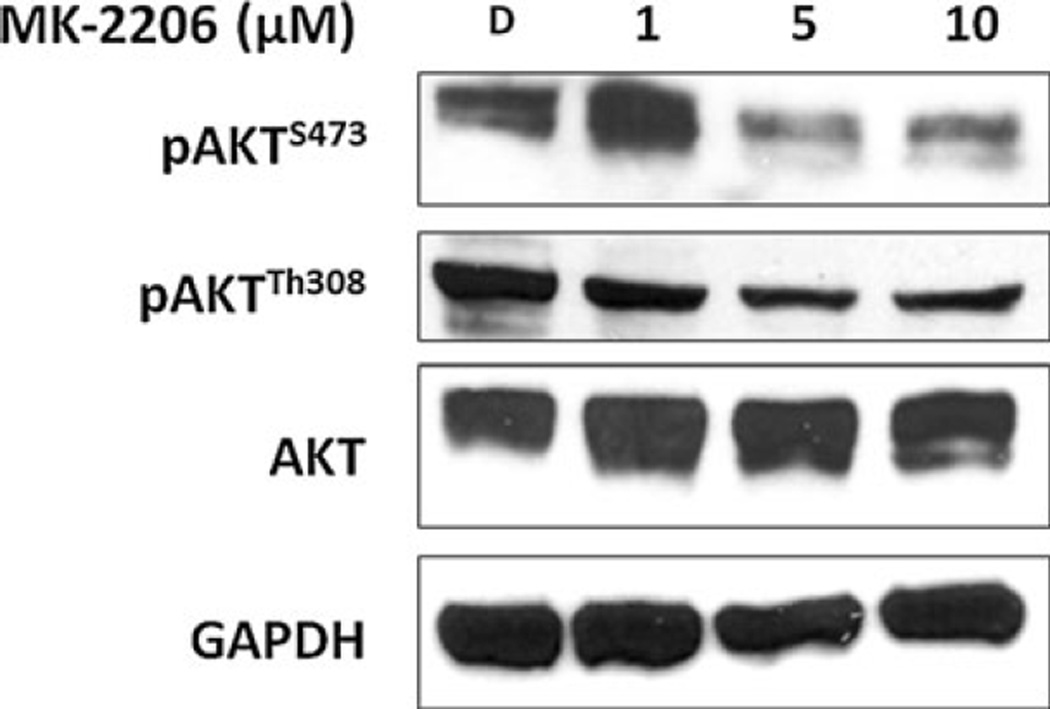
To investigate the method of growth reduction and suppression of neuroendocrine tumor marker production associated with MK-2206 treatment, we used Western blot analysis to assess the mechanism of action of MK-2206 in MTC-TT cells. Previous mechanistic studies of compounds targeted for Akt indicate that MK-2206 is an allosteric inhibitor of Akt phosphorylation [19, 27]. Therefore, the levels of Akt phosphorylated at serine 473 (pAktS473) and threonine 308 (pAktT308) residues were measured in protein lysates from MTC-TT cells treated for 4 days with 1 µM, 5 µM, and 10 µM of MK-2206, and compared to control cells incubated in media with DMSO only. The levels of pAktS473decreased significantly with increasing doses of MK-2206, along with a marginal decrease in pAktT308expression (Figure 2). Levels of total Akt remained the same at all treatment doses, indicating MK-2206 was successful at inhibiting activation of Akt without reducing the amount of total Akt.
Figure 2. MK-2206 inhibits phosphorylation of Akt.
Protein lysates of medullary thyroid cancer cells (MTC-TT) isolated after 4 days of treatment with MK-2206 (0–10 µM) demonstrate that increasing doses of MK-2206 reduce phosphorylation at the serine 473 residue of Akt (pAktS473), with marginal reduction at threonine 308 (pAktT308). Levels of total Akt protein remain stable with treatment. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control.
MK-2206 Induces Cleavage of Apoptotic Markers
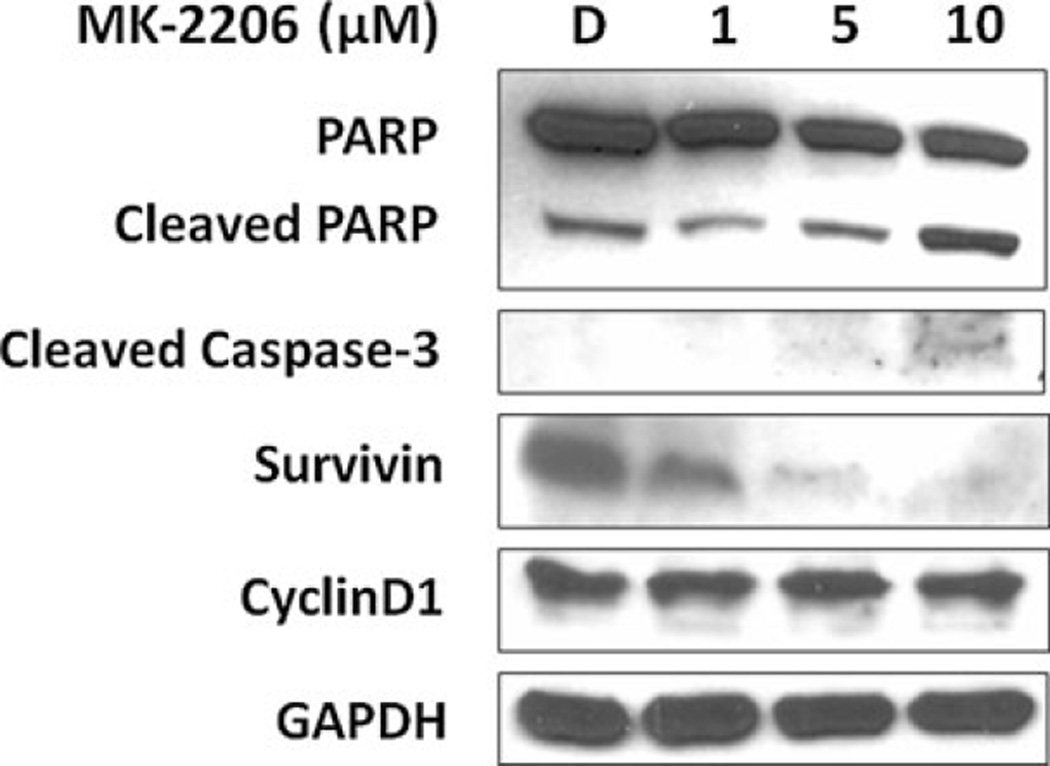
As demonstrated, the inhibition of Akt activation in MTC-TT cells by MK-2206 resulted in significant growth suppression. In order to determine if this suppression was due to cell cycle arrest or induction of apoptosis, we used Western blot analysis to investigate the expression levels of apoptotic proteins PARP and caspase-3 and cell cycle regulator cyclin D1. The protease caspase-3 is the common end target of both intrinsic and extrinsic apoptotic pathways, and cleavage of caspase-3 results in cleavage of other apoptotic markers, including PARP. Therefore, the dose-dependent increase in cleaved caspase-3 and cleaved PARP demonstrated in MTC-TT cells with MK-2206 treatment is evidence that growth suppression is due to apoptosis (Figure 3). To further support this finding, we evaluated expression levels of the apoptosis inhibitor, survivin. High levels of survivin expression have previously been associated with more aggressive tumor types and poorer prognosis in other tumor cells [28, 29]. The increase in cleaved apoptotic factors was accompanied by a significant decrease in survivin expression with increasing doses of MK-2206. We also wanted to evaluate whether there were changes in cyclin D1 expression due to MK-2206 that may indicate concomitant cell cycle regulation effects. Cyclin D1 is an established oncogene and of the three D-type cyclins, cyclin D1 is the one most commonly overexpressed in tumor cells [30, 31]. As shown in Figure 3, cyclin D1 levels remain unchanged with MK-2206 treatment, suggesting that the growth suppression of MTC-TT cells is independent of cell cycle regulation.
Figure 3. Growth suppression of medullary thyroid cancer cells (MTC-TT) by MK-2206 is mediated by apoptosis.
Treatment of MTC-TT cells with MK-2206 (0–10 µM) for four days resulted in increased expression of apoptotic proteins cleaved poly-ADP ribose polymerase (PARP) and cleaved caspase-3, with corresponding decrease in apoptosis inhibitor survivin. Stable cyclin D1 levels suggest there is no associated change in cell cycle signaling. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control.
MK-2206 Does Not Inhibit RET Activation
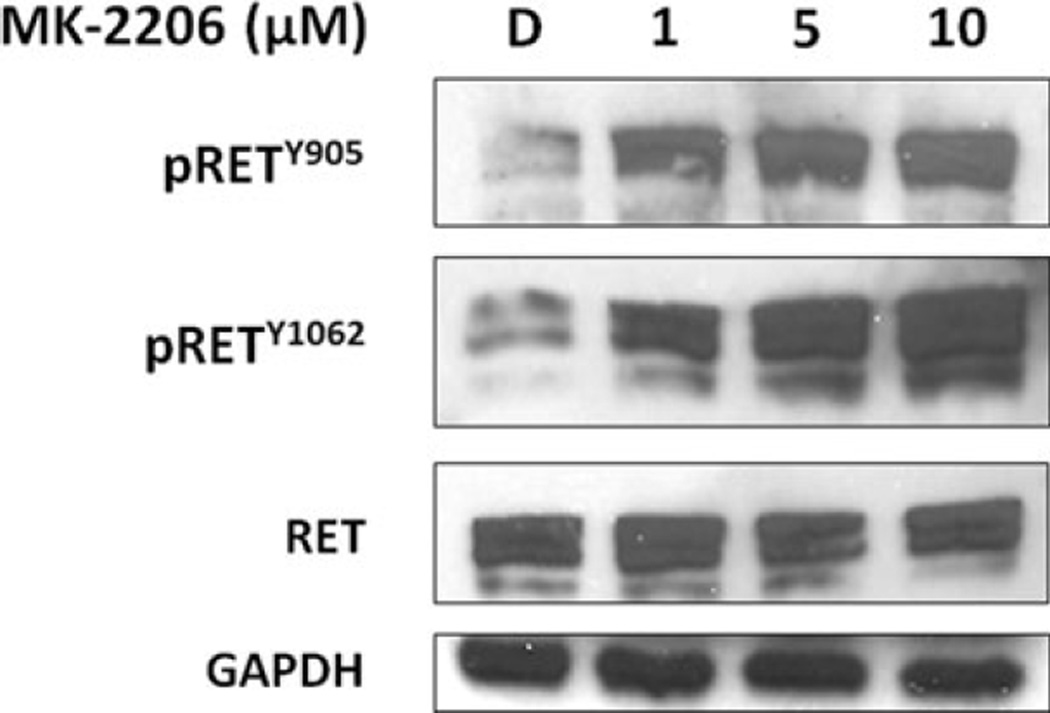
Finally, activation of the RET proto-oncogene can activate the PI3K/Akt pathway, and activating mutations of the RET protein are associated with both sporadic and hereditary MTC [32]. Many current investigative MTC treatments target RET for inhibition. We thus wanted to explore whether there was any crossover inhibition of RET with the Akt-specific inhibitor MK-2206. RET phosphorylated at the tyrosine 905 (pRETY905) residue is important for cell transforming activity [33], while autophosphorylation of the tyrosine 1062 (pRETY1062) residue is enhanced in MEN2B and activates PI3K through the p85 regulatory subunit [34, 35]. With increasing doses of MK-2206 in MTC-TT cells, total RET protein levels remained unchanged, and levels of both phosphorylated RET proteins actually increased (Figure 4). This demonstrates that targeted Akt inhibition with MK-2206 is specific in MTC-TT cells and does not cause any decrease in RET expression. As PI3K and Akt are downstream targets of RET, it is possible that this evident increase in phosphorylated RET is due to feedback from an upstream portion of the pathway in response to specific molecular inhibition of Akt.
Figure 4. Treatment with MK-2206 causes no concomitant inhibition of RET (Rearranged during Transfection) activity in medullary thyroid cancer cells (MTC-TT).
Total RET protein levels are stable in MTC-TT lysates isolated after 4 days of treatment with MK-2206 (0–10 µM). Select phosphorylation residues (pRETY905 and pRETY1062) actually increase with treatment, indicating RET activity is not blocked by MK-2206 treatment. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control.
Discussion
Due to its neuroendocrine origins, MTC is not responsive to the standard treatments for epithelial thyroid cancers, including radioactive iodine and TSH suppression, and thus is more difficult to treat and has lower overall survival rates [36]. In addition, patients with metastatic disease often have excess circulating calcitonin and other peptides, which cause debilitating symptoms such as dyspnea, dysphagia, diarrhea, and flushing. Standard chemotherapy has minimal efficacy in controlling these symptoms or slowing tumor growth [1]. Targeted treatments are therefore needed for metastatic and recurrent MTC. Due to the role of PI3K and Akt pathway activation in the oncogenic properties of MTC [4], these proteins represent promising targets for directed inhibition. Recent studies have demonstrated reduction in tumor cell growth with inhibition of these proteins in thyroid cancers and other solid tumors [12, 15, 17]. The novel allosteric Akt inhibitor, MK-2206, has been effective at suppressing tumor growth in multiple solid tumor cell lines in vitro [19–21], and passed a phase I clinical trial with tolerable side effects [22]. Due to these promising results, we hypothesized that treatment of MTC cells with MK2206 would suppress tumor cell growth through Akt inhibition. We also proposed to establish the mechanism of cell growth suppression and evaluate the inhibition of tumor function, shown by the decrease in NET marker production.
Our study supported previous findings that MTC-TT cells express high levels of phosphorylated Akt at baseline [12]. As predicted, MK-2206 effectively inhibited phosphorylation of Akt at the serine 473 residue and, to a lesser degree, the threonine 308 residue. This inhibition resulted in significant suppression of tumor cell growth in a dose-dependent manner. MTC-TT cells treated with MK-2206 also showed a reduction in neuroendocrine tumor marker production, with decreasing expression of ASCL1 and CgA. We demonstrated that cell growth inhibition was due to activation of apoptotic pathways, while treatment made no evident change in cyclinD signaling. Finally, MK-2206 did not show any crossover inhibition of RET protein activation, but instead showed a modest increase in activated RET phosphorylated at tyrosine residues 905 and 1062. We have not yet elaborated the mechanism by which this activation occurs, but these findings indicate that cell growth and functional suppression with MK-2206 is still possible even with activated RET proteins present.
RET has been targeted recently for new MTC therapies. Several tyrosine kinase inhibitors that include RET in their inhibitory profile have passed phase II and III clinical trials, such as Vandetanib (ZD6474) [37] and Sorafenib (BAY 43-9006) [38]. However, while dominant-activating RET mutations are present in 95% of hereditary MTC, these cases represent only 25% of all those diagnosed with MTC [4]. The rate of RET mutations in sporadic MTC cases is far lower [4], suggesting a significant portion of patients may be resistant to RET inhibitors. Akt is a downstream target of RET, but it can also be activated by multiple receptor proteins. It is therefore a valid candidate for targeted MTC therapy, and may be even more favorable, especially in RET-inhibitor resistant MTC. Our results demonstrate that no concomitant RET inhibition is necessary to achieve growth suppression and reduction in NET marker production with MK-2206 in MTC cells. This finding highlights the possibility for compound therapy targeting both of these important proteins, as represented in Figure 5. Specifically, future work could investigate the efficacy of treating MTC-TT cells with a receptor tyrosine kinase inhibitor that includes RET in its inhibitory profile, such as Sorafenib, in combination with MK-2206.
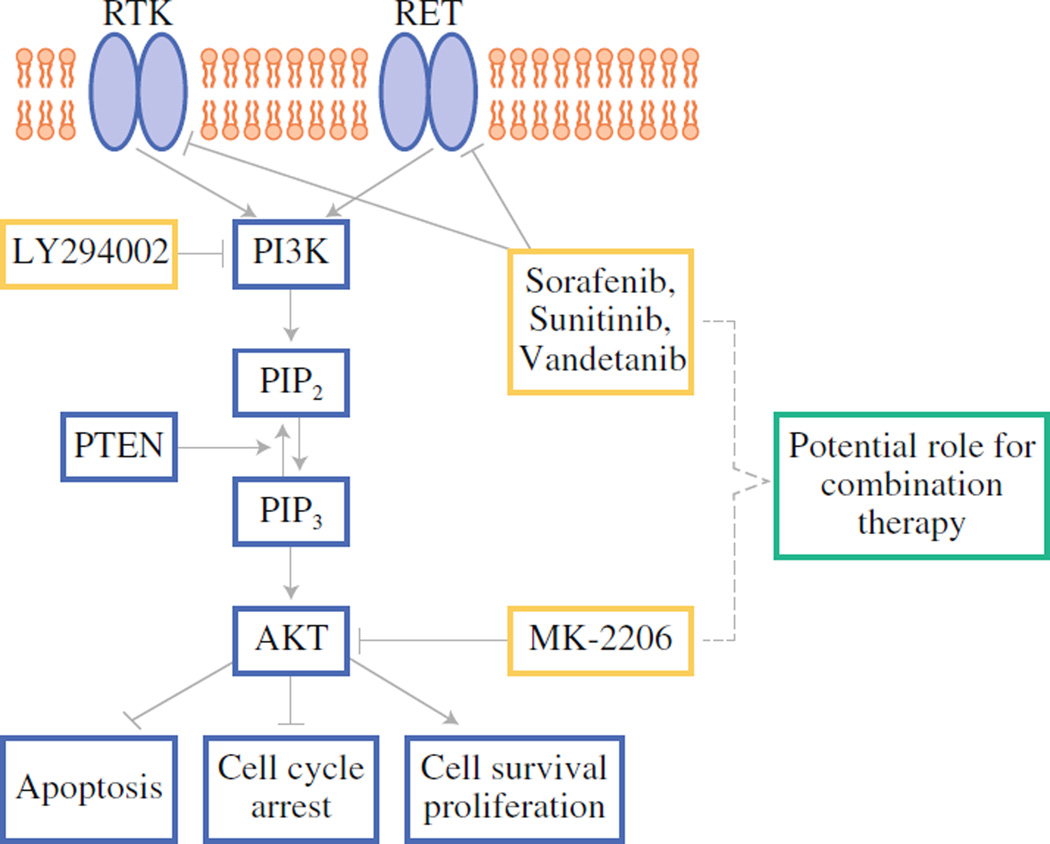
Figure 5. Schematic of potential combination treatment.
The phosphoinositide-3-kinase (PI3K)/Akt pathway is highly active in many tumor cells, including medullary thyroid cancer. The pathway is activated by one of many receptor tyrosine kinases (RTK). Akt is activated downstream of PI3K, and then can activate or inhibit multiple targets that lead to cell proliferation and inhibition of cell cycle arrest and apoptosis. Activation of RET (Rearranged during Transfection) can also activated PI3K. Certain compounds, including LY294002, Sorafenib, Sunitinib, Vandetanib, and MK-2206 (in black circles) effectively inhibit proteins in these pathways, and thus combinations of compounds that target different components could be tested. PTEN, Phosphate and Tensin Homolog; PIP2, phosphatidylinositol-3,4-diphosphate; PIP3, phosphatidylinositol-3,4,5-triphosphate.
In conclusion, MK-2206 is an orally active compound that has been shown to effectively inhibit Akt phosphorylation in humans with a tolerable side effect profile. Considering the evidence presented in this study that Akt inhibition reduces growth and NET marker production in MTC, MK-2206 is a good candidate for further investigation as a treatment for medullary thyroid cancer in phase II trials. Our findings also support that the inhibition of Akt results in successful inhibition of pro-survival pathways with activated RET still present upstream. This suggests that Akt inhibition could be used alone or in combination with tyrosine kinase inhibitors for treatment of MTC, especially in tumors resistant to RET inhibitors.
Acknowledgements
This work was supported in part by grants from the American College of Surgeons Resident Research Fellowship (J. Burke), NIH grant RO1 CA121115 (H. Chen), NIH T32 Research Grant in Surgical Oncology CA090217 (H. Chen), the American Cancer Society Research Scholars Grant (H. Chen), and the American Cancer Society MEN2 Professorship (H. Chen). We thank the Merck Corporation for supplying the MK-2206 used in this study and Maria Georgen for creating the Figure artwork.
Footnotes
Presented in part at the American Association of Endocrine Surgeons Annual Meeting, Iowa City, Iowa, April 2012
References
- 1.Sippel RS, Kunnimalaiyaan M, Chen H. Current management of medullary thyroid cancer. Oncologist. 2008;13:539–547. doi: 10.1634/theoncologist.2007-0239. [DOI] [PubMed] [Google Scholar]
- 2.Pitt SC, Moley JF. Medullary, anaplastic, and metastatic cancers of the thyroid. Semin Oncol. 2010;37:567–579. doi: 10.1053/j.seminoncol.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Sippel RS, O’Dorisio MS, Vinik AI, Lloyd RV, Pacak K. North American Neuroendocrine Tumor Society. The North American Neuroendocrine Tumor Society consensus guideline for the diagnosis and management of neuroendocrine tumors: pheochromocytoma, paraganglioma, and medullary thyroid cancer. Pancreas. 2010;39:775–783. doi: 10.1097/MPA.0b013e3181ebb4f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pitt SC, Chen H. The phosphatidylinositol 3-kinase/Akt signaling pathway in medullary thyroid cancer. Surgery. 2008;144:721–724. doi: 10.1016/j.surg.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitt SC, Chen H, Kunnimalaiyaan M. Inhibition of phosphatidylinositol 3-kinase/Akt signaling suppresses tumor cell proliferation and neuroendocrine marker expression in GI carcinoid tumors. Ann Surg Oncol. 2009;16:2936–2942. doi: 10.1245/s10434-009-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitt SC, Chen H, Kunnimalaiyaan M. Phosphatidylinositol 3-kinase-Akt signaling in pulmonary carcinoid cells. J Am Coll Surg. 2009;209:82–88. doi: 10.1016/j.jamcollsurg.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adler JT, Hottinger DG, Kunnimalaiyaan M, Chen H. Inhibition of the PI3K pathway suppresses hormonal secretion and limits growth in pheochromocytoma cells. World J Surg. 2009;33:2452–2457. doi: 10.1007/s00268-009-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaskula-Sztul R, Pisarnturakit P, Landowski M, Chen H, Kunnimalaiyaan M. Expression of the active Notch1 decreases MTC tumor growth in vivo. J Surg Res. 2011;171:23–27. doi: 10.1016/j.jss.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunnimalaiyaan M, Ndiaye M, Chen H. Neuroendocrine tumor cell growth inhibition by ZM336372 through alterations in multiple signaling pathways. Surgery. 2007;142:959–964. doi: 10.1016/j.surg.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sippel RS, Carpenter JE, Kunnimalaiyaan M, Lagerholm S, Chen H. Raf-1 activation suppresses neuroendocrine marker and hormone levels in human gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G245–G254. doi: 10.1152/ajpgi.00420.2002. [DOI] [PubMed] [Google Scholar]
- 11.Sippel RS, Carpenter JE, Kunnimalaiyaan M, Chen H. The role of human achaete-scute homolog-1 in medullary thyroid cancer cells. Surgery. 2003;134:866–871. doi: 10.1016/s0039-6060(03)00418-5. [DOI] [PubMed] [Google Scholar]
- 12.Kunnimalaiyaan M, Ndiaye M, Chen H. Apoptosis-mediated medullary thyroid cancer growth suppression by the PI3K inhibitor LY294002. Surgery. 2006;140:1009–1014. doi: 10.1016/j.surg.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 13.Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, Chen H. Over-expression of the NOTCH1 intracellular domain inhibits cell proliferation and alters the neuroendocrine phenotype of medullary thyroid cancer cells. J Biol Chem. 2006;281:39819–39830. doi: 10.1074/jbc.M603578200. [DOI] [PubMed] [Google Scholar]
- 14.Segouffin-Cariou C, Billaud M. Transforming ability of MEN2ARET requires activation of the phosphatidylinositol 3-kinase/ AKT signaling pathway. J Biol Chem. 2000;275:3568–3576. doi: 10.1074/jbc.275.5.3568. [DOI] [PubMed] [Google Scholar]
- 15.Mandal M, Kim S, Younes MN, et al. The Akt inhibitor KP372-1 suppresses Akt activity and cell proliferation and induces apoptosis in thyroid cancer cells. Br J Cancer. 2005;92:1899–1905. doi: 10.1038/sj.bjc.6602595. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Rodriguez-Viciana P, Warne PH, Dhand R, et al. Phosphatidylinositol- 3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 17.Wallin JJ, Edgar KA, Guan J, et al. GDC-0980 is a novel class I PI3K/mTOR kinase inhibitor with robust activity in cancer models driven by the PI3K pathway. Mol Cancer Ther. 2011;10:2426–2536. doi: 10.1158/1535-7163.MCT-11-0446. [DOI] [PubMed] [Google Scholar]
- 18.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 19.Hirai H, Sootome H, Nakatsuru Y, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 20.Liu R, Liu D, Trink E, Bojdani E, Ning G, Xing M. The Aktspecific inhibitor MK2206 selectively inhibits thyroid cancer cells harboring mutations that can activate the PI3K/Akt pathway. J Clin Endocrinol Metab. 2011;96:E577–E585. doi: 10.1210/jc.2010-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knowles JA, Golden B, Yan L, Carroll WR, Helman EE, Rosenthal EL. Disruption of the AKT pathway inhibits metastasis in an orthotopic model of head and neck squamous cell carcinoma. Laryngoscope. 2011;121:2359–2365. doi: 10.1002/lary.22180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yap TA, Yan L, Patnaik A, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 23.Kunnimalaiyaan M, Traeger K, Chen H. Conservation of the Notch1 signaling pathway in gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G636–G642. doi: 10.1152/ajpgi.00146.2005. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Udelsman R, Zeiger M, Ball D. Human achaete-scute homolog-1 is highly expressed in a subset of neuroendocrine tumors. Oncol Rep. 1997;4:775–778. doi: 10.3892/or.4.4.775. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Kunnimalaiyaan M, Van Gompel JJ. Medullary thyroid cancer: the functions of Raf-1 and human achaete-scute homologue-1. Thyroid. 2005;15:511–521. doi: 10.1089/thy.2005.15.511. [DOI] [PubMed] [Google Scholar]
- 26.Zarebczan B, Pinchot SN, Kunnimalaiyaan M, Chen H. Hesperetin, a potential therapy for carcinoid cancer. Am J Surg. 2011;201:329–332. doi: 10.1016/j.amjsurg.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindsley CW, Barnett SF, Layton ME, Bilodeau MT. The PI3K/ Akt pathway: recent progress in the development of ATP-competitive and allosteric Akt kinase inhibitors. Curr Cancer Drug Targets. 2008;8:7–18. doi: 10.2174/156800908783497096. [DOI] [PubMed] [Google Scholar]
- 28.Chu XY, Chen LB, Wang JH, et al. Overexpression of survivin is correlated with increased invasion and metastasis of colorectal cancer. J Surg Oncol. 2012;105:520–528. doi: 10.1002/jso.22134. [DOI] [PubMed] [Google Scholar]
- 29.Ekeblad S, Lejonklou MH, Stålberg P, Skogseid B. Prognostic relevance of survivin in pancreatic endocrine tumors. World J Surg. 2012;36:1411–1418. doi: 10.1007/s00268-011-1345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stamatakos M, Palla V, Karaiskos I, et al. Cell cyclins: triggering elements of cancer or not? World J Surg Oncol. 2010;8:111. doi: 10.1186/1477-7819-8-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Cyclin D1: normal and abnormal functions [minireview] Endocrinology. 2004;145:5439–5447. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- 32.Mologni L, Sala E, Riva B, et al. Expression, purification, and inhibition of human RET tyrosine kinase. Protein Expr Purif. 2005;41:177–185. doi: 10.1016/j.pep.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Kato M, Takeda K, Kawamoto Y, et al. Repair by Src kinase of function-impaired RET with multiple endocrine neoplasia type 2A mutation with substitutions of tyrosines in the COOH-terminal kinase domain for phenylalanine. Cancer Res. 2002;62:2414–2422. [PubMed] [Google Scholar]
- 34.Prazeres H, Torres J, Rodrigues F, et al. How to treat a signal? Current basis for RET-genotype-oriented choice of kinase inhibitors for the treatment of medullary thyroid cancer. J Thyroid Res. 2011;2011:678357. doi: 10.4061/2011/678357. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, Chen H. Inactivation of glycogen synthase kinase-3beta, a downstream target of the raf-1 pathway, is associated with growth suppression in medullary thyroid cancer cells. Mol Cancer Ther. 2007;6:1151–1158. doi: 10.1158/1535-7163.MCT-06-0665. [DOI] [PubMed] [Google Scholar]
- 36.Bhattacharyya N. A population-based analysis of survival factors in differentiated and medullary thyroid carcinoma. Otolaryngol Head Neck Surg. 2003;128:115–123. doi: 10.1067/mhn.2003.2. [DOI] [PubMed] [Google Scholar]
- 37.Wells SA, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30:134–141. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider T, Abdulrahman R, Corssmit EP, Morreau H, Smit JW, Kapiteijn E. Long term analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: final results of a phase II trial. Eur J Endocrinol. 2012;167:643–650. doi: 10.1530/EJE-12-0405. [DOI] [PubMed] [Google Scholar]