Abstract
Significance: Integrins are bidirectional signaling receptors for extracellular matrix that regulate both inside-out signaling that controls keratinocyte-mediated changes to the wound microenvironment and outside-in signaling that controls keratinocyte responses to microenvironmental changes. As such, integrins represent attractive therapeutic targets for treatment of chronic wounds or general promotion of wound healing. Advances in wound management are particularly important as the elderly and diabetic populations within the United States continue to grow.
Recent Advances: Although integrins are best known for mediating cell adhesion and migration, integrins in wound epidermis also control cell survival, proliferation, matrix remodeling, and paracrine crosstalk to other cellular compartments of the wound. Importantly, the concept of targeting integrins in the clinic has been established for treatment of certain cancers and other diseases, laying the groundwork for similar exploitation of integrins as targets to treat chronic wounds.
Critical Issues: Despite their attractiveness as therapeutic targets, integrins have complex roles in wound healing that are impacted by both their own expression and a highly dynamic wound microenvironment that determines ligand availability. Therefore, identifying relevant integrin ligands in the wound and understanding both distinct and overlapping functions that different integrins play in the epidermis will be critical to determine their precise roles in wound healing.
Future Directions: Future research should focus on gaining a thorough understanding of the highly coordinated functions of different integrins in wound epidermis, and on determining which of these functions go awry in pathological wounds. This focus should facilitate development of integrin-targeting therapeutics for treating chronic wounds.

C. Michael DiPersio, PhD
Scope and Significance
Integrins are bidirectional signaling receptors that serve as an interface between extracellular matrix and the intracellular milieu. While it is well known that integrins are the major receptors for keratinocyte adhesion to the basement membrane that underlies the epidermis in skin, roles for epidermal integrins in wound healing expand far beyond adhesion. This review will address autonomous keratinocyte functions that are regulated by integrins during wound healing, including survival and proliferation. We will also discuss roles for epidermal integrins in modulating the wound microenvironment through local matrix remodeling, as well as paracrine crosstalk to other cells within the wound.
Translational Relevance
Chronic wounds are characterized by defects in skin, including impaired re-epithelialization. In their roles as signaling receptors that regulate keratinocyte functions including proliferation and migration, integrins are attractive targets for more efficacious drug therapies to treat chronic wounds. However, exploiting integrins as therapeutic targets will require a better understanding of how they coordinately regulate epidermal functions during wound healing and how these functions are altered in pathological wounds. Recent studies shed light on the complexity of integrin signaling and extracellular matrix ligation and on the various and far-reaching roles that epidermal integrins play in wound healing.
Clinical Relevance
The ever-growing elderly and diabetic populations in the United States create a demand for more effective wound healing therapies. For these groups in particular, chronic wounds are a major impediment to overall patient quality of life. Moreover, chronic wounds are a major clinical problem that translates to a substantial financial burden to the United States as healthcare costs associated with wound management continue to rise. The development of novel therapies to restore normal epidermal functions to chronic wounds, including those that target integrins expressed on keratinocytes, is crucial to enhance patient quality of life and reduce healthcare costs.
Introduction
Basal keratinocytes in the stratified epidermis of skin are adherent to a specialized form of extracellular matrix (ECM) known as the basement membrane (BM), which physically separates the epidermis from the underlying connective tissue of the dermis. Regeneration of the BM is essential during wound re-epithelialization to restore tissue compartmentalization and provide structural support to the neo-epidermis. In addition, newly deposited BM proteins, together with ECM and matricellular proteins that appear in the wound bed, provide signals to the regenerating epidermis that are essential for normal wound healing. Importantly, altered mechanical properties or composition of the ECM are well known to contribute to the pathogenesis of chronic wounds.1 Integrins are the major cell surface receptors for adhesion to the ECM, and they can mediate both inside-out and outside-in signal transduction pathways that control a wide variety of cell functions that contribute to both normal and pathological tissue remodeling processes, including proliferation, survival, ECM remodeling, migration, and gene expression.2–4 In this review, we will provide an overview of what is currently known about the regulatory roles that epidermal integrins play in cutaneous wound healing. We will briefly cover what might be considered as “classical” roles that integrins play in the regulation of cell adhesion, migration, survival, and proliferation. In addition, we will discuss recently discovered roles for certain integrins in controlling the ability of the epidermis to modulate the wound microenvironment, through either effects on ECM or intercellular crosstalk to other cellular compartments. As space limitations preclude an exhaustive discussion of the numerous publications in this field, we direct the reader to several excellent reviews for further coverage of relevant topics.5–8
Discussion of Findings and Relevant Literature
Integrins: regulators of cell adhesion and signal transduction
All members of the integrin family are heterodimeric, transmembrane glycoproteins that consist of an α and a β subunit, each with a large extracellular domain, a single-pass transmembrane domain, and a cytoplasmic domain. Most α and β subunit cytoplasmic domains are relatively short (∼20–70 amino acids), with the exception of the β4 cytoplasmic domain (over 1,000 amino acids).3 Eighteen α subunits and eight β subunits can dimerize in different combinations to form 24 different integrins with distinct, although often overlapping ligand-binding specificities. As a group, integrins can interact with a wide variety of ECM proteins, to which they bind via their extracellular domains.3 Simultaneously, integrins interact via their cytoplasmic domains with cytoskeletal proteins to mediate a transmembrane linkage of the ECM to the cytoskeleton, which is critical for controlling cell shape, polarization, and motility.3,4,9–11
Although integrins lack intrinsic enzymatic activity, they can interact directly or indirectly with a wide variety of signaling effectors, thereby functioning as conduits of bidirectional signal transduction across the cell membrane.3,9,12,13 Cytoplasmic interactions can regulate the activation state of an integrin, thereby modulating its affinity for extracellular ligands during “inside-out” signaling.3,14 In addition, integrins can regulate numerous intracellular pathways in response to ECM binding or other extracellular cues during “outside-in” signaling.12 Indeed, as integrins are clustered at sites of cell adhesion, their cytoplasmic domains function as docking sites to recruit signaling and adaptor proteins that regulate a wide range of downstream pathways. In addition, some integrins undergo lateral interactions with other cell surface proteins, such as growth factor receptors, tetraspanins, urokinase receptor (uPAR), syndecan, or caveolin, which may occur at sites of cell-ECM adhesion or from within specialized membrane microdomains to modulate integrin signaling or adhesive functions.2,15–23 Thus, signaling functions of an individual integrin may be regulated through subcellular localization that controls its association with distinct binding partners, or vice-versa.
The full range of signal transduction pathways that can be regulated by integrins is too extensive to discuss here and has been reviewed extensively elsewhere.2–4,12 However, the adhesion-dependent activation of focal adhesion kinase (FAK) serves as an instructive example of outside-in integrin signaling.24,25 Following integrin-mediated cell adhesion, FAK is auto-phosphorylated on Y397, creating a high-affinity binding site for the SRC-homology 2 (SH2) domain of SRC (or another SRC-family kinase). Once bound, SRC phosphorylates additional FAK tyrosines to create docking sites for other kinases or adaptors, such as GRB2, phosphatidylinositol 3-kinase (PI3-K), and p130CAS. In this way, the FAK/SRC complex can link integrins to downstream signaling effectors that include mitogen-activated protein kinases (e.g., ERK, JNK, and p38), certain Rho family guanosine triphophatases (GTPases; e.g., CDC42, Rho, RAC1), and the serine/threonine kinase AKT.2,24,25
FAK activation often occurs as a result of cooperative signaling from integrins and growth factors and has been linked to several cell functions with relevance to wound healing, including formation of polarized lamellipodia, migration, proliferation, survival, and expression or activity of ECM-degrading proteases.26–31 These functional linkages suggest that some epidermal wound functions might be controlled by the temporal appearance during wound healing of ligands for integrins that are known to activate FAK (for example, α3β1 and α5β1). Interestingly, FAK, RAC1, and integrin α3β1 have each been be linked to the maintenance of the epidermal stem cell compartment that resides in the hair follicle bulge,30,32,33 and α3β1 binding to laminin-332 can activate FAK/SRC-to-RAC1 signaling in cultured keratinocytes,26,34 suggesting that this signaling axis might control expansion of stem cells that contribute to skin tumorigenesis or wound re-epithelialization. However, keratinocyte-specific knockout studies revealed that while α3β1 and FAK each contribute to development of squamous cell carcinoma (SCC),29,33 neither is essential for wound re-epithelialization,29,30,35,36 indicating that elements of the wound microenvironment that are absent from cell culture may compensate for FAK signaling deficiencies in vivo. Indeed, it should be noted that many other integrin-linked signaling molecules, such as integrin-linked kinase (ILK) and phospholipase C (PLC), have also been shown to play important roles in the epidermis, as reviewed elsewhere.28
Epidermal integrins
The stratified epidermis of the skin is a continually regenerating tissue wherein the loss of dead keratinocytes from the outermost layer is balanced by keratinocyte proliferation in the basal layer.37–39 In normal resting epidermis, integrin expression is restricted to the basal keratinocytes that are attached to the BM, and it is down-regulated as differentiating keratinocytes detach from the BM and are displaced upwards into the suprabasal layers.37 In the epidermis of a healing wound, keratinocytes acquire new adhesion/migration properties as a result of either changes in integrin expression or altered adhesion/signaling functions of integrins that were already expressed prior to wounding. Constitutively expressed integrins that persist or are upregulated in wound epidermis include α3β1 and α6β4 (both laminin-332 receptors), α2β1 (a collagen receptor), α9β1 (a receptor for cellular fibronectin, tenascin, and other ligands), and αvβ5 (a vitronectin receptor).37,40 Integrins that are expressed de novo in healing wounds include α5β1 (a fibronectin receptor) and αvβ6 (a fibronectin and tenascin receptor). These epidermal integrins can bind numerous ligands present in the provisional wound ECM, including fibronectin (α5β1, α9β1, αvβ6), vitronectin (αvβ5), and tenascin (α9β1, αvβ6), as well as laminin-332 (α3β1, α6β4) that is deposited by migrating keratinocytes.8,37,40,41
Numerous studies in both human and rodent models have documented the various expression patterns and functions of individual integrins in the epidermis during skin development, in adult skin, and during wound repair.8,37 Of course, there is a need for caution when extrapolating results obtained in murine models to human wound healing, as there are species-specific differences that include hair follicle density, skin thickness, and relative importance of wound contraction. Nevertheless, many aspects of human wound healing are recapitulated in murine models.42 Moreover, many integrin functions have been evolutionarily conserved in mice and humans, and genetic studies in global or epidermis-specific knockout mice have been instructive in determining the relative importance of individual integrins for specific epidermal functions in both resting skin and during wound healing.8 The general importance of β1 integrins is illustrated by the phenotypes of mice that harbor epidermis-specific null mutations in the Itgb1 gene (encoding the integrin β1 subunit), which display an array of defects that includes impaired proliferation, loss of sebaceous glands and hair follicles, organizational defects in the BM, and impaired wound re-epithelialization.43–45 Importantly, knockout of individual β1 integrins through null mutation of specific α subunits leads to only a subset of the β1-null phenotypes in each case, indicating unique functions for different integrins. Nevertheless, some integrins have overlapping functions, such as in the regulation of epidermal migration (see Epidermal migration). Interestingly, while early studies in cultured keratinocytes implicated β1 integrins in the control of cell differentiation, the effects of either epidermis-specific β1 subunit deletion or knockout of individual integrins (α3β1, α6β4, α2β1, α9β1, or αvβ5) on epidermal stratification and differentiation were mild or absent,43,44,46–51 indicating that adhesion-dependent regulation of keratinocyte differentiation in vivo is not dependent on any particular integrin(s).
In the sections that follow, we will discuss what is currently known about the roles of individual integrins in the regulation of distinct epidermal/keratinocyte functions, many of which have been elucidated through studies utilizing both cell culture and genetic models. Expression patterns, potential ligands, and known functions of individual integrins in the unwounded and wounded epidermis are summarized in Table 1 and described in detail in the corresponding subsections. However, it is important to consider that the repertoire of integrins expressed on wound epidermis suggests complex interactions, where interplay between different integrins with synergistic or opposing effects may be necessary for proper regulation of keratinocyte wound functions. This concept is supported by observations that different integrin ligands present in the wound bed can have either cumulative or dominant effects on keratinocyte function.34,41,52–56 Thus, the precise temporal and spatial control of integrin–ECM engagement, perhaps dictated by the temporal appearance or accessibility of ECM ligands in the wound bed, is likely to be critically important for coordinate regulation of different integrins during wound healing.
Table 1.
Epidermal integrins, their known ligands, and summary of their known functions in unwounded or wounded skin
| Integrin | ECM Ligands in Skin | Known Functions in Unwounded Epidermis | Known Functions in Wound Epidermis |
|---|---|---|---|
| α3β1 | Laminins (LN-332 and LN-511) | Expressed constitutively. Required for BM assembly during skin development and epidermal-dermal adhesion in neonates. | Required for stability of nascent BM and epidermal-dermal adhesion. Promotes wound angiogenesis through crosstalk to vasculature. May negatively control epidermal migration. |
| α6β4 | Laminins (mainly LN-332 in hemidesmosomes) | Expressed constitutively. Function within hemidesmosomes is essential for epidermal-dermal adhesion. | Presumably required for hemidesmosome assembly and epidermal adhesion following re-epithelialization. Roles in epidermal migration are unclear. |
| α9β1 | FN, TN, OPN, VEGF, TSP, ADAMs, EMILIN1, and others | Expressed constitutively at low levels. Nonessential for epidermal development or adhesion. | Required for normal keratinocyte proliferation during wound re-epithelialization. |
| α2β1 | Collagens | Expressed constitutively. Nonessential for epidermal development or adhesion. | No essential roles identified. May contribute to epidermal migration over collagen. |
| α5β1 | FN (via RGD) | Expressed at very low levels. Nonessential for epidermal development or adhesion. | No essential roles reported. May contribute to epidermal migration over fibronectin. |
| αvβ6 | FN, TN, LAP of TGFβ-1 and -3 (via RGD) | Not expressed in interfollicular epidermis. Nonessential for epidermal development or adhesion, although required in juvenile mice for normal hair growth. | No essential roles identified, although may promote keratinocyte-mediated activation of latent TGFβ. Also implicated in ECM proteolysis and keratinocyte survival. |
| αvβ5 | VN (via RGD) | Expressed at very low levels. Nonessential for epidermal development or adhesion. | No essential roles identified; may contribute weakly to keratinocyte-mediated activation of latent TGFβ. |
See text for expanded discussions and supporting literature.
LN, laminin; BM, basement membrane; FN, fibronectin; TN, tenascin, OPN, osteopontin; VEGF, vascular endothelial cell growth factor; TSP, thrombospondin; ADAM, a disintegrin and metalloproteinase; EMILIN, elastin microfibril interfacer; RGD, arginylglycylaspartic acid; LAP, latency-associated proteins; TGFβ, transforming growth factor beta; ECM, extracellular matrix; VN, vitronectin.
Finally, it is worth noting there are compelling similarities between wound healing and development of squamous cell carcinoma (SCC),57 and that some integrin expression patterns in SCC mirror those that occur in cutaneous wound healing.37,40 Consistently, integrins regulate a number of epithelial cell functions that are important in both processes, including migration, proliferation, survival, matrix remodeling, and the production of pro-angiogenic factors. Therefore, we will occasionally refer to studies of certain integrins in SCC or other carcinomas, where results from these models may offer clues regarding integrin expression or function during wound healing.
The laminin-binding integrins (α3β1 and α6β4)
Laminin-332, the main adhesive ligand in the resting epidermis, is composed of three distinct chains designated α3, β3, and γ2.58,59 The effects of laminin-332 on keratinocyte behavior are mediated mainly through its two integrin receptors, α3β1 and α6β4,41,54–56 although other receptors such as syndecan-1 also contribute.60 Integrin α6β4 is a major transmembrane component of hemidesmosomes, which are the intermediate filament-associated structures on the basal surfaces of keratinocytes that anchor the epidermis to the dermis.10,61 In this context, α6β4 is thought to play primarily a structural role with little or no signal transduction function.8 In contrast, α3β1-mediated adhesions are associated with the actin cytoskeleton and manifest as focal adhesions in cultured keratinocytes.10,61 Moreover, α3β1 can initiate adhesion-dependent signal transduction in keratinocytes through activation of FAK, SRC, and other signaling molecules.26,34,62 As receptors for a common BM ligand, it seems likely that α3β1 and α6β4 function coordinately (either cooperatively or in opposition) with regard to some keratinocyte processes. However, since these two integrins differ considerably with regard to both cytoskeletal interactions and signaling capacity, their combined effects on keratinocyte function are likely to occur through convergence of their distinct functions, rather than as redundant functions. Correspondingly, there is evidence that distinct proteolysis of laminin-332 (LN-332) may differentiate between α3β1 and α6β4 binding. For example, an N-terminal proteolytic cleavage fragment of the full-length laminin α3B chain can preferentially ligate integrin α3β1.63 Moreover, laminin-α3 chain processing within the C-terminal globular domains appears to modulate whether LN-332 localizes to α6β4-containing hemidesmosomes or α3β1-mediated adhesions, suggesting differential integrin interaction.64
Although the epidermis stratifies normally in mice that lack α3β1 and α6β4, either alone or in combination, these integrins play essential but distinguishable roles in maintaining the epidermal–dermal junction through their interactions with laminin-332 in the BM.50,51,65–67 Indeed, human gene mutations in the relevant integrin subunits, or in the individual subunits of laminin-332 itself, lead to variants of the human blistering skin disease junctional epidermolysis bullosa (JEB). However, differences in the details of these JEB variants, which are mirrored in the corresponding knockout mice, illustrate the very different roles that α3β1 and α6β4 play in epidermal–dermal adhesion. Indeed, the essential adhesion role for α6β4 in hemidesmosomes is revealed by the extensive epidermal blistering that occurs in mice that harbor a null mutation in either the Itga6 or Itgb4 gene (encoding the α6 or β4 subunit, respectively),65–67 and in human patients with loss-of-function mutations in α6β4.68,69 In contrast, the absence of α3β1 (through null, or loss-of-function, mutation of the Itga3 gene encoding the α3 subunit) causes much less severe epidermal blistering in newborn mice50 or young patients,70 and hemidesmosomes are left intact in non-blistered regions of α3β1-deficient skin. Moreover, blisters in α3β1-deficient skin form via a mechanism of BM rupture that is distinct from loss of epidermal attachment to laminin-332 per se, which persists in these mice via α6β4.50,51
α6β4
Hemidesmosomes are essential for stable epidermal adhesion, and their dissolution is necessary for epidermal migration during wound healing.10 Hemidesmosome disassembly involves the SRC-mediated phosphorylation of the β4 cytoplasmic domain, possibly triggered by endothelial growth factor (EGF) or other signals from the wound microenvironment.71 In this sense, the function of α6β4 from within hemidesmosomes can be viewed as a brake to epidermal migration that must be released for wound re-epithelialization to proceed. Following wound healing, hemidesmosomes are re-assembled to restore stable adhesion of the neo-epidermis.10
While the hemidesmosomal role for α6β4 in stable epidermal adhesion is well established, whether this integrin also regulates signal transduction pathways that control keratinocyte functions (e.g., survival, proliferation, migration) is less clear.8 Indeed, pro-migratory/pro-invasive roles for integrin α6β4 have been described for carcinoma cells, where this integrin may cooperate with receptor tyrosine kinases such as EGFR, RON, and MET to activate migration-promoting signals, as reviewed elsewhere.5 However, the extent to which these roles extend to keratinocyte migration during wound re-epithelialization remains uncertain. Noteably, some studies do support a pro-migratory role for α6β4 in keratinocytes, possibly through antagonism of α3β172 and/or through regulation of laminin-332 matrix deposition that directs keratinocyte migration.73
α3β1
In vivo and in vitro studies have identified several roles for integrin α3β1 in regulating keratinocyte functions that may contribute to wound healing, including ECM deposition and organization,74,75 cell polarization and migration,34,53 cell survival,62 cell proliferation,76 and secretion of ECM proteases and pro-angiogenic factors.36,77–79 Many of these processes depend on α3β1 binding to laminin-332, although some may involve ECM-independent functions of α3β1 that are modulated by lateral interactions (direct or indirect) with other cell membrane proteins, such as tetraspanins,77,80 uPAR,81 or adherens junction proteins.82,83 For example, α3β1 binding to the tetraspanin CD15184 can regulate α3β1-mediated signaling85 and epithelial cell motility.86 Interestingly, CD151 is upregulated in wound epidermis, and deletion of CD151 leads to wound healing defects,87 suggesting that CD151 might modulate α3β1 function during wound repair.
Roles for α3β1 in keratinocyte migration have been controversial. On one hand, studies using either human or murine keratinocytes support a role for α3β1 in regulating front–back polarization and promoting processive migration on laminin-332,34,53 which occurs in part through regulation of a FAK/SRC–RAC1 signaling axis.26,34 In contrast, other studies report that α3β1-deficient keratinocytes display enhanced motility and directional migration, although in some cases this appears to involve compensatory upregulation of other integrins such as α6β1.35,74 These discordant results may stem in part from differences in the cell culture models used, including species from which cells were derived and the differential deposition of ECM ligands for other integrins.41,52,54–56 Indeed, α3β1 may been have trans-dominant inhibitory effects on other keratinocyte integrins.35,36,88,89 In any case, in vivo studies performed by separate groups using similar epidermis-specific α3-knockout models have shown that α3β1 is not essential for efficient wound closure35,36 and may even slightly inhibit re-epithelialization, at least in adult mice.35,36 Interestingly, absence of α3β1 reduced efficient wound closure in skin grafts from neonatal α3-null mice,90 which might suggest a role in wound re-epithelialization at earlier developmental stages, or that compensatory mechanisms present in adult skin are absent from neonatal skin. Alternatively, absence of α3β1 from other cellular compartments may have caused reduced wound closure in the latter study, since skin grafts were derived from mice with a global α3-null mutation.90 These questions might be resolved in future studies using mice with compound, epidermis-specific deletion of multiple integrins.
While the dispensability of α3β1 for epidermal migration was unexpected, in vivo and cell culture studies have revealed other roles for this integrin in allowing the epidermis to modulate the tissue microenvironment; for example, through alterations of the ECM or paracrine crosstalk to other cellular compartments. Indeed, epidermis-specific deletion of α3β1 was associated with reduced wound angiogenesis, and α3-null keratinocytes showed reduced secretion of factors that stimulate endothelial cell migration.36 In addition, our group recently identified a novel role for α3β1 in promoting the stability of nascent BM that forms during wound healing, as we discuss later (see Local basement membrane deposition and assembly).91 Moreover, α3β1 has been shown to control laminin-332 deposition and organization in cultured keratinocytes,74 and we recently observed that proteolytic processing of the laminin-γ2 chain is delayed in wounds of α3β1-deficient epidermis and impaired in α3-null keratinocytes.91
Other β1 integrins (α9β1, α2β1, and α5β1)
Integrin α9β1 is expressed constitutively in the epidermis and is upregulated during wound healing, where it may bind to several ECM proteins or other ligands in the wound, including the EIIIA/EDA segment in cellular fibronectin, tenascin, EMILIN1, osteopontin, thrombospondin, certain ADAM (a disintegrin and metalloprotease domain) family members, and VEGF (vascular endothelial growth factor).8,92,93 Distinct binding motifs for α9β1 have been identified, including the AEIDGIEL in tenascin-C, the PEDGIHE motif that occurs within an exposed loop of the fibronectin EIIIA domain, and distinct motifs within ADAM family members.93,94 While α9β1 has been studied less extensively than other integrins, its roles are likely to be complex given the wide range of potential ligands for this integrin that are present in wounds. Genetic studies have revealed an important role for α9β1 in wound re-epithelialization. Indeed, keratinocyte proliferation was significantly impaired in wounds of mice with epidermis-specific deletion of α9β1, while the rate of re-epithelialization was unaffected, resulting in diminished thickness of the neo-epidermis that was presumably due to a reduced number of migrating keratinocytes.49 Thus, α9β1 contributes to establishing integrity of the neoepidermis by promoting proliferation of wound keratinocytes, rather than by driving epidermal migration per se.
The collagen-binding integrin, α2β1, is also expressed constitutively in epidermis and upregulated in wounds. Remarkably, however, mice with a global α2-null mutation display normal skin development with no obvious defects in wound re-epithelialization or contraction, indicating that this integrin is not essential for wound closure.47,95 However, α2β1-deficient mice did display enhanced angiogenesis, reduced infiltration of mast cells, and (in one study) reduced tensile strength of healed wounds, indicating complex roles in wound healing.47,48 While many of the wound healing defects in the α2-null mice are probably caused by absence of α2β1 from the involved cells, it remains to be determined whether some of these defects reflect the loss of integrin-dependent crosstalk between different cellular compartments of the wound, as mentioned above for α3β1. Moreover, there is the possibility that loss of α2β1–collagen interactions that promote keratinocyte migration are compensated by other integrin–ECM interactions. Future studies in which α2β1 is deleted from epidermis, alone or in combination with other integrins, may address these questions.
Integrin α5β1 is largely absent from resting epidermis and expressed de novo in wound epidermis, where it presumably contributes to epidermal migration over fibronectin in the provisional matrix of the wound.96 However, in vivo roles for epidermal α5β1 during wound healing are not defined, since the α5-null mutation is embryonic lethal,97 and studies in epidermis-specific α5 knockout mice have not been reported. Thus, it remains possible that loss of α5β1 accounts for much of the epidermal migration defect that was reported in mice lacking β1,45 since other α subunit knockouts did not phenocopy this defect.
The αv integrins (αvβ5 and αvβ6)
The integrins αvβ5 and αvβ8 are expressed at low levels in resting epidermis, with the latter being expressed suprabasally, while αvβ6 is restricted to hair follicle stem cells and is not normally expressed in the interfollicular epidermis of unwounded skin.8,98–100 However, both αvβ6 and αvβ5 are upregulated during wound healing,8,98–100 where each may bind to several ECM ligands through the arginine-glycine-aspartate (RGD) motif (e.g., fibronectin, tenascin, vitronectin).3,40 Given the diversity of ligands that αv integrins can bind, their upregulation in wound epidermis may be important for modulating keratinocyte motility over the complex matrix of the wound bed. Moreover, αvβ6 has been shown to regulate the expression of extracellular proteases, including matrix metalloprotease (MMP)-9,101 MMP-3,102 and uPA,103 suggesting roles in ECM remodeling. Finally, as discussed below (in epidermal survival section), αvβ6 might promote cell survival and prevent keratinocytes from differentiating.
The de novo induction of αvβ6 during wound healing might also provide temporal control over local transforming growth factor-β (TGFβ) activation, since this integrin can activate the ECM-bound pool of latent TGFβ.40,104,105 Each of the mammalian TGFβ isoforms (TGFβ-1, 2, and 3) is secreted as an inactive complex consisting of the latency-associated protein (LAP) and the latent TGFβ binding protein (LTBP), which is covalently linked to the ECM through fibronectin.104,106 Integrin αvβ6 (and perhaps to a lesser extent αvβ5) can bind to the RGD motif within the LAP, resulting in a conformational change in the complex that activates latent TGFβ1 or TGFβ3.104,105,107 Although this mechanism has been best characterized in carcinoma cells, it also occurs in keratinocytes and may initiate αvβ6-dependent TGFβ signaling pathways that are important for wound healing.105
Functions of wound epidermis that are regulated by integrins
A number of diverse keratinocyte functions are regulated by adhesion to the ECM, and epidermis-specific gene knockout studies have revealed roles for individual integrins in distinct keratinocyte functions that are important for normal wound healing, including migration, proliferation, ECM remodeling/BM regeneration, stable attachment of the neo-epidermis, and induction of angiogenesis. In some cases, however, there is considerable discordance between results from cell culture studies and those from in vivo studies. For example, studies in cultured keratinocytes have clearly implicated certain integrins in the control of cell migration, while studies in knockout mice have so far failed to identify individual integrin–ECM interactions that are essential for wound re-epithelialization in adult skin (discussed below).
The complex process of wound healing is further regulated by growth factors, some of which work in concert or collaboration with integrins to control epidermal functions. Two growth factors of particular importance in wound healing, VEGF and TGFβ,108 have each been functionally linked to integrins that are expressed in wound epidermis. For instance, α6β4 was shown to enhance VEGF translation in carcinoma cells,109 and VEGF expression can upregulate αvβ6 in some carcinoma cells,110 raising the intriguing possibility of dynamic integrin–growth factor feedback loops that might extend to wound keratinocytes. Additionally, αvβ6-mediated activation of latent TGFβ (see previous section) might stimulate the expression of α5β1 and αvβ5 in keratinocytes.111 However, while TGFβ stimulates pro-migratory integrins, it has also been shown to inhibit keratinocyte proliferation, so there are conflicting reports with regard to the net effect of TGFβ signaling on re-epithelialization.112 In any case, it is clear that the repertoire of integrins expressed in wound epidermis, in combination with growth factors present in the wound microenvironment, is important for coordinating diverse keratinocyte functions that collectively ensure efficient wound repair and epidermal regeneration.
In the following sections, we will discuss diverse functions of the wound epidermis that are regulated by keratinocyte integrins (summarized in Fig. 1). We will begin with a discussion of the roles that integrins play in autonomous keratinocyte functions that promote wound healing, followed by a discussion of more recently appreciated roles for certain epidermal integrins in the regulation of paracrine crosstalk to other cell types in the wound.
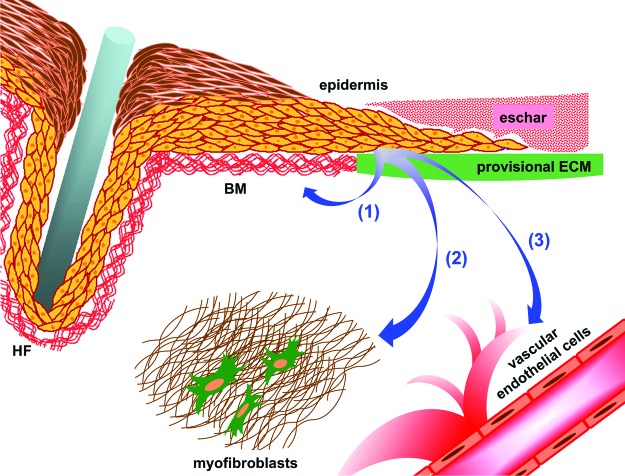
Figure 1.
A model depicting functions of wound epidermis that may be controlled by keratinocyte integrins. Arrow (1) indicates autonomous regulation of keratinocyte functions such as migration, proliferation, local matrix remodeling, and stable adhesion of the neo-epidermis. Arrows (2) and (3) indicate regulation of paracrine crosstalk from the epidermis to other cellular compartments, including myofibroblasts to control wound contraction (2) and vascular endothelial cells to promote wound angiogenesis (3). Components of the wound microenvironment are indicated. BM, basement membrane; HF, hair follicle; ECM, extracellular matrix. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Autonomous keratinocyte functions
Epidermal migration
Given their global importance in cell adhesion and motility, it seems intuitive that a major function of integrins during wound healing is the regulation of epidermal migration. Consistently, deletion of β1 integrins from the epidermis resulted in severely compromised wound re-epithelialization,45 and numerous cell culture studies have shown that individual integrins can regulate keratinocyte adhesion and migration on their respective ECM ligands.34,45,53,61,73,113,114 Nevertheless, results from in vivo wound healing studies in integrin knockout mice are surprisingly discordant with findings from cell culture models, and the roles for individual integrins in epidermal migration remain unclear. For example, αvβ6 and αvβ5 can each mediate keratinocyte migration on their respective ligands in vitro, yet absence of either or both integrins in vivo did not cause impaired wound healing in young adult mice (although deletion of αvβ6 caused delayed wound healing in older mice).46,115,116 Similarly, integrins α3β1, α2β1, and α9β1 can each mediate keratinocyte migration on relevant ligands in vitro, but their ablation in vivo had remarkably mild or no effects on wound re-epithelialization in adult mice.35,47–49 As already mentioned, in a striking example of discordance, α3β1 has been reported to promote migration of cultured keratinocytes on laminin-332,34,53 yet it is not essential for wound closure in vivo, at least in adult mice.35 In fact, deletion of α3β1 from the epidermis led to a slightly increased rate of wound closure,35 consistent with other studies using anti-α3β1 blocking antibodies that indicated a suppressive role in migration.89 Together, these disparate findings indicate a complex role for α3β1 in epidermal migration that is sensitive to ECM composition and may include modulation of the rate of re-epithelialization.
In some cases, these discrepancies between cell culture models and in vivo wound models may reflect the greater complexity of ECM in vivo, where multiple ligands in the wound bed might provide opportunity for compensation between integrins with overlapping migration functions, as discussed at length elsewhere.8 Such overlap makes sense from an evolutionary standpoint, given the importance of rapidly restoring epidermal barrier function after wounding. Consistent with this idea, mice with epidermis-specific deletion of all β1 integrins (i.e., β1-null) showed impaired wound re-epithelialization,45 while deletion of individual integrins did not,8,35,47–49 suggesting that there is considerable overlap in the ability of distinct β1 integrins to promote epidermal migration.
Although α6β4 can regulate the motility of cultured keratinocytes,72,73 it remains uncertain whether this integrin has important pro-migratory roles in wound re-epithelialization. It is clear that α6β4 is not sufficient for normal wound re-epithelialization in the absence of β1 integrins, since β1-null epidermis shows severe migration defects.45 Nevertheless, it remains possible that some pro-migratory/pro-invasive roles for α6β4 that have been described in carcinoma cells (mentioned previously) may extend to wound keratinocytes. In any case, a role for α6β4 in epidermal migration cannot yet be ruled out, since the severe epidermal blistering that occurs in α6β4-deficient mice presents a formidable challenge to wound healing experiments. More detailed discussions of potential roles for α6β4 in keratinocyte migration can be found in separate reviews8 (also, see the review by Hopkinson et al.117 in this issue).
Epidermal proliferation
Epidermal homeostasis is maintained by a resident population of stem cells within the basal cell layer that gives rise to committed progenitor cells, or transit-amplifying cells, which in turn replenish the differentiated keratinocytes that are eventually shed from the outer layer of the skin.37–39 While most of the proliferative cells that maintain homeostasis are thought to reside in the interfollicular epidermis, stem cells in the bulge of the hair follicle make the most substantial contribution to the replenishment of keratinocytes that accompanies wound re-epithelialization.118,119 β1 integrins as well as α6β4 are expressed at higher levels in epidermal stem cells, where they are thought to help control the balance between stem cell renewal and terminal differentiation.5,37,39,120–123 It follows that changes in integrin signaling or adhesion functions that shift this balance are likely to be important for wound healing. Consistently, integrin signaling through mitogen-activated protein kinases (MAPKs) or the Rho family GTPase, RAC1, has been linked to the maintenance of epidermal stem cells.32,33,123 As discussed in the section on other β1 integrins, integrin α9β1 is essential to maintain proper levels of keratinocyte proliferation during wound healing.49 Interestingly, a recent study in an SCC model revealed a novel role for α3β1 in the retention of the slow-cycling cells in the hair follicle bulge, where deletion of this integrin allowed these cells to detach from the BM and emigrate into the suprabasal layers where they terminally differentiated. This loss of slow-cycling cells resulted in reduced skin tumor formation, presumably because these are the same cell that would otherwise accumulate oncogenic or tumor suppressor mutations that eventually give rise to tumors.124 These findings might reflect similar functions for α3β1 or other integrins within the hair follicle stem cells that participate in epidermal regeneration during wound healing.
Epidermal survival
Presumably, wound keratinocytes must somehow maintain appropriate survival signals that prevent them from undergoing anoikis (i.e., apoptosis that is induced by reduced or inappropriate adhesion) as they lose contact with damaged BM and encounter the dramatically altered ECM of the wound bed. As mentioned above, the de novo expression of αvβ6 may prevent wound keratinocytes from undergoing anoikis, as this integrin is known to activate AKT survival pathways in SCC cells.125 In addition, α3β1-mediated adhesion has been shown to promote the survival of keratinocyte cell lines through pathways that involve FAK and ERK, suggesting a possible role in survival of wound keratinocytes.62 Adhesion through α6β4 has also been linked to epidermal cell survival,67 and it is conceivable that this integrin contributes to adhesion-dependent survival of the neo-epidermis when hemidesmosomes are assembled following wound closure. However, mice lacking both α3β1 and α6β4 (e.g., through combined α3-null and β4-null mutations) displayed apoptotic basal keratinocytes only in detached regions of epidermis, but not in non-blistered epidermis,51 indicating that other adhesion mechanisms are sufficient to protect from anoikis.
Local basement membrane deposition and assembly
The cutaneous BM not only provides a physical separation between the epidermal and dermal compartments of the skin, but it also provides cues for signaling pathways that regulate a variety of epidermal cell functions including polarization, differentiation, survival, tissue structure, and migration. Indeed, many of the keratinocyte functions discussed above are regulated by keratinocyte-mediated changes to the local ECM, including the BM, which as discussed here may occur through either deposition of ECM proteins or matrix proteolysis. Early studies in β1 integrin-deficient mice or embryoid bodies have revealed essential roles for certain β1integrins in proper BM formation in embryonic or adult tissues.50,126,127 In vivo and in vitro studies from our group and others collectively support roles for integrin α3β1 in several steps that may be important for the regeneration of a stable BM during wound healing, providing an example of how some integrins may regulate the ability of the epidermis to regulate ECM organization and assembly (Fig. 2). Indeed, as already mentioned, α3β1 regulates laminin-332 organization in cultured keratinocytes.74 Moreover, we recently demonstrated that mice lacking epidermal α3β1 display blisters in newly regenerated wound epidermis,91 which are characterized by BM rupture that phenocopies the epidermal blisters in neonatal α3-null mice,50 indicating that the developmental role for α3β1 in maintaining BM integrity is recapitulated during adult wound healing. Interestingly, this blistering phenotype was linked to reduced fibulin-2 levels in both neonatal skin and early wounds, and genetic deletion of fibulin-2 (i.e., in the presence of α3β1) was sufficient to generate neonatal skin blisters.91 Since fibulin-2 binds to the laminin-γ2 chain within the L4 module near the N-terminus,128 and this interaction has been implicated in stable incorporation of laminin-332 into the developing BM,129 these findings suggest an important role for α3β1-dependent fibulin-2 expression in BM maturation (Fig. 2).
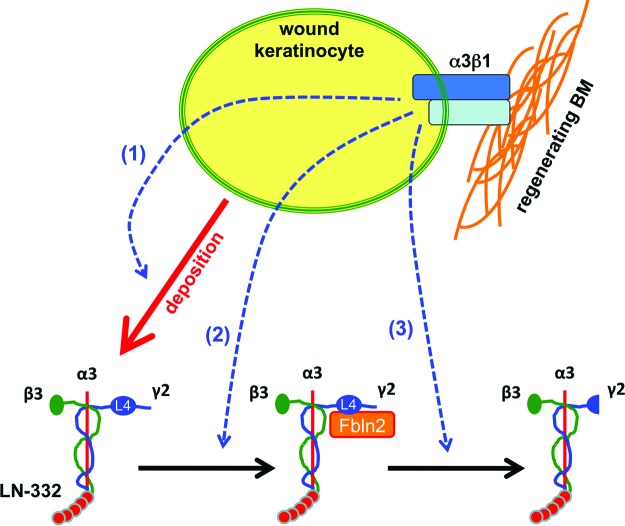
Figure 2.
A model depicting possible roles for epidermal integrin α3β1 in regeneration of BM during wound healing. In vivo and cell culture studies have implicated α3β1 in several different steps that may impact the stable assembly of laminin-332 (LN-332) into BM, including (1) LN-332 deposition, (2) expression of the LN-332-binding protein fibulin-2 (Fbln2), and (3) proteolytic processing of the LN-332 γ2 chain (see text for detailed discussion and supporting references). Other epidermal integrins may have similar roles in the incorporation of LN-332 or other BM proteins. The α3, β3, and γ2 chains of LN-332 trimer, as well as the L4 module on the γ2 chain, are indicated. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Integrins can also control matrix proteolysis by the epidermis, as supported by the ability of several integrins to control the expression or functions of MMPs or other ECM-degrading extracellular proteases (discussed below). While a variety of ECM proteins present in the provisional wound matrix undergo proteolysis, we will focus on laminin-332 as an example since both its α3 and γ2 chains undergo extensive proteolytic processing.130–133 Although the importance of these processing events in vivo are not yet fully understood, some have been shown to influence the migratory behaviors of cultured keratinocytes. For example, differential processing of the laminin α3 chain, regulated by tissue-type plasminogen activator and plasmin, was shown to alter epithelial cell motility.64,134 Laminin-332 proteolysis is also likely to be an important mode of regulating BM architecture through modulation of key linkages with other ECM proteins, such as the interaction of fibulin-2 with laminin-332 described above.58 Indeed, the L4 module to which fibulin-2 binds is lost upon proteolytic processing of γ2, which might be an important step for BM maturation.128,129,132 Interestingly, recent data from our laboratory indicates a role for epidermal α3β1 in the regulation of laminin-γ2 chain processing, both in vivo during wound healing and in keratinocytes cultured under conditions of high calcium.91 Thus, α3β1 may have a dual role in BM assembly that involves both matrix protein deposition and proteolytic processing of matrix proteins (Fig. 2).
Epidermis-mediated modulation of the wound microenvironment
Most studies of keratinocyte integrins have focused on their “classical” roles in regulating cell-autonomous processes such as motility, proliferation, local matrix assembly/remodeling, and survival, as discussed above. However, epidermal integrins may have far-reaching effects on other aspects of the wound microenvironment, including modification of the ECM in distal parts of the wound, and crosstalk to distal cells with essential roles in wound healing (i.e., endothelial cells, fibroblasts). Consistently, some keratinocyte integrins can modulate the expression of genes that are implicated in paracrine crosstalk to other cells.36,47,135,136 Indeed, our own studies have identified α3β1-dependent regulation of a number of genes in keratinocyte cell lines that encode growth factors, extracellular proteases, or ECM/matricellular proteins with known roles in modulating the microenvironment of wounds or tumors36,79,137 (Missan and DiPersio, unpublished observation). In this section, we will discuss these more recently appreciated roles for certain epidermal integrins in modification of the wound microenvironment, including paracrine functions that influence extra-epidermal compartments of the wound.
Proteolysis and remodeling of the wound extracellular matrix
While the effects of local matrix remodeling on epidermal functions are clear, long-range effects of altered wound ECM are also important. Indeed, controlled ECM remodeling is an essential feature of normal wound healing,138 and defects in ECM organization are associated with chronic wounds.139 It has long been known that changes in matrix composition or structure can act upstream of integrins through ligation that turns on intracellular signaling pathways. However, integrin–ECM signals should more accurately be considered as bidirectional, or even as signaling loops, as many integrins have been shown to alter ECM composition/structure at the levels of both gene expression and matrix assembly. For example, integrin α2β1 has been shown to be a positive regulator of type-1 collagen gene expression,140 while α1β1 suppresses collagen synthesis in the dermis.141 Furthermore, both fibronectin-binding integrins and ILK have been shown to support fibronectin matrix assembly and fibrillogenesis.142,143
The dynamic regulation of MMPs and other extracellular proteases can also contribute to changes in ECM that are crucial for successful wound healing. Indeed, MMPs are involved in all stages of wound resolution, from early removal of damaged ECM to late stage scar remodeling,144 and defects in MMP expression or function contribute to the pathogenesis of chronic wounds.139,145 Furthermore, MMPs are increasingly credited with the proteolytic release of important growth factors from the ECM or cell surface,144,146 thereby supplying the wound microenvironment with the appropriate effector molecules to promote healing. Not surprisingly, several integrins that are expressed during wound healing (Table 1) have been implicated in modulating MMP expression in keratinocytes or other cells, suggesting similar roles in the wound microenvironment. Examples include α3β1-mediated induction of MMP-9 or uPA in keratinocytes,79,137 α2β1-mediated induction of MMP-1 in osteogenic cells,140 and α5β1-mediated regulation of MMP-3 and MMP-9 in some cells.147
Paracrine crosstalk to other cellular compartments
It is well known that a complex network of communication exists between the various cell types that reside in the wound microenvironment. The epidermis can send paracrine signals to other cellular compartments that contribute to wound healing, including the vasculature and the stroma (as depicted in Fig. 1), and recent evidence suggests that some of these signals are regulated by keratinocyte integrins.32,62,122,123 Such intercellular signals may be propagated from the epidermis to other cells through physical changes in the ECM (i.e., mechanical signaling), through diffusible growth factors that are secreted by the epidermis, or through generation of bioactive fragments following ECM proteolysis.
An important mode of intercellular crosstalk within the wound microenvironment is growth factor–mediated communication. Although keratinocytes reside in the epidermal compartment of skin and are spatially separated from the stromal compartment, it has long been known that growth factors and cytokines produced de novo by the epidermis can diffuse to other cellular compartments of the wound.148 Thus, the epidermis is able to influence other cell types (i.e., endothelial cells, fibroblasts, immune cells) by regulating the availability of growth factors such as VEGF, TGFβ, and KGF. Some integrins can regulate the expression of growth factors by keratinocytes, which can then mediate paracrine stimulation of other cell types. For instance, deletion of α3β1 from cultured keratinocytes or epidermis reduced the expression of the pro-angiogenic factor mitogen-regulated protein 3 (MRP-3), which contributed to reduced stimulation of endothelial cell migration in vitro and impaired wound angiogenesis in vivo.36 Conversely, it has been shown in α2-null mice that ablation of α2β1 (albeit in all cell types at once) results in neovascular enhancement in both wounds and sponge implants, indicating an anti-angiogenic role for this integrin that might involve intercellular crosstalk.47 These findings demonstrate that epidermal integrins may regulate keratinocyte-to-endothelial cell crosstalk to modulate wound angiogenesis, and that coordinated activities of different integrins may be important for proper outcome. As integrin–ECM interactions are likely to be regulated temporally during wound healing, we speculate that integrins might regulate the ability of epidermis to influence not only angiogenic growth, but also blood vessel regression and vascular normalization at later stages of wound healing.
There is also published evidence to support a role for crosstalk from keratinocytes to mesenchymal fibroblasts during wound healing. Indeed, delays in wound re-epithelialization were associated with enhanced wound fibrosis, possibly due to a lack of signaling from epidermis, which may play a role in hypertrophic scar development.135,149 Studies in co-culture models have demonstrated that many fibroblast genes are regulated by keratinocyte-derived factors, including genes coding for growth factors, ECM components, and MMPs,136 all vital constituents for successful wound healing. Integrin α3β1 is an intriguing candidate for influencing keratinocyte-to-fibroblast crosstalk in cutaneous wounds, given its above described role in promoting keratinocyte-to-endothelial cell crosstalk.36 For example, in a murine model of lung fibrosis, ablation of α3β1 in lung epithelial cells resulted in reduced β-catenin/Smad signaling, accompanied by decreased accumulation of lung myofibroblasts.83
Some epidermal integrins may control the bioavailability of ECM-bound growth factors or bioactive ECM fragments, either directly or indirectly, that influence the behaviors of other cell types. A direct role for certain αv integrins in the local activation of the ECM-associated latent TGFβ complex has already been discussed in an earlier section.104 While the extent to which the latter mechanism might render an activated growth factor available to a distal cellular compartment remains unclear, there are also examples of integrin-mediated liberation and diffusion of ECM-bound growth factors. For example, certain integrins (i.e., αvβ6 and α3β1) can induce expression of MMP-9, uPA, or other extracellular proteases79,101,137,150 that can degrade ECM and release reservoirs of ECM-associated growth factors (i.e., VEGF) to promote angiogenesis.151,152 In addition, degradation of laminins, collagens, or other ECM proteins by some of these extracellular proteases may lead to the generation of bioactive matrix fragments that can directly stimulate cell growth or motility.152,153
Finally, changes in ECM composition or structure that alter the physical properties (i.e., stiffness) of a tissue can influence cell function through mechanical signals. It follows that integrin-dependent changes in ECM, brought about by deposition of matrix proteins or matrix proteolysis, might mediate intercellular crosstalk from the epidermis to other wound cells through mechanical signaling. Mechanisms whereby different cell types use mechanical signals to communicate with one another are still poorly understood. However, mounting evidence supports an important role for alterations in matrix compliance and mechanical stress in the pathogenesis of chronic wound healing,1 and the current use of topical negative pressure devices as an adjuvant therapy for chronic wounds may promote healing through increased mechanical tension in the wound.154,155 Moreover, defects in ECM deposition or proteolytic processing that are likely to abberantly alter the mechanical properties of the ECM are thought to contribute to the pathogenesis of chronic wounds.156,157
Future Directions
Exploiting integrins as therapeutic targets in wound healing
The ever-growing elderly and diabetic populations in the United States create a continuously increasing demand for effective wound healing therapies, which have yet to be identified. Impaired re-epithelialization is a hallmark of chronic wounds and poses a major clinical concern due to increased susceptibility of patients to infection. Prolonged treatment of chronic wounds, as well as infection due to slow healing, places a substantial financial burden on the U.S., and negatively impacts quality of life for the patient. Mounting evidence supports the idea that changes or defects in integrin-dependent keratinocyte functions contribute to the pathogenesis of chronic wounds, providing a strong rationale for exploiting epidermal integrins as therapeutic targets for their treatment.
Chronic wounds are often characterized by persistent inflammation, unhealthy granulation tissue, and impaired re-epithelialization.139 The mechanisms that prevent the healing of chronic wounds are unknown. However, it is clear that the ECM is compromised in chronic wounds, and aberrant changes in ECM or its receptors are implicated in the pathology of chronic wounds, as reviewed elsewhere.139 For example, it has been postulated that elevated levels of pro-inflammatory cytokines in chronic wounds results in atypical secretion/activation of extracellular proteases, which in turn may lead to “suboptimal ECM composition” and reduced integrin ligation that does not support normal growth factor activity.139 In addition, diabetic foot ulcers often display up-regulation of MMPs 1, 2, 8, and 9, and concurrently reduced levels of tissue inhibitor of metalloprotease-2 (TIMP-2) compared to traumatic wounds.139,145 Moreover, deregulation of syndecan-1 and syndecan-4 have been detected in venous leg ulcers.158 Fibronectin deficiency, most likely resulting from enhanced ECM degradation, has been reported to occur in chronic wounds where it may impair cell migration.139,159,160 Moreover, fibronectin fragments (generated by fibronectin degradation) have been shown to modulate levels of MMPs and TIMPs in some models,139,161 and have been reported to occur in chronic wound fluids.162–164
As bidirectional signaling receptors that regulate both keratinocyte-mediated changes to the wound microenvironment, and keratinocyte responses to those microenvironmental changes, integrins are attractive targets for therapeutic strategies to promote wound healing or to treat chronic wounds. The general concept of therapeutically targeting integrin function is already well established. For example, the integrin-blocking agent Cilengitide, an RGD mimetic, has shown promise in clinical trials involving the treatment of recurrent glioma.165,166 It is well known that wound healing and skin carcinogenesis share similarities regarding both epidermal cell function and microenvironmental factors that drive each process,57 and integrin-targeting therapies are relevant to the treatment of chronic wounds for many of the same reasons that they are relevant to the treatment of cancer. For example, we have already discussed how roles for some integrins within the stem cell compartment may be important during both wound re-epithelialization and skin carcinogenesis (see Epidermal proliferation).
Integrins that are upregulated in chronic wounds might serve as particularly attractive therapeutic targets. For example, integrin αvβ6 is not normally expressed in epidermis but is induced during normal wound healing and has been shown to be strongly upregulated in chronic wounds of human patients.167 In the same study, transgenic mice that constitutively over-expressed β6 integrin in the epithelium showed no change in wound closure rate but spontaneously developed chronic fibrotic ulcers,167 while a separate study showed that diabetic β6-null mice showed delays in early wound closure.168 These findings indicate complex roles for integrin αvβ6 in wound healing that are likely to be precisely timed and may require the correct extracellular milieu. More work in this area must be done to tease apart and fully understand the precise functions of particular integrins in normal wound healing, then identify which of these functions are deficient in chronic wounds, in order to fully exploit integrins as therapeutic targets.
In addition to direct targeting of integrins, rational wound healing therapies might exploit integrins as a means of targeted therapeutic delivery. For instance, one group recently generated an injectable complex containing platelet derived growth factor B (PDGF-B) plasmid DNA and an integrin-selective RGDK-lipopeptide, towards the goal of delivering PDGF-B directly to relevant integrin-decorated cells within the wound bed, thereby circumventing the modest efficacy achieved through repeated topical application.169 A single, subcutaneous injection of this complex was demonstrated to promote wound healing in a streptozotocin-induced diabetic rat model of chronic wounds, resulting in enhanced re-epithelialization, collagen fibrillogenesis, and blood vessel formation.169 Thus, it is possible that similar strategies can be developed to direct therapeutic agents to wound epidermis using lipopeptides that recognize keratinocyte integrins.
In summary, while the field has made good progress in identifying functions of individual keratinocyte integrins, and understanding how their coordinated activities might control the range of epidermal functions that are required for normal wound healing, some formidable challenges lie ahead as we attempt to translate this knowledge into therapeutic approaches for wound healing deficiencies in human patients. Considering that several epidermal integrins are required for different epidermal functions, one challenge will be to develop multicombinatorial strategies to target several integrins. An additional challenge is that the relevant ligands in the wound microenvironment have not yet been identified for all epidermal integrins. Finally, as already mentioned, it is likely that the control of diverse epidermal functions by individual integrins is precisely timed, in order to maintain properly coordinated regulation of epidermal wound functions.
Take-Home Messages.
• Integrins are bidirectional signaling receptors that regulate both keratinocyte-mediated changes to the wound microenvironment and keratinocyte responses to microenvironmental changes.
• In addition to controlling many cell-autonomous keratinocyte functions such as migration, proliferation, and survival, some integrins can regulate the ability of epidermis to cross-talk to other cellular compartments, such as endothelial cells and fibroblasts, in a paracrine fashion.
• The dynamic regulation of ECM composition during wound healing is critical to coordinate integrin activity in a spatiotemporal manner in order to successfully promote wound healing. Alternatively, inappropriate ECM composition/mechanics are known to contribute to the pathogenesis of chronic wounds.
• Chronic wounds display altered integrin expression, as well as changes in the extracellular milieu, which can both be caused by, and contribute to, inappropriate integrin signaling or aberrant MMP activity.
• Integrins expressed on wound epidermis represent potential therapeutic targets for the treatment of chronic wounds, although some formidable challenges lie ahead before we can fully exploit integrins as clinical targets.
Abbreviations and Acronyms
- BM
basement membrane
- ECM
extracellular matrix
- EGF
epidermal growth factor
- FAK
focal adhesion kinase
- GTPase
guanosine triphosphatase
- ILK
integrin-linked kinase
- JEB
junctional epidermolysis bullosa
- LAP
latency-associated protein
- LTBP
latent TGFβ binding protein
- MAPK
mitogen-activated protein kinases
- MMP
matrix metalloprotease
- PDGF-B
platelet derived growth factor B
- PI3-K
phosphatidylinositol 3-kinase
- PLC
phospholipase C
- SCC
squamous cell carcinoma
- SH2
SRC-homolog 2
- TGFβ
transforming growth factor beta
- TIMP
tissue inhibitor of metalloprotease
- uPAR
urokinase receptor
- VEGF
vascular endothelial growth factor
Acknowledgments
The authors are grateful to Dr. Livingston Van De Water for critical reading of the manuscript, as well as to other colleagues at Albany Medical College and members of the DiPersio laboratory for valuable discussions and insights. Research in the DiPersio laboratory is supported by NIH grants from NCI (R01CA129637 to C.M. DiPersio) and NIAMS (R01AR063778 to C.M. DiPersio and L. Van De Water). Whitney Longmate is supported by an NRSA predoctoral fellowship from the National Institutes of Health (F31CA174198). We offer our apologies to the many researchers whose valuable contributions to the field could not be cited due to space constraints.
Author Disclosure and Ghostwriting Statement
The authors declare no competing financial interests or other conflicts of interest. No ghostwriters were involved in the preparation of this manuscript.
About the Authors
The authors are members of the Center for Cell Biology and Cancer Research (CCBCR) at Albany Medical College, Albany, New York. Michael DiPersio is a Professor in CCBCR whose research program is focused on investigating molecular mechanisms whereby integrins regulate extracellular matrix remodeling and gene expression in epidermal keratinocytes and carcinoma cells. Whitney Longmate is a predoctoral student in the DiPersio laboratory.
References
- 1.Wong VW, Gurtner GC, and Longaker MT: Wound healing: a paradigm for regeneration. Mayo Clin Proc 2013; 88:1022. [DOI] [PubMed] [Google Scholar]
- 2.Giancotti FG. and Ruoslahti E: Integrin signaling. Science 1999; 285:1028. [DOI] [PubMed] [Google Scholar]
- 3.Hynes RO: Integrins: bidirectional, allosteric signaling machines. Cell 2002; 110:673. [DOI] [PubMed] [Google Scholar]
- 4.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. : Cell migration: integrating signals from front to back. Science 2003; 302:1704. [DOI] [PubMed] [Google Scholar]
- 5.Janes SM. and Watt FM: New roles for integrins in squamous-cell carcinoma. Nat Rev Cancer 2006; 6:175. [DOI] [PubMed] [Google Scholar]
- 6.Ziober BL, Silverman SS, Jr, and Kramer RH: Adhesive mechanisms regulating invasion and metastasis in oral cancer. Crit Rev Oral Biol Med 2001; 12:499. [DOI] [PubMed] [Google Scholar]
- 7.Kramer RH, Shen X, and Zhou H: Tumor cell invasion and survival in head and neck cancer. Cancer Metastasis Rev 2005; 24:35. [DOI] [PubMed] [Google Scholar]
- 8.Margadant C, Charafeddine RA, and Sonnenberg A: Unique and redundant functions of integrins in the epidermis. FASEB J 2010; 24:4133. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Calderwood DA, and Ginsberg MH: Integrin cytoplasmic domain-binding proteins. J Cell Sci 2000; 113:3563. [DOI] [PubMed] [Google Scholar]
- 10.Litjens SH, de Pereda JM, and Sonnenberg A: Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol 2006; 16:376. [DOI] [PubMed] [Google Scholar]
- 11.Delon I. and Brown NH: Integrins and the actin cytoskeleton. Curr Opin Cell Biol 2007; 19:43. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz MA. and Ginsberg MH: Networks and crosstalk: integrin signalling spreads. Nat Cell Biol 2002; 4:E65. [DOI] [PubMed] [Google Scholar]
- 13.Legate KR. and Fassler R: Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci 2009; 122:187. [DOI] [PubMed] [Google Scholar]
- 14.Askari JA, Buckley PA, Mould AP, and Humphries MJ: Linking integrin conformation to function. J Cell Sci 2009; 122:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porter JC. and Hogg N: Integrins take partners: cross-talk between integrins and other membrane receptors. Trends Cell Biol 1998; 8:390. [DOI] [PubMed] [Google Scholar]
- 16.Chapman HA, Wei Y, Simon DI, and Waltz DA: Role of urokinase receptor and caveolin in regulation of integrin signaling. Thromb Haemost 1999; 82:291. [PubMed] [Google Scholar]
- 17.Berditchevski F: Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci 2001; 114:4143. [DOI] [PubMed] [Google Scholar]
- 18.Hemler ME: Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol 2005; 6:801. [DOI] [PubMed] [Google Scholar]
- 19.Del Pozo MA. and Schwartz MA: Rac, membrane heterogeneity, caveolin and regulation of growth by integrins. Trends Cell Biol 2007; 17:246. [DOI] [PubMed] [Google Scholar]
- 20.Salanueva IJ, Cerezo A, Guadamillas MC, and del Pozo MA: Integrin regulation of caveolin function. J Cell Mol Med 2007; 11:969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comoglio PM, Boccaccio C, and Trusolino L: Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr Opin Cell Biol 2003; 15:565. [DOI] [PubMed] [Google Scholar]
- 22.Ffrench-Constant C. and Colognato H: Integrins: versatile integrators of extracellular signals. Trends Cell Biol 2004; 14:678. [DOI] [PubMed] [Google Scholar]
- 23.Guo W. and Giancotti FG: Integrin signalling during tumour progression. Nat Rev Mol Cell Biol 2004; 5:816. [DOI] [PubMed] [Google Scholar]
- 24.Cary LA. and Guan JL: Focal adhesion kinase in integrin-mediated signaling. Front Biosci 1999; 4:D102. [DOI] [PubMed] [Google Scholar]
- 25.Mitra SK. and Schlaepfer DD: Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol 2006; 18:516. [DOI] [PubMed] [Google Scholar]
- 26.Choma DP, Milano V, Pumiglia KM, and DiPersio CM: Integrin alpha3beta1-dependent activation of FAK/Src regulates Rac1-mediated keratinocyte polarization on laminin-5. J Invest Dermatol 2007; 127:31. [DOI] [PubMed] [Google Scholar]
- 27.Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, et al. : FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol 2000; 2:249. [DOI] [PubMed] [Google Scholar]
- 28.Gilcrease MZ: Integrin signaling in epithelial cells. Cancer Lett 2007; 247:1. [DOI] [PubMed] [Google Scholar]
- 29.McLean GW, Komiyama NH, Serrels B, Asano H, Reynolds L, Conti F, et al. : Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev 2004; 18:2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Essayem S, Kovacic-Milivojevic B, Baumbusch C, McDonagh S, Dolganov G, Howerton K, et al. : Hair cycle and wound healing in mice with a keratinocyte-restricted deletion of FAK. Oncogene 2006; 25:1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsia DA, Mitra SK, Hauck CR, Streblow DN, Nelson JA, Ilic D, et al. : Differential regulation of cell motility and invasion by FAK. J Cell Biol 2003; 160:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haase I, Hobbs RM, Romero MR, Broad S, and Watt FM: A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis. J Clin Invest 2001; 108:527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benitah SA, Frye M, Glogauer M, and Watt FM: Stem cell depletion through epidermal deletion of Rac1. Science 2005; 309:933. [DOI] [PubMed] [Google Scholar]
- 34.Choma DP, Pumiglia K, and DiPersio CM: Integrin alpha3beta1 directs the stabilization of a polarized lamellipodium in epithelial cells through activation of Rac1. J Cell Sci 2004; 117:3947. [DOI] [PubMed] [Google Scholar]
- 35.Margadant C, Raymond K, Kreft M, Sachs N, Janssen H, and Sonnenberg A: Integrin alpha3beta1 inhibits directional migration and wound re-epithelialization in the skin. J Cell Sci 2009; 122:278. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell K, Szekeres C, Milano V, Svenson KB, Nilsen-Hamilton M, Kreidberg JA, et al. : Alpha3beta1 integrin in epidermis promotes wound angiogenesis and keratinocyte-to-endothelial-cell crosstalk through the induction of MRP3. J Cell Sci 2009; 122:1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watt FM: Role of integrins in regulating epidermal adhesion, growth and differentiation. Embo J 2002; 21:3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owens DM. and Watt FM: Contribution of stem cells and differentiated cells to epidermal tumours. Nat Rev Cancer 2003; 3:444. [DOI] [PubMed] [Google Scholar]
- 39.Fuchs E: Skin stem cells: rising to the surface. J Cell Biol 2008; 180:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas GJ, Nystrom ML, and Marshall JF: Alphavbeta6 integrin in wound healing and cancer of the oral cavity. J Oral Pathol Med 2006; 35:1. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen BP, Ryan MC, Gil SG, and Carter WG: Deposition of laminin 5 in epidermal wounds regulates integrin signaling and adhesion. Curr Opin Cell Biol 2000; 12:554. [DOI] [PubMed] [Google Scholar]
- 42.Chen JS, Longaker MT, and Gurtner GC: Murine models of human wound healing. Methods Mol Biol 2013; 1037:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano JL, Pirro A, et al. : Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. Embo J 2000; 19:3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raghavan S, Bauer C, Mundschau G, Li Q, and Fuchs E: Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol 2000; 150:1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grose R, Hutter C, Bloch W, Thorey I, Watt FM, Fassler R, et al. : A crucial role of beta 1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development 2002; 129:2303. [DOI] [PubMed] [Google Scholar]
- 46.Huang X, Griffiths M, Wu J, Farese RV, Jr, and Sheppard D: Normal development, wound healing, and adenovirus susceptibility in beta5-deficient mice. Mol Cell Biol 2000; 20:755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zweers MC, Davidson JM, Pozzi A, Hallinger R, Janz K, Quondamatteo F, et al. : Integrin alpha2beta1 is required for regulation of murine wound angiogenesis but is dispensable for reepithelialization. J Invest Dermatol 2007; 127:467. [DOI] [PubMed] [Google Scholar]
- 48.Grenache DG, Zhang Z, Wells LE, Santoro SA, Davidson JM, and Zutter MM: Wound healing in the alpha2beta1 integrin-deficient mouse: altered keratinocyte biology and dysregulated matrix metalloproteinase expression. J Invest Dermatol 2007; 127:455. [DOI] [PubMed] [Google Scholar]
- 49.Singh P, Chen C, Pal-Ghosh S, Stepp MA, Sheppard D, and Van De Water L: Loss of integrin alpha9beta1 results in defects in proliferation, causing poor re-epithelialization during cutaneous wound healing. J Invest Dermatol 2009; 129:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DiPersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, and Hynes RO: α3β1 integrin is required for normal development of the epidermal basement membrane. J Cell Biol 1997; 137:729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DiPersio CM, van der Neut R, Georges-Labouesse E, Kreidberg JA, Sonnenberg A, and Hynes RO: alpha3beta1 and alpha6beta4 integrin receptors for laminin-5 are not essential for epidermal morphogenesis and homeostasis during skin development. J Cell Sci 2000; 113:3051. [DOI] [PubMed] [Google Scholar]
- 52.Hamill KJ, Hopkinson SB, Hoover P, Todorovic V, Green KJ, and Jones JC: Fibronectin expression determines skin cell motile behavior. J Invest Dermatol 2012; 132:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frank DE. and Carter WG: Laminin 5 deposition regulates keratinocyte polarization and persistent migration. J Cell Sci 2004; 117:1351. [DOI] [PubMed] [Google Scholar]
- 54.Carter WG, Ryan MC, and Gahr PJ: Epiligrin, a new cell adhesion ligand for integrin α3β1 in epithelial basement membranes. Cell 1991; 65:599. [DOI] [PubMed] [Google Scholar]
- 55.Giannelli G, Astigiano S, Antonaci S, Morini M, Barbieri O, Noonan DM, et al. : Role of the alpha3beta1 and alpha6beta4 integrins in tumor invasion. Clin Exp Metastasis 2002; 19:217. [DOI] [PubMed] [Google Scholar]
- 56.Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, Russell AJ, et al. : NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature 2003; 421:639. [DOI] [PubMed] [Google Scholar]
- 57.Dvorak HF: Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986; 315:1650. [DOI] [PubMed] [Google Scholar]
- 58.Aumailley M, El Khal A, Knoss N, and Tunggal L: Laminin 5 processing and its integration into the ECM. Matrix Biol 2003; 22:49. [DOI] [PubMed] [Google Scholar]
- 59.Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, et al. : A simplified laminin nomenclature. Matrix Biol 2005; 24:326. [DOI] [PubMed] [Google Scholar]
- 60.Okamoto O, Bachy S, Odenthal U, Bernaud J, Rigal D, Lortat-Jacob H, et al. : Normal human keratinocytes bind to the alpha3LG4/5 domain of unprocessed laminin-5 through the receptor syndecan-1. J Biol Chem 2003; 278:44168. [DOI] [PubMed] [Google Scholar]
- 61.Carter WG, Kaur P, Gil SG, Gahr PJ, and Wayner EA: Distinct functions for integrins α3β1 in focal adhesions and α6β4/bullous antigen in a new stable anchoring contact (SAC) of keratinocytes: relation to hemidesmosomes. J Cell Biol 1990; 111:3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manohar A, Shome SG, Lamar J, Stirling L, Iyer V, Pumiglia K, et al. : Alpha 3 beta 1 integrin promotes keratinocyte cell survival through activation of a MEK/ERK signaling pathway. J Cell Sci 2004; 117:4043. [DOI] [PubMed] [Google Scholar]
- 63.Kariya Y, Yasuda C, Nakashima Y, Ishida K, Tsubota Y, and Miyazaki K: Characterization of laminin 5B and NH2-terminal proteolytic fragment of its alpha3B chain: promotion of cellular adhesion, migration, and proliferation. J Biol Chem 2004; 279:24774. [DOI] [PubMed] [Google Scholar]
- 64.Goldfinger LE, Stack MS, and Jones JCR: Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J Cell Biol 1998; 141:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, and Le Meur M: Absence of integrin α6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet 1996; 13:370. [DOI] [PubMed] [Google Scholar]
- 66.van der Neut R, Krimpenfort P, Calafat J, Niessen CM, and Sonnenberg A: Epithelial detachment due to absence of hemidesmosomes in integrin β4 null mice. Nat Genet 1996; 13:366. [DOI] [PubMed] [Google Scholar]
- 67.Dowling J, Yu Q-C, and Fuchs E: β4 integrin is required for hemidesmosomal formation, cell adhesion and cell survival. J Cell Biol 1996; 134:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vidal F, Aberdam D, Miquel C, Christiano AM, Pulkkinen L, Uitto J, et al. : Integrin beta 4 mutations associated with junctional epidermolysis bullosa with pyloric atresia. Nat Genet 1995; 10:229. [DOI] [PubMed] [Google Scholar]
- 69.Ruzzi L, Gagnoux-Palacios L, Pinola M, Belli S, Meneguzzi G, D'Alessio M, et al. : A homozygous mutation in the integrin alpha6 gene in junctional epidermolysis bullosa with pyloric atresia. J Clin Invest 1997; 99:2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Has C, Sparta G, Kiritsi D, Weibel L, Moeller A, Vega-Warner V, et al. : Integrin alpha3 mutations with kidney, lung, and skin disease. N Engl J Med 2012; 366:1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mariotti A, Kedeshian PA, Dans M, Curatola AM, Gagnoux-Palacios L, Giancotti FG: EGF-R signaling through Fyn kinase disrupts the function of integrin alpha6beta4 at hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J Cell Biol 2001; 155:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Russell AJ, Fincher EF, Millman L, Smith R, Vela V, Waterman EA, et al. : Alpha 6 beta 4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of alpha 3 beta 1 integrin. J Cell Sci 2003; 116:3543. [DOI] [PubMed] [Google Scholar]
- 73.Sehgal BU, DeBiase PJ, Matzno S, Chew TL, Claiborne JN, Hopkinson SB, et al. : Integrin beta4 regulates migratory behavior of keratinocytes by determining laminin-332 organization. J Biol Chem 2006; 281:35487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.deHart GW, Healy KE, and Jones JC.: The role of alpha3beta1 integrin in determining the supramolecular organization of laminin-5 in the extracellular matrix of keratinocytes. Exp Cell Res 2003; 283:67. [DOI] [PubMed] [Google Scholar]
- 75.Hamelers IH, Olivo C, Mertens AE, Pegtel DM, van der Kammen RA, Sonnenberg A, et al. : The Rac activator Tiam1 is required for (alpha)3(beta)1-mediated laminin-5 deposition, cell spreading, and cell migration. J Cell Biol 2005; 171:871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gonzales M, Haan K, Baker SE, Fitchmun M, Todorov I, Weitzman S, et al. : A cell signal pathway involving laminin-5, α3β1 integrin, and mitogen-activated protein kinase can regulate epithelial cell proliferation. Mol Biol Cell 1999; 10:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sugiura T. and Berditchevski F: Function of α3β1-tetraspan protein complexes in tumor cell invasion. Evidence for the role of the complexes in production of matrix metalloproteinase 2 (MMP-2). J Cell Biol 1999; 146:1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DiPersio CM, Shao M, Di Costanzo L, Kreidberg JA, and Hynes RO: Mouse keratinocytes immortalized with large T antigen acquire α3β1 integrin-dependent secretion of MMP-9/gelatinase B. J Cell Sci 2000; 113:2909. [DOI] [PubMed] [Google Scholar]
- 79.Ghosh S, Brown R, Jones JC, Ellerbroek SM, and Stack MS: Urinary-type plasminogen activator (uPA) expression and uPA receptor localization are regulated by alpha 3 beta 1 integrin in oral keratinocytes. J Biol Chem 2000; 275:23869. [DOI] [PubMed] [Google Scholar]
- 80.Berditchevski F, Zutter MM, and Hemler ME: Characterization of novel complexes on the cell surface between integrins and proteins with 4 transmembrane domains (TM4 proteins). Mol Biol Cell 1996; 7:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wei Y, Eble JA, Wang Z, Kreidberg JA, and Chapman HA: Urokinase receptors promote beta1 integrin function through interactions with integrin alpha3beta1. Mol Biol Cell 2001; 12:2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chattopadhyay N, Wang Z, Ashman LK, Brady-Kalnay SM, and Kreidberg JA: Alpha3beta1 integrin-CD151, a component of the cadherin-catenin complex, regulates PTPmu expression and cell-cell adhesion. J Cell Biol 2003; 163:1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim Y, Kugler MC, Wei Y, Kim KK, Li X, Brumwell AN, et al. : Integrin alpha3beta1-dependent beta-catenin phosphorylation links epithelial Smad signaling to cell contacts. J Cell Biol 2009; 184:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yauch RL, Kazarov AR, Desai B, Lee RT, and Hemler ME: Direct extracellular contact between integrin α3β1 and TM4SF protein CD151. J Biol Chem 2000; 275:9230. [DOI] [PubMed] [Google Scholar]
- 85.Yauch RL, Berditchevsky F, Harler MB, Reichner J, and Hemler ME: Highly stoichiometric, stable, and specific association of integrin α3β1 with CD151 provides a major link to phosphatidylinositol 4-kinase, and may regulate cell migration. Mol Biol Cell 1998; 9:2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Winterwood NE, Varzavand A, Meland MN, Ashman LK, and Stipp CS: A critical role for tetraspanin CD151 in alpha3beta1 and alpha6beta4 integrin-dependent tumor cell functions on laminin-5. Mol Biol Cell 2006; 17:2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cowin AJ, Adams D, Geary SM, Wright MD, Jones JC, and Ashman LK: Wound healing is defective in mice lacking tetraspanin CD151. J Invest Dermatol 2006; 126:680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hodivala-Dilke KM, DiPersio CM, Kreidberg JA, and Hynes RO: Novel roles for α3β1 integrin as a regulator of cytoskeletal assembly and as a transdominant inhibitor of integrin receptor function in keratinocytes. J Cell Biol 1998; 142:1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim JP, Zhang K, Kramer RH, Schall TJ, and Woodley DT: Integrin receptors and RGD sequences in human keratinocyte migration: unique anti-migratory function of alpha 3 beta 1 epiligrin receptor. J Invest Dermatol 1992; 98:764. [DOI] [PubMed] [Google Scholar]
- 90.Reynolds LE, Conti FJ, Silva R, Robinson SD, Iyer V, Rudling R, et al. : alpha3beta1 integrin-controlled Smad7 regulates reepithelialization during wound healing in mice. J Clin Invest 2008; 118:965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Longmate WM, Monichan R, Chu ML, Tsuda T, Mahoney MG, and DiPersio CM: Reduced fibulin-2 contributes to loss of basement membrane integrity and skin blistering in mice lacking integrin α3β1 in the epidermis. J Invest Dermatol 2014January3[Epub ahead of print]; DOI: 10.1038/jid.2014.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singh P, Reimer CL, Peters JH, Stepp MA, Hynes RO, and Van De Water L: The spatial and temporal expression patterns of integrin alpha9beta1 and one of its ligands, the EIIIA segment of fibronectin, in cutaneous wound healing. J Invest Dermatol 2004; 123:1176. [DOI] [PubMed] [Google Scholar]
- 93.Hoye AM, Couchman JR, Wewer UM, Fukami K, and Yoneda A: The newcomer in the integrin family: integrin alpha9 in biology and cancer. Adv Biol Regul 2012; 52:326. [DOI] [PubMed] [Google Scholar]
- 94.Shinde AV, Bystroff C, Wang C, Vogelezang MG, Vincent PA, Hynes RO, et al. : Identification of the peptide sequences within the EIIIA (EDA) segment of fibronectin that mediate integrin alpha9beta1-dependent cellular activities. J Biol Chem 2008; 283:2858. [DOI] [PubMed] [Google Scholar]
- 95.Chen J, Diacovo TG, Grenache DG, Santoro SA, and Zutter MM: The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol 2002; 161:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim JP, Zhang K, Chen JD, Wynn KC, Kramer RH, and Woodley DT: Mechanism of human keratinocyte migration on fibronectin: unique roles of RGD site and integrins. J Cell Physiol 1992; 151:443. [DOI] [PubMed] [Google Scholar]
- 97.Yang JT, Rayburn H, and Hynes RO: Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development 1993; 119:1093. [DOI] [PubMed] [Google Scholar]
- 98.Jones J, Watt FM, and Speight PM: Changes in the expression of alpha v integrins in oral squamous cell carcinomas. J Oral Pathol Med 1997; 26:63. [DOI] [PubMed] [Google Scholar]
- 99.Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, et al. : Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci 1995; 108:2241. [DOI] [PubMed] [Google Scholar]
- 100.Hamidi S, Salo T, Kainulainen T, Epstein J, Lerner K, and Larjava H: Expression of alpha(v)beta6 integrin in oral leukoplakia. Br J Cancer 2000; 82:1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thomas GJ, Lewis MP, Hart IR, Marshall JF, and Speight PM: AlphaVbeta6 integrin promotes invasion of squamous carcinoma cells through up-regulation of matrix metalloproteinase-9. Int J Cancer 2001; 92:641. [DOI] [PubMed] [Google Scholar]
- 102.Ramos DM, But M, Regezi J, Schmidt BL, Atakilit A, Dang D, et al. : Expression of integrin beta 6 enhances invasive behavior in oral squamous cell carcinoma. Matrix Biol 2002; 21:297. [DOI] [PubMed] [Google Scholar]
- 103.Ahmed N, Pansino F, Clyde R, Murthi P, Quinn MA, Rice GE, et al. : Overexpression of alpha(v)beta6 integrin in serous epithelial ovarian cancer regulates extracellular matrix degradation via the plasminogen activation cascade. Carcinogenesis 2002; 23:237. [DOI] [PubMed] [Google Scholar]
- 104.Sheppard D: Integrin-mediated activation of latent transforming growth factor beta. Cancer Metastasis Rev 2005; 24:395. [DOI] [PubMed] [Google Scholar]
- 105.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. : The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999; 96:319. [DOI] [PubMed] [Google Scholar]
- 106.Taipale J, Miyazono K, Heldin CH, and Keski-Oja J: Latent transforming growth factor-beta 1 associates to fibroblast extracellular matrix via latent TGF-beta binding protein. J Cell Biol 1994; 124:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Annes JP, Chen Y, Munger JS, and Rifkin DB: Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol 2004; 165:723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barrientos S, Stojadinovic O, Golinko MS, Brem H, and Tomic-Canic M: Growth factors and cytokines in wound healing. Wound Repair Regen 2008; 16:585. [DOI] [PubMed] [Google Scholar]
- 109.Chung J, Bachelder RE, Lipscomb EA, Shaw LM, and Mercurio AM: Integrin (alpha 6 beta 4) regulation of eIF-4E activity and VEGF translation: a survival mechanism for carcinoma cells. J Cell Biol 2002; 158:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao R, Liu XQ, Wu XP, Liu YF, Zhang ZY, Yang GY, et al. : Vascular endothelial growth factor (VEGF) enhances gastric carcinoma invasiveness via integrin alpha(v)beta6. Cancer Lett 2010; 287:150. [DOI] [PubMed] [Google Scholar]
- 111.Gailit J, Welch MP, and Clark RA: TGF-beta 1 stimulates expression of keratinocyte integrins during re-epithelialization of cutaneous wounds. J Invest Dermatol 1994; 103:221–7 [DOI] [PubMed] [Google Scholar]
- 112.Margadant C. and Sonnenberg A: Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep 2010; 11:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carter WG, Wayner EA, Bouchard TS, and Kaur P: The role of integrins α2β1 and α3β1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol 1990; 110:1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, and Parks WC: The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol 1997; 137:1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, et al. : Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol 1996; 133:921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.AlDahlawi S, Eslami A, Hakkinen L, and Larjava HS: The alphavbeta6 integrin plays a role in compromised epidermal wound healing. Wound Repair Regen 2006; 14:289. [DOI] [PubMed] [Google Scholar]
- 117.Hopkinson SB, Hamill KJ, Wu Y, Eisenberg JL, Hiroyasu S, and Jones JCR: Focal contact and hemidesmosomal proteins in keratinocyte migration and wound repair. Advances in Wound Care [Epub ahead of print]; DOI: 10.1089/wound.2013.0489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mascre G, Dekoninck S, Drogat B, Youssef KK, Brohee S, Sotiropoulou PA, et al. : Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 2012; 489:257. [DOI] [PubMed] [Google Scholar]
- 119.Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, et al. : Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 2005; 11:1351. [DOI] [PubMed] [Google Scholar]
- 120.Jones PH. and Watt FM: Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 1993; 73:713. [DOI] [PubMed] [Google Scholar]
- 121.Jones PH, Harper S, and Watt FM: Stem cell patterning and fate in human epidermis. Cell 1995; 80:83. [DOI] [PubMed] [Google Scholar]
- 122.Terunuma A, Kapoor V, Yee C, Telford WG, Udey MC, and Vogel JC: Stem cell activity of human side population and alpha6 integrin-bright keratinocytes defined by a quantitative in vivo assay. Stem Cells 2007; 25:664. [DOI] [PubMed] [Google Scholar]
- 123.Zhu AJ, Haase I, and Watt FM: Signaling via beta1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proc Natl Acad Sci USA 1999; 96:6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sachs N, Secades P, van Hulst L, Kreft M, Song JY, and Sonnenberg A: Loss of integrin alpha3 prevents skin tumor formation by promoting epidermal turnover and depletion of slow-cycling cells. Proc Natl Acad Sci USA 2012; 109:21468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Janes SM. and Watt FM: Switch from alphavbeta5 to alphavbeta6 integrin expression protects squamous cell carcinomas from anoikis. J Cell Biol 2004; 166:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aumailley M, Pesch M, Tunggal L, Gaill F, and Fassler R: Altered synthesis of laminin 1 and absence of basement membrane component deposition in (beta)1 integrin-deficient embryoid bodies. J Cell Sci 2000; 113:259. [DOI] [PubMed] [Google Scholar]
- 127.Henry MD. and Campbell KP: A role for dystroglycan in basement membrane assembly. Cell 1998; 95:859. [DOI] [PubMed] [Google Scholar]
- 128.Utani A, Nomizu M, and Yamada Y: Fibulin-2 binds to the short arms of laminin-5 and laminin-1 via conserved amino acid sequences. J Biol Chem 1997; 272:2814. [DOI] [PubMed] [Google Scholar]
- 129.Gagnoux-Palacios L, Allegra M, Spirito F, Pommeret O, Romero C, Ortonne JP, et al. : The short arm of the laminin gamma2 chain plays a pivotal role in the incorporation of laminin 5 into the extracellular matrix and in cell adhesion. J Cell Biol 2001; 153:835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Amano S, Scott IC, Takahara K, Koch M, Champliaud MF, Gerecke DR, et al. : Bone morphogenetic protein 1 is an extracellular processing enzyme of the laminin 5 gamma 2 chain. J Biol Chem 2000; 275:22728. [DOI] [PubMed] [Google Scholar]
- 131.Marinkovich MP, Lunstrum GP, and Burgeson RE: The anchoring filament protein kalinin is synthesized and secreted as a high molecular weight protein precursor. J Biol Chem 1992; 267:17900. [PubMed] [Google Scholar]
- 132.Sasaki T, Gohring W, Mann K, Brakebusch C, Yamada Y, Fassler R, et al. : Short arm region of laminin-5 gamma2 chain: structure, mechanism of processing and binding to heparin and proteins. J Mol Biol 2001; 314:751. [DOI] [PubMed] [Google Scholar]
- 133.Tsubota Y, Mizushima H, Hirosaki T, Higashi S, Yasumitsu H, and Miyazaki K: Isolation and activity of proteolytic fragment of laminin-5 alpha3 chain. Biochem Biophys Res Commun 2000; 278:614. [DOI] [PubMed] [Google Scholar]
- 134.Goldfinger LE, Hopkinson SB, deHart GW, Collawn S, Couchman JR, and Jones JCR: The α3 laminin subunit, α6β4 and α3β1 integrin coordinately regulate wound healing in cultured epithelial cells in the skin. J Cell Sci 1999; 112:2615. [DOI] [PubMed] [Google Scholar]
- 135.Ghahary A. and Ghaffari A: Role of keratinocyte-fibroblast cross-talk in development of hypertrophic scar. Wound Repair Regen 2007; 15 (Suppl 1):S46. [DOI] [PubMed] [Google Scholar]
- 136.Nowinski D, Lysheden AS, Gardner H, Rubin K, Gerdin B, and Ivarsson M: Analysis of gene expression in fibroblasts in response to keratinocyte-derived factors in vitro: potential implications for the wound healing process. J Invest Dermatol 2004; 122:216. [DOI] [PubMed] [Google Scholar]
- 137.Iyer V, Pumiglia K, and DiPersio CM: Alpha3beta1 integrin regulates MMP-9 mRNA stability in immortalized keratinocytes: a novel mechanism of integrin-mediated MMP gene expression. J Cell Sci 2005; 118:1185. [DOI] [PubMed] [Google Scholar]
- 138.Steffensen B, Hakkinen L, and Larjava H: Proteolytic events of wound-healing–coordinated interactions among matrix metalloproteinases (MMPs), integrins, and extracellular matrix molecules. Crit Rev Oral Biol Med 2001; 12:373. [DOI] [PubMed] [Google Scholar]
- 139.Agren MS. and Werthen M: The extracellular matrix in wound healing: a closer look at therapeutics for chronic wounds. Int J Low Extrem Wounds 2007; 6:82. [DOI] [PubMed] [Google Scholar]
- 140.Riikonen T, Westermarck J, Koivisto L, Broberg A, Kahari VM, and Heino J: Integrin alpha 2 beta 1 is a positive regulator of collagenase (MMP-1) and collagen alpha 1(I) gene expression. J Biol Chem 1995; 270:13548. [DOI] [PubMed] [Google Scholar]
- 141.Gardner H, Broberg A, Pozzi A, Laato M, and Heino J: Absence of integrin alpha1beta1 in the mouse causes loss of feedback regulation of collagen synthesis in normal and wounded dermis. J Cell Sci 1999; 112:263. [DOI] [PubMed] [Google Scholar]
- 142.Wu C, Keivens VM, O'Toole TE, McDonald JA, and Ginsberg MH: Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell 1995; 83:715. [DOI] [PubMed] [Google Scholar]
- 143.Wu C, Keightley SY, Leung-Hagesteijn C, Radeva G, Coppolino M, Goicoechea S, et al. : Integrin-linked protein kinase regulates fibronectin matrix assembly, E-cadherin expression, and tumorigenicity. J Biol Chem 1998; 273:528. [DOI] [PubMed] [Google Scholar]
- 144.Gill SE. and Parks WC: Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol 2008; 40:1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, and Lehnert H: Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia 2002; 45:1011. [DOI] [PubMed] [Google Scholar]
- 146.Page-McCaw A, Ewald AJ, and Werb Z: Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 2007; 8:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Huhtala P, Humphries MJ, McCarthy JB, Tremble PM, Werb Z, and Damsky CH: Cooperative signaling by alpha 5 beta 1 and alpha 4 beta 1 integrins regulates metalloproteinase gene expression in fibroblasts adhering to fibronectin. J Cell Biol 1995; 129:867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Singer AJ. and Clark RA: Cutaneous wound healing. N Engl J Med 1999; 341:738. [DOI] [PubMed] [Google Scholar]
- 149.Yates CC, Krishna P, Whaley D, Bodnar R, Turner T, and Wells A: Lack of CXC chemokine receptor 3 signaling leads to hypertrophic and hypercellular scarring. Am J Pathol 2010; 176:1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ghosh S, Johnson JJ, Sen R, Mukhopadhyay S, Liu Y, Zhang F, et al. : Functional relevance of urinary-type plasminogen activator receptor-alpha3beta1 integrin association in proteinase regulatory pathways. J Biol Chem 2006; 281:13021. [DOI] [PubMed] [Google Scholar]
- 151.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. : Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2000; 2:737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McCawley LJ. and Matrisian LM: Matrix metalloproteinases: they're not just for matrix anymore! Curr Opin Cell Biol 2001; 13:534. [DOI] [PubMed] [Google Scholar]
- 153.Schenk S. and Quaranta V: Tales from the crypt[ic] sites of the extracellular matrix. Trends Cell Biol 2003; 13:366. [DOI] [PubMed] [Google Scholar]
- 154.Shirakawa M. and Isseroff RR: Topical negative pressure devices: use for enhancement of healing chronic wounds. Arch Dermatol 2005; 141:1449. [DOI] [PubMed] [Google Scholar]
- 155.Lancerotto L, Bayer LR, and Orgill DP: Mechanisms of action of microdeformational wound therapy. Semin Cell Dev Biol 2012; 23:987. [DOI] [PubMed] [Google Scholar]
- 156.Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, et al. : Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 1999; 7:442. [DOI] [PubMed] [Google Scholar]
- 157.Hermes O, Schlage P, and auf dem Keller U: Wound degradomics: current status and future perspectives. Biol Chem 2011; 392:949. [DOI] [PubMed] [Google Scholar]
- 158.Lundqvist K. and Schmidtchen A: Immunohistochemical studies on proteoglycan expression in normal skin and chronic ulcers. Br J Dermatol 2001; 144:254. [DOI] [PubMed] [Google Scholar]
- 159.Herrick SE, Sloan P, McGurk M, Freak L, McCollum CN, and Ferguson MW: Sequential changes in histologic pattern and extracellular matrix deposition during the healing of chronic venous ulcers. Am J Pathol 1992; 141:1085. [PMC free article] [PubMed] [Google Scholar]
- 160.Herrick SE, Ireland GW, Simon D, McCollum CN, and Ferguson MW: Venous ulcer fibroblasts compared with normal fibroblasts show differences in collagen but not fibronectin production under both normal and hypoxic conditions. J Invest Dermatol 1996; 106:187. [DOI] [PubMed] [Google Scholar]
- 161.Kapila YL, Kapila S, and Johnson PW: Fibronectin and fibronectin fragments modulate the expression of proteinases and proteinase inhibitors in human periodontal ligament cells. Matrix Biol 1996; 15:251. [DOI] [PubMed] [Google Scholar]
- 162.Grinnell F, Ho CH, and Wysocki A: Degradation of fibronectin and vitronectin in chronic wound fluid: analysis by cell blotting, immunoblotting, and cell adhesion assays. J Invest Dermatol 1992; 98:410. [DOI] [PubMed] [Google Scholar]
- 163.Wysocki AB. and Grinnell F: Fibronectin profiles in normal and chronic wound fluid. Lab Invest 1990; 63:825. [PubMed] [Google Scholar]
- 164.Stanley CM, Wang Y, Pal S, Klebe RJ, Harkless LB, Xu X, et al. : Fibronectin fragmentation is a feature of periodontal disease sites and diabetic foot and leg wounds and modifies cell behavior. J Periodontol 2008; 79:861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nabors LB, Mikkelsen T, Rosenfeld SS, Hochberg F, Akella NS, Fisher JD, et al. : Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol 2007; 25:1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Subbaram S. and Dipersio CM: Integrin alpha3beta1 as a breast cancer target. Expert Opin Ther Targets 2011; 15:1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hakkinen L, Koivisto L, Gardner H, Saarialho-Kere U, Carroll JM, Lakso M, et al. : Increased expression of beta6-integrin in skin leads to spontaneous development of chronic wounds. Am J Pathol 2004; 164:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jacobsen JN, Steffensen B, Hakkinen L, Krogfelt KA, and Larjava HS: Skin wound healing in diabetic beta6 integrin-deficient mice. APMIS 2010; 118:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bhattacharyya J, Mondal G, Madhusudana K, Agawane SB, Ramakrishna S, Gangireddy SR, et al. : Single subcutaneous administration of RGDK-lipopeptide:rhPDGF-B gene complex heals wounds in streptozotocin-induced diabetic rats. Mol Pharm 2009; 6:918. [DOI] [PubMed] [Google Scholar]